扩展功能
文章信息
- 许蓉, 谢孟乐, 苏文鑫, 田上卿, 李玉, Chayanard Phukhamsakda
- XU Rong, XIE Mengle, SU Wenxin, TIAN Shangqing, LI Yu, Chayanard Phukhamsakda
- 采自文冠果葡萄座腔菌科的4个新记录种
- Four new records of botryosphaerialean fungi from Xanthoceras sorbifolium
- 微生物学通报, 2024, 51(6): 2065-2080
- Microbiology China, 2024, 51(6): 2065-2080
- DOI: 10.13344/j.microbiol.china.230855
-
文章历史
- 收稿日期: 2023-10-17
- 接受日期: 2023-11-25
- 网络首发日期: 2024-01-05
2. 吉林农业大学食药用菌教育部工程研究中心, 吉林 长春 130118;
3. Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand
2. Engineering Research Center of Chinese Ministry of Education for Edible and Medicinal Fungi, Jilin Agricultural University, Changchun 130118, Jilin, China;
3. Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand
文冠果(Xanthoceras sorbifolium Bunge)为无患子科(Sapindaceae)、文冠果属植物,又名崖木瓜、僧灯毛道、温旦革子,属于灌木或小乔木,是中国北方特有树种,主要分布于华北、东北、西北等地区,因其具有耐寒、耐旱、耐贫瘠等特点,常被用于水土保护、造林绿化和防风固沙等方面[1]。同时,作为油料树种,文冠果种仁含油量高达66%,富含的植物源神经酸对心血管和自身免疫疾病有良好的效果[2],其中亚油酸是中药益寿宁的主要成分,具有降血压的作用。然而,目前文冠果上的微型子囊菌鲜有研究,只有零星报道[3-4],其真菌多样性及种质资源仍有待挖掘。
葡萄座腔菌科(Botryosphaeriaceae)由Theissen和Sydow于1918建立,葡萄座腔菌属(Botryosphaeria)为模式属[5]。该科真菌多为农业和林业上主要的病原菌,主要引起树木溃疡病;也有生于腐烂植物的腐生菌,或与植物共生的内生真菌[6-12]。葡萄座腔菌科真菌可寄生于多种寄主植物,在世界各地均有分布[13-17]。目前,葡萄座腔菌科共包含22属,其中葡萄座腔菌属(Botryosphaeria)是引起多种果树和林木枝干溃疡病和果实腐烂病的病原菌,在河南、河北、北京、天津、辽宁、山东、山西、陕西、江西、贵州、江苏、安徽、浙江、湖北、福建等地常有报道;其寄主范围较广,常见寄主有苹果、梨、桃、柑橘、葡萄、蓝莓、樱桃、猕猴桃、桉树、茶树、杨树等[18-31],但文冠果上尚无该属真菌报道[3-4]。暗葡萄腔菌属(Phaeobotryon)真菌于2015年在中国首次报道,引起鹿角漆树(Rhus typhina)枯死病和溃疡病[32]。截至2023年,在我国北京市怀柔区、门头沟区、延庆区及宁夏回族自治区银川市共记录4种暗葡萄腔菌属真菌:Phaeobotryon aplosporum[33]、P. rhoinum[34]、P. rhois[32]和P. spiraeae[35],寄主分别为绣线菊(Spiraea salicifolia)、鹿角漆树(Rhus typhina)、丁香(Syzygium aromaticum)、穿龙薯蓣(Dioscorea nipponica)、侧柏(Platycladus orientalis)和鼠李(Rhamnus davurica)。
本研究对在吉林省长春市和四平市采集获得的文冠果枝干标本进行分类学鉴定,旨在明确吉林省文冠果微型子囊菌物种资源多样性及分布特性,以期为微型子囊菌资源利用和保护提供重要理论依据和经验积累。
1 材料与方法 1.1 样品文冠果枯枝采自吉林省长春市吉林农业大学校园内及吉林省四平市梨树县国有林总场文冠果种植基地,均保存于吉林农业大学菌物标本馆(The Herbarium of Mycology, Jilin Agricultural University, HMJAU)。采用单孢分离法获得纯培养物[36],挑取萌发的孢子转移至PDA培养基中,于25 ℃黑暗条件下培养,分离纯化后的菌株保存于吉林农业大学食药用菌教育部工程研究中心(The International Cooperation Research Center of China for New Germplasm Breeding of Edible Mushrooms Culture Collection, CCMJ)。
1.2 培养基PDA培养基(g/L):马铃薯200.0,葡萄糖20.0,琼脂粉16.0,121 ℃灭菌30 min。
1.3 主要试剂和仪器基因组DNA提取试剂盒和2×Es Taq Master Mix (Dye),江苏康为世纪生物科技股份有限公司。立体显微镜和光学显微镜,卡尔蔡司光学(中国)有限公司;PCR仪,Bio-Rad公司。
1.4 形态学特征使用立体显微镜观察子囊果和分生孢子器的形态特征。显微结构的观察是以蒸馏水为浮载剂进行手工切片,使用光学显微镜对子囊、子囊孢子、产孢细胞及分生孢子的形态、颜色、表面纹饰、大小等特征进行观察并拍照。
1.5 分子系统学特征分离纯化获得的菌株于25 ℃培养2周后,利用基因组DNA提取试剂盒提取基因组总DNA,扩增内转录间隔区(internal transcribed spacer, ITS)、核糖体大亚基(ribosomal large subunit, LSU)和翻译延伸因子(translation elongation factor 1-alpha, tef1-α),所用引物序列如表 1所示。PCR反应体系:上、下游引物(10 μmol/L) 0.3 μL,2×Es Taq Master Mix (Dye) 10 μL,模板(10 ng/μL) 1 μL,无菌水补足20 μL。PCR反应条件(ITS/LSU/tef1-α):94 ℃ 5 min;94 ℃ 30 s,53 ℃ 45 s,72 ℃ 90 s,35个循环;72 ℃ 10 min。PCR扩增产物经1%琼脂糖凝胶电泳检测,确认合格后由生工生物工程(上海)股份有限公司进行测序。
使用BioEdit v.7.2.5[40]读取测序所获得的序列峰图,将自测序列在NCBI公共数据库(https://blast.ncbi.nlm.nih.gov/)进行BLAST在线比对,选取并下载同源序列。使用MAFFT v7.0 (https://mafft.cbrc.jp/alignment/server)对序列进行自动对齐[41],并用AliView软件手动矫正无法对齐的序列[42]。利用PAUP 4.0进行最大简约(maximum parsimony, MP)分析[43],在CIPRES门户网站上分别使用RAxML-HPC2和MrBayes v3.2.6进行最大似然(maximum likelihood, ML)分析[44-45]和贝叶斯推导(Bayesian inference, BI)分析[46]。使用FigTree v1.4软件(http://tree.bio.ed.ac.uk/software/fgtree/)构建系统发育树。
2 结果与分析 2.1 中华葡萄座腔菌Botryosphaeria sinensis Y.P. Zhou & Y. Zhang ter in Phytotaxa 245(1): 45 (2016).
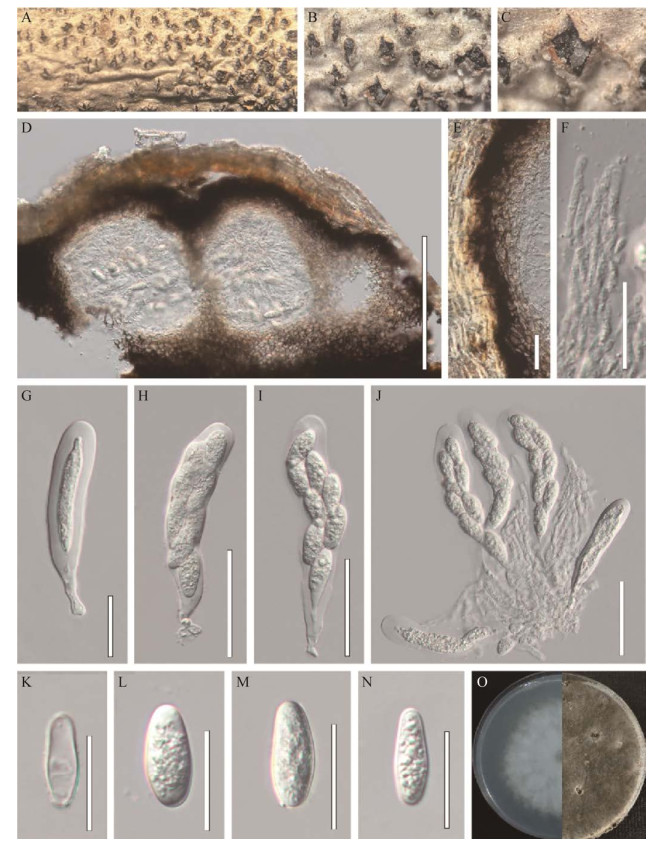
|
| 图 1 中华葡萄座腔菌的有性型形态图(HMJAU 64805) Figure 1 Sexual morphology of Botryosphaeria sinensis (HMJAU 64805). A–C:文冠果枯枝上的子囊果. D:子囊果的纵切面. E:子囊果的壁. F:拟侧丝. G–J:子囊. K–N:子囊孢子. O:中华葡萄座腔文冠果枯枝上的子囊果菌在PDA上的培养形态(左侧培养4 d,右侧培养20 d). 标尺:D为200 μm;H–J为50 μm;E–G和K–N为20 μm A–C: Appearance of ascomata on host substrate. D: Vertical section of ascomata. E: Partial peridium wall. F: Pseudoparaphyses. G–J: Asci. K–N: Ascospores. O: Culture characteristics on PDA after 4 d (left) and 20 d (right). Scale bars: D, 200 μm; H–J, 50 μm; E–G and K–N, 20 μm. |
|
|
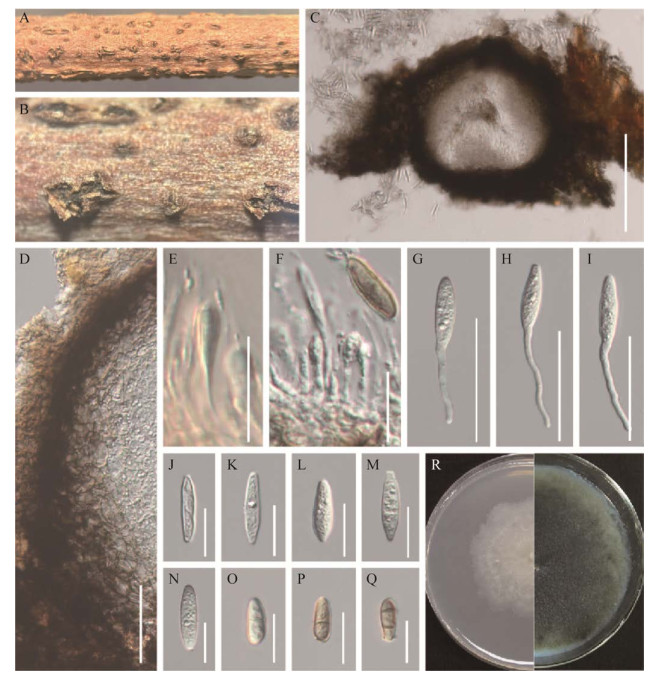
|
| 图 2 中华葡萄座腔菌的无性型形态图(HMJAU 64804) Figure 2 Asexual morphology of Botryosphaeria sinensis (HMJAU 64804). A和B:文冠果枯枝上的分生孢子器. C:分生孢子器的纵切面. D:分生孢子器的壁. E–I:产孢细胞及分生孢子. J–Q:分生孢子. R:中华葡萄座腔菌在PDA上的培养形态(左侧培养4 d,右侧培养20 d). 标尺:C为200 μm;D、G–I为50 μm;E、F和J–Q为20 μm A and B: Appearance of conidiomata on host substrate. C: Vertical section of conidiomata. D: Section of conidioma wall. E–I: Conidiogenous cells and conidia. J–Q: Conidia. R: Culture characteristics on PDA after 4 d (left) and 20 d (right). Scale bars: C, 200 μm; D and G–I, 50 μm; E–F and J–Q, 20 μm. |
|
|
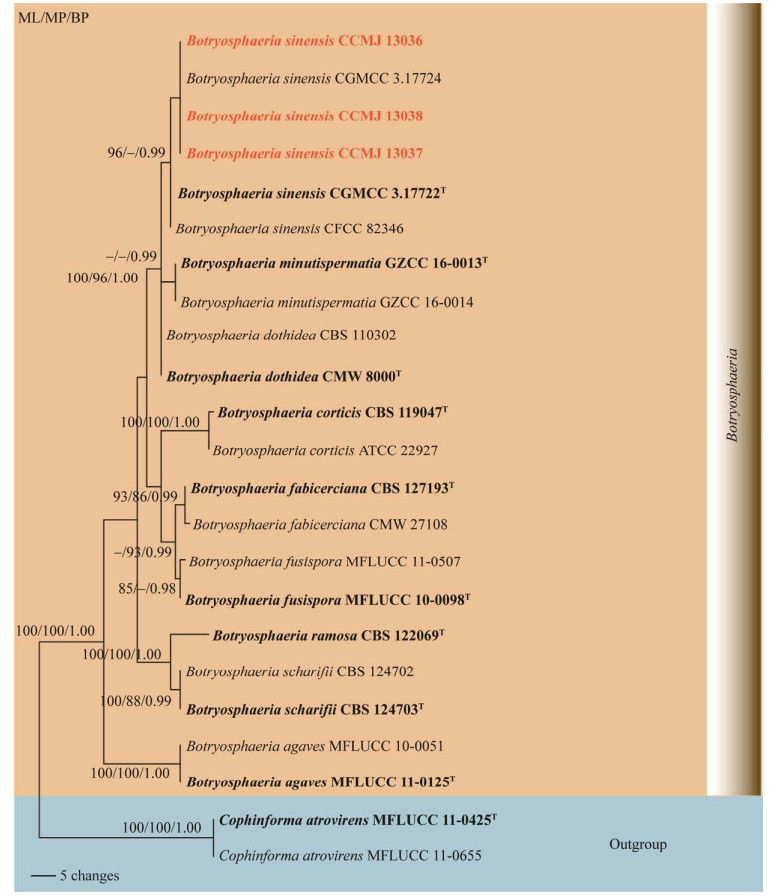
|
| 图 3 基于ITS+LSU+tef1-α序列的葡萄座腔菌属系统发育树最大似然法支持率≥70%、最大简约法支持率≥70%及贝叶斯后验概率≥0.9标示在分支节点 Figure 3 Phylogenetic analysis of Botryosphaeria based on ITS, LSU and tef1-α genes. Maximum likelihood bootstrap support values ≥70% (ML), maximum parsimony bootstrap support values ≥70% (MP) and Bayesian posterior probabilities ≥0.9 (BYPP) are given at the nodes as ML/MP/BYPP. |
|
|
有性型:腐生于文冠果枯枝上,子囊果半埋生,成熟后撑破枝干表皮;子囊果(204–240) µm×(178–242) µm (
无性型:腐生于文冠果枯枝上,半埋生,成熟后撑破枝干表皮,球形至近球形,聚生或单生,褐色至黑色,直径为330–370 µm;子实体包被厚25–41 μm,类皮质,由5–15层浅棕色至深棕色细胞按照角胞组织的形式排列构成,细胞由内向外颜色逐渐变深;分生孢子梗不明显或退化为产孢细胞,产孢细胞(9–32) µm×(1.0–2.7) μm (
有性型菌落特征:菌落生长速度较快,在PDA培养基上25 ℃条件下培养,生长第4天直径达6 cm。菌落初期呈白色,气生菌丝,边缘整齐;后期菌丝呈墨绿色至黑色,有油滴产生,菌落反面呈放射状,中间呈黑色,边缘菌丝墨绿色。
无性型菌落特征:菌落生长速度一般,在PDA培养基上25 ℃条件下培养,生长4 d直径达3.7 cm。菌落初期呈白色,菌丝稀疏;后期菌丝呈墨绿色至黑色,菌落反面呈放射状,中间呈黑色,边缘菌丝墨绿色。
标本信息:采集时间:2021年8月12日,采集人:许蓉,采集地点:吉林省四平市梨树县国有林总场文冠果科研基地,采自文冠果枯枝,标本号为HMJAU 64804,菌株号为CCMJ 13036 (ITS、LSU和tef1-α的GenBank登录号分别为OQ846791、OQ845867和OQ980395);采集时间:2021年10月18日;采集人:许蓉;采集地点:吉林省长春市净月潭国家森林公园,采自文冠果枯枝;标本号为HMJAU 64805;菌株号为CCMJ 13037 (ITS、LSU和tef1-αGenBank登录号分别为OQ846792、OQ845868和OQ980396);采集时间:2021年10月31日;采集人:许蓉;采集地点:吉林省长春市吉林农业大学校园内,采自文冠果枯枝;标本号为HMJAU 64806;菌株号为CCMJ 13038 (ITS、LSU和tef1-α的GenBank登录号分别为OQ846793、OQ845869和OQ980397)。
世界分布:中国。
2.2 无隔孢暗葡萄腔菌Phaeobotryon aplosporum M. Pan & X.L. Fan in Mycol. Progr. 18(11): 1356 (2019).
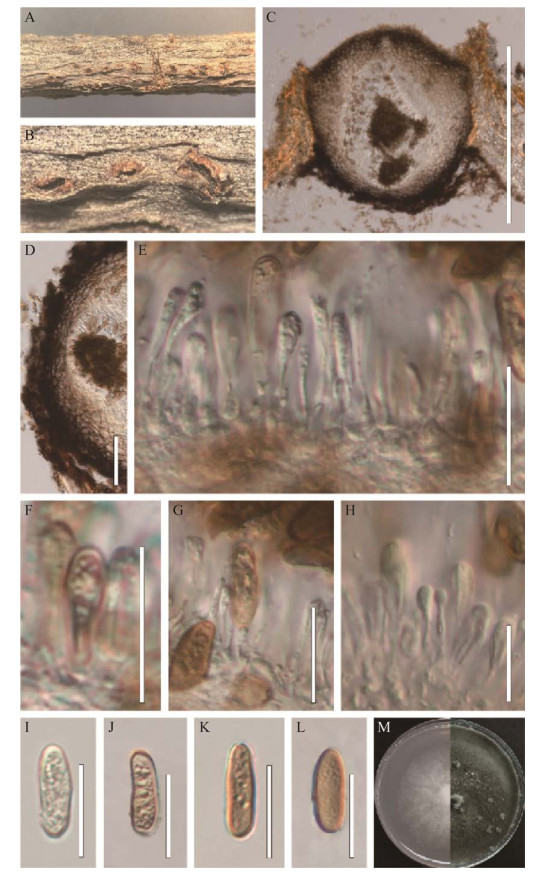
|
| 图 4 无隔孢暗葡萄腔菌(HMJAU 64807) Figure 4 Morphology of Phaeobotryon aplosporum (HMJAU 64807). A和B:文冠果枯枝上的分生孢子器. C:分生孢子器的纵切面. D:分生孢子器的壁. E–H:产孢细胞及分生孢子. I–L:分生孢子. M:无隔孢暗葡萄腔菌在PDA上的培养形态(左侧培养4 d,右侧培养20 d). 标尺:C为500 μm;D为50 μm;E–L为20 μm A and B: Appearance of conidiomata on host substrate. C: Vertical section of conidiomata. D: Section of conidioma wall. E–H: Conidiogenous cells and conidia. I–L: Conidia. M: Culture characteristics on PDA after 4 d (left) and 20 d (right). Scale bars: C, 500 μm; D, 50 μm; E–L, 20 μm. |
|
|
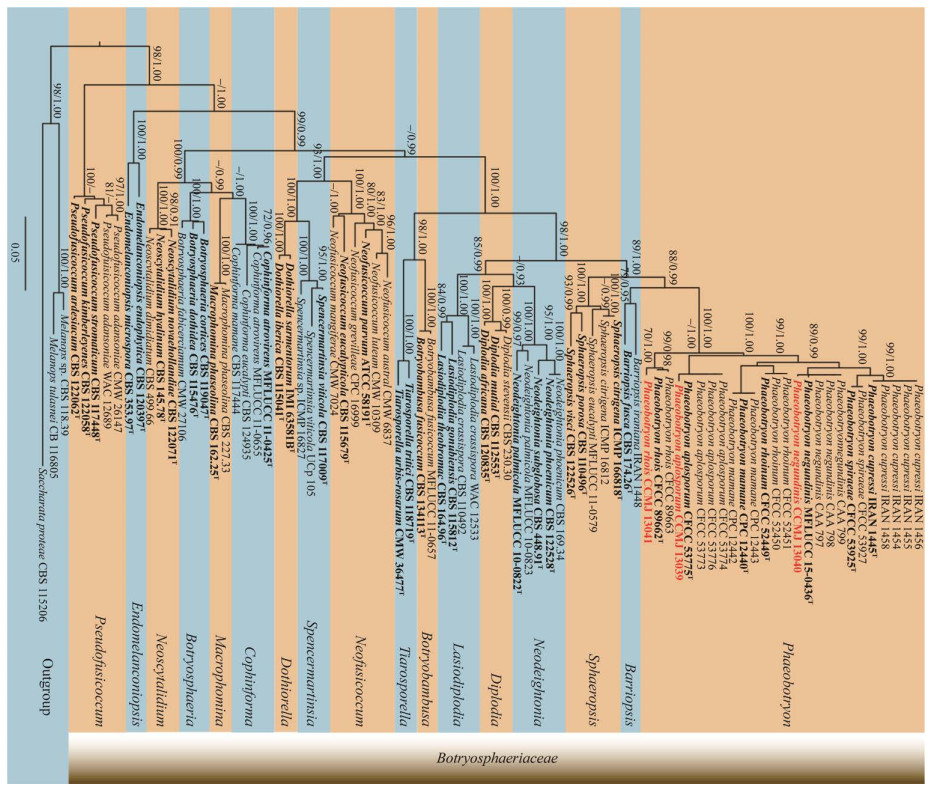
|
| 图 5 基于ITS+LSU+tef1-α序列的暗葡萄腔菌属系统发育树 Figure 5 Phylogenetic analysis of Phaeobotryon based on ITS, LSU and tef1-α genes. 最大似然法支持率≥70%及贝叶斯后验概率≥0.9标示在分支节点,模式菌株用粗体和“T”标记,本研究分离的菌株用粗体标记 Maximum likelihood bootstrap support values ≥70% (ML) and Bayesian posterior probabilities ≥0.9 (BYPP) are given at the nodes as ML/MP/BYPP. The type strains are in bold and marked with "T". The new isolates are in bold. |
|
|
有性型:未发现。
无性型:腐生于文冠果枯枝上,分生孢子器(160.5–248.0) μm×(122–238) μm (
菌落特征:菌落生长速度较快,在PDA培养基上25 ℃条件下,生长4 d直径达4.8 cm。菌落初期呈白色,边缘整齐,后期菌丝呈灰褐色,菌丝柔软致密,形成白色无菌贴伏菌丝垫,菌落反面同样呈灰褐色。
标本信息:采集时间:2021年8月12日;采集人:许蓉;采集地点:吉林省四平市梨树县国有林总场文冠果科研基地,采自文冠果枯枝;标本号为HMJAU 64807;菌株号为CCMJ 13039 (ITS、LSU的GenBank登录号分别为OQ846794、OQ845870)。
世界分布:中国。
国内分布:北京市[33]、吉林省。
2.3 楲生暗葡萄腔菌Phaeobotryon negundinis Daranag., Bulgakov & K.D. Hyde, in Mycosphere 7(7): 936 (2016).
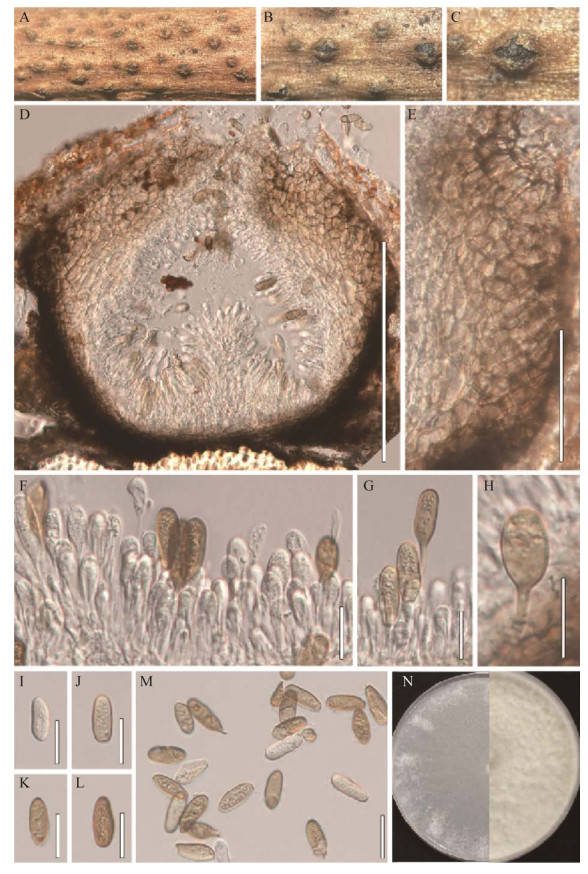
|
| 图 6 楲生暗葡萄腔菌的形态图(HMJAU 64808) Figure 6 Morphology of Phaeobotryon negundinis (HMJAU 64808). A–C:文冠果枯枝上的分生孢子器. D:分生孢子器的纵切面. E:分生孢子器的壁. F–H:产孢细胞及分生孢子. I–M:分生孢子. N:楲生暗葡萄腔菌在PDA上的培养形态(左侧培养4 d,右侧培养20 d). 标尺:D为200 μm;E为50 μm;F–M为20 μm A–C: Appearance of conidiomata on host substrate. D: Vertical section of conidiomata. E: Section of conidioma wall. F–H: Conidiogenous cells and conidia. I–M: Conidia. N: Culture characteristics on PDA after 4 d (left) and 20 d (right). Scale bars: D, 200 μm; E, 50 μm; F–M, 20 μm. |
|
|
有性型:未发现。
无性型:腐生于文冠果枯枝上,分生孢子器(175–343)×(250–400) μm (
菌落特征:菌落生长速度较快,在PDA培养基上25 ℃条件下,生长4 d直径达6.7 cm。菌落初期呈白色,中间菌丝稀疏,边缘整齐、较密;后期菌丝乳白色,致密,棉絮状。
标本信息:采集时间:2022年7月2日;采集人:许蓉;采集地点:吉林省长春市吉林农业大学校园内,采自文冠果枯枝;标本号为HMJAU 64808;菌株号为CCMJ 13040 (ITS、LSU和tef1-α的GenBank登录号分别为OQ846795、OQ845871和OQ980398)。
世界分布:中国、俄罗斯[47]。
国内分布:吉林省。
2.4 火炬树生暗葡萄腔菌Phaeobotryon rhois C.M. Tian, X.L. Fan & K.D. Hyde, in Phytotaxa 205(2): 95 (2015).

|
| 图 7 火炬树生暗葡萄腔菌(HMJAU 64809) Figure 7 Morphology of Phaeobotryon rhois (HMJAU 64809). A–C:文冠果枯枝上的分生孢子器. D:分生孢子器的纵切面. E:分生孢子器的壁. F:产孢细胞及分生孢子. G:分生孢子. H:火炬树生暗葡萄腔菌在PDA上的培养形态(左侧培养4 d,右侧培养20 d). 标尺:D为100 μm;E为50 μm;F–G为20 μm A–C: Appearance of conidiomata on host substrate. D: Vertical section of conidiomata. E: Section of conidioma wall. F: Conidiogenous cells and conidia. G: Conidia. H: Culture characteristics on PDA after 4 d (left) and 20 d (right). Scale bars: D, 100 μm; E, 50 μm; F–G, 20 μm. |
|
|
有性型:未发现。
无性型:腐生于文冠果枯枝上,分生孢子器(150–190)×(140–160) μm (
菌落特征:菌落生长速度较快,在PDA培养基上25 ℃条件下,生长4 d直径达7 cm。菌落初期呈白色,菌丝稀疏,边缘整齐;后期菌丝呈深绿色至黑色,气生菌丝,中间有贴伏的菌丝垫。
标本信息:采集时间:2022年7月2日;采集人:许蓉;采集地点:吉林省长春市吉林农业大学校园内,采自文冠果枯枝;标本号为HMJAU 64809;菌株号为CCMJ 13041 (ITS、LSU和tef1-α的GenBank登录号分别为OQ846796、OQ845872和OQ980399)。
世界分布:中国。
国内分布:宁夏回族自治区[32]、北京市[33-34]和吉林省。
3 讨论与结论中华葡萄座腔菌(B. sinensis)最早由Zhou等[31]报道,采集自河南省的桃树、桑树和杨树上。2020年,陈娅娅在贵州省的桃树和禾本科植物标本上分离得到中华葡萄座腔菌[18]。本研究从吉林省文冠果上采集的标本与陈娅娅[18]、Zhou等[31]描述的形态相似,根据系统发育树,本研究分离到的3株菌株与B. sinensis聚为一支且具有较高的支持率(图 3),因此鉴定为中华葡萄座腔菌(B. sinensis)。该种首次在吉林省的文冠果上发现,扩大了中华葡萄座腔菌在中国的分布范围。
无隔孢暗葡萄腔菌(P. aplosporum)是Pan等[33]于2019年报道的一个新种,引起鹿角漆树(Rhus typhina)溃疡病害,系统发育分析显示,菌株CCMJ 13039与无隔孢暗葡萄腔菌聚集在一个分支,与模式菌株(CFCC 53775)的ITS序列(446/446)和LSU序列完全一致(814/814)。从形态上看,CCMJ 13039与无隔孢暗葡萄腔菌的分生孢子大小相似(17–19) μm×(5.5–7.0) μm vs. (15.8–21.5) μm×(4.5–9.0) μm,且均无隔膜[46]。因此,将其鉴定为无隔孢暗葡萄腔菌(P. aplosporum)。
Daranagama等在俄罗斯罗斯托夫地区的梣叶槭(Acer negundo)、金钟连翘(Forsythia intermedia)和女贞(Ligustrum vulgare)上首次报道了槭生暗葡萄腔菌(P. negundinis),虽然该菌与植株枝干、叶片上的病害症状有关,但致病性尚未得到证实[47]。系统发育分析显示,菌株CCMJ 13040与楲生暗葡萄腔菌以较高的支持率(89/0.99)聚为一支,且形态特征相似。Daranagama等[47]认为(P. cupressi)与(P. negundinis)二者的主要区别在于(P. negundinis)的分生孢子略小(24.1–25.0) μm×(12.2–12.5) μm vs. (16.0–24.5) μm×(7.9–11.5) μm,本研究分离得到的CCMJ 13040符合这一特征[(19–25) μm×(7.7–10.7) μm],依据其主要形态特征及系统分析结果鉴定该种为槭生暗葡萄腔菌(P. negundinis),本研究新增了该种在中国的地理分布。
目前,火炬树生暗葡萄腔菌(P. rhois)主要引起鹿角漆树、穿龙薯蓣、侧柏和鼠李的溃疡病和枯死病,且仅在中国有报道[32-34]。菌株CCMJ 13041与火炬树生暗葡萄腔菌的形态相似,分生孢子初期透明,成熟后变为深棕色,具1个隔膜。系统发育分析结果显示,菌株CCMJ 13041以较高的支持率(ML/BI=70/1.00)与模式菌株(P. rhois) CFCC 89662聚为“姊妹”支。因而,将其鉴定为火炬树生暗葡萄腔菌(P. rhois)。
除了中华葡萄座腔菌(B. sinensis)和槭生暗葡萄腔菌(P. negundinis)外,另外两种真菌均可引起树木溃疡病或枯死病,这与前人报道的葡萄座腔菌科大多为农业和林业上重要的病原菌且主要为树木溃疡病病原菌的报道一致[6-12]。本文从文冠果枝干上分离到这4种真菌,对其分类学进行深入研究,丰富了文冠果上微型子囊菌物种多样性及种质资源,扩大了葡萄座腔菌科物种的地理分布。后续研究还需要通过致病性测定验证4种真菌是否具有致病力,明确真菌的生物学特征,为文冠果溃疡病的发生及防治提供理论依据。
| [1] |
WANG YL, LÜ D, LIU JH, ZHAO GS. Planting effect of Xanthoceras sorbifolia from different provenances in Hexi Corridor of Gansu Province arid desert area[J]. Hebei Journal of Forestry and Orchard Research, 2016, 31(4): 370-373. (in Chinese) 王艺林, 吕东, 刘建海, 赵国生. 甘肃省河西走廊干旱荒漠区文冠果不同种源造林效果[J]. 河北林果研究, 2016, 31(4): 370-373. |
| [2] |
ZANG EH, QIU B, CHEN N, LI CF, LIU Q, ZHANG M, LIU YC, LI MH. Xanthoceras sorbifolium Bunge: a review on botany, phytochemistry, pharmacology, and applications[J]. Frontiers in Pharmacology, 2021, 12: 708549. DOI:10.3389/fphar.2021.708549 |
| [3] |
ZHANG BH. Isolation and identification of pathogenic bacteria causing crown rot of Xanthoceras sorbifolium Bunge[D]. Tai'an: Master's Thesis of Shandong Agricultural University, 2021 (in Chinese). 张丙红. 文冠果颈腐病病原菌的分离鉴定[D]. 泰安: 山东农业大学硕士论文, 2021. |
| [4] |
XU R, SU WX, TIAN SQ, BHUNJUN CS, TIBPROMMA S, HYDE KD, LI Y, PHUKHAMSAKDA C. Synopsis of Leptosphaeriaceae and introduction of three new taxa and one new record from China[J]. Journal of Fungi (Basel, Switzerland), 2022, 8(5): 416. |
| [5] |
THEISSEN F, SYDOW H. Vorentwürfe zu den Pseudosphaeriales[J]. Annales Mycologici, 1918, 16: 1-34. |
| [6] |
WU XQ, HE YQ, LIU ZH. Occurrence and progress on tree cankers caused by Botryosphaeria spp.[J]. Journal of Nanjing Forestry University (Natural Sciences Edition), 2001, 25(1): 61-66. (in Chinese) 吴小芹, 何月秋, 刘忠华. 葡萄座腔菌属所致树木溃疡病发生与研究进展[J]. 南京林业大学学报(自然科学版), 2001, 25(1): 61-66. |
| [7] |
LI WY, LI X, XIE KZ, DENG WQ, ZHUANG WY. Systematics and species diversity of botryosphaeriaceous fungi[J]. Biodiversity Science, 2017, 25(8): 874-885. (in Chinese) 李文英, 李夏, 解开治, 邓旺秋, 庄文颖. 葡萄座腔菌科真菌的系统学和多样性探讨[J]. 生物多样性, 2017, 25(8): 874-885. |
| [8] |
SLIPPERS B, WINGFELD MJ. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact[J]. Fungal Biology Reviews, 2007, 21(2/3): 90-106. |
| [9] |
CHENG YL, LIANG J, LÜ Q, ZHANG XY. Advances in Botryosphaeriaceae: identification, phylogeny and molecular ecology[J]. Acta Ecologica Sinica, 2011, 31(11): 3197-3207. (in Chinese) 程燕林, 梁军, 吕全, 张星耀. 葡萄座腔菌科研究进展: 鉴定, 系统发育学和分子生态学[J]. 生态学报, 2011, 31(11): 3197-3207. |
| [10] |
MEHL JWM, SLIPPERS B, ROUX J, WINGFIELD MJ. Botryosphaeriaceae associated with die-back of Schizolobium parahyba trees in South Africa and Ecuador[J]. Forest Pathology, 2014, 44(5): 396-408. DOI:10.1111/efp.12116 |
| [11] |
MANAWASINGHE IS, PHILLIPS AJL, HYDE KD, CHETHANA KWT, ZHANG W, ZHAO WS, YAN JY, LI XH. Mycosphere essays 14: assessing the aggressiveness of plant pathogenic Botryosphaeriaceae[J]. Mycosphere, 2016, 7(7): 883-892. DOI:10.5943/mycosphere/si/1b/7 |
| [12] |
TIBPROMMA S, HYDE KD, MCKENZIE EHC, BHAT DJ, PHILLIPS AJL, WANASINGHE DN, SAMARAKOON MC, JAYAWARDENA RS, DISSANAYAKE AJ, TENNAKOON DS, DOILOM M, PHOOKAMSAK R, TANG AMC, XU JC, MORTIMER PE, PROMPUTTHA I, MAHARACHCHIKUMBURA SSN, KHAN S, KARUNARATHNA SC. Fungal diversity notes 840-928: micro-fungi associated with Pandanaceae[J]. Fungal Diversity, 2018, 93(1): 1-160. DOI:10.1007/s13225-018-0408-6 |
| [13] |
HYDE KD, JONES EBG, LIU JK, ARIYAWANSA H, BOEHM E, BOONMEE S, BRAUN U, CHOMNUNTI P, CROUS PW, DAI DQ, DIEDERICH P, DISSANAYAKE A, DOILOM M, DOVERI F, HONGSANAN S, JAYAWARDENA R, LAWREY J, LI YM, LIU YX, LÜCKING R, et al. Families of Dothideomycetes[J]. Fungal Diversity, 2013, 63: 1-313. DOI:10.1007/s13225-013-0263-4 |
| [14] |
PHILLIPS ALL, ALVES A, ABDOLLAHZADEH J, SLIPPERS B, WINGFIELD MJ, GROENEWALD JZ, CROUS PW. The Botryosphaeriaceae: genera and species known from culture[J]. Studies in Mycology, 2013, 76(1): 51-167. |
| [15] |
PHILLIPS AJL, HYDE KD, ALVES A, LIU JK. Families in Botryosphaeriales: a phylogenetic, morphological and evolutionary perspective[J]. Fungal Diversity, 2019, 94(1): 1-22. DOI:10.1007/s13225-018-0416-6 |
| [16] |
SLIPPERS B, BOISSIN E, PHILLIPS AJL, GROENEWALD JZ, LOMBARD L, WINGFIELD MJ, POSTMA A, BURGESS T, CROUS PW. Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework[J]. Studies in Mycology, 2013, 76(1): 31-49. |
| [17] |
DISSANAYAKE AJ, CAMPORESI E, HYDE KD, PHILLIPS AJL, FU CY, YAN JY, LIU XH. Dothiorella species associated with woody hosts in Italy[J]. Mycosphere, 2016, 7(1): 51-63. DOI:10.5943/mycosphere/7/1/6 |
| [18] |
CHEN YY. Taxonomic and Phylogenetic studies of microfungi from Guizhou Karst areas[D]. Guiyang: Doctoral Dissertation of Guizhou University, 2020 (in Chinese). 陈娅娅. 贵州喀斯特地区微型真菌分类与系统发育研究[D]. 贵阳: 贵州大学博士学位论文, 2020. |
| [19] |
LI L, ZHANG B, FAN L, LIAO T, HONG YC. Isolation, identification and antagonistic bacterium screening of a fungal pathogen Botryosphaeria dothidea isolate infecting tea plant leaves[J]. Journal of Ningde Teachers College (Natural Science Edition), 2023, 35(1): 75-80. (in Chinese) 李力, 张渤, 范俐, 廖婷, 洪永聪. 侵染茶树的葡萄座腔菌分离鉴定及其拮抗菌筛选[J]. 宁德师范学院学报(自然科学版), 2023, 35(1): 75-80. |
| [20] |
LI GQ, LIU FF, LI JQ, LIU QL, CHEN SF. Botryosphaeriaceae from Eucalyptus plantations and adjacent plants in China[J]. Persoonia, 2018, 40: 63-95. DOI:10.3767/persoonia.2018.40.03 |
| [21] |
WANG YX, ZHOU T, ZHOU YW, LI QS, WANG H, SHAN HY. Isolation and identification of a pathogen causing pear ring spot in Tianjin[J]. Tianjin Agricultural Sciences, 2023, 29(9): 42-46, 52. (in Chinese) 汪宇轩, 周涛, 周逸文, 李齐升, 王卉, 单宏英. 天津地区梨轮纹病病原菌的分离与鉴定[J]. 天津农业科学, 2023, 29(9): 42-46, 52. |
| [22] |
WANG YF. Study on the diversity of pathogenic fungi and mycovirues in the Boryosphaeriaceae from fruit tree branches[D]. Zhengzhou: Doctoral Dissertation of Henan Agricultural University, 2023 (in Chinese). 王彦芬. 果树枝干葡萄座腔菌科病原真菌及其真菌病毒多样性研究[D]. 郑州: 河南农业大学博士学位论文, 2023. |
| [23] |
XIAO F. Gene function of Bklip1 and identification of Botryosphaeria causing pear ring rot in China[D]. Wuhan: Doctoral Dissertation of Huazhong Agricultural University, 2022 (in Chinese). 肖峰. 我国梨轮纹病病原鉴定与BkLiP1基因功能研究[D]. 武汉: 华中农业大学博士学位论文, 2022. |
| [24] |
XIAO XE. Diversity of fungi causing Citrus branch diseases in China[D]. Hangzhou: Doctoral Dissertation of Zhejiang University, 2022 (in Chinese). 肖小娥. 中国柑橘枝干真菌性病害病原多样性[D]. 杭州: 浙江大学博士学位论文, 2022. |
| [25] |
CHU RT, DOU ZP, HE W, ZHANG Y. Two novel species of Botryosphaeria causing stem canker of blueberries from China (in English)[J]. Mycosystema, 2021, 40(3): 473-486. 褚睿天, 豆志鹏, 贺伟, 张英. 引起蓝莓茎溃疡病的葡萄座腔菌属Botryosphaeria二新种(英文)[J]. 菌物学报, 2021, 40(3): 473-486. |
| [26] |
ZHOU YY, ZHANG W, LI XH, JIA JY, WANG J, ZHANG KC, YAN JY. Identification of Botryosphaeria dothidea associated with cherry leaf spot disease[J]. Acta Phytopathologica Sinica, 2021, 51(4): 636-640. (in Chinese) 周悦妍, 张玮, 李兴红, 贾静怡, 王晶, 张开春, 燕继晔. 引起甜樱桃叶斑病的葡萄座腔菌的鉴定[J]. 植物病理学报, 2021, 51(4): 636-640. |
| [27] |
WANG F, ZHAO L, LI GH, HUANG JB, HSIANG T. Identification and characterization of Botryosphaeria spp. causing gummosis of peach trees in Hubei Province, central China[J]. Plant Disease, 2011, 95(11): 1378-1384. DOI:10.1094/PDIS-12-10-0893 |
| [28] |
YAN JY, XIE Y, ZHANG W, WANG Y, LIU JK, HYDE KD, SEEM RC, ZHANG GZ, WANG ZY, YAO SW, BAI XJ, DISSANAYAKE AJ, PENG YL, LI XH. Species of Botryosphaeriaceae involved in grapevine dieback in China[J]. Fungal Diversity, 2013, 61(1): 221-236. DOI:10.1007/s13225-013-0251-8 |
| [29] |
TANG W, DING Z, ZHOU ZQ, WANG YZ, GUO LY. Phylogenetic and pathogenic analyses show that the causal agent of apple ring rot in China is Botryosphaeria dothidea[J]. Plant Disease, 2012, 96(4): 486-496. DOI:10.1094/PDIS-08-11-0635 |
| [30] |
XU C, WANG CS, JU LL, ZHANG R, BIGGS AR, TANAKA E, LI BZ, SUN GY. Multiple locus genealogies and phenotypic characters reappraise the causal agents of apple ring rot in China[J]. Fungal Diversity, 2015, 71(1): 215-231. DOI:10.1007/s13225-014-0306-5 |
| [31] |
ZHOU YP, DOU ZP, HE W, ZHANG XD, ZHANG Y. Botryosphaeria sinensia sp. nov., a new species from China[J]. Phytotaxa, 2016, 245(1): 43-50. DOI:10.11646/phytotaxa.245.1.4 |
| [32] |
FAN XL, HYDE KD, LIU JK, LIANG YM, TIAN CM. Multigene phylogeny and morphology reveal Phaeobotryon rhois sp. nov. (Botryosphaeriales, Ascomycota)[J]. Phytotaxa, 2015, 205(2): 90-98. DOI:10.11646/phytotaxa.205.2.2 |
| [33] |
PAN M, ZHU HY, BEZERRA JDP, BONTHOND G, TIAN CM, FAN XL. Botryosphaerialean fungi causing canker and dieback of tree hosts from Mount Yudu in China[J]. Mycological Progress, 2019, 18(11): 1341-1361. DOI:10.1007/s11557-019-01532-z |
| [34] |
ZHU HY, TIAN CM, FAN XL. Studies of Botryosphaerialean fungi associated with canker and dieback of tree hosts in Dongling Mountain of China[J]. Phytotaxa, 2018, 348(2): 63-76. DOI:10.11646/phytotaxa.348.2.1 |
| [35] |
WIJAYAWARDENE NN, PHILLIPS AJL, TIBPROMMA S, DAI DQ, SELBMANN L, MONTEIRO JS, APTROOT A, FLAKUS A, RAJESHKUMAR KC, COLEINE C, PEREIRA DS, FAN X, ZHANG L, MAHARACHCHIKUMBURA SSN, SOUZA MF, KUKWA M, SUWANNARACH N, RODRIGUEZ-FLAKUS P, ASHTEKAR N, DAUNER L, et al. Looking for the undiscovered asexual taxa: case studies from lesser studied life modes and habitats[J]. Mycosphere, 2021, 12(1): 1290-1333. |
| [36] |
SENANAYAKE IC, RATHNAYAKA AR, MARASINGHE DS, CALABON MS, GENTEKAKI E, LEE HB, HURDEAL VG, PEM D, DISSANAYAKE LS, WIJESINGHE SN, BUNDHUN D, NGUYEN TT, GOONASEKARA ID, ABEYWICKRAMA PD, BHUNJUN CS, JAYAWARDENA RS, WANASINGHE DN, JEEWON R, BHAT DJ, XIANG MM. Morphological approaches in studying fungi: collection, examination, isolation, sporulation and preservation[J]. Mycosphere, 2020, 11(1): 2678-2754. |
| [37] |
WHITE TJ, BRUNS TD, LEE SB, TAYLOR JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics[A]//INNI MA, GELFAND DH, SNINSKY JJ, WHITE TJ (eds. ) PCR Protocols: A Guide to Methods and Applications[M]. San Diego, USA: Academic Press, 1990: 315-322.
|
| [38] |
VILGALYS R, HESTER M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species[J]. Journal of Bacteriology, 1990, 172(8): 4238-4246. |
| [39] |
ALVES A, CROUS PW, CORREIA A, PHILLIPS AJL. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae[J]. Fungal Diversity, 2008, 28(2): 1-13. |
| [40] |
HALL TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT[J]. Nucleic Acids Symposium Series, 1999, 41(41): 95-98. |
| [41] |
KATOH K, STANDLEY DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability[J]. Molecular Biology and Evolution, 2013, 30(4): 772-780. |
| [42] |
LARSSON A. AliView: a fast and lightweight alignment viewer and editor for large datasets[J]. Bioinformatics, 2014, 30(22): 3276-3278. |
| [43] |
SWOFFORD DL. PAUP: Phylogenetic Analysis Using Parsimony (and Other Methods), Version 4.0b10[M]. Sunderland, Massachusetts: Sinauer Associates Inc., 2002.
|
| [44] |
STAMATAKIS A. RAxML-Ⅵ-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models[J]. Bioinformatics, 2006, 22(21): 2688-2690. |
| [45] |
STAMATAKIS A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies[J]. Bioinformatics, 2014, 30(9): 1312-1313. |
| [46] |
RONQUIST F, HUELSENBECK JP. MrBayes 3: Bayesian phylogenetic inference under mixed models[J]. Bioinformatics, 2003, 19(12): 1572-1574. |
| [47] |
DARANAGAMA DA, THAMBUGALA KM, CAMPINO B, ALVES A, BULGAKOV TS, PHILLIPS AJL, LIU XZ, HYDE KD. Phaeobotryon negundinis sp. nov. (Botryosphaeriales) from Russia[J]. Mycosphere, 2016, 7(7): 933-941. |
 2024, Vol. 51
2024, Vol. 51




