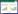扩展功能
文章信息
- 林杨轩, 陈芳艳, 韩黎
- LIN Yangxuan, CHEN Fangyan, HAN Li
- 烟曲霉黑色素的研究进展
- Research progress in melanins of Aspergillus fumigatus
- 微生物学通报, 2023, 50(8): 3688-3702
- Microbiology China, 2023, 50(8): 3688-3702
- DOI: 10.13344/j.microbiol.china.221259
-
文章历史
- 收稿日期: 2022-12-29
- 接受日期: 2023-04-13
- 网络首发日期: 2023-06-01
2. 安徽医科大学公共卫生学院, 安徽 合肥 230032
2. School of Public Health, Anhui Medical University, Hefei 230032, Anhui, China
自然界真菌共有600多万种,其中曲霉属有800多种,仅有少数可引起疾病[1-2]。烟曲霉(Aspergillus fumigatus)是一种广泛存在于自然界的腐生真菌,是曲霉属的一种,主要生态位是土壤;烟曲霉能够产生大量的分生孢子,其直径只有2‒3 μm,且其表面具有疏水性,可通过扩散到空气中而广泛传播;正常人每天约吸入几百个甚至上千个烟曲霉分生孢子,被吸入后可到达人体肺泡中,所以疾病主要发生于肺部,而有免疫功能缺陷的人群会增加烟曲霉相关疾病的发病率[3]。烟曲霉引发的疾病主要包括侵袭性曲霉菌病、慢性肺曲霉病、曲霉肿,以及过敏性疾病如哮喘和过敏性支气管肺曲霉病,其中最严重的是侵袭性曲霉菌病[4]。全球因血液系统疾病、遗传免疫缺陷、实体器官移植等相关免疫抑制引起的侵袭性曲霉菌病已超过20万例/年[5]。
烟曲霉之所以能够在各种环境下生存,黑色素是一个重要因素[6]。人们最早对黑色素的关注始于对黑色素瘤的研究[7]。由于黑色素瘤对放化疗的不敏感,猜测黑色素在其中发挥了作用,自此揭开了黑色素的神秘面纱。黑色素这个词是用来描述生物圈中发现的一种独特的色素,从黑色、棕色到黄色和红色。黑色素被广泛定义为由酚类前体氧化聚合而产生的棕色到黑色的高分子量色素;黑色素类型可以根据其来源(动物、植物、真菌或细菌)或物理和化学特征(真黑素、褐黑素、神经黑素、异黑素和脓黑素)进行分类;这些色素具有保护生物体免受不同的外界压力,包括紫外线照射、高温、活性氧(reactive oxygen species, ROS),还可以结合金属离子和自由电子,从而发挥氧化还原作用[8]。在真菌中,黑色素通常位于细胞外,甚至合成通常也不会发生在细胞质中,因为中间体产生对菌体本身具有潜在的毒性[9]。真菌可以合成3种不同类型的黑色素:多巴黑色素、DHN-黑色素和脓黑素,烟曲霉能够产生后两种[10]。本文主要综述烟曲霉产生的两种黑色素,详细描述它们的代谢、遗传和功能,以及它们与宿主免疫系统的相互作用。
1 黑色素的遗传代谢烟曲霉可以合成DHN-黑色素和脓黑素,其中DHN-黑色素的合成过程严重依赖酶的催化和聚合,这是其与多巴黑色素合成最大的区别;此外,DHN-黑色素利用内源性底物合成,不需要外源添加黑色素前体,而多巴黑色素合成有时需要外源性添加黑色素前体,如新生隐球菌(Cryptococcus neoformans)培养过程中需要添加酪氨酸[11]。烟曲霉脓黑素合成前体则与多巴黑色素类似,大部分来源于酪氨酸降解途径的产物,其与多巴黑色素或DHN-黑色素不同的是其高度溶于水,因此也被称为水溶性黑色素[10]。
目前关于真菌黑色素合成的调控机制研究并不多,近年来有研究表明白假丝酵母菌(Candida albicans)中控制white-opaque表型转换的核心调节因子白-灰转换因子1 (white-opaque regulator 1, Wor1)和调节碱性反应的rim101信号转导途径可分别在opaque和gray表型中通过上调铁氧化酶基因fets表达促进黑色素的产生[12-13]。在新生隐球菌中,Lee等[14]研究发现了调控黑色素合成的复杂信号网络,该网络由4个调节黑色素核心转录因子(melanin-regulating core transcription factors, MRC-TF) (Bzp4、Hob1、Usv101和Mbs1)和两个激酶(Gsk3和Kic1)组成,其中Mbs1能够独立调节lac1基因的表达,而Hob1通过调控Bzp4和Usv101进而调控lac1基因,上述两条通路均受Gsk3和Kic1的调节,进一步研究发现环磷酸腺苷(cyclic adenosine monophosphate, cAMP)信号途径、RAM (regulation of Ace2 and morphogenesis)信号途径以及高渗甘油(high osmolarity glycerol, HOG)信号途径也能通过Hob1和Bzp4调节该信号网络。而在烟曲霉中,环磷酸腺苷/蛋白激酶A (cyclic adenosine monophosphate/protein kinase A, cAMP/PKA)通路和丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)信号级联通路主要负责调控DHN-黑色素的产生。下文将详细介绍烟曲霉DHN-黑色素与脓黑素的合成途径及其合成调控机制。
1.1 DHN-黑色素 1.1.1 DHN-黑色素的合成烟曲霉产生的最常见的黑色素是DHN-黑色素,编码DHN-黑色素的生物合成基因簇由位于烟曲霉2号染色体上的6个基因(ayg1、arp1、arp2、abr1、abr2和pksP/alb1)组成,基因簇大小为19 kb[15]。其中pksP是第一个被鉴定为分生孢子色素形成所必需的基因,编码聚酮合酶,是DHN-黑色素生物合成簇的核心基因[16]。
首先由pksP/alb1基因编码的聚酮合酶(PksP/Alb1)参与乙酰辅酶A (Acetyl-CoA)和丙二酰辅酶A (Malonyl-CoA)的缩合反应,生成YWA1 (heptaketide napthopyrone);第2步是由ayg1基因编码的酶(Ayg1)催化YWA1水解生成1, 3, 6, 8-四羟基萘(1, 3, 6, 8-THN);第3步是由arp2基因编码的1, 3, 6, 8-THN还原酶(Arp2)将1, 3, 6, 8-THN转化为小柱孢酮(scytalone);第4步由arp1基因编码的脱水酶(Arp1)将其脱水成1, 3, 8-三羟基萘(1, 3, 8-THN);第5步是由Arp2还原酶将1, 3, 8-THN还原为柱酮醌(vermelone);第6步是由abr1基因编码的氧化酶(Abr1)将柱酮醌转化为1, 8-DHN;最后在abr2基因编码的漆酶(Abr2)作用下聚合生成DHN-黑色素(图 1)[10]。DHN-黑色素沉积在分生孢子表面疏水蛋白层以下,该层由RodA和RodB疏水蛋白组成[10]。这也是此前DHN-黑色素被认为是烟曲霉孢子呈典型灰绿色的原因。然而,近来有研究认为烟曲霉分生孢子颜色不能单独用DHN-黑色素来解释,YWA1衍生的色素也可能导致其呈蓝绿色[17]。
1.1.2 DHN-黑色素合成的调控机制调控烟曲霉DHN-黑色素合成的上游信号途径大致分为两条,即cAMP/PKA通路和MAPK信号级联通路。研究者最初发现acyA、gpaB或pkaC1基因的缺失导致烟曲霉的毒力急剧减弱,就有人猜测pksP基因的表达至少部分受cAMP/PKA信号传导途径控制[18-19]。Grosse等[20]通过构建pkaC1过表达菌株发现该菌株表现出高PKA活性从而导致pksP基因表达增加;而Kim等[21]发现G蛋白的信号转导调节因子D (regulator of G protein signaling protein D, RgsD)可以通过抑制Gα蛋白家族中的GpaB的活化从而抑制cAMP/PKA信号通路,进而减少烟曲霉黑色素的生成。
MAPK通路对信号的传递、整合和放大至关重要,是真核生物多种生物学过程中的重要组成部分[22]。与哺乳动物MAPK通路不同,关于烟曲霉MAPK信号级联通路的研究并不多,烟曲霉含有4种可编码MAPKs的基因,分别是MpkA、MpkB、MpkC和SakA[23]。Valiante等[24]发现烟曲霉黑色素的产生受双功能转录因子DevR和RlmA的调控,DevR和RlmA可识别并作用于pksP-arp1基因间区域中的特定DNA结合位点。此外,有研究阐明了MpkB与MpkA信号通路的相互作用,并揭示MpkA在分生孢子形成、发育以及DHN-黑色素合成中的作用,而且发现G蛋白偶联受体(G protein-coupled receptors, GPCRs)中的GprM和GprJ可以通过MpkB抑制MpkA,从而实现对黑色素的合成产生负调控作用[23, 25]。不仅如此,另一种G蛋白信号转导调节因子Rax1也被发现通过SakA通路对黑色素合成产生负调控[26]。
近期有研究发现一个新的碱性螺旋-环-螺旋(basic helix-loop-helix, bHLH)蛋白编码基因ecdR可能通过控制brlA、abaA和wetA基因(产孢过程的中央调控通路)的表达参与调控烟曲霉黑色素的产生,但具体机制还有待发掘[27]。详细通路如图 2所示。
烟曲霉产生的另一种黑色素是脓黑素,是一种棕黑色的色素。与DHN-黑色素不同,脓黑素并无自己的生物合成途径,而是由一种来自左旋酪氨酸和左旋苯丙氨酸降解过程的子产物尿黑酸聚合产生的。该过程同样是由烟曲霉2号染色体上6个基因(hppD、hmgX、hmgA、fahA、maiA和hmgR)参与合成,苯丙氨酸羟化酶(PhhA)将左旋苯丙氨酸(l-phenylalanine)转化为左旋酪氨酸(l-tyrosine),然后酪氨酸氨基转移酶(Tat)将左旋酪氨酸转化为4-羟基苯丙酮酸(4-hydroxyphenylpyruvate),之后由hppD基因编码的双加氧酶(HppD)介导4-羟基苯丙酮酸氧化脱羧生成尿黑酸(homogentisic acid, HGA),尿黑酸的积累会使得其自发氧化转化为苯醌醋酸酯(benzoquinone acetate),苯醌醋酸酯通过氧化聚合反应生成脓黑素(图 3)[28]。hmgX可能是HppD酶的辅助因子,而第6个基因hmgR则作为该基因簇的转录激活因子[29]。正常情况下,由hmgA基因编码的双加氧酶(HmgA)会继续氧化尿黑酸生成4-顺丁烯二酸单酰乙酰乙酸(4-maleylacetoacetate),随后被maiA基因编码的酶(MaiA)异构成4-延胡索酰乙酰乙酸(4-fumarylacetoacetate),最后,fahA编码的水解酶(FahA)将4-延胡索酰乙酰乙酸水解成乙酰乙酸(acetoacetate)和延胡索酸(fumarate),该产物可用于三羧酸循环(tricarboxylic acid cycle)[28]。
2 黑色素的功能 2.1 DHN-黑色素的功能DHN-黑色素的功能对于保护烟曲霉免受外界环境干扰至关重要,能够在干燥环境中保持分生孢子不失水[30],避免光照和紫外照射及电离辐射造成的损害[31-32],保护分生孢子免受土壤阿米巴的捕食[33-34],至于DHN-黑色素的其他功能,如掩盖病原体相关分子模式(pathogen- associated molecular patterns, PAMPs)、抵抗ROS、抑制LC3-相关吞噬作用(LC3-associated phagocytosis, LAP)等将在与宿主相互作用中详细描述。当生长条件合适时,烟曲霉分生孢子逐渐膨胀和萌发,形成菌丝,在此过程中其表面的黑色素和疏水蛋白逐渐脱落,但是否完全脱落视环境条件而不同,因此烟曲霉菌丝的细胞壁可能含有黑色素[4],具体的脱落过程还需要更多研究证实。失去黑色素和疏水蛋白层会使得真菌亲水,方便其黏附于物体表面或组织,有利于分生孢子的结合和萌发[35]。此外,DHN-黑色素对于烟曲霉分生孢子细胞壁的正确组装是必不可少的,缺乏DHN-黑色素合成的分生孢子会产生无最外层电子致密层的光滑细胞壁[35-36]。因此,DHN-黑色素不仅是一种毒力因子,还会影响细胞壁其他毒力因子如疏水蛋白、黏附素的正常功能。DHN-黑色素还能够调节分生孢子对存在于肺部的纤维连接蛋白和层粘连蛋白的结合能力[35, 37],从而影响烟曲霉的黏附与定殖,进而影响其内化及侵袭力,提高其在上皮细胞中的存活率[38]。另外,分生孢子与肺纤维连接蛋白的黏附不但具有依赖性,还有饱和性[39]。
DHN-黑色素在其他真菌病原体中也具有重要作用,一些真菌,如单梗着色霉(Fonsecaea monophora)其白化突变菌株被证实对氧化应激、极端pH环境和抗真菌药物包括伊曲康唑、特比萘芬和两性霉素B更敏感[40]。申克氏孢子丝菌(Sporothrix schenckii)黑色素能显著降低宿主炎症因子的释放,尤其是TNF-α和IL-6[41]。然而裴氏着色霉(Fonsecaea pedrosoi)黑色素能通过替代途径激活补体系统,这可能在真菌感染与宿主细胞反应间发挥重要作用[42]。
2.2 脓黑素的功能脓黑素的功能在其他病原中已有相关描述,如脓黑素增加了嗜肺军团菌(Legionella pneumophila)的光抵抗性[43],其分泌的脓黑素在铁限制条件下能促进细菌对铁的吸收和生长[44],海藻希瓦氏菌(Shewanella algae)产生的脓黑素也被证实有相似功能[45];霍乱弧菌(Vibrio cholerae)产生的脓黑素可抵抗棘阿米巴(Acanthamoeba castellanii)的捕食[46];而在青枯雷尔氏菌(Ralstonia solanacearum)中,脓黑素则具有抗氧化应激的作用[47];解脂假交替单胞菌(Pseudoalteromonas lipolytica)的脓黑素还有减少生物污染的作用[48];此外,在慢性感染期间产生脓黑素的绿脓杆菌(Pseudomonas aeruginosa)对pyocins有显著抗性,对其在宿主中的存活发挥关键作用[49]。在烟曲霉中,与DHN-黑色素相反,关于脓黑素的功能研究寥寥无几,其合成途径与分生孢子萌发和外界环境应激有关,细胞壁的机械或化学压力通过对hmgA基因的转录后修饰,提高了左旋酪氨酸降解基因的表达,进而增加了脓黑素的积累,以此保护在压力条件下出现的菌丝;这个过程似乎依赖于表面传感器,可能与左旋酪氨酸或左旋苯丙氨酸和细胞壁完整性(cell wall integrity, CWI)途径相关,而且该过程受MpkA的调控[24, 50]。
3 黑色素与宿主相互作用 3.1 DHN-黑色素与宿主相互作用DHN-黑色素最重要的特征之一是其掩盖烟曲霉分生孢子表面的PAMPs,包括细胞壁主要成分β-1, 3-葡聚糖、几丁质、甘露聚糖及其他衍生物,阻止其与淋巴细胞和单核巨噬细胞表面的模式识别受体(pattern recognition receptor, PRR)结合,如树突状细胞相关性C型植物凝集素-1 (dendritic cell-associated C-type lectin-1, dectin-1)受体、Toll样受体4 (Toll-like receptor 4, TLR4)和甘露糖受体(mannose receptors, MR),从而调节宿主促炎反应[51]。
尽管真菌有逃避免疫的策略,宿主免疫系统同样具有识别休眠孢子中DHN-黑色素并促进免疫应答的机制,黑色素感应C型凝集素受体(melanin-sensing C-type lectin receptor, MelLec)存在于内皮细胞和髓系细胞中,能够通过萘二醇单元特异性识别DHN-黑色素,但随着分生孢子的萌发膨胀,宿主受体MelLeC不再具有其识别能力[52]。但也有研究发现MelLeC加重了宿主细胞因子反应以及真菌负担,小鼠感染试验发现失去MelLec可以防止小鼠体重减轻[53]。此外,可溶性C型凝集素受体(C-type lectin receptors, CLRs)和表面活性蛋白D (surfactant protein D, SP-D)也能够识别和调理DHN-黑色素,从而促进抗炎细胞因子的产生,增加分生孢子的吞噬作用[54]。不仅如此,SP-D通过一系列机制发挥抗烟曲霉作用,包括菌丝生长限制、菌丝表面修饰、菌丝表面多糖的掩蔽,从而改变菌丝免疫特性[55]。
DHN-黑色素不仅能够干扰宿主对分生孢子的识别,还能抑制吞噬溶酶体膜上脂筏(lipid raft)的组装从而抑制吞噬溶酶体的形成和酸化,使吞噬细胞中的分生孢子存活;不仅如此,烟曲霉DHN-黑色素在吞噬小体内的Ca2+螯合作用还能抑制钙调素信号[作为含有RUN结构域和富含半胱氨酸结构域的Beclin1相互作用蛋白(RUN domain and cysteine-rich domain containing, Beclin 1-interacting protein, Rubicon)、NADPH氧化酶和LAP及其他分子组分的主要调节器]的激活,从而抑制LAP的发生[56-59]。此外,DHN-黑色素还能降低中性粒细胞的清除作用[60],这在斑马鱼模型试验中已得到证实[61]。
DHN-黑色素还能影响宿主相关生物学效应,包括:(1) 抑制宿主巨噬细胞内源性和外源性凋亡:DHN-黑色素通过激活磷脂酰肌醇3激酶/蛋白激酶B (phosphoinositide 3-kinase/protein kinase B, PI3K/Akt)信号通路抑制巨噬细胞内源性和外源性凋亡通路,从而抑制真菌凋亡,烟曲霉可以利用这一重要的毒力机制实现长期的细胞内存活[62]。(2) 干扰人单核细胞的凋亡:DHN-黑色素能够使凋亡的单核细胞从线粒体酸化中恢复并继续细胞周期[63]。(3) 影响宿主糖酵解过程:糖酵解是巨噬细胞代谢所必需的,巨噬细胞内烟曲霉黑色素的清除是启动葡萄糖代谢的关键因子,研究发现真菌黑色素可以通过哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)和缺氧诱导因子-1α (hypoxia- inducible factor-1 alpha, HIF-1α)信号通路重组宿主代谢,为巨噬细胞的代谢转向糖酵解和高效清除真菌提供必要的信号,这是一个双向机制,离不开钙信号的重塑[64]。由于mTOR是细胞代谢的主要调节因子,因此需要更多的研究来阐明黑色素对宿主免疫细胞代谢的影响。(4) 清除宿主细胞产生的ROS:DHN-黑色素此前被认为可以保护烟曲霉免受过氧化氢的侵害是基于烟曲霉pksP突变体(白化株,不产生DHN-黑色素)对ROS的敏感性增加,但是最近有研究发现烟曲霉DHN-黑色素对过氧化氢和超氧化物无保护作用,研究者认为因试验方法的原因导致cat1基因发生了突变从而出现了对ROS敏感性增加的现象,并推断DHN-黑色素中苯二酚和吲哚结构的缺失导致了与多巴黑色素的差异[65]。多巴黑色素可以抑制ROS产生的自由基,如构巢曲霉提取的多巴黑色素能够抑制脂多糖激活巨噬细胞的NO、TNF-α的产生,发挥抗氧化、抗炎作用[66]。此前也有研究发现该现象[23]。
除单核细胞和巨噬细胞外,黑色素与其他免疫和非免疫细胞的相互作用基本未知,本研究团队一直从事烟曲霉与肺泡上皮细胞相互作用的机制研究,发现烟曲霉细胞壁主要成分β-1, 3-glucan和分泌毒素gliotoxin通过诱导宿主磷脂酶D (phospholipase D, PLD)活化和调控胞内cofilin-Cdc42/RhoA-LIMK1信号通路等方式,在烟曲霉内化侵入肺泡上皮细胞过程中发挥重要作用[67-70]。同时首次发现肺泡上皮细胞表面表达补体受体3 (complement receptor, CR3),而且CR3通过激活FAK信号介导烟曲霉内化侵入肺泡上皮细胞[71]。后续将深入研究烟曲霉黑色素与宿主细胞相互作用信号机制。
此外,DHN-黑色素还被证明具有激活血小板的作用,当其他先天免疫细胞受到抑制时,血小板可以发挥抗真菌反应,血小板减少已被确认是侵袭性曲霉菌病的危险因素之一,因此有必要进一步阐明血小板在真菌感染中的确切作用[72-73]。
3.2 脓黑素与宿主相互作用一些体外数据表明,脓黑素可以参与真菌对免疫反应和ROS的保护。如上所述,在细胞壁压力应激下,酪氨酸降解基因簇的转录增加,并使真菌在细胞壁应激下积累脓黑素作为一种防御化合物,因此脓黑素的形成可能参与了真菌从免疫系统逃逸和生存[28]。然而葡萄糖和氮饥饿条件也被发现足以诱导酪氨酸降解相关基因的表达,但是却并未导致脓黑素的积累,可能是因为脓黑素的积累会抑制酪氨酸的降解,或者酪氨酸的降解主要用于合成延胡索酸和乙酰乙酸,进而用于三羧酸循环[29]。此外,在小鼠肺部侵袭性曲霉病感染模型中发现了与脓黑素合成相关基因表达的上调,表明烟曲霉在肺部感染时可能通过合成脓黑素保护真菌,但需要进一步的试验来证实[74]。因此,在感染过程中发现的酪氨酸降解簇基因在体内转录,并不一定表明脓黑素在真菌致病中发挥重要作用,因为这种代谢途径被发现可能参与真菌生长的调节,如马尔尼菲篮状菌(Penicillium marneffei)需要该途径将菌丝形态转换为有感染功能的酵母形态[75]。
目前还不清楚烟曲霉是否能够在感染部位产生足够数量的脓黑素,以保护其免受宿主免疫系统的攻击。在使用皮质类固醇治疗的肺曲霉病感染模型小鼠中,与野生分生孢子相比,hppD和hmgR基因缺失突变体感染导致的小鼠死亡率无显著差异,这表明脓黑素的毒力对烟曲霉是可有可无的[29]。因此,关于脓黑素的功能及其与宿主相互作用机制还需要更多更深入的研究来阐明。
4 总结与展望DHN-黑色素生成的代谢途径在孢子形成过程中被激活,涉及包括cAMP和MAPK途径在内的多条信号机制。黑化孢子的DHN-黑色素具有抵抗宿主固有免疫反应的功能,主要影响巨噬细胞的活性,包括抑制吞噬作用、吞噬溶酶体的形成、吞噬溶酶体的酸化、抑制凋亡等,有利于孢子在感染期间的定殖。同时,宿主可通过特异性PRR识别黑色素产生有效的抗真菌免疫反应,以抵消黑色素的抑制作用。而随着孢子的萌发膨胀,DHN-黑色素将逐渐脱落,此时,真菌与巨噬细胞相互作用期间的代谢重组可被视为宿主控制真菌疾病的替代免疫效应;当DHN-黑色素消失时,脓黑素的产生被认为是一种保护萌发菌丝的替代机制,尽管目前尚无证据表明其在抗应激中的作用。另外,在一项烟曲霉和绿脓杆菌体外培养试验中发现在细菌和真菌菌落之间的连接处有黑色素的存在,或许烟曲霉黑色素的抗菌功能也值得深入探讨[76]。不仅如此,特定条件培养下的链格孢(Alternaria alternata)分泌的脓黑素对DHN-黑色素还有抑制作用,从而减少细胞壁几丁质含量,进而影响细胞壁厚度[77]。烟曲霉中的2种黑色素是否存在关联也还需要证实;此外,烟曲霉黑色素与细胞壁其他成分的相互作用也尚未可知。深入阐明烟曲霉黑色素合成代谢机制及黑色素与宿主互作机制有助于为开发烟曲霉所致疾病的相关药物和疫苗提供理论基础。
当前限制烟曲霉黑色素研究的一个比较大的问题是缺乏体内黑色素的特异性检测方法,目前黑色素的检测方法主要是基于黑色素表面形态和理化性质进行检测,包括紫外-可见光谱法、比较傅里叶变换红外光谱法、核磁共振法,也可以通过上述方法与人工合成的黑色素相互比较进行定量或半定量检测,还可通过检测黑色素合成过程中的中间产物来间接确定黑色素分型[78-79]。这些方法均是将黑色素从真菌体内提纯进行体外检测分析,很难用于真菌在细胞内或细胞外生命周期中的黑色素功能研究,不仅如此,以上分析工具只提供了关于黑色素样本的一些特定信息,还需要综合上述所有技术方法来确定黑色素类型。随着生物技术的发展,相信在未来可开发出体内直接分析黑色素的工具,届时不仅能进一步分析黑色素的结构组成,还能直接进行黑色素的体内研究,以此探究黑色素及其上游合成通路作为抗烟曲霉治疗和疫苗的潜在靶点的可能性将大大提升。
| [1] |
JIAN Y. Study on the localization mechanism of melanocytes in Aspergillus fumigatus[D]. Nanchang: Master's Thesis of Nanchang University, 2020 (in Chinese). 简勇. 烟曲霉菌中的黑色素合酶细胞定位机制的探索[D]. 南昌: 南昌大学硕士学位论文, 2020. |
| [2] |
STRICKLAND AB, SHI MQ. Mechanisms of fungal dissemination[J]. Cellular and Molecular Life Sciences, 2021, 78(7): 3219-3238. DOI:10.1007/s00018-020-03736-z |
| [3] |
LATGÉ JP. Aspergillus fumigatus and aspergillosis[J]. Clinical Microbiology Reviews, 1999, 12(2): 310-350. DOI:10.1128/CMR.12.2.310 |
| [4] |
van de VEERDONK FL, GRESNIGT MS, ROMANI L, NETEA MG, LATGÉ JP. Aspergillus fumigatus morphology and dynamic host interactions[J]. Nature Reviews Microbiology, 2017, 15(11): 661-674. DOI:10.1038/nrmicro.2017.90 |
| [5] |
BROWN GD, DENNING DW, GOW NAR, LEVITZ SM, NETEA MG, WHITE TC. Hidden killers: human fungal infections[J]. Science Translational Medicine, 2012, 4(165): 165rv13. |
| [6] |
BURKHART CG, BURKHART CN. The mole theory: primary function of melanocytes and melanin may be antimicrobial defense and immunomodulation (not solar protection)[J]. International Journal of Dermatology, 2005, 44(4): 340-342. DOI:10.1111/j.1365-4632.2004.02556.x |
| [7] |
SONG Y, LIU J, CHEN JH, LI WJ, HU W, LIU L. Progress in fungi melanin radioprotection[J]. Journal of Radiation Research and Radiation Processing, 2016, 34(6): 1-6. (in Chinese) 宋缘, 刘敬, 陈积红, 李文建, 胡伟, 刘璐. 真菌黑色素辐射防护作用综述[J]. 辐射研究与辐射工艺学报, 2016, 34(6): 1-6. |
| [8] |
CORDERO RJB, CASADEVALL A. Melanin[J]. Current Biology: CB, 2020, 30(4): R142-R143. DOI:10.1016/j.cub.2019.12.042 |
| [9] |
HEINEKAMP T, THYWIßEN A, MACHELEIDT J, KELLER S, VALIANTE V, BRAKHAGE AA. Aspergillus fumigatus melanins: interference with the host endocytosis pathway and impact on virulence[J]. Frontiers in Microbiology, 2013, 3: 440. |
| [10] |
PEREZ-CUESTA U, APARICIO-FERNANDEZ L, GURUCEAGA X, MARTIN-SOUTO L, ABAD-DIAZ-DE-CERIO A, ANTORAN A, BULDAIN I, HERNANDO FL, RAMIREZ-GARCIA A, REMENTERIA A. Melanin and pyomelanin in Aspergillus fumigatus: from its genetics to host interaction[J]. International Microbiology, 2020, 23(1): 55-63. DOI:10.1007/s10123-019-00078-0 |
| [11] |
SMITH DFQ, CASADEVALL A. The role of melanin in fungal pathogenesis for animal hosts[M]//Fungal Physiology and Immunopathogenesis. Cham: Springer International Publishing, 2019: 1-30.
|
| [12] |
DAI BD, XU YX, GAO N, CHEN JY. Wor1-regulated ferroxidases contribute to pigment formation in opaque cells of Candida albicans[J]. FEBS Open Bio, 2021, 11(3): 598-621. DOI:10.1002/2211-5463.13070 |
| [13] |
DAI BD, XU YX, WU HY, CHEN JY. Rim101-upregulated Fets contribute to dark pigment formation in gray cells of Candida albicans[J]. Acta Biochimica et Biophysica Sinica, 2021, 53(12): 1723-1730. DOI:10.1093/abbs/gmab142 |
| [14] |
LEE D, JANG EH, LEE M, KIM SW, LEE Y, LEE KT, BAHN YS. Unraveling melanin biosynthesis and signaling networks in Cryptococcus neoformans[J]. mBio, 2019, 10(5): e02267-e02219. |
| [15] |
TSAI HF, WHEELER MH, CHANG YC, KWON-CHUNG KJ. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus[J]. Journal of Bacteriology, 1999, 181(20): 6469-6477. DOI:10.1128/JB.181.20.6469-6477.1999 |
| [16] |
LANGFELDER K, JAHN B, GEHRINGER H, SCHMIDT A, WANNER G, BRAKHAGE AA. Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence[J]. Medical Microbiology and Immunology, 1998, 187(2): 79-89. DOI:10.1007/s004300050077 |
| [17] |
NAMBU N, TSAI HF, CHANG YC, KWON-CHUNG KJ, YOSHIDA T, TANAKA N, TOMODA H, EBIZUKA Y, FUJII I. Novel angular naphthopyrone formation by Arp1p dehydratase involved in Aspergillus fumigatus melanin biosynthesis[J]. Environmental Microbiology Reports, 2021, 13(6): 822-829. DOI:10.1111/1758-2229.13013 |
| [18] |
LIEBMANN B, MÜLLER M, BRAUN A, BRAKHAGE AA. The cyclic AMP-dependent protein kinase a network regulates development and virulence in Aspergillus fumigatus[J]. Infection and Immunity, 2004, 72(9): 5193-5203. DOI:10.1128/IAI.72.9.5193-5203.2004 |
| [19] |
BRAKHAGE AA, LIEBMANN B. Aspergillus fumigatus conidial pigment and cAMP signal transduction: significance for virulence[J]. Medical Mycology, 2005, 43(Supplement_1): S75-S82. |
| [20] |
GROSSE C, HEINEKAMP T, KNIEMEYER O, GEHRKE A, BRAKHAGE AA. Protein kinase a regulates growth, sporulation, and pigment formation in Aspergillus fumigatus[J]. Applied and Environmental Microbiology, 2008, 74(15): 4923-4933. DOI:10.1128/AEM.00470-08 |
| [21] |
KIM Y, LEE MW, JUN SC, CHOI YH, YU JH, SHIN KS. RgsD negatively controls development, toxigenesis, stress response, and virulence in Aspergillus fumigatus[J]. Scientific Reports, 2019, 9: 811. DOI:10.1038/s41598-018-37124-2 |
| [22] |
PEARSON G, ROBINSON F, BEERS GIBSON T, XU BG, KARANDIKAR M, BERMAN K, COBB MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions[J]. Endocrine Reviews, 2001, 22(2): 153-183. |
| [23] |
MANFIOLLI AO, SIQUEIRA FS, dos REIS TF, van DIJCK P, SCHREVENS S, HOEFGEN S, FÖGE M, STRAßBURGER M, de ASSIS LJ, HEINEKAMP T, ROCHA MC, JANEVSKA S, BRAKHAGE AA, MALAVAZI I, GOLDMAN GH, VALIANTE V. Mitogen-activated protein kinase cross-talk interaction modulates the production of melanins in Aspergillus fumigatus[J]. mBio, 2019, 10(2): e00215-e00219. |
| [24] |
VALIANTE V, BALDIN C, HORTSCHANSKY P, JAIN R, THYWIßEN A, STRAßBURGER M, SHELEST E, HEINEKAMP T, BRAKHAGE AA. The Aspergillus fumigatus conidial melanin production is regulated by the bifunctional bHLH DevR and MADS-box RlmA transcription factors[J]. Molecular Microbiology, 2016, 102(2): 321-335. DOI:10.1111/mmi.13462 |
| [25] |
da COSTA FILHO AP, BRANCINI GTP, ALVES de CASTRO P, VALERO C, ALVES FERREIRA FILHO J, SILVA LP, ROCHA MC, MALAVAZI I, de MORAES PONTES JG, FILL T, SILVA RN, ALMEIDA F, STEENWYK JL, ROKAS A, dos REIS TF, RIES LNA, GOLDMAN GH. Aspergillus fumigatus G-protein coupled receptors GprM and GprJ are important for the regulation of the cell wall integrity pathway, secondary metabolite production, and virulence[J]. mBio, 2020, 11(5): e02458-e02420. |
| [26] |
IGBALAJOBI OA, YU JH, SHIN KS. Characterization of the rax1 gene encoding a putative regulator of G protein signaling in Aspergillus fumigatus[J]. Biochemical and Biophysical Research Communications, 2017, 487(2): 426-432. DOI:10.1016/j.bbrc.2017.04.079 |
| [27] |
HE C, WEI Q, XU J, CAI RH, KONG QT, CHEN PY, LU L, SANG H. bHLH transcription factor EcdR controls conidia production, pigmentation and virulence in Aspergillus fumigatus[J]. Fungal Genetics and Biology, 2023, 164: 103751. DOI:10.1016/j.fgb.2022.103751 |
| [28] |
SCHMALER-RIPCKE J, SUGAREVA V, GEBHARDT P, WINKLER R, KNIEMEYER O, HEINEKAMP T, BRAKHAGE AA. Production of pyomelanin, a second type of melanin, via the tyrosine degradation pathway in Aspergillus fumigatus[J]. Applied and Environmental Microbiology, 2009, 75(2): 493-503. DOI:10.1128/AEM.02077-08 |
| [29] |
KELLER S, MACHELEIDT J, SCHERLACH K, SCHMALER-RIPCKE J, JACOBSEN ID, HEINEKAMP T, BRAKHAGE AA. Pyomelanin formation in Aspergillus fumigatus requires HmgX and the transcriptional activator HmgR but is dispensable for virulence[J]. PLoS One, 2011, 6(10): e26604. DOI:10.1371/journal.pone.0026604 |
| [30] |
GOW NAR, LATGE JP, MUNRO CA. The fungal cell wall: structure, biosynthesis, and function[J]. Microbiology Spectrum, 2017, 5(3). |
| [31] |
FULLER KK, CRAMER RA, ZEGANS ME, DUNLAP JC, LOROS JJ. Aspergillus fumigatus photobiology illuminates the marked heterogeneity between isolates[J]. mBio, 2016, 7(5): e01517-e01516. |
| [32] |
BLACHOWICZ A, RAFFA N, BOK JW, CHOERA T, KNOX B, LIM FY, HUTTENLOCHER A, WANG CCC, VENKATESWARAN K, KELLER NP. Contributions of spore secondary metabolites to UV-C protection and virulence vary in different Aspergillus fumigatus strains[J]. mBio, 2020, 11(1): e03415-e03419. |
| [33] |
FERLING I, DAN DUNN J, FERLING A, SOLDATI T, HILLMANN F. Conidial melanin of the human-pathogenic fungus Aspergillus fumigatus disrupts cell autonomous defenses in amoebae[J]. mBio, 2020, 11(3): e00862-e00820. |
| [34] |
HILLMANN F, NOVOHRADSKÁ S, MATTERN DJ, FORBERGER T, HEINEKAMP T, WESTERMANN M, WINCKLER T, BRAKHAGE AA. Virulence determinants of the human pathogenic fungus Aspergillus fumigatus protect against soil amoeba predation[J]. Environmental Microbiology, 2015, 17(8): 2858-2869. DOI:10.1111/1462-2920.12808 |
| [35] |
PIHET M, VANDEPUTTE P, TRONCHIN G, RENIER G, SAULNIER P, GEORGEAULT S, MALLET R, CHABASSE D, SYMOENS F, BOUCHARA JP. Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia[J]. BMC Microbiology, 2009, 9: 177. DOI:10.1186/1471-2180-9-177 |
| [36] |
VALSECCHI I, DUPRES V, MICHEL JP, DUCHATEAU M, MATONDO M, CHAMILOS G, SAVEANU C, GUIJARRO JI, AIMANIANDA V, LAFONT F, LATGÉ JP, BEAUVAIS A. The puzzling construction of the conidial outer layer of Aspergillus fumigatus[J]. Cellular Microbiology, 2019, 21(5): e12994. DOI:10.1111/cmi.12994 |
| [37] |
BAYRY J, BEAUSSART A, DUFRÊNE YF, SHARMA M, BANSAL K, KNIEMEYER O, AIMANIANDA V, BRAKHAGE AA, KAVERI SV, KWON-CHUNG KJ, LATGÉ JP, BEAUVAIS A. Surface structure characterization of Aspergillus fumigatus conidia mutated in the melanin synthesis pathway and their human cellular immune response[J]. Infection and Immunity, 2014, 82(8): 3141-3153. DOI:10.1128/IAI.01726-14 |
| [38] |
AMIN S, THYWISSEN A, HEINEKAMP T, SALUZ HP, BRAKHAGE AA. Melanin dependent survival of Apergillus fumigatus conidia in lung epithelial cells[J]. International Journal of Medical Microbiology, 2014, 304(5/6): 626-636. |
| [39] |
XU CY, WU JH, WEN H, CHEN JH, XU H, MENG YJ, LI XY. Preliminary study on the interaction of adhesion between fibronectin and Aspergillus fumigatus conidia[J]. Journal of Clinical Dermatology, 2003, 32(2): 61-64. (in Chinese) 徐赤宇, 吴建华, 温海, 陈江汉, 徐红, 孟玉景, 李小英. 纤连蛋白与烟曲霉分生孢子相互黏附作用的初步研究[J]. 临床皮肤科杂志, 2003, 32(2): 61-64. DOI:10.3969/j.issn.1000-4963.2003.02.002 |
| [40] |
XIAO X, LI Y, LAN Y, ZHANG J, HE Y, CAI WY, CHEN ZW, XI LY, ZHANG JM. Deletion of pksA attenuates the melanogenesis, growth and sporulation ability and causes increased sensitivity to stress response and antifungal drugs in the human pathogenic fungus Fonsecaea monophora[J]. Microbiological Research, 2021, 244: 126668. DOI:10.1016/j.micres.2020.126668 |
| [41] |
GUAN MQ, YAO L, ZHEN Y, SONG Y, CUI Y, LI SS. Melanin of Sporothrix globosa affects the function of THP-1 macrophages and modulates the expression of TLR2 and TLR4[J]. Microbial Pathogenesis, 2021, 159: 105158. DOI:10.1016/j.micpath.2021.105158 |
| [42] |
PINTO L, GRANJA LFZ, de ALMEIDA MA, ALVIANO DS, da SILVA MH, EJZEMBERG R, ROZENTAL S, ALVIANO CS. Melanin particles isolated from the fungus Fonsecaea pedrosoi activates the human complement system[J]. Memórias Do Instituto Oswaldo Cruz, 2018, 113(8): e180120. |
| [43] |
STEINERT M, ENGELHARD H, FLÜGEL M, WINTERMEYER E, HACKER J. The Lly protein protects Legionella pneumophila from light but does not directly influence its intracellular survival in Hartmannella vermiformis[J]. Applied and Environmental Microbiology, 1995, 61(6): 2428-2430. DOI:10.1128/aem.61.6.2428-2430.1995 |
| [44] |
ZHENG HX, CHATFIELD CH, LILES MR, CIANCIOTTO NP. Secreted pyomelanin of Legionella pneumophila promotes bacterial iron uptake and growth under iron-limiting conditions[J]. Infection and Immunity, 2013, 81(11): 4182-4191. DOI:10.1128/IAI.00858-13 |
| [45] |
TURICK CE, CACCAVO F, TISA LS. Pyomelanin is produced by Shewanella algae BrY and affected by exogenous iron[J]. Canadian Journal of Microbiology, 2008, 54(4): 334-339. DOI:10.1139/W08-014 |
| [46] |
NOORIAN P, HU J, CHEN ZL, KJELLEBERG S, WILKINS MR, SUN SY, MCDOUGALD D. Pyomelanin produced by Vibrio cholerae confers resistance to predation by Acanthamoeba castellanii[J]. FEMS Microbiology Ecology, 2017, 93(12): fix147. |
| [47] |
AHMAD S, LEE SY, KONG HG, JO EJ, CHOI HK, KHAN R, LEE SW. Genetic determinants for pyomelanin production and its protective effect against oxidative stress in Ralstonia solanacearum[J]. PLoS One, 2016, 11(8): e0160845. DOI:10.1371/journal.pone.0160845 |
| [48] |
ZENG ZS, GUO XP, CAI XS, WANG PX, LI BY, YANG JL, WANG XX. Pyomelanin from Pseudoalteromonas lipolytica reduces biofouling[J]. Microbial Biotechnology, 2017, 10(6): 1718-1731. DOI:10.1111/1751-7915.12773 |
| [49] |
HOCQUET D, PETITJEAN M, ROHMER L, VALOT B, KULASEKARA HD, BEDEL E, BERTRAND X, PLÉSIAT P, KÖHLER T, PANTEL A, JACOBS MA, HOFFMAN LR, MILLER SI. Pyomelanin-producing Pseudomonas aeruginosa selected during chronic infections have a large chromosomal deletion which confers resistance to pyocins[J]. Environmental Microbiology, 2016, 18(10): 3482-3493. DOI:10.1111/1462-2920.13336 |
| [50] |
VALIANTE V, JAIN R, HEINEKAMP T, BRAKHAGE AA. The MpkA MAP kinase module regulates cell wall integrity signaling and pyomelanin formation in Aspergillus fumigatus[J]. Fungal Genetics and Biology, 2009, 46(12): 909-918. DOI:10.1016/j.fgb.2009.08.005 |
| [51] |
CHAI LYA, NETEA MG, SUGUI J, VONK AG, van de SANDE WWJ, WARRIS A, KWON-CHUNG KJ, KULLBERG BJ. Aspergillus fumigatus conidial melanin modulates host cytokine response[J]. Immunobiology, 2010, 215(11): 915-920. DOI:10.1016/j.imbio.2009.10.002 |
| [52] |
STAPPERS MHT, CLARK AE, AIMANIANDA V, BIDULA S, REID DM, ASAMAPHAN P, HARDISON SE, DAMBUZA IM, VALSECCHI I, KERSCHER B, PLATO A, WALLACE CA, YUECEL R, HEBECKER B, da GLÓRIA TEIXEIRA SOUSA M, CUNHA C, LIU Y, FEIZI T, BRAKHAGE AA, CHUNG KJK, et al. Recognition of DHN-melanin by a C-type lectin receptor is required for immunity to Aspergillus[J]. Nature, 2018, 555(7696): 382-386. DOI:10.1038/nature25974 |
| [53] |
TONE K, STAPPERS MHT, HATINGUAIS R, DAMBUZA IM, SALAZAR F, WALLACE C, YUECEL R, MORVAY PL, KUWANO K, WILLMENT JA, BROWN GD. MelLec exacerbates the pathogenesis of Aspergillus fumigatus-induced allergic inflammation in mice[J]. Frontiers in Immunology, 2021, 12: 675702. DOI:10.3389/fimmu.2021.675702 |
| [54] |
WONG SSW, RANI M, DODAGATTA-MARRI E, IBRAHIM-GRANET O, KISHORE U, BAYRY J, LATGÉ JP, SAHU A, MADAN T, AIMANIANDA V. Fungal melanin stimulates surfactant protein d-mediated opsonization of and host immune response to Aspergillus fumigatus spores[J]. Journal of Biological Chemistry, 2018, 293(13): 4901-4912. DOI:10.1074/jbc.M117.815852 |
| [55] |
WONG SSW, DELLIÈRE S, SCHIEFERMEIER- MACH N, LECHNER L, PERKHOFER S, BOMME P, FONTAINE T, SCHLOSSER AG, SORENSEN GL, MADAN T, KISHORE U, AIMANIANDA V. Surfactant protein D inhibits growth, alters cell surface polysaccharide exposure and immune activation potential of Aspergillus fumigatus[J]. The Cell Surface, 2022, 8: 100072. DOI:10.1016/j.tcsw.2022.100072 |
| [56] |
THYWIßEN A, HEINEKAMP T, DAHSE HM, SCHMALER-RIPCKE J, NIETZSCHE S, ZIPFEL PF, BRAKHAGE AA. Conidial dihydroxynaphthalene melanin of the human pathogenic fungus Aspergillus fumigatus interferes with the host endocytosis pathway[J]. Frontiers in Microbiology, 2011, 2: 96. |
| [57] |
AKOUMIANAKI T, KYRMIZI I, VALSECCHI I, GRESNIGT MS, SAMONIS G, DRAKOS E, BOUMPAS D, MUSZKIETA L, PREVOST MC, KONTOYIANNIS DP, CHAVAKIS T, NETEA MG, van de VEERDONK FL, BRAKHAGE AA, EL-BENNA J, BEAUVAIS A, LATGE JP, CHAMILOS G. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity[J]. Cell Host & Microbe, 2016, 19(1): 79-90. |
| [58] |
KYRMIZI I, FERREIRA H, CARVALHO A, ALBERTO LANDERO FIGUEROA J, ZARMPAS P, CUNHA C, AKOUMIANAKI T, STYLIANOU K, DEEPE GS JR, SAMONIS G, LACERDA JF, CAMPOS A JR, KONTOYIANNIS DP, MIHALOPOULOS N, KWON-CHUNG KJ, EL-BENNA J, VALSECCHI I, BEAUVAIS A, BRAKHAGE AA, NEVES NM, et al. Calcium sequestration by fungal melanin inhibits calcium-calmodulin signalling to prevent LC3-associated phagocytosis[J]. Nature Microbiology, 2018, 3(7): 791-803. DOI:10.1038/s41564-018-0167-x |
| [59] |
SCHMIDT F, THYWIßEN A, GOLDMANN M, CUNHA C, CSERESNYÉS C, SCHMIDT H, RAFIQ M, GALIANI S, GRÄLER MH, CHAMILOS G, Lacerda JF, CAMPOS A Jr, EGGELING C, FIGGE MT, HEINEKAMP T, FILLER SG, CARVALHO A, BRAKHAGE AA. Flotillin-dependent membrane microdomains are required for functional phagolysosomes against fungal infections[J]. Cell Reports, 2020, 32(7): 108017. DOI:10.1016/j.celrep.2020.108017 |
| [60] |
ESCOBAR N, ORDONEZ SR, WÖSTEN HAB, HAAS PJ A, de COCK H, HAAGSMAN HP. Hide, keep quiet, and keep low: properties that make Aspergillus fumigatus a successful lung pathogen[J]. Frontiers in Microbiology, 2016, 7: 438. |
| [61] |
FORN-CUNÍ G, WELVAARTS L, STEL F, van den HONDEL C, ARENTSHORST M, RAM A, MEIJER A. Stimulating the autophagic-lysosomal axis enhances host defense against fungal infection in a zebrafish model of invasive Aspergillosis[J]. Autophagy, 2023, 19(1): 324-337. DOI:10.1080/15548627.2022.2090727 |
| [62] |
VOLLING K, THYWISSEN A, BRAKHAGE AA, SALUZ HP. Phagocytosis of melanized Aspergillus conidia by macrophages exerts cytoprotective effects by sustained PI3K/Akt signalling[J]. Cellular Microbiology, 2011, 13(8): 1130-1148. |
| [63] |
MOHEBBI S, ERFURTH F, HENNERSDORF P, BRAKHAGE AA, SALUZ HP. Hyperspectral imaging using intracellular spies: quantitative real-time measurement of intracellular parameters in vivo during interaction of the pathogenic fungus Aspergillus fumigatus with human monocytes[J]. PLoS One, 2016, 11(10): e0163505. |
| [64] |
GONÇALVES SM, DUARTE-OLIVEIRA C, CAMPOS CF, AIMANIANDA V, TER HORST R, LEITE L, MERCIER T, PEREIRA P, FERNÁNDEZ-GARCÍA M, ANTUNES D, RODRIGUES CS, BARBOSA-MATOS C, GAIFEM J, MESQUITA I, MARQUES A, OSÓRIO NS, TORRADO E, RODRIGUES F, COSTA S, JOOSTEN LA, et al. Phagosomal removal of fungal melanin reprograms macrophage metabolism to promote antifungal immunity[J]. Nature Communications, 2020, 11: 2282. |
| [65] |
KEIZER EM, VALDES ID, McCANN BL, BIGNELL EM, WÖSTEN HAB, de COCK H. The protective role of 1, 8-dihydroxynaphthalene-melanin on conidia of the opportunistic human pathogen Aspergillus fumigatus revisited: no role in protection against hydrogen peroxide and superoxides[J]. mSphere, 2022, 7(1): e00874-e00821. |
| [66] |
de CÁSSIA RIBEIRO GONÇALVES R, KITAGAWA RR, STELLA GONÇALVES RADDI M, CARLOS IZ, POMBEIRO-SPONCHIADO SR. Inhibition of nitric oxide and tumour necrosis factor-α production in peritoneal macrophages by Aspergillus nidulans melanin[J]. Biological and Pharmaceutical Bulletin, 2013, 36(12): 1915-1920. |
| [67] |
HAN XL, YU RT, ZHEN DY, TAO S, SCHMIDT M, HAN L. β-1, 3-glucan-induced host phospholipase D activation is involved in Aspergillus fumigatus internalization into type II human pneumocyte A549 cells[J]. PLoS One, 2011, 6(7): e21468. |
| [68] |
JIA XD, CHEN FY, PAN WH, YU RT, TIAN SG, HAN GG, FANG HQ, WANG S, ZHAO JY, LI XP, ZHENG DY, TAO S, LIAO WQ, HAN XL, HAN L. Gliotoxin promotes Aspergillus fumigatus internalization into type II human pneumocyte A549 cells by inducing host phospholipase D activation[J]. Microbes and Infection, 2014, 16(6): 491-501. |
| [69] |
BAO ZY, HAN XL, CHEN FY, JIA XD, ZHAO JY, ZHANG CJ, YONG C, TIAN SG, ZHOU X, HAN L. Evidence for the involvement of cofilin in Aspergillus fumigatus internalization into type II alveolar epithelial cells[J]. BMC Microbiology, 2015, 15(1): 1-11. |
| [70] |
ZHANG CJ, CHEN FY, LIU XY, HAN XL, HU YS, SU XT, CHEN Y, SUN YS, HAN L. Gliotoxin induces cofilin phosphorylation to promote actin cytoskeleton dynamics and internalization of Aspergillus fumigatus into type II human pneumocyte cells[J]. Frontiers in Microbiology, 2019, 10: 1345. |
| [71] |
HAN XL, SU XT, LI ZQ, LIU YX, WANG S, ZHU M, ZHANG CJ, YANG F, ZHAO JY, LI XP, CHEN FY, HAN L. Complement receptor 3 mediates Aspergillus fumigatus internalization into alveolar epithelial cells with the increase of intracellular phosphatidic acid by activating FAK[J]. Virulence, 2021, 12(1): 1980-1996. |
| [72] |
RAMBACH G, BLUM G, LATGÉ JP, FONTAINE T, HEINEKAMP T, HAGLEITNER M, JECKSTRÖM H, WEIGEL G, WÜRTINGER P, PFALLER K, KRAPPMANN S, LÖFFLER J, LASS-FLÖRL C, SPETH C. Identification of Aspergillus fumigatus surface components that mediate interaction of conidia and hyphae with human platelets[J]. The Journal of Infectious Diseases, 2015, 212(7): 1140-1149. |
| [73] |
SONG L, ZHAO YJ, WANG G, ZOU WL, SAI LT. Investigation of predictors for invasive pulmonary aspergillosis in patients with severe fever with thrombocytopenia syndrome[J]. Scientific Reports, 2023, 13: 1538. |
| [74] |
GURUCEAGA X, EZPELETA G, MAYAYO E, SUEIRO-OLIVARES M, ABAD-DIAZ-DE-CERIO A, AGUIRRE URÍZAR JM, LIU HG, WIEMANN P, BOK JW, FILLER SG, KELLER NP, HERNANDO FL, RAMIREZ-GARCIA A, REMENTERIA A. A possible role for fumagillin in cellular damage during host infection by Aspergillus fumigatus[J]. Virulence, 2018, 9(1): 1548-1561. |
| [75] |
BOYCE KJ, McLAUCHLAN A, SCHREIDER L, ANDRIANOPOULOS A. Intracellular growth is dependent on tyrosine catabolism in the dimorphic fungal pathogen Penicillium marneffei[J]. PLoS Pathogens, 2015, 11(3): e1004790. |
| [76] |
BRIARD B, RASOLDIER V, BOMME P, ElAOUAD N, GUERREIRO C, CHASSAGNE P, MUSZKIETA L, LATGÉ JP, MULARD L, BEAUVAIS A. Dirhamnolipids secreted from Pseudomonas aeruginosa modify anjpegungal susceptibility of Aspergillus fumigatus by inhibiting β1, 3 glucan synthase activity[J]. The ISME Journal, 2017, 11(7): 1578-1591. |
| [77] |
FERNANDES C, MOTA M, BARROS L, DIAS MI, FERREIRA ICFR, PIEDADE AP, CASADEVALL A, GONÇALVES T. Pyomelanin synthesis in Alternaria alternata inhibits DHN-melanin synthesis and decreases cell wall chitin content and thickness[J]. Frontiers in Microbiology, 2021, 12: 691433. |
| [78] |
RAMAN NM, RAMASAMY S. Genetic validation and spectroscopic detailing of DHN-melanin extracted from an environmental fungus[J]. Biochemistry and Biophysics Reports, 2017, 12: 98-107. |
| [79] |
SINGH S, NIMSE SB, ELZE MATHEW D, DHIMMAR A, SAHASTRABUDHE H, GAJJAR A, GHADGE VA, KUMAR P, SHINDE PB. Microbial melanin: recent advances in biosynthesis, extraction, characterization, and applications[J]. Biotechnology Advances, 2021, 53: 107773. |
 2023, Vol. 50
2023, Vol. 50