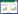扩展功能
文章信息
- 李菲菲, 杨文迪, 陈吕军, 温东辉
- LI Feifei, YANG Wendy, CHEN Lyujun, WEN Donghui
- 环境抗生素耐药性风险评价中最小抑菌浓度的研究进展
- Advancements in minimum inhibitory concentration (MIC) for risk assessment of environmental antimicrobial resistance
- 微生物学通报, 2024, 51(12): 4984-5005
- Microbiology China, 2024, 51(12): 4984-5005
- DOI: 10.13344/j.microbiol.china.240589
-
文章历史
- 收稿日期: 2024-07-14
- 接受日期: 2024-11-28
- 网络首发日期: 2024-12-05
2. Faculty of Science, University of British Columbia, Vancouver V6T 1Z4, Canada;
3. 北京大学 环境科学与工程学院, 北京 100087
2. Faculty of Science, University of British Columbia, Vancouver V6T 1Z4, Canada;
3. College of Environmental Sciences and Engineering, Peking University, Beijing 100087, China
抗生素的大量生产及使用造成了环境中抗生素耐药菌(antibiotic resistant bacteria, ARB)和耐药基因(antibiotic resistance gene, ARG)日益增加,导致抗生素耐药性(antimicrobial resistance, AMR)问题成为全球公共卫生最严重的威胁之一[1]。因此,目前对AMR问题的研究热点不局限于医学领域,已经引起各研究领域的广泛关注。人类医疗、动物养殖、农业和环境被确定为与AMR产生密切相关的4个领域[2]。在环境领域,受到抗生素选择压力的介质包括污水处理系统、河流、土壤和沉积物等,这些介质中细菌种类丰富,数量极高,加上环境中抗生素浓度较低,不足以完全抑制或杀死细菌,细菌长期处于低浓度抗生素的亚抑制作用下,非常有利于ARB和ARG的产生、富集和传播[1, 3]。
为了控制环境中的AMR风险,首先需要表征和量化在环境抗生素浓度水平下细菌被诱导产生、富集和传播ARG的可能性大小,即进行AMR风险评估。AMR风险评估在极大程度上依赖不同抗生素对细菌的最小抑菌浓度(minimum inhibitory concentration, MIC)。利用MIC监测数据有助于了解环境中AMR现状及趋势,确定需要干预的重点区域和领域等。然而,世界许多地区缺乏适当的MIC监测,导致AMR的基础数据信息存在巨大缺口,共享和比较耐药性信息的能力极度欠缺。本文经过文献调研和数据库分析,旨在全面总结不同测试方法获得的MIC数据差异性及可比性,梳理与AMR风险评估直接相关的MIC基础数据研究现状,讨论不同类别细菌对抗生素MIC数据的异同,分析不同种类抗生素MIC数据的差异和缺陷等,以期为MIC研究及环境中抗生素AMR风险评价提供参考和支持。
1 环境成为抗生素耐药性问题的热点研究领域抗生素耐药性的形成是每种药物均会产生的自然生物过程,也是微生物防御机制的形成过程[4]。为应对抗生素的胁迫,细菌通过新基因获取或基因突变,对某些抗生素由本来的敏感状态自发地转变为耐受状态[5]。然而,抗生素在人类医疗、畜牧养殖等大量使用,并持续向环境中排放,实际上加速了ARG的产生和传播[6-7]。
随着抗生素生产、动物养殖等活动的规模化发展,大量抗生素、ARB和ARG通过污/废水、粪便等形式排入环境中,对环境微生物造成了极大的选择压力。研究证明含抗生素废水的输入导致环境中ARB的比例增大[8],说明抗生素在诱导细菌产生耐药性方面起到了至关重要的作用。另有研究发现,在接受抗生素生产废水排放的河流中,筛选出的细菌40%以上具有多重耐药性[9]。Li等[10]证明环境中抗生素浓度通常低于抑菌浓度,更容易诱发产生ARG。环境中微生物种类繁多,细菌量极高,ARB和ARG来源丰富、分布广泛,通过多种暴露途径危害人体健康的不确定性极高。尤其是整合子、转座子、质粒和噬菌体等可移动遗传元件(mobile genetic element, MGE)介导的水平基因转移(horizontal gene transfer, HGT),极大地驱动了ARG在不同微生物间传播扩散的频率[11]。加之,ARG兼具“可复制或传播”的生物特性和“不易消亡或环境持久”的物理化学特性,因此,环境成为了一个巨大的ARG库,ARG在环境中不断传递和循环,并转移到人类共生微生物和病原体上,威胁人类健康[12-13]。
近年来,临床及环境中致病菌耐药性不断增长和扩散,如,耐甲氧西林金黄色葡萄球菌(methicillin-resistant Staphylococcus aureus, MRSA)、泛耐药鲍曼不动杆菌(pandrug-resistant Acinetobacter baumannii, PRAB)、碳青霉烯类耐药铜绿假单胞菌(carbapenem-resistant Pseudomonas aeruginosa, CRPA)等典型多重耐药菌的出现,使公共卫生安全面临着巨大挑战[11]。据调查,欧洲地区每年约有2.5万因多重耐药性感染而死亡的案例[14];在非洲坦桑尼亚某地医院,死于ARB感染的血液病例高达40%[15]。因此,2015年,世界卫生组织(World Health Organization, WHO)将AMR问题列为21世纪人类在健康领域面临的最大挑战之一。2017年联合国环境规划署(United Nations Environment Programme, UNEP)将AMR问题列为六大新兴环境问题之首[16],环境成为抗生素AMR问题的热点研究领域。
2 环境中抗生素耐药性风险评价现状环境中通常野生型和耐药突变型菌株同时存在,当抗生素浓度低于最小抑菌浓度MIC时,耐药突变菌株生长速度高于野生菌株并成为优势菌[17]。在低于MIC的浓度中,Andersson等[18]认为,有一个最小选择浓度(minimal selective concentration, MSC)会触发细菌的耐药性,即抗生素浓度高于MSC时,便可选择性扩增耐药突变菌株,使其成为优势菌群,进而产生细菌耐药性。环境介质中抗生素浓度一般比较低,通常大部分抗生素浓度低于MIC,因此,在长期低水平暴露中,细菌被诱导产生耐药性的风险是一个亟须关注的问题。
由于缺乏关键的变量数据,目前多针对特定的ARB或者ARG利用模型获取关键数据,开展以定性为主的风险评估[9]。有研究者基于MSC尝试外推了风险熵(risk quotient, RQ)评价法,得到抗生素AMR风险熵(RQR),即RQR= MEC/MSC,其中MEC为检测的环境浓度(measured environmental concentration, MEC)[19]。我们以往的研究利用该方法进行了抗生素的AMR风险评价,证明了耐药性风险是中国海洋环境中抗生素的最大风险,50%以上的抗生素具有一定耐药性风险,其中阿莫西林(amoxicillin)、环丙沙星(ciprofloxacin)、恩诺沙星(enrofloxacin)、诺氟沙星(norfloxacin)、氧氟沙星(ofloxacin)、克林霉素(clindamycin)、氧四环素(oxytetracycline)、四环素(tetracycline)及红霉素(erythromycin)具有高耐药性风险[20]。另有研究指出,环丙沙星、恩诺沙星、四环素、阿莫西林和诺氟沙星在水产养殖水体中具有高耐药性风险(RQR > 1)[21-22]。
耐药性风险熵评价法依赖每种抗生素对细菌的MSC值,但是通过试验获得MSC值的过程比较烦琐且耗时长。研究指出,根据抗生素和细菌耐药突变类型的不同,MSC与MIC的比值大约介于1/4−1/230,例如链霉素(streptomycin)的MSC值为敏感菌株MIC值的1/4,四环素的MSC值为MIC的1/100,环丙沙星的MSC值为MIC的1/10−1/230[23]。也有研究认为MSC与MIC比值集中在1/16−1/32[19]。由于环境中检出的抗生素种类多,且大量种类细菌共存,有研究建议取MIC的1/16作为MSC值[20]。此外,以往在临床及环境等领域关于抗生素AMR的研究还暴露出很多问题:MIC数据量有限,已测试的抗生素种类不足,受试细菌数量也有限,不同抗生素的MIC因受试细菌不同而可比性差。这些问题导致MIC基础数据无法满足研究需求,因此,有必要全面梳理目前关于细菌对抗生素MIC的研究现状,为后续相关研究提供支持和建议。
3 不同测试方法对MIC的影响为了更真实地反映AMR的出现和发展趋势,有必要将现有的表征方法统一并标准化,以便与国家或国际监测数据比较。目前常用的抗生素敏感性试验方法主要有试管倍比稀释法、纸片琼脂扩散法(K-B法)、牛津杯法、琼脂稀释法、肉汤微稀释法及梯度扩散法(E-test)等。本研究通过文献调研发现,琼脂稀释法、肉汤微稀释法和E-test法是目前最常用的3种定量测试方法,大肠杆菌(Escherichia coli) (G−)、铜绿假单胞菌(P. aeruginosa) (G−)、金黄色葡萄球菌(S. aureus) (G+)及肺炎葡萄球菌(S. pneumoniae) (G+)在3种常用测试方法下的MIC数据量最多。图 1展示了分别利用琼脂稀释法[24-59]、肉汤微稀释法[31, 55, 60-148]和E-test法[32, 61, 134, 148-152]测试上述4种细菌的MIC所获得的结果。
|
| 图 1 基于不同测试方法的典型细菌MIC分布及E. coli (A)、P. aeruginosa (B)、S. aureus (C)、S. pneumoniae (D) MIC测试方法比例 Figure 1 MIC distribution of typical bacteria based on different test methods and the proportion of MIC test methods for E. coli (A), P. aeruginosa (B), S. aureus (C), and S. pneumoniae (D). |
|
|
由图 1A–1D可见,一半以上(55%−71%)的MIC数据由肉汤微稀释法测得,约21%−33%由琼脂稀释法测得,采用E-test法进行MIC数据测试相对较少(8%−12%)。有研究表明与琼脂稀释法相比,E-test法测试出的MIC值一般较低[24]。也有研究证明肉汤微稀释法测出的MIC值一般比E-test法高[25]。还有研究发现E-test法测出的万古霉素MIC值始终比肉汤或琼脂稀释法确定的MIC高1倍[26]。另有研究表明,E-test和琼脂稀释法之间的一致性水平取决于所测试的抗生素种类[24]。然而,本研究图 1显示,同一种细菌分别采用3种方法测试所得MIC数据离散度有所差异,但中位数没有数量级上的差别,这与已有研究一致[153-154]。因此,不同方法所测得的MIC值均可以用于AMR风险评价中。表 1总结了3种方法的基本步骤和优缺点。
由表 1可见,肉汤微稀释法优势明显,可对多种抗生素或多种细菌同时进行试验,可手动或自动化,测试精度高且费用低,该技术已被推荐用于大肠杆菌的抗菌药物敏感性测试[24]。琼脂稀释法可以准确测定MIC值,可同时测试多种生物体对单一抗菌剂耐受能力,被称为抗菌药物敏感性测试的金标准技术。然而,肉汤微稀释法和琼脂稀释法都需要大量的手动操作,需要对人员进行广泛的培训,并且相对耗时耗力。E-test法操作简单,MIC值判读清晰,更适于临床实验室进行MIC值的判定应用。综上所述,3种方法都是测定细菌对抗生素药物敏感性的有效方法,建议根据试验条件选择合适的测试方法。琼脂稀释法适用于需要高精度和可靠结果的研究,肉汤微稀释法适用于快速筛查大量样本,E-test法适合快速获得试验结果。
| 方法Method | 基本介绍Basic introduction | 优缺点Merit and demerit |
| 肉汤微稀释法 Broth microdilution |
肉汤微稀释法是指在96孔细胞培养板里用液体肉汤连续两倍稀释某种抗生素,然后将标准化数量的细菌注入到对应微孔里。经过一夜培养后,无细菌生长痕迹的(颜色或者黏稠度变化),且含抗生素浓度最低的微孔即为最低抑菌浓度 Broth microdilution entails continuous double dilution of an antibiotic in liquid broth within a 96-well cell culture plate, followed by the injection of a standardized number of bacteria into the corresponding micropores. After an overnight culture, the micropores showing no sign of bacterial growth (such as color or viscosity change) and having the lowest concentration of antibiotics represent the minimum inhibitory concentration |
优点: 药品种类或浓度选择自由度高; 成本低,无须大量试剂; 可对多种抗生素和细菌同时试验; 可手动或自动化,如MBD Sensititre System 缺点: 操作复杂; 耗时、耗力 Merit: High freedom of choice of drug type or concentration; Low cost, no need for a large number of reagents; Simultaneous testing of multiple antibiotics and bacteria; Manual or automated, such as MBD Sensititre System Demerit: Complex operation; Time-consuming and labor-intensive |
| 琼脂稀释法 Agar dilution |
琼脂稀释法是指将不同浓度的抗生素(通常采用连续两倍稀释法)加入到未固化的琼脂培养基,然后将标准化数量的细菌按点添加到琼脂板表面。经过一夜培养后,无细菌生长痕迹且含抗生素浓度最低的琼脂板即为最低抑菌浓度 The agar dilution method implies the addition of antibiotics at varying concentrations (typically through the continuous double dilution approach) to the uncured agar medium. Subsequently, a standardized quantity of bacteria is applied to the surface of the agar plate by spotting. After overnight culture, the agar plate showing no evidence of bacterial growth and with the lowest concentration of antibiotics constitutes the minimum inhibitory concentration |
优点: 药品种类或浓度选择自由度高; 可同时测试不同菌株; 适用于会以其颜色掩盖液体培养基生物生长的药物[155] 缺点: 不能同时测多种抗生素; 操作复杂; 连续稀释耗时、耗力、耗试剂[24] Merit: High freedom of choice of drug type or concentration; Different strains can be tested simultaneously; For drugs that mask biological growth in liquid medium with their color[155] Demerits: Cannot test for multiple antibiotics at the same time; Complex operation; Continuous dilution consumes time, power and reagents[24] |
| E-test | E-test是将多个涂有预定抗生素浓度梯度的试条放置在已接种了测试菌的琼脂表面上。经过一夜培养后,会在试条周围形成椭圆形抑菌区。抑菌区与试条上最小刻度的交叉点即为最低抑菌浓度 E-test involves placing multiple strips coated with a predetermined antibiotic concentration gradient on an agar surface that has been inoculated with the test bacteria. After overnight culture, an oval antibacterial zone is formed around the test strip. The intersection point between the inhibitory zone and the minimum scale on the test strip is the minimum inhibitory concentration |
优点: 简单且易操作; 可测两种不同抗生素的联合作用; 对细菌耐药表型敏感度高[156] 缺点: 精确度相对低[152]; 仍缺乏很多种类抗生素的试条; 价格贵[153] Merits: Simple and easy to operate; The combined action of two different antibiotics can be measured; High sensitivity to bacterial resistance phenotypes[156] Demerits: Relatively low accuracy[152]; There is still a lack of test strips for many types of antibiotics; Expensive[153] |
在医学领域,MIC是抗生素对细菌的最小抑菌浓度,二者一一对应。与医学领域不同,实际环境中众多抗生素与细菌共存。即每种细菌承受多种抗生素的选择压力,同时每种抗生素对多种细菌产生胁迫。因此,从环境实际情况出发,我们统计了多种抗生素对一种细菌,以及多种细菌对一种抗生素MIC值的分布范围和特征,旨在厘清不同细菌对抗生素耐药性的差异,以及细菌对不同抗生素的耐药性强弱。
4.1 不同类别细菌对抗生素的耐药性分析由于细菌细胞壁的成分和结构不同,将细菌分为革兰氏阳性菌(G+)和革兰氏阴性菌(G−)。根据细菌分类的不同,比较分析不同类别细菌对抗生素的MIC数据,有助于指导抗生素的临床使用及环境耐药性风险评估。MIC数据来源于EUCAST数据库(https://mic.eucast.org/),该数据库MIC数值来自全球各个国家的耐药性研究和监测项目、制药行业、兽医项目及个别实验室。在接收MIC数据时,EUCAST数据库并不考虑所使用的测试方法。另外,如果某些细菌对某种或某类抗生素具有内在耐药性,则EUCAST系统里没有其抗菌敏感性实验数据。例如G−对糖肽类(如万古霉素)具有内在耐药性,而G+对其无内在耐药性[157],所以EUCAST数据库里只有G+对万古霉素的MIC数据。
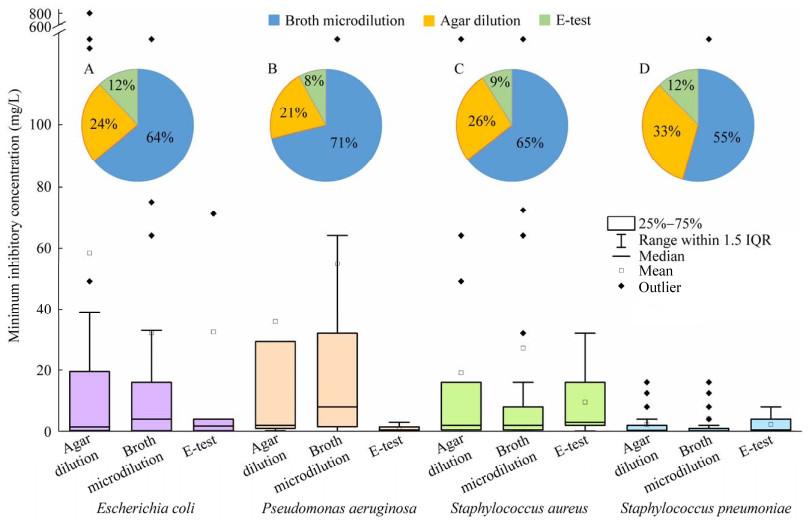
经统计,EUCAST数据库共收集了136种G−的MIC数据,G−的平均数据量为35 742条,其中大肠杆菌的测试数据量最多,为1 140 155条。该数据库收集了132种G+的MIC数据,G+的平均数据量为22 825条,其中金黄色葡萄球菌(S. aureus)的测试数据量最多,为949 203条。总体来说,G−的MIC数据量远大于G+,说明目前的研究更多关注G−的耐药性问题。图 2总结了G−和G+中数据量位列前15的细菌MIC分布。
|
| 图 2 典型革兰氏阴性菌和阳性菌的MIC分布 Figure 2 MIC distribution of typical Gram-negative and positive bacteria. |
|
|
由图 2可见,G+的MIC中位数的平均值(1.51 mg/L)高于G− (1.14 mg/L)。G−来自的属比较多样化,其中鲍曼不动杆菌(Acinetobacter baumannii)的MIC中位数最高,为8 mg/L,对抗生素的耐药性较强,其他细菌的MIC中位数在0.125−2.000 mg/L。鲍曼不动杆菌MIC的四分位距(interquartile range, IQR)跨度也最大,区间范围为1−32 mg/L,MIC数值较离散。其他菌种MIC的IQR跨度较小,区间范围均小于0−8 mg/L,MIC数值相对集中。G+主要包含葡萄球菌属(Staphylococcus) (6种)和链球菌属(Streptococcus) (3种),其中屎肠球菌(Enterococcus faecium)的MIC中位数最高,为8 mg/L,对抗生素的耐药性较强,其他细菌的MIC中位数均介于0.06−4.00 mg/L。屎肠球菌MIC的IQR跨度也最大,区间范围为1−64 mg/L,MIC数值较离散;其次为粪肠球菌(E. faecalis)、溶血葡萄球菌(S. haemolyticus),伪中间葡萄球菌(S. pseudintermedius)和鸟类分枝杆菌(Mycobacterium avium),区间范围均为0−16 mg/L;其他菌种MIC的IQR跨度较小,区间范围均小于0−8 mg/L,MIC数值相对集中。
综上所述,细菌中耐药性最强的G−和G+分别是鲍曼不动杆菌和屎肠球菌,这意味着在临床治疗中,针对鲍曼不动杆菌和屎肠球菌的抗生素有效性降低,治疗难度大;在环境中,它们具备高度适应抗生素的能力,比环境中其他细菌的竞争力强,AMR传播风险更大。大多数细菌对抗生素的MIC中位数不超过2 mg/L,并且对抗生素的MIC值IQR跨度集中在0−8 mg/L。这些细菌对抗生素敏感性较强,临床治疗效果好,但是随着抗生素的持续使用,无论是医疗环境还是自然环境,它们产生AMR的风险较大。
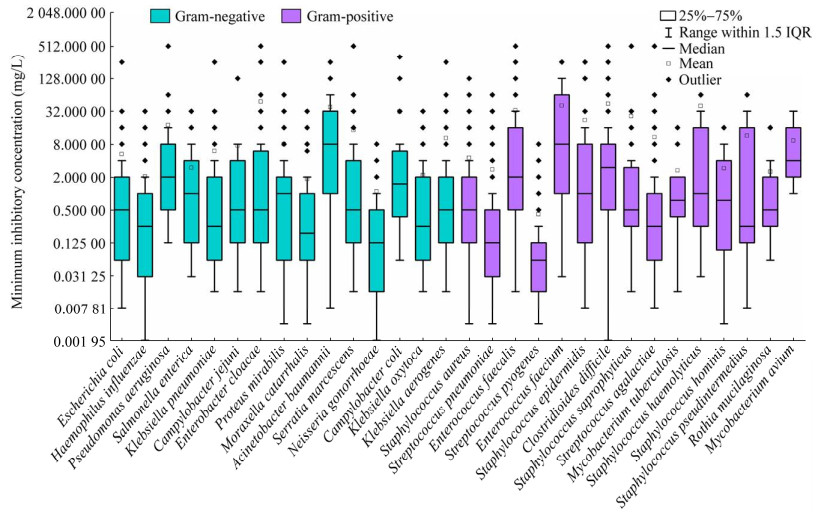
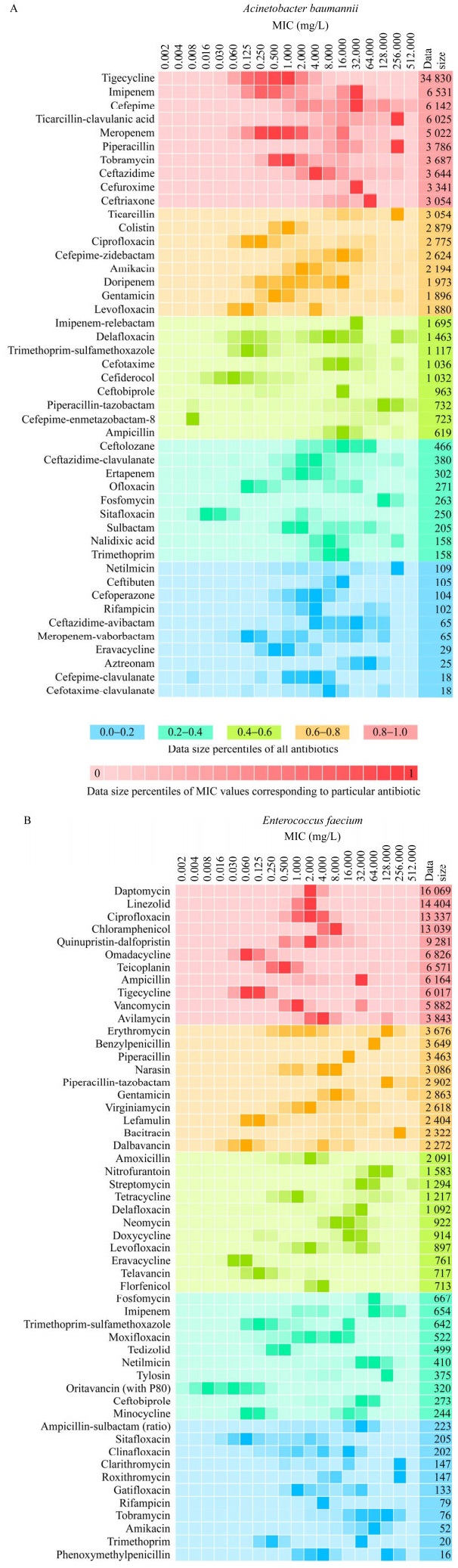
4.2 典型细菌对抗生素的MIC分析鲍曼不动杆菌和屎肠球菌分别是耐药性最强的G−和G+,图 3展示了2种细菌对不同抗生素的MIC数据的详细研究情况。
|
| 图 3 鲍曼不动杆菌(A)和屎肠球菌(B)对抗生素的MIC数据分布 Figure 3 MIC distribution of antibiotics for A. baumannii (A) and E. faecium (B). The MIC data is organized from high to low, with the red group representing the top 20%, followed by the orange group (20%−40%), the light green group (40%−60%), the dark green group (60%−80%), and the blue group representing the bottom 20%. The shade of each color within these groups indicates the volume of data, with darker shades representing larger amounts of data. MIC数据总量按照从高到低排列,红色系是前20%,其次是橙色系(20%−40%)、草绿色系(40%−60%)、蓝绿色系(60%−80%)、蓝色系是后20%. 各个色系内部颜色的深浅表示数据量的多少,颜色越深则数据量越大 |
|
|
由图 3可见,鲍曼不动杆菌有46种抗生素的MIC数据,主要集中于β-内酰胺类抗生素,例如亚胺培南(imipenem)、头孢吡肟(cefepime)、替卡西林-克拉维酸(ticarcillin-clavulanic acid)、美罗培南(meropenem)、哌拉西林(piperacillin)、头孢他啶(ceftazidime)、头孢呋辛(cefuroxime)、头孢曲松(ceftriaxone)。鲍曼不动杆菌对替卡西林-克拉维酸、哌拉西林、替卡西林(ticarcillin)、奈替米星(netilmicin)的MIC最高,可达256 mg/L,而对复合头孢吡肟(cefepime-enmetazobactam)的耐药性最低,MIC仅为0.008 mg/L。屎肠球菌有53种抗生素的MIC数据,它对杆菌肽(bacitracin)、克拉霉素(clarithromycin)和罗红霉素(roxithromycin)耐药性最强,MIC为256 mg/L,而对奥利万星(oritavancin)耐药性最弱,MIC为0.008 mg/L。因此,当以鲍曼不动杆菌和屎肠球菌为目标细菌进行环境抗生素耐药性风险评价时,应分别优先考虑复合头孢吡肟、奥利万星等MIC低的抗生素,它们未来可能产生较高的细菌耐药性风险。
针对鲍曼不动杆菌MIC研究最多的是替加环素(tigecycline),它是一种四环素的半合成衍生物,于2005年首次在美国上市。针对屎肠球菌研究最多的是达托霉素(daptomycin),它是继万古霉素之后的第二代糖肽类抗生素。为了确保抗生素的治疗效果,2003年底,美国食品与药物管理局(Food and Drug Administration, FDA)经过快速审理程序批准注射用达托霉素。在数据量居前25%的11种抗生素中,利奈唑胺(linezolid)、奎奴普丁-达福普汀(quinupristin-dalfopristin)、替加环素和甲苯磺酸奥玛环素(omadacycline)分别于2000年、1999年、2005年和2020年上市。由此可见,目前对新抗生素的细菌耐药性研究关注度较大。旧抗生素在长期使用过程中,细菌已经对其产生了广泛的耐药性,因此更多的研究者将精力转向新抗生素。由于新抗生素具有独特的化学结构和作用机制,充分研究新抗生素的MIC分布特征,可以预测其排入环境后产生的AMR风险,以应对现有的环境抗生素耐药性问题。
4.3 不同种类抗生素对细菌的MIC分析EUCAST数据库每种抗生素数据是独立的,共收录了149种抗生素的MIC数据。将已被测试的抗生素按结构划分成喹诺酮类(quinolones)、β-内酰胺类(β-lactams)、大环内酯类(macrolides)、四环素类(tetracyclines)、氨基糖苷类(aminoglycosides)、糖肽类(glycopeptides)、脂糖肽类(lipoglycopeptides)、多肽类(polypeptides)、酰胺醇类(amphenicols)、磺胺类(sulfonamides)及其他类(others),这些类别涵盖了目前市面上常见的抗生素种类。根据统计需求,筛选出数据量高于30 000条的54种抗生素,作为该类别的代表性抗生素被纳入图 4,从而对比不同抗生素类别之间MIC的异同。
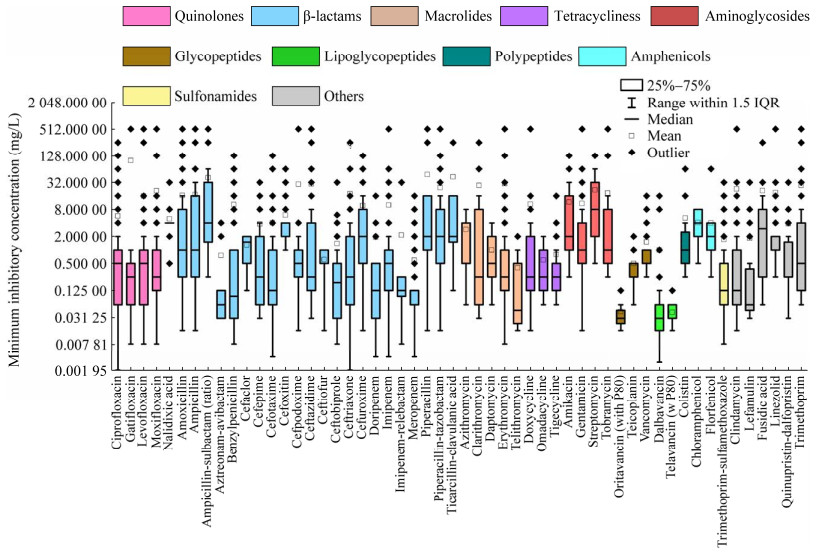
|
| 图 4 不同类别抗生素MIC的分布 Figure 4 Distribution of MIC values across different classes of antibiotics. |
|
|
由图 4所示,目前针对β-内酰胺类抗生素的研究数据量最多,54种抗生素中有22种属于β-内酰胺类。β-内酰胺类抗生素具有广谱抗菌作用,对多种常见细菌有效,副作用较少相对安全,是临床上广泛使用的抗生素之一。据报道,病原菌对青霉素类抗生素(属于β-内酰胺类)的耐药率高达70%以上[158]。研究数量最少的是磺胺类和多肽类,分别只有磺胺甲噁唑(sulfamethoxazole)和黏菌素(colistin)的MIC数据。磺胺类抗生素种类多,它们的结构中都含有磺胺基团(−SO2NH2),磺胺基团是磺胺类抗生素的关键结构特征和主要药理活性部位,因此,当其他磺胺类抗生素MIC数据缺失时,已有研究建议用磺胺甲噁唑的MIC替代[20]。多肽类抗生素对G−杆菌抗菌作用强,通常通过破坏细菌膜结构或细胞壁来起到杀菌作用,而其他抗生素可能通过不同的机制,比如抑制蛋白质合成、阻断核酸合成等[158]。因此,相较于其他抗生素,细菌对多肽类产生抗药性的可能性较低,这有可能是多肽类抗生素MIC数据少的主要原因。
氨苄西林钠(ampicillin-sulbactam)、链霉素和夫西地酸(fusidic acid)的MIC中位数高于其他抗生素,分别为4、8和4 mg/L,细菌对它们的耐药性较强,而对其他抗生素的MIC值均不超过2 mg/L。氨苄西林钠和链霉素的MIC值的IQR跨度最大,分别为1.75−32.00 mg/L和2−32 mg/L,MIC值离散。其次为氨苄西林(ampicillin)、哌拉西林、替卡西林-克拉维酸和阿米卡星(amikacin),MIC值的IQR跨度范围约0−16 mg/L,离散度较高。其他抗生素的MIC值IQR跨度不超过0−8 mg/L,MIC数值相对集中。
综上所述,目前针对MIC的研究集中在β-内酰胺类抗生素,磺胺类和多肽类抗生素研究最少。其中,细菌对氨苄西林钠、链霉素和夫西地酸的耐药性强,MIC中位数均不低于4 mg/L,因此上述抗生素在临床有效性减低,在环境中相关的ARG容易富集,具有较高的传播风险。细菌对其他抗生素的MIC值中位数不超过2 mg/L,因此其他抗生素可能会继续诱导产生ARG,进而在临床及环境中产生较高的AMR风险。
5 结论与展望(1) 在MIC测试方法中,肉汤微稀释法最常用,其次是琼脂稀释法和E-test法,3种测试方法对MIC值的影响不明显。
(2) 现有MIC研究更多关注G−的AMR问题,G−的MIC数据量远大于G+,但G+对抗生素的耐药性比G−更强,其中鲍曼不动杆菌和屎肠球菌分别是G−和G+中耐药性最强的细菌。
(3) 针对细菌MIC的研究集中在β-内酰胺类抗生素,磺胺类和多肽类研究最少。在所有抗生素中,细菌对氨苄西林钠、链霉素和夫西地酸的耐药性最强。
(4) EUCAST数据库中包含的细菌及抗生素数量远远不够,MIC基础数据远远不足,大量MIC数据缺失或单薄,极大地限制了医疗及环境耐药性风险评估的可靠性。
(5) 建议研究者或相关机构持续扩大MIC研究覆盖面,及时纳入已发现的细菌和已使用的抗生素,补充完善耐药性监测系统里缺失的MIC数据,积极进行耐药性信息数据收集和分析,促进耐药信息共享。
| [1] |
BERENDONK TU, MANAIA CM, MERLIN C, FATTA-KASSINOS D, CYTRYN E, WALSH F, BÜRGMANN H, SØRUM H, NORSTRÖM M, PONS MN, KREUZINGER N, HUOVINEN P, STEFANI S, SCHWARTZ T, KISAND V, BAQUERO F, MARTINEZ JL. Tackling antibiotic resistance: the environmental framework[J]. Nature Reviews Microbiology, 2015, 13(5): 310-317. |
| [2] |
PRESTINACI F, PEZZOTTI P, PANTOSTI A. Antimicrobial resistance: a global multifaceted phenomenon[J]. Pathogens and Global Health, 2015, 109(7): 309-318. |
| [3] |
MARTÍNEZ JL. Antibiotics and antibiotic resistance genes in natural environments[J]. Science, 2008, 321(5887): 365-367. |
| [4] |
LIU CX. Global concern: Strategies for antibiotic development and risk of resistance[J]. Chinese Journal of Antibiotics, 2019, 44(1): 1-8. (in Chinese) 刘昌孝. 全球关注: 重视抗生素发展与耐药风险的对策[J]. 中国抗生素杂志, 2019, 44(1): 1-8. |
| [5] |
LIU H, ZHANG GL, XU SY, ZHOU D. The research development of biotic resistance against bacteria in chickens[J]. Shandong Poultry, 2001(2): 32-34. (in Chinese) 刘辉, 张供领, 许胜勇, 周迪. 细菌对抗生素耐药性的国内外研究现状[J]. 山东家禽, 2001(2): 32-34. |
| [6] |
LI HN, YANG ZZ, HE XJ. Risk assessment of antimicrobial resistance[J]. China Environmental Science, 2024, 44(9): 5209-5221. (in Chinese) 李红娜, 杨珍珍, 何霄嘉. 抗微生物药物耐药性风险评估研究进展[J]. 中国环境科学, 2024, 44(9): 5209-5221. |
| [7] |
ZHANG T, LI B. Antibiotic resistance in water environment: frontiers of fundamental research, risk assessment and control strategies[J]. Chinese Science Bulletin, 2020, 65(24): 2543-2554. (in Chinese) 张彤, 李炳. 水环境中抗生素耐药性的科学研究前沿、环境健康风险评估和控制阻断策略[J]. 科学通报, 2020, 65(24): 2543-2554. |
| [8] |
LI D, YANG M, HU JY, ZHANG J, LIU RY, GU X, ZHANG Y, WANG ZY. Antibiotic-resistance profile in environmental bacteria isolated from penicillin production wastewater treatment plant and the receiving river[J]. Environmental Microbiology, 2009, 11(6): 1506-1517. |
| [9] |
SIDRACH-CARDONA R, HIJOSA-VALSERO M, MARTI E, BALCÁZAR JL, BECARES E. Prevalence of antibiotic-resistant fecal bacteria in a river impacted by both an antibiotic production plant and urban treated discharges[J]. Science of the Total Environment, 2014, 488: 220-227. |
| [10] |
LI S, ZHANG RJ, HU JR, SHI WZ, KUANG YZ, GUO XY, SUN WL. Occurrence and removal of antibiotics and antibiotic resistance genes in natural and constructed riverine wetlands in Beijing, China[J]. Science of the Total Environment, 2019, 664: 546-553. |
| [11] |
SU ZG, ZHANG Y, DAI TJ, CHEN JY, ZHANG YM, WEN DH. Antibiotic resistance genes and class 1 integron in the environment: research progress[J]. Microbiology China, 2018, 45(10): 2217-2233. (in Chinese) 苏志国, 张衍, 代天娇, 陈嘉瑜, 张永明, 温东辉. 环境中抗生素抗性基因与Ⅰ型整合子的研究进展[J]. 微生物学通报, 2018, 45(10): 2217-2233. |
| [12] |
SU JQ, HUANG FY, ZHU YG. Antibiotic resistance genes in the environment[J]. Biodiversity Science, 2013, 21(4): 481-487. (in Chinese) 苏建强, 黄福义, 朱永官. 环境抗生素抗性基因研究进展[J]. 生物多样性, 2013, 21(4): 481-487. |
| [13] |
KÜMMERER K. Antibiotics in the aquatic environment: a review-Part I[J]. Chemosphere, 2009, 75(4): 417-434. |
| [14] |
GYSSENS IC, van der MEER JWM. Required actions to control antimicrobial resistant healthcare-associated infections[M]//GOULD IM, van der MEER JWM, eds. Antibiotic Policies. New York, NY: Springer New York, 2011: 183-202.
|
| [15] |
BLOMBERG B, MANJI KP, URASSA WK, TAMIM BS, MWAKAGILE DSM, JUREEN R, MSANGI V, TELLEVIK MG, HOLBERG-PETERSEN M, HARTHUG S, MASELLE SY, LANGELAND N. Antimicrobial resistance predicts death in Tanzanian children with bloodstream infections: a prospective cohort study[J]. BMC Infectious Diseases, 2007, 7: 43. |
| [16] |
UNEP. Frontiers 2017 Emerging Issues of Environmental Concern[R]. United Nations Environment Programme, Nairobi, 2017.
|
| [17] |
WANG FZ, NI WT, CUI JC. Sub-inhibitory concentration of antimicrobial agents and its clinical implications[J]. Chinese Journal of Infection and Chemotherapy, 2017, 17(5): 597-601. (in Chinese) 王方舟, 倪文涛, 崔俊昌. 抗菌药物亚抑菌浓度及其临床意义[J]. 中国感染与化疗杂志, 2017, 17(5): 597-601. |
| [18] |
ANDERSSON DI, HUGHES D. Evolution of antibiotic resistance at non-lethal drug concentrations[J]. Drug Resistance Updates, 2012, 15(3): 162-172. |
| [19] |
BENGTSSON-PALME J, JOAKIM LARSSON DG. Concentrations of antibiotics predicted to select for resistant bacteria: proposed limits for environmental regulation[J]. Environment International, 2016, 86: 140-149. |
| [20] |
LI FF, BAO YY, CHEN LJ, SU ZG, TANG YS, WEN DH. Screening of priority antibiotics in Chinese seawater based on the persistence, bioaccumulation, toxicity and resistance[J]. Environment International, 2023, 179: 108140. |
| [21] |
HAN QF, ZHAO S, ZHANG XR, WANG XL, SONG C, WANG SG. Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China[J]. Environment International, 2020, 138: 105551. |
| [22] |
SUN M, CHANG ZQ, van den BRINK PJ, LI J, ZHAO FZ, RICO A. Environmental and human health risks of antimicrobials used in Fenneropenaeus chinensis aquaculture production in China[J]. Environmental Science and Pollution Research, 2016, 23(15): 15689-15702. |
| [23] |
GULLBERG E, CAO S, BERG OG, ILBÄCK C, SANDEGREN L, HUGHES D, ANDERSSON DI. Selection of resistant bacteria at very low antibiotic concentrations[J]. PLoS Pathogens, 2011, 7(7): e1002158. |
| [24] |
VARELA NP, FRIENDSHIP R, DEWEY C, VALDIVIESO A. Comparison of agar dilution and E-test for antimicrobial susceptibility testing of Campylobacter coil isolates recovered from 80 Ontario swine farms[J]. Canadian Journal of Veterinary Research, 2008, 72(2): 168-174. |
| [25] |
HAQ SU, WANG L, GUO WZ, AQIB AI, MUNEER A, SAQIB M, AHMAD S, GHAFOOR M, IFTIKHAR A, CHEN KY, LIANG JP. Enhancing activity of β-lactam and fluoroquinolones antibiotics by artemisinin and its derivatives against MDR Escherichia coli[J]. Frontiers in Veterinary Science, 2022, 9: 1048531. |
| [26] |
PRAKASH V, LEWIS JS 2nd, JORGENSEN JH. Vancomycin MICs for methicillin-resistant Staphylococcus aureus isolates differ based upon the susceptibility test method used[J]. Antimicrobial Agents and Chemotherapy, 2008, 52(12): 4528. |
| [27] |
WANG H, HUEBNER R, CHEN M, KLUGMAN K. Antibiotic susceptibility patterns of Streptococcus pneumoniae in China and comparison of MICs by agar dilution and E-test methods[J]. Antimicrobial Agents and Chemotherapy, 1998, 42(10): 2633-2636. |
| [28] |
SEIFERT H. Comparative in-vitro activities of trovafloxacin, ciproflaxacin, ofloxacin, fleroxacin and broad-spectrum β-lactams against aerobe blood culture isolates[J]. Zentralblatt für Bakteriologie, 1998, 288: 509-518. |
| [29] |
CLARK CL, JACOBS MR, APPELBAUM PC. Activities of clinafloxacin, alone and in combination with other compounds, against 45 gram-positive and-negative organisms for which clinafloxacin MICs are high[J]. Antimicrobial Agents and Chemotherapy, 1999, 43(9): 2295-2298. |
| [30] |
FUNG-TOMC J, GRADELSKI E, HUCZKO E, MINASSIAN B, BONNER DP. Activity of gatifloxacin against strains resistant to ofloxacin and ciprofloxacin and its ability to select for less susceptible bacterial variants[J]. International Journal of Antimicrobial Agents, 2001, 18(1): 77-80. |
| [31] |
YAMAKAWA T, MITSUYAMA J, HAYASHI K. In vitro and in vivo antibacterial activity of T-3912, a novel non-fluorinated topical quinolone[J]. Journal of Antimicrobial Chemotherapy, 2002, 49(3): 455-465. |
| [32] |
BOLMSTROM A, KARLSSON A. Influence of CO2 incubation on quinolone activity against Streptococcus pneumoniae and Haemophilus influenzae[J]. Diagnostic Microbiology and Infectious Disease, 2002, 42(1): 65-69. |
| [33] |
FÉRIA C, FERREIRA E, CORREIA JD, GONÇALVES J, CANIÇA M. Patterns and mechanisms of resistance to beta-lactams and beta-lactamase inhibitors in uropathogenic Escherichia coli isolated from dogs in Portugal[J]. Journal of Antimicrobial Chemotherapy, 2002, 49(1): 77-85. |
| [34] |
KERRN MB, KLEMMENSEN T, FRIMODT- MØLLER N, ESPERSEN F. Susceptibility of Danish Escherichia coli strains isolated from urinary tract infections and bacteraemia, and distribution of Sul genes conferring sulphonamide resistance[J]. Journal of Antimicrobial Chemotherapy, 2002, 50(4): 513-516. |
| [35] |
RIBES S, TABERNER F, DOMENECH A, CABELLOS C, TUBAU F, LIÑARES J, VILADRICH PF, GUDIOL F. Evaluation of fosfomycin alone and in combination with ceftriaxone or vancomycin in an experimental model of meningitis caused by two strains of cephalosporin-resistant Streptococcus pneumoniae[J]. Journal of Antimicrobial Chemotherapy, 2006, 57(5): 931-936. |
| [36] |
MAZZARIOL A, KONCAN R, VITALI LA, CORNAGLIA G. Activities of 16-membered ring macrolides and telithromycin against different genotypes of erythromycin-susceptible and erythromycin-resistant Streptococcus pyogenes and Streptococcus pneumoniae[J]. Journal of Antimicrobial Chemotherapy, 2007, 59(6): 1171-1176. |
| [37] |
MARÍN P, FERNÁNDEZ-VARÓN E, ESCUDERO E, VANCRAEYNEST D, CÁRCELES CM. Pharmacokinetic-pharmacodynamic integration of orbifloxacin in rabbits after intravenous, subcutaneous and intramuscular administration[J]. Journal of Veterinary Pharmacology and Therapeutics, 2008, 31(1): 77-82. |
| [38] |
IZAWA A, KISAKI Y, IRIE K, EDA Y, NAKAGOME T, KOMATSU T. Antibacterial activity of miloxacin[J]. Antimicrobial Agents and Chemotherapy, 1980, 18(1): 37-40. |
| [39] |
WOOTTON M, WALSH TR, MACFARLANE L, HOWE RA. Activity of mecillinam against Escherichia coli resistant to third-generation cephalosporins[J]. Journal of Antimicrobial Chemotherapy, 2010, 65(1): 79-81. |
| [40] |
HARADA K, SHIMIZU T, KATAOKA Y, TAKAHASHI T. Post-antibiotic effect of orbifloxacin against Escherichia coli and Pseudomonas aeruginosa isolates from dogs[J]. Acta Veterinaria Scandinavica, 2012, 54(1): 16. |
| [41] |
BECNEL BOYD L, MAYNARD MJ, MORGAN- LINNELL SK, HORTON LB, SUCGANG R, HAMILL RJ, JIMENEZ JR, VERSALOVIC J, STEFFEN D, ZECHIEDRICH L. Relationships among ciprofloxacin, gatifloxacin, levofloxacin, and norfloxacin MICs for fluoroquinolone-resistant Escherichia coli clinical isolates[J]. Antimicrobial Agents and Chemotherapy, 2009, 53(1): 229-234. |
| [42] |
HUCZYŃSKI A, JANCZAK J, STEFAŃSKA J, ANTOSZCZAK M, BRZEZINSKI B. Synthesis and antimicrobial activity of amide derivatives of polyether antibiotic-salinomycin[J]. Bioorganic & Medicinal Chemistry Letters, 2012, 22(14): 4697-4702. |
| [43] |
BUCKLEY LM, McEWAN NA, NUTTALL T. Tris-EDTA significantly enhances antibiotic efficacy against multidrug-resistant Pseudomonas aeruginosa in vitro[J]. Veterinary Dermatology, 2013, 24(5): 519-e122. |
| [44] |
PORTER MC, HENDERSON BA, HEALY PE, COOMBS GW, INGRAM PR, McLELLAN D, CLARK B. Can interchangeability of lincosamides be assumed in clinical practice? Comparative MICs of clindamycin and lincomycin for Streptococcus pyogenes, Streptococcus agalactiae and Staphylococcus aureus[J]. Journal of Antimicrobial Chemotherapy, 2014, 69(3): 856-857. |
| [45] |
MATUSCHEK E, BROWN DFJ, KAHLMETER G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories[J]. Clinical Microbiology and Infection, 2014, 20(4): O255-O266. |
| [46] |
VELLUTI F, MOSCONI N, ACEVEDO A, BORTHAGARAY G, CASTIGLIONI J, FACCIO R, BACK DF, MOYNA G, RIZZOTTO M, TORRE MH. Synthesis, characterization, microbiological evaluation, genotoxicity and synergism tests of new nano silver complexes with sulfamoxole X-ray diffraction of [Ag2(SMX)2]·DMSO[J]. Journal of Inorganic Biochemistry, 2014, 141: 58-69. |
| [47] |
CLARK SM, LOEFFLER A, BOND R. Susceptibility in vitro of canine methicillin-resistant and-susceptible staphylococcal isolates to fusidic acid, chlorhexidine and miconazole: opportunities for topical therapy of canine superficial pyoderma[J]. Journal of Antimicrobial Chemotherapy, 2015, 70(7): 2048-2052. |
| [48] |
LEI ZX, LIU QY, XIONG JC, YANG B, YANG SK, ZHU QQ, LI K, ZHANG SS, CAO JY, HE QG. Pharmacokinetic and pharmacodynamic evaluation of marbofloxacin and PK/PD modeling against Escherichia coli in pigs[J]. Frontiers in Pharmacology, 2017, 8: 542. |
| [49] |
HODILLE E, BADIOU C, BOUVEYRON C, BES M, TRISTAN A, VANDENESCH F, LINA G, DUMITRESCU O. Clindamycin suppresses virulence expression in inducible clindamycin-resistant Staphylococcus aureus strains[J]. Annals of Clinical Microbiology and Antimicrobials, 2018, 17(1): 38. |
| [50] |
BARMPA A, FROUSIOU O, KALOGIANNIS S, PERDIH F, TUREL I, PSOMAS G. Manganese(II) complexes of the quinolone family member flumequine: structure, antimicrobial activity and affinity for albumins and calf-Thymus DNA[J]. Polyhedron, 2018, 145: 166-175. |
| [51] |
RUBIO-LANGRE S, AGUILAR-SOLA S, LORENZUTTI AM, SAN ANDRÉS MI, de LUCAS JJ, LITTERIO NJ. Pharmacokinetic evaluation of marbofloxacin after intravenous administration at different ages in llama crias, and pharmacokinetic/ pharmacodynamic analysis by Monte Carlo simulation[J]. Journal of Veterinary Pharmacology and Therapeutics, 2018, 41(6): 861-870. |
| [52] |
ZHANG XX, ZHANG YZ, WANG F, WANG C, CHEN LJ, LIU HY, LU H, WEN H, ZHOU TL. Unravelling mechanisms of nitrofurantoin resistance and epidemiological characteristics among Escherichia coli clinical isolates[J]. International Journal of Antimicrobial Agents, 2018, 52(2): 226-232. |
| [53] |
NOEL AR, ATTWOOD M, BOWKER KE, KIM A, KRAUSE KM, MacGOWAN AP. Pharmacodynamics of plazomicin and a comparator aminoglycoside, amikacin, studied in an in vitro pharmacokinetic model of infection[J]. International Journal of Antimicrobial Agents, 2019, 54(5): 626-632. |
| [54] |
de LYRA ACF, dos SANTOS SILVA AL, dos SANTOS ECL, LÓPEZ AMQ, Da SILVA JCS, FIGUEIREDO IM, SANTOS JCC. Molecular interaction of sulfonamides and ovalbumin, an allergenic egg protein, exploring biophysical, theoretical and biological studies[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2020, 228: 117747. |
| [55] |
NOEL A, ATTWOOD M, BOWKER K, MacGOWAN A. The pharmacodynamics of fosfomycin against Staphylococcus aureus studied in an in vitro model of infection[J]. International Journal of Antimicrobial Agents, 2020, 56(1): 105985. |
| [56] |
ZHANG HL, WU SL, FU JL, JIANG HX, DING HZ. Research Note: Epidemiological cutoff values and acquired resistance mechanisms of three veterinary antibiotics against Escherichia coli from chicken respiratory tract infections[J]. Poultry Science, 2021, 100(2): 1093-1097. |
| [57] |
NOEL AR, ATTWOOD M, BOWKER KE, MacGOWAN AP. The pharmacodynamics of minocycline alone and in combination with rifampicin against Staphylococcus aureus studied in an in vitro pharmacokinetic model of infection[J]. Journal of Antimicrobial Chemotherapy, 2021, 76(7): 1840-1844. |
| [58] |
ASADI S, NAYERI-FASAEI B, ZAHRAEI-SALEHI T, YAHYA-RAYAT R, SHAMS N, SHARIFI A. Antibacterial and anti-biofilm properties of carvacrol alone and in combination with cefixime against Escherichia coli[J]. BMC Microbiology, 2023, 23(1): 55. |
| [59] |
DiVINCENZO CA, SHATZER KL, VENEZIO FR. In vitro activity of lomefloxacin against multiply resistant Pseudomonas aeruginosa, Enterobacter cloacae, and Staphylococcus epidermidis[J]. Diagnostic Microbiology and Infectious Disease, 1989, 12(3 Suppl): 13S-16S. |
| [60] |
JOHNSON RM, LI KL, CHEN XY, MORGAN GL, AUBÉ J, LI B. The hybrid antibiotic thiomarinol A overcomes intrinsic resistance in Escherichia coli using a privileged dithiolopyrrolone moiety[J]. ACS Infectious Diseases, 2024, 10(2): 582-593. |
| [61] |
STONE TJ, KILIC A, WILLIAMSON JC, PALAVECINO EL. In vitro activity of omadacycline and comparator antibiotics against extended-spectrum Beta-Lactamase-producing Escherichia coli and Klebsiella pneumoniae urinary isolates[J]. Antibiotics, 2023, 12(6): 953. |
| [62] |
SATRIA D, HARAHAP U, DALIMUNTHE A, SEPTAMA AW, HERTIANI T, NASRI N. Synergistic antibacterial effect of ethyl acetate fraction of Vernonia amygdalina delile leaves with tetracycline against clinical isolate Methicillin-resistant Staphylococcus aureus (mrsa) and Pseudomonas aeruginosa[J]. Advances in Pharmacological and Pharmaceutical Sciences, 2023, 2023(1): 2259534. |
| [63] |
WANG JL, LAI CC, KO WC, HSUEH PR. Geographical patterns of in vitro susceptibilities to tigecycline and colistin among worldwide isolates of Acinetobacter baumannii, Escherichia coli and Klebsiella pneumoniae: data from the antimicrobial testing leadership and surveillance (ATLAS) programme, 2016–2021[J]. International Journal of Antimicrobial Agents, 2023, 62(3): 106930. |
| [64] |
van der MEIJDEN A, ARANZANA-CLIMENT V, van der SPEK H, de WINTER BCM, COUET W, MELETIADIS J, MULLER AE, van den BERG S. Pharmacokinetic and pharmacodynamic properties of polymyxin B in Escherichia coli and Klebsiella pneumoniae murine infection models[J]. Journal of Antimicrobial Chemotherapy, 2023, 78(3): 832-839. |
| [65] |
HUANG CF, WANG JT, CHUANG YC, SHENG WH, CHEN YC. In vitro susceptibility of common Enterobacterales to eravacycline in Taiwan[J]. Journal of Microbiology, Immunology and Infection, 2023, 56(2): 358-366. |
| [66] |
MESSELE YE, ALKHALLAWI M, VELTMAN T, TROTT DJ, McMENIMAN JP, KIDD SP, LOW WY, PETROVSKI KR. Phenotypic and genotypic analysis of antimicrobial resistance in Escherichia coli recovered from feedlot beef cattle in Australia[J]. Animals, 2022, 12(17): 2256. |
| [67] |
CHEN PH, SUNG LK, HEGEMANN JD, CHU J. Disrupting transcription and folate biosynthesis leads to synergistic suppression of Escherichia coli growth[J]. ChemMedChem, 2022, 17(10): e202200075. |
| [68] |
BAGDATLI E, CIL E. Sulfa drugs-based norbornenyl imides and reductive heck reactions: synthesis and antimicrobial screening[J]. Journal of Heterocyclic Chemistry, 2022, 59(2): 264-274. |
| [69] |
MA YB, PIROLO M, SUBRAMANI P, GEHRING R, DAMBORG P, FRANZYK H, GUARDABASSI L. Macrolide resistance and in vitro potentiation by peptidomimetics in porcine clinical Escherichia coli[J]. mSphere, 2022, 7(5): e0040222. |
| [70] |
GALECIO JS, ESCUDERO E, CORRALES JC, GARCÍA-ROMERO E, de la Fe C, HERNANDIS V, MARIN P. Susceptibility of caprine mastitis pathogens to tildipirosin, gamithromycin, oxytetracycline, and danofloxacin: effect of serum on the in vitro potency of current macrolides[J]. World Journal of Microbiology & Biotechnology, 2022, 38(12): 221. |
| [71] |
XIAO X, CHEN XJ, YAN KX, JIANG LJ, LI RC, LIU Y, WANG MZ, WANG ZQ. PK/PD integration and pharmacodynamic cutoff of cefquinome against cow mastitis due to Escherichia coli[J]. Journal of Veterinary Pharmacology and Therapeutics, 2022, 45(1): 83-91. |
| [72] |
FRATONI AJ, AVERY LM, NICOLAU DP, ASEMPA TE. In vivo pharmacokinetics and pharmacodynamics of ceftibuten/ledaborbactam, a novel oral β-lactam/ β-lactamase inhibitor combination[J]. Journal of Antimicrobial Chemotherapy, 2022, 78(1): 93-100. |
| [73] |
NORM/NORM-VET. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway[R]. Norway, 2022.
|
| [74] |
KHALIL A, JARADAT N, HAWASH M, ISSA L. In vitro biological evaluation of benzodioxol derivatives as antimicrobial and antioxidant agents[J]. Arabian Journal for Science and Engineering, 2021, 46(6): 5447-5453. |
| [75] |
STEWART AG, COTTRELL K, HENDERSON A, VEMURI K, BAUER MJ, PATERSON DL, HARRIS PNA. In vitro activity of cefotetan against ESBL-producing Escherichia coli and Klebsiella pneumoniae bloodstream isolates from the merino trial[J]. Microbiology Spectrum, 2021, 9(1): e0022621. |
| [76] |
ÖZTÜRK F, AYCAN T, ÇON AH. Spectroscopic, structural characterization and magnetic studies of Cu(II)-sulfathiazole complex with 1, 10-phenanthroline and N-(2-hydroxyethyl)-ethylenediamine ligands[J]. Journal of Molecular Structure, 2020, 1202: 127220. |
| [77] |
MERKATORIS P, SCHLEINING J, KRULL A, BORTS D, FAJT V. In vitro elution of penicillin, ampicillin, tetracycline, tulathromycin, and florfenicol from plaster of Paris beads[J]. Frontiers in Veterinary Science, 2020, 7: 585423. |
| [78] |
JOHNSTON BD, THURAS P, PORTER SB, ANACKER M, VonBANK B, VAGNONE PS, WITWER M, CASTANHEIRA M, JOHNSON JR. Activity of imipenem-relebactam against carbapenem- resistant Escherichia coli isolates from the United States in relation to clonal background, resistance genes, coresistance, and region[J]. Antimicrobial Agents and Chemotherapy, 2020, 64(5): e02408-19. |
| [79] |
TUON FF, CIESLINSKI J, RODRIGUES SDS, SERRA FB, PAULA MD. Evaluation of in vitro activity of ceftolozane-tazobactam against recent clinical bacterial isolates from Brazil-the EM200 study[J]. The Brazilian Journal of Infectious Diseases, 2020, 24(2): 96-103. |
| [80] |
BHOWMICK T, WEINSTEIN MP. Microbiology of meropenem-vaborbactam: a novel carbapenem beta-lactamase inhibitor combination for carbapenem- resistant enterobacterales infections[J]. Infectious Diseases and Therapy, 2020, 9(4): 757-767. |
| [81] |
ROSTAMIZADEH S, DANESHFAR Z, MOGHIMI H. Synthesis of sulfamethoxazole and sulfabenzamide metal complexes; evaluation of their antibacterial activity[J]. European Journal of Medicinal Chemistry, 2019, 171: 364-371. |
| [82] |
CHAN WY, HICKEY EE, KHAZANDI M, PAGE SW, TROTT DJ, HILL PB. In vitro antimicrobial activity of narasin against common clinical isolates associated with canine otitis externa[J]. Veterinary Dermatology, 2018, 29(2): 149-e57. |
| [83] |
MIZDAL CR, STEFANELLO ST, NOGARA PA, ANTUNES SOARES FA, de LOURENÇO MARQUES L, de CAMPOS MMA. Molecular docking, and anti-biofilm activity of gold-complexed sulfonamides on Pseudomonas aeruginosa[J]. Microbial Pathogenesis, 2018, 125: 393-400. |
| [84] |
DOMALAON R, SANCHAK Y, KOSKEI LC, LYU YF, ZHANEL GG, ARTHUR G, SCHWEIZER F. Short proline-rich lipopeptide potentiates minocycline and rifampin against multidrug- and extensively drug-resistant Pseudomonas aeruginosa[J]. Antimicrobial Agents and Chemotherapy, 2018, 62(4): e02374-17. |
| [85] |
PFALLER MA, FLAMM RK, DUNCAN LR, STREIT JM, CASTANHEIRA M, SADER HS. Antimicrobial activity of ceftobiprole and comparator agents when tested against contemporary Gram-positive and-negative organisms collected from Europe (2015)[J]. Diagnostic Microbiology and Infectious Disease, 2018, 91(1): 77-84. |
| [86] |
BAI PY, QIN SS, CHU WC, YANG Y, CUI DY, HUA YG, YANG QQ, ZHANG E. Synthesis and antibacterial bioactivities of cationic deacetyl linezolid amphiphiles[J]. European Journal of Medicinal Chemistry, 2018, 155: 925-945. |
| [87] |
CHANG PC, CHEN CC, LU YC, LAI CC, HUANG HL, CHUANG YC, TANG HJ. The impact of inoculum size on the activity of cefoperazone-sulbactam against multidrug resistant organisms[J]. Journal of Microbiology, Immunology and Infection, 2018, 51(2): 207-213. |
| [88] |
CIEMNIAK K, CIELECKA-PIONTEK J, SZYMANOWSKA D, WIERGOWSKA G. Intereactions between doripenem and clavulanate: application of minimal inhibitory concentration analysis and cytometry flow for bactericidal studies[J]. Electronic Journal of Biotechnology, 2018, 32: 41-46. |
| [89] |
LI H, ESTABROOK M, JACOBY GA, NICHOLS WW, TESTA RT, BUSH K. In vitro susceptibility of characterized β-lactamase-producing strains tested with avibactam combinations[J]. Antimicrobial Agents and Chemotherapy, 2015, 59(3): 1789-1793. |
| [90] |
CHEN LZ, YANG DX, PAN ZK, LAI LH, LIU JH, FANG BH, SHI SN. Synthesis and antimicrobial activity of the hybrid molecules between sulfonamides and active antimicrobial pleuromutilin derivative[J]. Chemical Biology & Drug Design, 2015, 86(2): 239-245. |
| [91] |
PAPP-WALLACE KM, BAJAKSOUZIAN S, ABDELHAMED AM, FOSTER AN, WINKLER ML, GATTA JA, NICHOLS WW, TESTA R, BONOMO RA, JACOBS MR. Activities of ceftazidime, ceftaroline, and aztreonam alone and combined with avibactam against isogenic Escherichia coli strains expressing selected single β-lactamases[J]. Diagnostic Microbiology and Infectious Disease, 2015, 82(1): 65-69. |
| [92] |
FERNANDES P, SOUSA I, CUNHA-SILVA L, FERREIRA M, de CASTRO B, PEREIRA EF, FEIO MJ, GAMEIRO P. Synthesis, characterization and antibacterial studies of a copper(II) lomefloxacin ternary complex[J]. Journal of Inorganic Biochemistry, 2014, 131: 21-29. |
| [93] |
ALHAJLAN M, ALHARIRI M, OMRI A. Efficacy and safety of liposomal clarithromycin and its effect on Pseudomonas aeruginosa virulence factors[J]. Antimicrobial Agents and Chemotherapy, 2013, 57(6): 2694-2704. |
| [94] |
FERRARESI C, LUCATELLO L, MEUCCI V, INTORRE L, GRILLI G, PICCIRILLO A, RUSSO E, VILLA R, MONTESISSA C, CAGNARDI P. Pharmacokinetic/pharmacodynamic evaluation of the efficacy of flumequine in treating colibacillosis in turkeys[J]. Poultry Science, 2013, 92(12): 3158-3165. |
| [95] |
BUYCK JM, PLÉSIAT P, TRAORE H, VANDERBIST F, TULKENS PM, van BAMBEKE F. Increased susceptibility of Pseudomonas aeruginosa to macrolides and ketolides in eukaryotic cell culture media and biological fluids due to decreased expression of oprM and increased outer-membrane permeability[J]. Clinical Infectious Diseases, 2012, 55(4): 534-542. |
| [96] |
GEBRU E, DAMTE D, CHOI MJ, LEE SJ, KIM YH, PARK SC. Mutant prevention concentration and phenotypic and molecular basis of fluoroquinolone resistance in clinical isolates and in vitro-selected mutants of Escherichia coli from dogs[J]. Veterinary Microbiology, 2012, 154(3/4): 384-394. |
| [97] |
QIAN LQ, MA JL, QUAN JX, ZHENG BL, LI SN, YAO JL, JIANG LM. Antibacterial activity of medicinal extract for exterior application of BaifDai and its synergy effects with benzylpenicillin in vitro[J]. The Chinese Journal of Clinical Pharmacology, 2012, 28(10): 765-766. (in Chinese) 钱丽旗, 马建丽, 权菊香, 郑彬丽, 李素那, 姚九莲, 姜玲敏. 柏黛膏的体外抗菌活性及其与青霉素的协同抑菌作用[J]. 中国临床药理学杂志, 2012, 28(10): 765-766. |
| [98] |
BHAGUNDE P, SINGH R, LEDESMA KR, CHANG KT, NIKOLAOU M, TAM VH. Modelling biphasic killing of fluoroquinolones: guiding optimal dosing regimen design[J]. Journal of Antimicrobial Chemotherapy, 2011, 66(5): 1079-1086. |
| [99] |
ZHANEL GG, DeCORBY M, NICHOL KA, WIERZBOWSKI A, BAUDRY PJ, TAILOR F, LAGACÉ-WIENS P, WALKTY A, FANELLA S, LARIOS O, MULVEY MR, McCRACKEN M, KARLOWSKY JA, The Canadian Antimicrobial Resistance Alliance cara, HOBAN DJ. Antimicrobial susceptibility of 6685 organisms isolated from Canadian hospitals: CANWARD 2007[J]. Canadian Journal of Infectious Diseases and Medical Microbiology, 2009, 20: 20A-30A. |
| [100] |
EFTHIMIADOU EK, KATSAROU ME, KARALIOTA A, PSOMAS G. Copper(II) complexes with sparfloxacin and nitrogen-donor heterocyclic ligands: structure-activity relationship[J]. Journal of Inorganic Biochemistry, 2008, 102(4): 910-920. |
| [101] |
ACHARD A, GUÉRIN-FAUBLÉE V, PICHEREAU V, VILLERS C, LECLERCQ R. Emergence of macrolide resistance gene mph(B) in Streptococcus uberis and cooperative effects with rdmC-like gene[J]. Antimicrobial Agents and Chemotherapy, 2008, 52(8): 2767-2770. |
| [102] |
SAWANT AA, HEGDE NV, STRALEY BA, DONALDSON SC, LOVE BC, KNABEL SJ, JAYARAO BM. Antimicrobial-resistant enteric bacteria from dairy cattle[J]. Applied and Environmental Microbiology, 2007, 73(1): 156-163. |
| [103] |
DONALDSON SC, STRALEY BA, HEGDE NV, SAWANT AA, DebROY C, JAYARAO BM. Molecular epidemiology of ceftiofur-resistant Escherichia coli isolates from dairy calves[J]. Applied and Environmental Microbiology, 2006, 72(6): 3940-3948. |
| [104] |
STUBBINGS W, BOSTOCK J, INGHAM E, CHOPRA I. Mechanisms of the post-antibiotic effects induced by rifampicin and gentamicin in Escherichia coli[J]. Journal of Antimicrobial Chemotherapy, 2006, 58(2): 444-448. |
| [105] |
HERMSEN ED, HOVDE LB, SPRANDEL KA, RODVOLD KA, ROTSCHAFER JC. Levofloxacin plus metronidazole administered once daily versus moxifloxacin monotherapy against a mixed infection of Escherichia coli and Bacteroides fragilis in an in vitro pharmacodynamic model[J]. Antimicrobial Agents and Chemotherapy, 2005, 49(2): 685-689. |
| [106] |
BRAGA PC, DAL SM, WOODNUTT G. Staphylococcus aureus and Escherichia coli adhesion to human cells is reduced by sub-MICs of gemifloxacin[J]. Journal of Chemotherapy, 2002, 14(1): 41-46. |
| [107] |
OLIVER A, WEIGEL LM, RASHEED JK, McGOWAN JE Jr, RANEY P, TENOVER FC. Mechanisms of decreased susceptibility to cefpodoxime in Escherichia coli[J]. Antimicrobial Agents and Chemotherapy, 2002, 46(12): 3829-3836. |
| [108] |
FIRSOV AA, ZINNER SH, LUBENKO IY, VOSTROV SN. Gemifloxacin and ciprofloxacin pharmacodynamics in an in-vitro dynamic model: prediction of the equivalent AUC/MIC breakpoints and doses[J]. International Journal of Antimicrobial Agents, 2000, 16(4): 407-414. |
| [109] |
HUCZKO E, CONETTA B, BONNER D, VALERA L, STICKLE T, MACKO A, FUNG-TOMC J. Susceptibility of bacterial isolates to gatifloxacin and ciprofloxacin from clinical trials 1997–1998[J]. International Journal of Antimicrobial Agents, 2000, 16(4): 401-405. |
| [110] |
DESHPANDE LM, DIEKEMA DJ, JONES RN. Comparative activity of clinafloxacin and nine other compounds tested against 2000 contemporary clinical isolates from patients in United States hospitals[J]. Diagnostic Microbiology and Infectious Disease, 1999, 35(1): 81-88. |
| [111] |
COHEN MA, HUBAND MD, YODER SL, GAGE JW, ROLAND GE. Bacterial eradication by clinafloxacin, CI-990, and ciprofloxacin employing MBC test, in-vitro time-kill and in-vivo time-kill studies[J]. Journal of Antimicrobial Chemotherapy, 1998, 41(6): 605-614. |
| [112] |
SALMON SA, WATTS JL, CASE CA, HOFFMAN LJ, WEGENER HC, JR YANCEY RJ. Comparison of MICs of ceftiofur and other antimicrobial agents against bacterial pathogens of swine from the United States, Canada, and Denmark[J]. Journal of Clinical Microbiology, 1995, 33(9): 2435-2444. |
| [113] |
ONYEJI CO, NICOLAU DP, NIGHTINGALE CH, QUINTILIANI R. Optimal times above MICs of ceftibuten and cefaclor in experimental intra-abdominal infections[J]. Antimicrobial Agents and Chemotherapy, 1994, 38(5): 1112-1117. |
| [114] |
ARGUEDAS AG, AKANIRO JC, STUTMAN HR, MARKS MI. In vitro activity of tosufloxacin, a new quinolone, against respiratory pathogens derived from cystic fibrosis sputum[J]. Antimicrobial Agents and Chemotherapy, 1990, 34(11): 2223-2227. |
| [115] |
ROBBINS MJ, BASKERVILLE AJ, SANGHRAJKA M, MUMTAZ G, FELMINGHAM D, RIDGWAY GL, GRÜNEBERG RN. Comparative in vitro activity of lomefloxacin, a difluoro-quinolone[J]. Diagnostic Microbiology and Infectious Disease, 1989, 12(3): 65-76. |
| [116] |
KNAPP CC, SIERRA-MADERO J, WASHINGTON JA. Antibacterial activities of cefpodoxime, cefixime, and ceftriaxone[J]. Antimicrobial Agents and Chemotherapy, 1988, 32(12): 1896-1898. |
| [117] |
LIU PY, KO WC, LEE WS, LU PL, CHEN YH, CHENG SH, LU MC, LIN CY, WU TS, YEN MY, WANG LS, LIU CP, SHAO PL, LEE YL, SHI ZY, CHEN YS, WANG FD, TSENG SH, LIN CN, CHEN YH, et al. In vitro activity of cefiderocol, cefepime/enmetazobactam, cefepime/zidebactam, eravacycline, omadacycline, and other comparative agents against carbapenem-non-susceptible Pseudomonas aeruginosa and Acinetobacter baumannii isolates associated from bloodstream infection in Taiwan between 2018–2020[J]. Journal of Microbiology, Immunology and Infection, 2022, 55(5): 888-895. |
| [118] |
MEI JA, JOHNSON W, KINN B, LASKEY E, NOLIN L, BHAMARE P, STALKER C, DUNMAN PM, WOZNIAK RAF. Antimicrobial activity of a triple antibiotic combination toward ocular Pseudomonas aeruginosa clinical isolates[J]. Translational Vision Science & Technology, 2022, 11(5): 26. |
| [119] |
HEFFERNAN AJ, SIME FB, LIM SMS, ADIRAJU S, WALLIS SC, LIPMAN J, GRANT GD, ROBERTS JA. Pharmacodynamics of ceftriaxone for the treatment of methicillin‐susceptible Staphylococcus aureus: is it a viable treatment option?[J]. International Journal of Antimicrobial Agents, 2022, 59(3): 106537. |
| [120] |
RADWAN AA, AANAZI FK, AL-AGAMY M, MAHROUS GM. Design, synthesis and molecular modeling study of substituted indoline-2-ones and spiro[indole-heterocycles] with potential activity against Gram-positive bacteria[J]. Acta Pharmaceutica, 2022, 72(1): 79-95. |
| [121] |
SOUSA MGDC, XAVIER PD, CANTUÁRIA APC, AMORIM IA, ALMEIDA JA, FRANCO OL, REZENDE TMB. Antimicrobial and immunomodulatory in vitro profile of double antibiotic paste[J]. International Endodontic Journal, 2021, 54(10): 1850-1860. |
| [122] |
IVANCHIK NV, SUKHORUKOVA МV, CHAGARYAN АN, TRUSHIN IV, DEKHNICH AV, KOZLOV RS. In vitro activity of thiamphenicol against Haemophilus influenzae, Streptococcus pneumoniae and Streptococcus pyogenes clinical isolates[J]. Clinical Microbiology and Antimicrobial Chemotherapy, 2021, 23(1): 92-99. |
| [123] |
MOORE JA, MEAKIN M, EARL MH, KUMMER TM, McALEER JP, LONG TE. Effects of caspofungin, tolcapone and other FDA-approved medications on MRSA susceptibility to vancomycin[J]. Journal of Global Antimicrobial Resistance, 2020, 22: 283-289. |
| [124] |
LACOMA A, USÓN L, MENDOZA G, SEBASTIÁN V, GARCIA-GARCIA E, MURIEL-MORENO B, DOMÍNGUEZ J, ARRUEBO M, PRAT C. Novel intracellular antibiotic delivery system against Staphylococcus aureus: cloxacillin-loaded poly(d, l-lactide-co-glycolide) acid nanoparticles[J]. Nanomedicine, 2020, 15(12): 1189-1203. |
| [125] |
CARVALHAES CG, HUBAND MD, REINHART HH, FLAMM RK, SADER HS. Antimicrobial activity of omadacycline tested against clinical bacterial isolates from hospitals in China's mainland, Hong Kong, and Taiwan: results from the SENTRY antimicrobial surveillance program (2013–2016)[J]. Antimicrobial Agents and Chemotherapy, 2019, 63(3): e02262-18. |
| [126] |
WICHA WW, STRICKMANN DB, PAUKNER S. Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae[J]. Journal of Antimicrobial Chemotherapy, 2019, 74(Suppl 3): iii11-iii18. |
| [127] |
ILIZIROV Y, FORMANOVSKY A, MIKHURA I, PAITAN Y, NAKONECHNY F, NISNEVITCH M. Effect of photodynamic antibacterial chemotherapy combined with antibiotics on gram-positive and gram-negative bacteria[J]. Molecules, 2018, 23(12): 3152. |
| [128] |
ASEMPA T, NICOLAU D, KUTI J. 888: in vitro activity of imipenem/relebactam against pseudomonas aeruginosa isolates from U. S. hospitals[J]. Critical Care Medicine, 2019, 47(1): 423-423. |
| [129] |
TAHTACI H, KARACıK H, ECE A, ER M, ŞEKER MG. Design, synthesis, SAR and molecular modeling studies of novel imidazo[2, 1-b][1, 3, 4]thiadiazole derivatives as highly potent antimicrobial agents[J]. Molecular Informatics, 2018, 37(3): 1700083. |
| [130] |
PEHLIVAN V, BIÇER E, BEKIROĞLU YG, DEGE N. Electrochemical and spectroscopic studies on the interaction modes of calf Thymus DNA with antibacterial schiff bases obtained from substituted salicylaldehydes and sulfamethizole[J]. International Journal of Electrochemical Science, 2018, 13(11): 10700-10717. |
| [131] |
KIM JH, YU D, EOM SH, KIM SH, OH J, JUNG WK, KIM YM. Synergistic antibacterial effects of chitosan-caffeic acid conjugate against antibiotic- resistant acne-related bacteria[J]. Marine Drugs, 2017, 15(6): 167. |
| [132] |
BROWN-ELLIOTT BA, JR WALLACE RJ. In vitro susceptibility testing of tedizolid against nontuberculous mycobacteria[J]. Journal of Clinical Microbiology, 2017, 55(6): 1747-1754. |
| [133] |
LI SG, GUO Y, ZHAO CJ, CHEN HB, HU BJ, CHU YZ, ZHANG ZJ, HU YJ, LIU ZY, DU Y, GUI QD, JI P, ZENG J, CAO B, FU Q, ZHANG R, WANG ZX, ZHUO C, FENG XJ, JIA W, et al. In vitro activities of tedizolid compared with other antibiotics against Gram-positive pathogens associated with hospital- acquired pneumonia, skin and soft tissue infection and bloodstream infection collected from 26 hospitals in China[J]. Journal of Medical Microbiology, 2016, 65(10): 1215-1224. |
| [134] |
CANTÓN R, LIVERMORE DM, MOROSINI MI, DÍAZ-REGAÑÓN J, ROSSOLINI GM, GROUP PS. E-test® versus broth microdilution for ceftaroline MIC determination with Staphylococcus aureus: results from PREMIUM, a European multicentre study[J]. Journal of Antimicrobial Chemotherapy, 2017, 72(2): 431-436. |
| [135] |
WEI CF, CHANG SK, SHIEN JH, KUO HC, CHEN WY, CHOU CC. Synergism between two amphenicol of antibiotics, florfenicol and thiamphenicol, against Staphylococcus aureus[J]. Veterinary Record, 2016, 178(13): 319. |
| [136] |
YIM J, SMITH JR, BARBER KE, HALLESY JA, RYBAK MJ. Evaluation of pharmacodynamic interactions between telavancin and aztreonam or Piperacillin/Tazobactam against Pseudomonas aeruginosa, Escherichia coli and Methicillin-resistant Staphylococcus aureus[J]. Infectious Diseases and Therapy, 2016, 5(3): 367-377. |
| [137] |
McDOUGALL S, HUSSEIN H, PETROVSKI K. Antimicrobial resistance in Staphylococcus aureus, Streptococcus uberis and Streptococcus dysgalactiae from dairy cows with mastitis[J]. New Zealand Veterinary Journal, 2014, 62(2): 68-76. |
| [138] |
HADDADIN RNS, SALEH S, AL-ADHAM ISI, BUULTJENS TEJ, COLLIER PJ. The effect of subminimal inhibitory concentrations of antibiotics on virulence factors expressed by Staphylococcus aureus biofilms[J]. Journal of Applied Microbiology, 2010, 108(4): 1281-1291. |
| [139] |
AARESTRUP FM, SKOV RL. Evaluation of ceftiofur and cefquinome for phenotypic detection of methicillin resistance in Staphylococcus aureus using disk diffusion testing and MIC-determinations[J]. Veterinary Microbiology, 2010, 140(1/2): 176-179. |
| [140] |
SADER HS, BECKER HK, MOET GJ, JONES RN. Antimicrobial activity of daptomycin tested against Staphylococcus aureus with vancomycin MIC of 2 μg/mL isolated in the United States and European hospitals (2006–2008)[J]. Diagnostic Microbiology and Infectious Disease, 2010, 66(3): 329-331. |
| [141] |
HAAS W, PILLAR CM, HESJE CK, SANFILIPPO CM, MORRIS TW. Bactericidal activity of besifloxacin against staphylococci, Streptococcus pneumoniae and Haemophilus influenzae[J]. Journal of Antimicrobial Chemotherapy, 2010, 65(7): 1441-1447. |
| [142] |
FRITSCHE TR, SADER HS, STILLWELL MG, JONES RN. Antimicrobial activity of doripenem tested against prevalent gram-positive pathogens: results from a global surveillance study (2003–2007)[J]. Diagnostic Microbiology and Infectious Disease, 2009, 63(4): 440-446. |
| [143] |
GEORNARAS I, von HOLY A. Antimicrobial susceptibilities of isolates of Staphylococcus aureus, Listeria species and Salmonella serotypes associated with poultry processing[J]. International Journal of Food Microbiology, 2001, 70(1/2): 29-35. |
| [144] |
ODLAND BA, ERWIN ME, JONES RN. Quality control guidelines for disk diffusion and broth microdilution antimicrobial susceptibility tests with seven drugs for veterinary applications[J]. Journal of Clinical Microbiology, 2000, 38(1): 453-455. |
| [145] |
MacGOWAN AP, BOWKER KE, WOOTTON M, HOLT HA. Exploration of the in-vitro pharmacodynamic activity of moxifloxacin for Staphylococcus aureus and Streptococci of lancefield groups A and G[J]. The Journal of Antimicrobial Chemotherapy, 1999, 44(6): 761-766. |
| [146] |
JONES RN, ERWIN ME. In vitro susceptibility testing and quality control parameters for sarafloxacin (A-56620): a fluoroquinolone used for treatment and control of colibacillosis in poultry[J]. Diagnostic Microbiology and Infectious Disease, 1998, 32(1): 55-64. |
| [147] |
NORDEN CW, BUDINSKY A. Treatment of experimental chronic osteomyelitis due to staphylococcus aureus with ampicillin/sulbactam[J]. The Journal of Infectious Diseases, 1990, 161(1): 52-53. |
| [148] |
WADI MA. In vitro antibacterial activity of different honey samples against clinical isolates[J]. BioMed Research International, 2022, 2022: 1560050. |
| [149] |
OJDANA D, SIEŃKO A, SACHA P, MAJEWSKI P, WIECZOREK P, WIECZOREK A, TRYNISZEWSKA E. Genetic basis of enzymatic resistance of E. coli to aminoglycosides[J]. Advances in Medical Sciences, 2018, 63(1): 9-13. |
| [150] |
GRILLON A, SCHRAMM F, KLEINBERG M, JEHL F. Comparative activity of ciprofloxacin, levofloxacin and moxifloxacin against Klebsiella pneumoniae, Pseudomonas aeruginosa and Stenotrophomonas maltophilia assessed by minimum inhibitory concentrations and time-kill studies[J]. PLoS One, 2016, 11(6): e0156690. |
| [151] |
KIFFER CRV, KUTI JL, EAGYE KJ, MENDES C, NICOLAU DP. Pharmacodynamic profiling of imipenem, meropenem and ertapenem against clinical isolates of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella spp. from Brazil[J]. International Journal of Antimicrobial Agents, 2006, 28(4): 340-344. |
| [152] |
DILWORTH TJ, SLIWINSKI J, RYAN K, DODD M, MERCIER RC. Evaluation of vancomycin in combination with piperacillin-tazobactam or oxacillin against clinical methicillin-resistant Staphylococcus aureus isolates and vancomycin-intermediate S. aureus isolates in vitro[J]. Antimicrobial Agents and Chemotherapy, 2014, 58(2): 1028-1033. |
| [153] |
Di BONAVENTURA G, D'ANTONIO D, CATAMO G, BALLONE E, PICCOLOMINI R. Comparison of E-test, agar dilution, broth microdilution and disk diffusion methods for testing in vitro activity of levofloxacin against Staphylococcus spp. isolated from neutropenic cancer patients[J]. International Journal of Antimicrobial Agents, 2002, 19(2): 147-154. |
| [154] |
AMSLER K, SANTORO C, FOLENO B, BUSH K, FLAMM R. Comparison of broth microdilution, agar dilution, and E-test for susceptibility testing of doripenem against gram-negative and gram-positive pathogens[J]. Journal of Clinical Microbiology, 2010, 48(9): 3353-3357. |
| [155] |
BALOUIRI M, SADIKI M, IBNSOUDA SK. Methods for in vitro evaluating antimicrobial activity: a review[J]. Journal of Pharmaceutical Analysis, 2016, 6(2): 71-79. |
| [156] |
KHAN ZA, SIDDIQUI MF, PARK S. Current and emerging methods of antibiotic susceptibility testing[J]. Diagnostics, 2019, 9(2): 49. |
| [157] |
ZENG DN, DEBABOV D, HARTSELL TL, CANO RJ, ADAMS S, SCHUYLER JA, McMILLAN R, PACE JL. Approved glycopeptide antibacterial drugs: mechanism of action and resistance[J]. Cold Spring Harbor Perspectives in Medicine, 2016, 6(12): a026989. |
| [158] |
CUI XB. Isolation and identification, resistance analysis and combined medicine sensitive experiment research of avian Escherichia coli[D]. Tai'an: Master's Thesis of Shandong Agricultural University, 2014 (in Chinese). 崔笑博. 禽源大肠杆菌的分离鉴定, 耐药性分析及联合药敏试验的研究[D]. 泰安: 山东农业大学硕士学位论文, 2014. |
 2024, Vol. 51
2024, Vol. 51