扩展功能
文章信息
- 石静艺, 康晓静
- SHI Jingyi, KANG Xiaojing
- 人类疱疹病毒8型的免疫逃逸分子机制
- The molecular mechanism of immune evasion of human herpesvirus 8
- 微生物学通报, 2025, 52(5): 1929-1938
- Microbiology China, 2025, 52(5): 1929-1938
- CSTR: 32113.14.j.MC.240664
- DOI: 10.13344/j.microbiol.china.240664
-
文章历史
- 收稿日期: 2024-08-08
- 接受日期: 2024-11-11
- 网络首发日期: 2024-12-25
2. 新疆维吾尔自治区人民医院皮肤性病科 新疆皮肤性病临床医学研究中心 新疆皮肤病研究重点实验室, 新疆 乌鲁木齐 830001
2. Department of Dermatology and Venereology, People's Hospital of Xinjiang Uygur Autonomous Region, Xinjiang Clinical Research Center for Dermatology and Venereology, Xinjiang Key Laboratory of Dermatological Research, Urumqi 830001, Xinjiang, China
人类疱疹病毒8型(human herpesvirus 8, HHV-8)是一种基因组长约210 kb的DNA病毒,由140.5 kb的长特异编码区和801 bp的GC末端重复序列组成。在长特异编码区内存在超过81个开放阅读框(open reading frame, ORF),其中15个是卡波西肉瘤相关疱疹病毒(Kaposi’s sarcoma-associated herpesvirus, KSHV)特有的ORF。不同地区和人群中KSHV的感染率差异显著,在中国新疆地区HHV-8的感染率约为19.2%,明显高于其他地区,具有显著的民族和性别分布差异,并且人类免疫缺陷病毒(human immunodeficiency virus, HIV)感染人群HHV-8的感染率高达43.2%[1]。HHV-8感染与多种高度危害性的疾病密切相关,如卡波西肉瘤(Kaposi’s sarcoma, KS)、多中心性卡斯特莱曼病(multicenter Castleman’s disease, MCD)、原发性渗出性淋巴瘤(primary effusion lymphoma, PEL),以及最近报道的KSHV炎性细胞因子综合征(KSHV inflammatory cytokine syndrome, KICS),并且常与KS和PEL同时发生,可出现急性呼吸窘迫综合征等,死亡率高达60%[2]。其中,KS是最常见的HHV-8感染相关的恶性肿瘤,其患病率存在显著的地域和人群差异,在撒哈拉以南非洲高达30%–80%,地中海地区则为10%–20%,在中国超过90%的KS发生在新疆地区[1-3]。KS主要表现为皮肤、黏膜和内脏器官上的紫红色斑块或结节,具有侵袭性和高复发率[3]。MCD多发生于HIV感染者,病情进展迅速且复发风险高,严重病例可演变为B细胞增生性疾病,如弥漫性大B细胞淋巴瘤[4]。PEL主要发生在免疫功能低下的患者中,常出现体腔积液(如胸腔、腹腔),预后差[3]。鉴于目前的治疗手段有限,并且HHV-8在全球范围内带来了严峻的公共卫生挑战,深入研究HHV-8的致病机制以及免疫逃逸策略具有重要意义。
HHV-8的生命周期分为潜伏期和裂解期2个阶段[5]。在潜伏期,病毒基因组以质粒的形式游离于宿主细胞核内,通过潜伏期相关核抗原(latent associated nuclear antigen, LANA)与HHV-8基因组的保守末端重复序列结合形成LANA DNA复制元件,将HHV-8基因组整合至宿主细胞染色体上与其同步复制,逃避免疫系统的识别和清除。在裂解期,大量病毒基因表达启动病毒裂解复制,HHV-8通过分泌病毒趋化因子(如vCCL-1、vCCL-2)招募宿主的免疫细胞至感染部位,形成典型的免疫浸润现象。这种病毒和宿主的相互作用在某种程度上既促进了肿瘤细胞的增殖,又帮助病毒逃避宿主的免疫系统清除[6]。在HHV-8与人类宿主细胞长期共同进化的过程中,HHV-8进化出多种免疫逃逸策略以干扰宿主细胞的免疫应答效应。研究表明,HHV-8病毒主要通过干扰机体的固有免疫途径(图 1),调控适应性免疫信号通路,编码同源细胞因子,阻断宿主补体系统的激活,并表达病毒微小核糖核酸(microRNA, miRNA),从而破坏机体的免疫应答,致使HHV-8在宿主体内建立终身潜伏感染状态,并在特定条件下诱发恶性肿瘤[7]。本综述旨在探讨HHV-8的免疫逃逸机制,以期为HHV-8相关恶性肿瘤的免疫治疗及生物标志物提供思路。
|
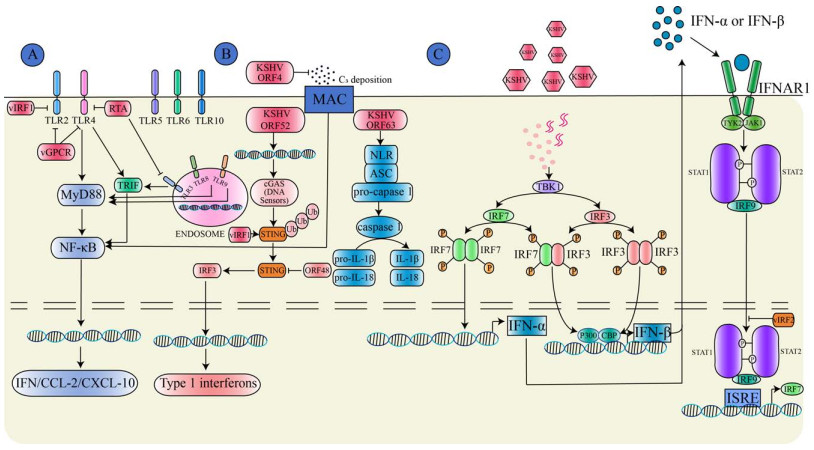
| 图 1 HHV-8病毒通过调控宿主固有免疫信号通路介导免疫逃逸机制示意图(由Adobe Illustrator软件绘制) Figure 1 HHV-8 mediates immune evasion mechanisms by modulating the host's innate immune signaling pathways (By Adobe Illustrator). A: HHV-8 evades immunity by inhibiting pattern recognition receptors (PRRs); B: HHV-8 evades immunity by modulating the complement system; C: HHV-8 evades immunity by promoting cytokine secretion. vIRFs: Viral interferon regulatory factors; RTA: Replication and transcription activator; TRIF: TIR domain-containing adaptor inducing interferon-β; vGPCR: Virus G-protein-coupled receptor; TLRs: Toll-like receptors; MyD88: Myeloid differentiation primary response protein 88; NF-κB: Nuclear factor kappa-B; IFN: Interferon; CCLs: C-C motif chemokine ligands; ORF: Open reading frame; MAC: Membrane attack complex; cGAS: Cyclic guanylate adenylate synthase; Ub: Ubiquitin; STING: stimulator of interferon genes; IRF: Interferon regulatory factor; NLR: Nucleotide-binding oligomerization domain-like receptor; ASC: Apoptosis-associated speck-like protein containing a CARD; TBK1: TANK-binding kinase 1; CBP: CREB-binding protein; IFNAR1: interferon receptor 1; TYK2: Tyrosine kinase 2; JAK1: Janus kinase 1; STATs: Signal transducer and activator of transcription; ISRE: Interferon-stimulated response element. A:HHV-8通过抑制模式识别受体发挥免疫逃逸;B:HHV-8通过调节补体系统发挥免疫逃逸;C:HHV-8通过促进细胞因子分泌发挥免疫逃逸。vIRFs:病毒干扰素调节因子;RTA:复制转录激活因子;TRIF:β干扰素TIR结构域衔接蛋白;vGPCR:病毒G蛋白偶联受体;TLRs:Toll样受体;MyD88:髓样分化因子88;NF-κB:核因子κB;IFN:干扰素;CCLs:C-C基序趋化因子配体;ORF:开放阅读框, MAC:膜攻击复合物;cGAS:环鸟苷酸-腺苷酸合酶;Ub:泛素;STING:干扰素基因刺激因子;IRF:干扰素调节因子;NLR:核苷酸结合寡聚化结构域样受体;ASC:凋亡相关颗粒样蛋白;TBK1:TANK结合激酶1;CBP:CREB结合蛋白;IFNAR1:干扰素受体1;TYK2:酪氨酸激酶2;JAK1:Janus激酶1;STATs:信号转导及转录激活蛋白;ISRE:干扰素刺激的反应元件。 |
|
|
固有免疫反应是机体抵御病毒感染的重要防线,当机体受到病原体刺激时首先通过编码模式识别受体(pattern recognition receptor, PRR)识别病原微生物表面的病原相关分子模式(pathogen-associated molecular pattern, PAMP)或损伤相关分子模式(damage-associated molecular pattern, DAMP)激活固有免疫信号通路[8]。目前发现6类PRR,包括Toll样受体(Toll-like receptor, TLR)、视黄酸诱导基因-1样受体(retinoic acid-inducible gene-1-like receptor, RLR)、黑色素瘤缺乏因子2样受体(absent in melanoma 2-like receptor, ALR)、核苷酸结合寡聚化结构域样受体(nucleotide-binding oligomerization domain-like receptor, NLR)、C型凝集素样受体(C-type lectin receptor, CLR)和环鸟苷酸-腺苷酸合酶/干扰素基因刺激因子(cyclic guanylate adenylate synthase/stimulator of interferon genes, cGAS/ STING)通路[9]。定位于细胞膜的TLR (包括TLR1/2/4/5/6/10)通常最先识别病毒糖蛋白,而位于细胞内囊泡上的TLR (包括TLR3/7/8/9)通常识别病毒DNA/RNA[10]。HHV-8感染单核细胞后引起TLR3蛋白表达上调,但HHV-8编码的病毒干扰素调节因子(viral interferon regulatory factor, vIRF)阻断了TLR3信号传导,导致干扰素-β (interferon-β, IFN-β)、C-C基序趋化因子配体2 (C-C motif chemokine ligand 2, CCL2)及C-X-C趋化因子配体10 (C-X-C motif chemokine ligand 10, CXCL10)分泌减少[11]。复制转录激活因子(replication and transcription activator, RTA)是HHV-8编码的一种关键裂解蛋白,研究发现RTA能够促进TLR3 β干扰素TIR结构域衔接蛋白(TIR domain-containing adaptor inducing interferon-β, TRIF)的蛋白酶体水解,从而阻断下游信号传导[12]。此外,RTA还通过促进编码配体蛋白的骨髓分化蛋白的mRNA水解来阻断TLR4信号传导[13]。HHV-8感染内皮细胞时通过编码病毒G蛋白偶联受体(virus G-protein- coupled receptor, vGPCR)和vIRF1抑制TLR4信号激活[14]。因此,HHV-8与TLR之间的相互作用对病毒免疫逃逸至关重要。
HHV-8还通过抑制其他PRR发挥免疫逃逸作用。NLR是胞质内重要的模式识别受体,活化的NLR会形成炎性复合体,并招募凋亡相关的Caspases,从而促进炎性细胞因子IL-1β和IL-18等分泌;KSHV ORF63与NLR家族结合而干扰炎性复合体形成及炎性细胞因子分泌[15]。RLR能够激活并上调Ⅰ型IFN水平并监测病毒DNA[16]。KSHV ORF37通过抑制ALR的激活,减少促炎细胞因子IL-1β的产生,从而逃避免疫监察[17]。此外,HHV-8还通过编码特定蛋白抑制宿主的cGAS/STING通路逃避免疫监察,促进其在宿主体内的长期潜伏感染状态;HHV-8分泌的关键蛋白ORF52通过抑制cGAS酶活性,抑制其与病毒DNA的识别,降低了cGAMP的生成,从而导致I型IFN合成受阻[18]。HHV-8分泌的vIRF1能够直接与STING结合阻断TANK结合激酶1 (TANK-binding kinase 1, TBK1)诱导的STING磷酸化,从而抑制IFN的信号传导[19]。而HHV-8分泌的ORF48通过抑制STING的活性降低I型IFN的激活[20]。总之,HHV-8通过抑制PRR与PAMP的相互作用,从而阻断PRR介导的信号转导,进而免疫逃逸,因此细胞免疫相关通路是预防和治疗HHV-8感染的潜在靶点。
1.2 HHV-8通过调节补体系统而免疫逃逸补体系统是抵御病原体入侵的第一道防线,由经典途径、替代途径和凝集素途径诱导补体C3激活形成膜攻击复合物(membrane attack complex, MAC)发挥免疫效应。人类疱疹病毒的ORF能够编码与机体补体激活调节因子(regulator of complement activation, RCA)结构相似的蛋白质。HHV-8编码的KSHV补体控制蛋白(KSHV complement control protein, KCP)又称KSHV ORF4,与RCA同源性高达44%−55%;KSHV ORF4全长约1 650 bp,编码550 aa的膜蛋白,通过可变剪接进一步表达膜结合蛋白和分泌蛋白[21]。在KSHV ORF4的N末端有4个补体控制蛋白结构域,在C末端存在跨膜结构域可将其附着到细胞或病毒表面。根据可变剪接方式不同,KSHV ORF4具有3种异构体,主要表达在裂解复制期的PEL细胞中[22]。有学者利用真核表达载体构建细胞模型表达KSHV ORF4后明显抑制C3沉积和补体介导的溶血反应,同时观察到KSHV ORF4能够抑制经典途径和替代途径介导的红细胞溶解反应,但替代途径的抑制程度远低于经典途径[23]。其作用机制可能是KSHV ORF4抑制经典途径中C3转化酶的活性及充当因子I介导的C3b和C4b裂解的辅助因子作用[24]。研究还发现ORF4不仅表达在HHV-8感染的宿主细胞中,而且还定位至HHV-8病毒表面,参与MAC引起的损伤防御机制[25]。此外,有学者从HHV-8感染的内皮细胞中分离出细胞囊泡,对其进行蛋白质组学测序分析,发现补体系统被激活,同时发现细胞囊泡利用内源性补体C3蛋白成为替代途径的有效激活剂,并激活NF-κB信号通路而促进HHV-8潜伏感染[26]。随后,有研究者将HHV-8合成的病毒囊泡免疫小鼠,能够激活特异性T细胞反应和补体系统发挥抗病毒免疫反应,同时在部分HHV-8感染人群的血清中检测到补体系统被激活,因此诱导抗体激活效应的疫苗接种可能增强HHV-8感染诱导的体液免疫[27]。总之,KSHV ORF4通过多种方式协助HHV-8逃避补体系统的免疫攻击,KSHV ORF4是预防和治疗HHV-8感染的潜在靶点。
1.3 HHV-8通过促进细胞因子分泌而免疫逃逸病毒感染机体后所促进的细胞因子分泌、激活免疫细胞功能和调节其细胞内定位,在介导免疫反应、炎症反应和血管生成中发挥关键作用[28]。研究发现,HHV-8感染单核细胞后促进IL-1α、IL-1β和IL-6等炎性细胞因子分泌[29]。在卡波西肉瘤患者血清中,IL-6和TNF-α水平显著升高,在MCD和KICS患者血清中IL-6和IL-10水平也显著升高,这提示HHV-8感染会导致机体出现慢性炎症反应[30]。HHV-8能够编码病毒IL-6 (virus IL-6, vIL-6),IL-6需要与IL-6R和gp130结合传递炎症信号,而vIL-6直接与gp130结合激活Janus激酶-STAT信号通路促进细胞增殖、血管生成和炎症反应等,而vIL-6能直接刺激IL-6分泌,从而促进卡波西肉瘤和MCD的疾病进展[31-32]。此外,病毒FLICE抑制蛋白(virus FLICE-Like inhibitory protein, vFLIP)和vGPCR激活NF-κB信号通路,促进IL-6和IL-8等炎性细胞因子分泌[33]。HHV-8编码潜伏相关核抗原(latency-associated nuclear antigen, LANA)蛋白激活IL-4/13-STAT6信号通路,诱导STAT6磷酸化后发生核转位,从而促进RTA的转录抑制因子分泌,抑制病毒潜伏期再激活从而发挥免疫逃逸作用[33]。因此,靶向细胞因子在治疗HHV-8感染性疾病中具有潜在的临床应用价值。此外对于临床表现相似、难以通过组织学鉴别的疾病可通过细胞因子作为疾病的生物标志物。目前抗IL-6抗体已被美国食品药品监督管理局(Food and Drug Administration, FDA)和欧洲药品管理局(European Medicines Agency, EMA)批准用于MCD的一线治疗[34]。
干扰素是一种分泌型细胞因子,当宿主细胞识别病原微生物入侵时会分泌干扰素调节机体免疫反应。干扰素有Ⅰ型、Ⅱ型和Ⅲ型。Ⅱ型干扰素仅由INF-γ组成,主要在免疫调节中发挥作用。Ⅰ型干扰素(INF-α/β)和Ⅲ型干扰素(INF-λ)能够与受体结合激活抗病毒免疫反应,增加主要组织相容性复合体-I (major histocompatibility complex-1, MHC-I)的表达,抑制病毒复制、促进细胞凋亡[35]。当病毒感染后,干扰素调节因子(interferon-regulatory factor, IRF)家族中的IRF-3和IRF-7是IFN-α/β基因表达的关键调节因子,IRF-3的C末端发生丝氨酸磷酸化后与组蛋白转乙酰化酶CBP/p300结合诱导IFN-β的表达,而IRF-7则通过负反馈方式激活IFN-α/β基因表达[36]。vIRF2通过与p300结合抑制由IRF1、IRF3和INF刺激基因因子3 (IFN-stimulated gene factor 3, ISGF3)介导的IFN诱导基因表达[37]。此外,研究显示HHV-8编码的vIRF1通过阻断沉默信息调节因子6 (silent information regulator 6, SIRT6)与泛素特异性蛋白酶10的相互作用,促进其赖氨酸残基发生丙酰化修饰,通过泛素-蛋白酶体降解途径显著抑制IFN-β和抗病毒免疫信号传导[38]。因此,靶向干扰素调节因子是治疗HHV-8相关疾病的潜在治疗靶点,目前重组IFN-α2a和IFN-α2b干扰素已用于治疗经典型卡波西肉瘤和艾滋病型卡波西肉瘤,具有良好的有效性和安全性[39]。
2 HHV-8调节适应性免疫反应MHC分子是适应性免疫的重要组成部分,主要通过抗原递呈中发挥重要作用。研究发现,HHV-8不仅通过表达E3泛素连接酶K3和K5抑制MHC-Ⅰ分子向CD8+ T细胞进行抗原递呈从而降低细胞毒性T淋巴细胞(cytotoxic T lymphocytes, CTL)的杀伤作用[40]。HHV-8还通过下调干扰素诱导的内皮细胞表面MHC-Ⅱ分子表达,从而抑制CD4+ T细胞激活实现免疫逃逸[41]。此外,HHV-8可通过下调宿主细胞表面MHC-Ⅰ分子,抑制CTL依赖穿孔素-颗粒酶途径的靶向杀伤作用,同时,CTL的免疫压力可能驱动HHV-8 ORFK1基因发生适应性突变,增强病毒免疫逃逸能力[42]。HHV-8感染原代成纤维细胞后通过下调MHC-Ⅰ、细胞间黏附分子-1 (intercellular cell adhesion molecule-1, ICAM-1)和人类白细胞抗原-E (human leukocyte antigen-E, HLA-E)抑制自然杀伤细胞的激活和杀伤作用[43]。HHV-8还能够下调属兔细胞特异性细胞间黏附分子3结合非整合素(dendritic cell-specific intercellular adhesion molecule-3 grabbing non-integrin, DC-SIGN),降低DC细胞的吞噬活性并抑制CD8+ T细胞的抗原特异性激活而免疫逃逸[44]。程序性细胞死亡配体1 (programmed cell death ligand 1, PD-L1)是一种由抗原呈递细胞表达的细胞表面糖蛋白,在调节T细胞和B细胞介导的免疫反应中发挥重要作用。研究发现,在HHV-8可通过激活丝裂原活化蛋白激酶(mitogen-activated protein kinases, MAPK)和磷脂酰肌醇3-激酶/蛋白激酶B (phosphatidylinositol 3-kinase/protein kinase B, PI3K/PKB)信号通路,诱导免疫细胞表面免疫检查点分子PD-L1和CTLA-4表达升高,进一步抑制T细胞的抗病毒活性及B细胞抗体合成能力,最终削弱宿主免疫系统对HHV-8的长期免疫监察功能[45]。此外,在艾滋病型卡波西肉瘤患者组织中PD-1/PD-L1/PD-L2表达升高[46]。目前开展的一项有关PD-1抑制剂(帕博利珠单抗)治疗卡波西肉瘤的多中心二期临床研究表明,帕博利珠单抗在治疗经典型和地方型卡波西肉瘤患者中具有良好的安全性和有效性[34]。
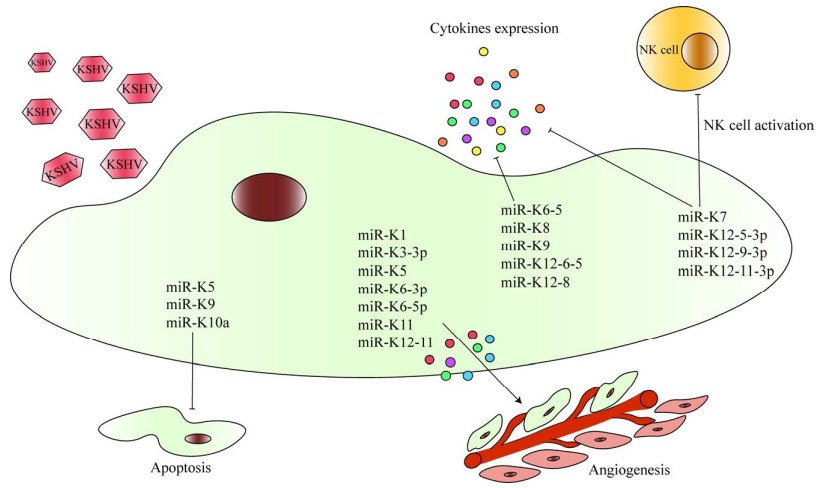
3 HHV-8调控病毒miRNA表达miRNA是一类长约19–22个核苷酸的非编码单链RNA,通过与目标基因的3ʹ非翻译区(3ʹ-untranslated region, 3ʹ-UTR)结合而抑制目标基因表达。HHV-8编码miRNA通过感染宿主细胞、抑制免疫系统和细胞凋亡等导致HHV-8相关疾病的发生发展(图 2)。对HHV-8感染细胞进行单细胞测序发现其可表达2 200个miRNA,其中与病毒免疫逃逸有关的KSHV miR-K12-9-3p、miR-K12-5-3p、miR-K12-11-3p显著升高。此外,miR-K12-11通过靶向抑制抗血管生成因子血小板反应蛋白-1 (thrombospondin-1, THBS-1)的表达,促进血管新生并重塑肿瘤微环境,为肿瘤发展提供有利的微环境[47]。研究发现KSHV miRNA-K6-5、miRNA-K8、miRNA-K9、miRNA-K12-6-5和miRNA-K12-8能够靶向抑制STAT3和STAT5磷酸化下调IFN和IL-6介导的抗病毒免疫反应[48]。KSHV miRNA-K7能够直接与活化的自然杀伤细胞(natural killer cell, NK cell)配体主要组织相容性复合体I类链相关基因B (main histocompatibility complex type I chain related gene B, MICB)的3ʹUTR结合逃避NK细胞的免疫攻击从而维持潜伏期感染[49]。此外,HHV-8 miRNA还能通过影响细胞因子分泌发挥免疫逃逸。IL-1R相关激酶和髓样分化因子88 (myeloid differentiation primary response protein 88, MyD88)参与TLR/IL-1R信号级联反应,是miR-K9和miR-K5的靶标;当人脐静脉内皮细胞(human umbilical vein endothelial cell, HUVEC)转染miR-K5或miR-K9后,细胞凋亡显著被抑制,同时IL-6和IL-8的分泌水平显著下降[50]。miR-K10a能够通过抑制TNF样细胞凋亡微弱抑制剂受体,抑制HUVEC分泌IL-8和单核细胞趋化因子-1从而抑制免疫反应[51]。此外,研究发现KSHV miR-K1、miR-K3-3p、miR-K5、MiR-K6-3p、MiR-K6-5p和miR-K11等具有促进血管生成和增殖的作用[52-53]。最近研究发现,HHV-8感染宿主细胞后,将其外泌体中携带的HHV-8 miRNA释放至感染部位附近,通过影响肿瘤微环境的糖代谢过程促进宿主细胞生长并逃避免疫系统攻击[54]。总之,HHV-8 miRNA以多个关键基因及其信号通路为靶标抑制宿主免疫系统从而免疫逃逸。HHV-8 miRNA可能成为HHV-8感染相关疾病的诊断、疾病监测和预后标志物。此外,针对HHV-8 miRNA的治疗方法,如miRNA抑制剂,可能为HHV-8相关肿瘤提供新的靶向治疗策略。
|
| 图 2 HHV-8病毒通过调控病毒miRNA表达介导免疫逃逸机制示意图 Figure 2 HHV-8 mediates immune evasion mechanisms by regulating the expression of viral miRNA. NK cell: Natural killer cell; miRs: microRNAs. NK cell:自然杀伤细胞;miRs:微小核糖核酸。 |
|
|
病毒免疫逃逸策略在病毒诱导的恶性肿瘤的发生发展过程中发挥重要作用,本文概述了HHV-8通过干扰宿主细胞固有免疫反应、适应性免疫反应、阻断宿主补体系统激活以及编码同源细胞因子分泌和miRNA表达等导致HHV-8感染相关疾病发生发展的机制。目前,靶向免疫逃逸通路的抗病毒策略在HHV-8相关疾病中进入临床试验阶段,如抗PD-1抑制剂、抗CD20抗体、抗IL-6抗体及重组干扰素等。此外,随着基因编辑技术如CRISPR-Cas9的迅猛发展,未来可能通过精准调控HHV-8的相关基因或宿主免疫相关基因实现对HHV-8感染的有效控制。
作者贡献声明
石静艺:方案设计与初稿写作;康晓静:审查和修改写作。
作者利益冲突公开声明
作者声明没有任何可能会影响本文所报告工作的已知经济利益或个人关系。
| [1] |
ZHANG X, FANG QW, ZHU SB, WU XF, YUAN HB, LIU ZQ, XU YY, CHEN T, ZENG Y, ZHANG TJ. Environmental risk factors and genetic markers of Kaposi's sarcoma-associated herpesvirus infection among Uygur population in Xinjiang, China[J]. Journal of Medical Virology, 2022, 94(6): 2755-2765. DOI:10.1002/jmv.27600 |
| [2] |
PRIETO-BARRIOS M, ARAGÓN-MIGUEL R, TARRAGÓ-ASENSIO D, LALUEZA A, ZARCO-OLIVO C. Human herpesvirus 8-associated inflammatory cytokine syndrome[J]. JAMA Dermatology, 2018, 154(2): 228-230. DOI:10.1001/jamadermatol.2017.5461 |
| [3] |
CESARMAN E, CHADBURN A, RUBINSTEIN PG. KSHV/HHV8-mediated hematologic diseases[J]. Blood, 2022, 139(7): 1013-1025. DOI:10.1182/blood.2020005470 |
| [4] |
RAMASWAMI R, LURAIN K, POLIZZOTTO MN, EKWEDE I, WALDON K, STEINBERG SM, MANGUSAN R, WIDELL A, RUPERT A, GEORGE J, GONÇALVES PH, MARSHALL VA, WHITBY D, WANG HW, PITTALUGA S, JAFFE ES, LITTLE RF, ULDRICK TS, YARCHOAN R. Characteristics and outcomes of KSHV-associated multicentric Castleman disease with or without other KSHV diseases[J]. Blood Advances, 2021, 5(6): 1660-1670. DOI:10.1182/bloodadvances.2020004058 |
| [5] |
BROUSSARD G, DAMANIA B. Regulation of KSHV latency and lytic reactivation[J]. Viruses, 2020, 12(9): 1034. DOI:10.3390/v12091034 |
| [6] |
CHOI YB, COUSINS E, NICHOLAS J. Novel functions and virus-host interactions implicated in pathogenesis and replication of human herpesvirus 8[J]. Recent Results in Cancer Research, 2021, 217: 245-301. |
| [7] |
BROUSSARD G, DAMANIA B. KSHV: immune modulation and immunotherapy[J]. Frontiers in Immunology, 2020, 10: 3084. DOI:10.3389/fimmu.2019.03084 |
| [8] |
AHMAD MALIK J, KAUR G, AGREWALA JN. Revolutionizing medicine with toll-like receptors: a path to strengthening cellular immunity[J]. International Journal of Biological Macromolecules, 2023, 253: 127252. DOI:10.1016/j.ijbiomac.2023.127252 |
| [9] |
TSUKIDATE T, HESPEN CW, HANG HC. Small molecule modulators of immune pattern recognition receptors[J]. RSC Chemical Biology, 2023, 4(12): 1014-1036. DOI:10.1039/D3CB00096F |
| [10] |
JAFARZADEH A, NEMATI M, SALARKIA E, YADAV S, AMINIZADEH N, JAFARZADEH S, YADAV M. Inflammatory responses during trichomoniasis: The role of Toll-like receptors and inflammasomes[J]. Parasite Immunology, 2023, 45(8): e13000. DOI:10.1111/pim.13000 |
| [11] |
JACOBS SR, GREGORY SM, WEST JA, WOLLISH AC, BENNETT CL, BLACKBOURN DJ, HEISE MT, DAMANIA B. The viral interferon regulatory factors of Kaposi's sarcoma-associated herpesvirus differ in their inhibition of interferon activation mediated by toll-like receptor 3[J]. Journal of Virology, 2013, 87(2): 798-806. DOI:10.1128/JVI.01851-12 |
| [12] |
JHA HC, BANERJEE S, ROBERTSON ES. The role of gammaherpesviruses in cancer pathogenesis[J]. Pathogens, 2016, 5(1): 18. DOI:10.3390/pathogens5010018 |
| [13] |
LINGEL A, EHLERS E, WANG QL, CAO MX, WOOD C, LIN RT, ZHANG LW. Kaposi's sarcoma-associated herpesvirus reduces cellular myeloid differentiation primary-response gene 88 (MyD88) expression via modulation of its RNA[J]. Journal of Virology, 2015, 90(1): 180-188. |
| [14] |
LAGOS D, VART RJ, GRATRIX F, WESTROP SJ, EMUSS V, WONG PP, ROBEY R, IMAMI N, BOWER M, GOTCH F, BOSHOFF C. Toll-like receptor 4 mediates innate immunity to kaposi sarcoma herpesvirus[J]. Cell Host & Microbe, 2008, 4(5): 470-483. |
| [15] |
GREGORY SM, DAVIS BK, WEST JA, TAXMAN DJ, MATSUZAWA SI, REED JC, TING JPY, DAMANIA B. Discovery of a viral NLR homolog that inhibits the inflammasome[J]. Science, 2011, 331(6015): 330-334. DOI:10.1126/science.1199478 |
| [16] |
ZHANG HR, NI GX, DAMANIA B. ADAR1 facilitates KSHV lytic reactivation by modulating the RLR-dependent signaling pathway[J]. Cell Reports, 2020, 31(4): 107564. DOI:10.1016/j.celrep.2020.107564 |
| [17] |
ZHANG XL, LAN QP, ZHANG MY, WANG F, SHI KY, LI XJ, KUANG ES. Inhibition of AIM2 inflammasome activation by SOX/ORF37 promotes lytic replication of Kaposi's sarcoma-associated herpesvirus[J]. Proceedings of the National Academy of Sciences of the United States of America, 2023, 120(27): e2300204120. |
| [18] |
NASH A, RYAN EJ. The oncogenic gamma herpesviruses Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV) hijack retinoic acid-inducible gene I (RIG-I) facilitating both viral and tumour immune evasion[J]. Tumour Virus Research, 2022, 14: 200246. DOI:10.1016/j.tvr.2022.200246 |
| [19] |
SRIKANTH S, WOO JS, WU BB, EL-SHERBINY YM, LEUNG J, CHUPRADIT K, RICE L, SEO GJ, CALMETTES G, RAMAKRISHNA C, CANTIN E, AN DS, SUN R, WU TT, JUNG JU, SAVIC S, GWACK Y. The Ca2+ sensor STIM1 regulates the type I interferon response by retaining the signaling adaptor STING at the endoplasmic reticulum[J]. Nature Immunology, 2019, 20(2): 152-162. DOI:10.1038/s41590-018-0287-8 |
| [20] |
VERONESE BHS, NGUYEN A, PATEL K, PAULSEN K, MA Z. ORF48 is required for optimal lytic replication of Kaposi's sarcoma-associated herpesvirus[J]. PLoS Pathogens, 2024, 20(8): e1012081. DOI:10.1371/journal.ppat.1012081 |
| [21] |
YOO SM, LEE MS. Kaposi's sarcoma-associated herpesvirus and host interaction by the complement system[J]. Pathogens, 2020, 9(4): 260. DOI:10.3390/pathogens9040260 |
| [22] |
YOO SM, JEON H, LEE S, LEE MS. Susceptibility of KSHV-infected PEL cell lines to the human complement system[J]. Journal of Microbiology and Biotechnology, 2016, 26(3): 618-626. DOI:10.4014/jmb.1512.12031 |
| [23] |
MULLICK J, BERNET J, SINGH AK, LAMBRIS JD, SAHU A. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) open reading frame 4 protein (kaposica) is a functional homolog of complement control proteins[J]. Journal of Virology, 2003, 77(6): 3878-3881. DOI:10.1128/JVI.77.6.3878-3881.2003 |
| [24] |
MARK L, LEE WH, SPILLER OB, PROCTOR D, BLACKBOURN DJ, VILLOUTREIX BO, BLOM AM. The Kaposi's sarcoma-associated herpesvirus complement control protein mimics human molecular mechanisms for inhibition of the complement system[J]. Journal of Biological Chemistry, 2004, 279(43): 45093-45101. DOI:10.1074/jbc.M407558200 |
| [25] |
SPILLER OB, MARK L, BLUE CE, PROCTOR DG, AITKEN JA, BLOM AM, BLACKBOURN DJ. Dissecting the regions of virion-associated Kaposi's sarcoma-associated herpesvirus complement control protein required for complement regulation and cell binding[J]. Journal of Virology, 2006, 80(8): 4068-4078. DOI:10.1128/JVI.80.8.4068-4078.2006 |
| [26] |
JEON H, YOO SM, CHOI HS, MUN JY, KANG HG, LEE J, PARK J, GAO SJ, LEE MS. Extracellular vesicles from KSHV-infected endothelial cells activate the complement system[J]. Oncotarget, 2017, 8(59): 99841-99860. DOI:10.18632/oncotarget.21668 |
| [27] |
LAM AK, ROSHAN R, MILEY W, LABO N, ZHEN J, KURLAND AP, CHENG C, HUANG HG, TENG PL, HARELSON C, GONG DY, TAM YK, RADU CG, EPELDEGUI M, JOHNSON JR, HONG ZHOU Z, WHITBY D, WU TT. Immunization of mice with virus-like vesicles of kaposi sarcoma-associated herpesvirus reveals a role for antibodies targeting ORF4 in activating complement-mediated neutralization[J]. Journal of Virology, 2023, 97(2): e0160022. DOI:10.1128/jvi.01600-22 |
| [28] |
CHAUHAN R, TIWARI M, CHAUDHARY A, SHARAN THAKUR R, PANDE V, DAS J. Chemokines: a key driver for inflammation in protozoan infection[J]. International Reviews of Immunology, 2024, 43(4): 211-228. DOI:10.1080/08830185.2023.2281566 |
| [29] |
HOST KM, JACOBS SR, WEST JA, ZHANG ZG, COSTANTINI LM, STOPFORD CM, DITTMER DP, DAMANIA B. Kaposi's sarcoma-associated herpesvirus increases PD-L1 and proinflammatory cytokine expression in human monocytes[J]. mBio, 2017, 8(5): e00917-17. |
| [30] |
CARO-VEGAS C, SELLERS S, HOST KM, SELTZER J, LANDIS J, FISCHER WA, DAMANIA B, DITTMER DP. Runaway Kaposi Sarcoma-associated herpesvirus replication correlates with systemic IL-10 levels[J]. Virology, 2020, 539: 18-25. DOI:10.1016/j.virol.2019.10.002 |
| [31] |
KANG SJ, TANAKA T, NARAZAKI M, KISHIMOTO T. Targeting interleukin-6 signaling in clinic[J]. Immunity, 2019, 50(4): 1007-1023. DOI:10.1016/j.immuni.2019.03.026 |
| [32] |
RIVERA-SOTO R, DISSINGER NJ, DAMANIA B. Kaposi's sarcoma-associated herpesvirus viral interleukin-6 signaling upregulates integrin β3 levels and is dependent on STAT3[J]. Journal of Virology, 2020, 94(5): e01384-19. |
| [33] |
LI X, HU YL. Attribution of NF-κB activity to CHUK/IKKα-involved carcinogenesis[J]. Cancers, 2021, 13(6): 1411. DOI:10.3390/cancers13061411 |
| [34] |
DELYON J, BIARD L, RENAUD M, RESCHE-RIGON M, Le GOFF J, DALLE S, HEIDELBERGER V, Da MEDA L, TOULLEC L, CARCELAIN G, MOURAH S, CAILLAT-ZUCMAN S, ALLAIN V, BATTISTELLA M, LEBBE C. PD-1 blockade with pembrolizumab in classic or endemic Kaposi's sarcoma: a multicentre, single-arm, phase 2 study[J]. The Lancet Oncology, 2022, 23(4): 491-500. DOI:10.1016/S1470-2045(22)00097-3 |
| [35] |
MESEV EV, LeDESMA RA, PLOSS A. Decoding type I and III interferon signalling during viral infection[J]. Nature Microbiology, 2019, 4(6): 914-924. DOI:10.1038/s41564-019-0421-x |
| [36] |
BARRETT L, DAI L, WANG SZ, QIN ZQ. Kaposi's sarcoma-associated herpesvirus and extracellular vesicles[J]. Journal of Medical Virology, 2021, 93(6): 3294-3299. DOI:10.1002/jmv.26780 |
| [37] |
MYOUNG J, LEE SA, LEE HR. Beyond viral interferon regulatory factors: immune evasion strategies[J]. Journal of Microbiology and Biotechnology, 2019, 29(12): 1873-1881. DOI:10.4014/jmb.1910.10004 |
| [38] |
SHI JL, JIA XM, HE YJ, MA XY, QI XY, LI W, GAO SJ, YAN Q, LU C. Immune evasion strategy involving propionylation by the KSHV interferon regulatory factor 1 (vIRF1)[J]. PLoS Pathogens, 2023, 19(4): e1011324. DOI:10.1371/journal.ppat.1011324 |
| [39] |
ROKX C, van der ENDE ME, VERBON A, RIJNDERS BJA. Peginterferon Alfa-2a for AIDS-associated kaposi sarcoma: experience with 10 patients[J]. Clinical Infectious Diseases, 2013, 57(10): 1497-1499. DOI:10.1093/cid/cit517 |
| [40] |
BRULOIS K, TOTH Z, WONG LY, FENG PH, GAO SJ, ENSSER A, JUNG JU. Kaposi's sarcoma- associated herpesvirus K3 and K5 ubiquitin E3 ligases have stage-specific immune evasion roles during lytic replication[J]. Journal of Virology, 2014, 88(16): 9335-9349. DOI:10.1128/JVI.00873-14 |
| [41] |
BUTLER LM, JEFFERY HC, WHEAT RL, LONG HM, RAE PC, NASH GB, BLACKBOURN DJ. Kaposi's sarcoma-associated herpesvirus inhibits expression and function of endothelial cell major histocompatibility complex class II via suppressor of cytokine signaling 3[J]. Journal of Virology, 2012, 86(13): 7158-7166. DOI:10.1128/JVI.06908-11 |
| [42] |
BELLON M, NICOT C. Feedback loop regulation between pim kinases and tax keeps human T-cell leukemia virus type 1 viral replication in check[J]. Journal of Virology, 2022, 96(3): e0196021. DOI:10.1128/jvi.01960-21 |
| [43] |
MÜNZ C. Natural killer cell responses during human γ-herpesvirus infections[J]. Vaccines, 2021, 9(6): 655. DOI:10.3390/vaccines9060655 |
| [44] |
HU ZT, USHERWOOD EJ. Immune escape of γ-herpesviruses from adaptive immunity[J]. Reviews in Medical Virology, 2014, 24(6): 365-378. DOI:10.1002/rmv.1791 |
| [45] |
ZHANG H, DAI ZY, WU WT, WANG ZY, ZHANG N, ZHANG LY, ZENG WJ, LIU ZX, CHENG Q. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer[J]. Journal of Experimental & Clinical Cancer Research, 2021, 40(1): 184. DOI:10.3760/cma.j.cn421213-20200918-00688 |
| [46] |
CHEN JG, del VALLE L, LIN HY, PLAISANCE-BONSTAFF K, FORREST JC, POST SR, QIN ZQ. Expression of PD-1 and PD-Ls in Kaposi's sarcoma and regulation by oncogenic herpesvirus lytic reactivation[J]. Virology, 2019, 536: 16-19. DOI:10.1016/j.virol.2019.07.024 |
| [47] |
CHEN SY, DENG Y, PAN DL. microRNA regulation of human herpesvirus latency[J]. Viruses, 2022, 14(6): 1215. DOI:10.3390/v14061215 |
| [48] |
RAMALINGAM D, ZIEGELBAUER JM. Viral microRNAs target a gene network, inhibit STAT activation, and suppress interferon responses[J]. Scientific Reports, 2017, 7: 40813. DOI:10.1038/srep40813 |
| [49] |
ABDALLA AE, MAHJOOB MO, ABOSALIF KOA, EJAZ H, ALI MOHAMMED ALAMEEN A, ELSAMAN T. Human cytomegalovirus-encoded microRNAs: a master regulator of latent infection[J]. Infection, Genetics and Evolution, 2020, 78: 104119. DOI:10.1016/j.meegid.2019.104119 |
| [50] |
ABEND JR, RAMALINGAM D, KIEFFER-KWON P, ULDRICK TS, YARCHOAN R, ZIEGELBAUER JM. Kaposi's sarcoma-associated herpesvirus microRNAs target IRAK1 and MYD88, two components of the toll-like receptor/interleukin-1R signaling cascade, to reduce inflammatory-cytokine expression[J]. Journal of Virology, 2012, 86(21): 11663-11674. DOI:10.1128/JVI.01147-12 |
| [51] |
ABEND JR, ULDRICK T, ZIEGELBAUER JM. Regulation of tumor necrosis factor-like weak inducer of apoptosis receptor protein (TWEAKR) expression by Kaposi's sarcoma-associated herpesvirus microRNA prevents TWEAK-induced apoptosis and inflammatory cytokine expression[J]. Journal of Virology, 2010, 84(23): 12139-12151. DOI:10.1128/JVI.00884-10 |
| [52] |
ALUIGI MG, ALBINI A, CARLONE S, REPETTO L, de MARCHI R, ICARDI A, MORO M, NOONAN D, BENELLI R. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic and Mediterranean forms of Kaposi's sarcoma[J]. Research in Virology, 1996, 147(5): 267-275. DOI:10.1016/0923-2516(96)82285-0 |
| [53] |
LI W, YAN Q, DING XY, SHEN CY, HU MM, ZHU Y, QIN D, LU HM, KRUEGER BJ, RENNE R, GAO SJ, LU C. The SH3BGR/STAT3 pathway regulates cell migration and angiogenesis induced by a gammaherpesvirus microRNA[J]. PLoS Pathogens, 2016, 12(4): e1005605. DOI:10.1371/journal.ppat.1005605 |
| [54] |
YOGEV O, HENDERSON S, HAYES MJ, MARELLI SS, OFIR-BIRIN Y, REGEV-RUDZKI N, HERRERO J, ENVER T. Herpesviruses shape tumour microenvironment through exosomal transfer of viral microRNAs[J]. PLoS Pathogens, 2017, 13(8): e1006524. DOI:10.1371/journal.ppat.1006524 |
 2025, Vol. 52
2025, Vol. 52