扩展功能
文章信息
- 李情情, 马青云, 阮志勇
- LI Qingqing, MA Qingyun, RUAN Zhiyong
- 三酮类除草剂微生物降解的研究进展
- Research progress in microbial degradation of triketone herbicides
- 微生物学通报, 2023, 50(2): 845-856
- Microbiology China, 2023, 50(2): 845-856
- DOI: 10.13344/j.microbiol.china.220898
-
文章历史
- 收稿日期: 2022-09-15
- 接受日期: 2022-11-19
- 网络首发日期: 2022-12-12
2. 中国农业科学院研究生院, 北京 100081;
3. 华中农业大学 农业微生物学国家重点实验室, 湖北 武汉 430070;
4. 西藏农牧学院资源与环境学院, 西藏 林芝 860000
2. Graduate School of Chinese Academy of Agricultural Sciences, Beijing 100081, China;
3. State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan 430070, Hubei, China;
4. College of Resources and Environment, Tibet Agriculture and Animal Husbandry University, Linzhi 860000, Tibet, China
除草剂的应用降低了人工除草的成本,同时有效地减少了因杂草导致的作物生产损失。近年来,我国化学除草面积以每年200 hm2的速度递增,除草剂市场潜力巨大,但是长期、广泛、大量地施用除草剂,导致抗性杂草数量不断增加,截至目前共有514种抗除草剂的杂草,其中仅抗乙酰乳酸合成酶(acetyl lactate synthase, ALS)抑制剂类除草剂的杂草数量就达到了170种之多[1],给农业生产带来了很大损失[2],而三酮类除草剂的应用大大降低了这种损失。三酮类除草剂最早是由先正达公司开发,起源于一种挥发性油类植物毒素纤精酮,该物质被敏感杂草吸收后,经由木质部和韧皮部被运输至叶片部位,通过抑制对羟基苯基丙酮酸双氧化酶(p-hydroxyphenylpyruvate dioxygenase, HPPD)活性,阻碍叶绿素合成,使杂草出现白化症状后缓慢死亡[3-4],一经上市广受好评,其中硝磺草酮2016年的销售额达到了6.50亿美元,占据全球除草剂销售额的第4位[5]。三酮类除草剂是继磺酰脲类、二硝基苯胺类除草剂等后出现的新兴除草剂,主要应用于玉米生产,因其抗性杂草少、杀草谱广,正在成为一类高效的“明星”除草剂,包括硝磺草酮、磺草酮、环磺酮、双环磺草酮等(表 1)。
| 除草剂类型 Type of herbicides |
结构式 Structure |
物质特性 Characteristics |
来源 References |
| 磺草酮 Sulcotrione |
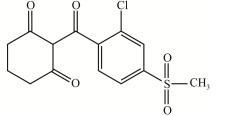 |
褐灰色固体,25 ℃时溶解度为 0.2 g/L,CAS:99105-77-8 Gray solid with solubility of 0.2 g/L at 25 ℃, CAS: 99105-77-8 |
[6] |
| 硝磺草酮 Mesotrione |
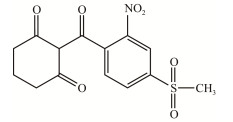 |
浅黄色固体,20 ℃时溶解度为 2.2–22.0 g/L,CAS:104206-82-8 Pale yellow solid with solubility of 2.2–22.0 g/L at 20 ℃, CAS: 104206-82-8 |
[6] |
| 环磺酮 Tembotrione |
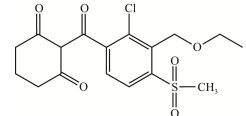 |
无臭固体,20 ℃时溶解度为 0.2–29.7 g/L, CAS:335104-84-2 Odorless solid with solubility of 0.2–29.7 g/L at 20 ℃, CAS: 335104-84-2 |
[6] |
| 双环磺草酮 Benzobicyclon |
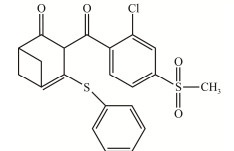 |
浅黄色结晶,20 ℃时溶解度为0.1 mg/L,CAS:156963-66-5 Light yellow crystal with solubility of 0.1 mg/L at 20 ℃, CAS: 156963-66-5 |
[7] |
尽管三酮类除草剂除草效果良好,对玉米安全性高[8-9],但由于其广泛、大量的施用,不可避免地造成了该类除草剂在土壤[10-11]、水体[12]中的残留积累,对环境造成的污染仍然需要持续关注。已有研究表明,残留在土壤、水体中的三酮类除草剂对生态环境会产生显著的影响。
首先,三酮类除草剂会影响土壤酶活和微生物群落的丰富度。土壤酶活和微生物群落是表征土壤健康非常重要的指标,对维持土壤生态系统稳定、植物健康生长具有至关重要的作用[13-14]。磺草酮和硝磺草酮均会对土壤的微生物群落产生一定的扰动,改变土壤物种丰富度,显著降低土壤中固氮根瘤菌的末端限制性片段(terminal restriction fragment, T-RF)丰度,减弱土壤固氮能力[15-16]。孙约兵等[17]发现在实验浓度(0–100 mg/kg)范围内,硝磺草酮对土壤中的过氧化氢酶和蔗糖酶活性存在一定的激活作用,且随浓度的增加呈现先增加后降低的趋势,而土壤中脲酶在硝磺草酮浓度 > 20 mg/kg时活性受到抑制,低浓度(5.0 g/kg)时相对稳定,β-葡萄糖苷酶活性等也受到一定程度的影响[16]。
其次,硝磺草酮、磺草酮及其降解产物在环境中性质稳定,对土壤造成污染,影响后茬作物的健康生长。磺草酮的喷施导致蚕豆、洋葱等敏感作物有丝分裂异常,死亡率随剂量的增加而增加,呈现剂量依赖性,表明该除草剂原药可能有强大的诱变效应[18],磺草酮的商业产品Mikado®显示出比原药更大的细胞毒性[19-20]。停用推荐剂量的硝磺草酮一年后的土壤仍会导致后茬作物金鱼草、卷心菜、甜椒、蔓越莓、芸豆出现发育迟缓、组织坏死、整体生长减少等损伤,造成产量下降[21-22]。土壤中残留的磺草酮和硝磺草酮在土壤微生物的作用下部分被转化为各自的降解产物,磺草酮的2种主要光解产物也会使洋葱有丝分裂指数显著降低,染色异常呈现剂量依赖性[23-24]。
再次,三酮类除草剂对动物和水生生物也会造成不同程度的毒伤。磺草酮显著增加了大鼠肝脏酪氨酸水平,5 mg/kg的磺草酮对大鼠的角膜造成18%以上的伤害,并呈现剂量依赖性[25]。Piancini等[26]研究发现,硝磺草酮增加了Oreochorimis niloticus和Geophagus brasiliensis的DNA损伤和氧化应激水平。另外,10 mg/L硝磺草酮能增加微囊藻的相对丰度,增加水华发生的风险[27];同时还能降低小球藻的叶绿素含量和光合作用相关基因转录水平,破坏小球藻的细胞膜和叶绿体结构的完整性,影响其光合作用能力,减少小球藻的生长量[28]。此外,Bonnet等[29]发现这2种除草剂的降解中间产物相较于母体分子,对2种代表性环境微生物Tetrahymena pyriformis和Vibrio fischeri表现出更强的毒性。
2 磺草酮和硝磺草酮的生物降解许多研究表明,三酮类除草剂在土壤中的降解受到多种因素的影响。例如,土壤水分、pH、微生物、土壤类型等,其中微生物是其降解的主要驱动力[30-31]。三酮类除草剂在玉米土壤中较为稳定[32-33],淋溶性强,其除草剂分子及降解产物易对后茬敏感作物产生毒害,对土壤、地下水有一定的污染风险。因此,研究三酮类除草剂的微生物降解、彻底矿化显得非常有必要[12]。
2.1 微生物降解资源生物降解是指微生物(包括一些其他生物)对物质(特别是环境污染物)的分解作用[34]。自然界中的微生物数量大、种类繁多、适应性强、繁殖速度快,修复环境污染时具有绿色、经济、效率高等优点。目前已有研究者从土壤、水体等环境中筛选出了部分以芽孢杆菌属(Bacillus)为主的三酮类除草剂降解微生物资源(表 2)。
| 除草剂类型 Type of herbicides |
降解菌株 Degrading strains |
浓度 Concentration (mg/L) |
降解率 Degradation rate (%) |
来源 References |
| 磺草酮 Sulcotrione |
Bradyrhizobium sp. SR1 | 35 | 100 (48 h) | [35] |
| Pseudomonas sp. 1OP | 34 | > 80 (30 d) | [36] | |
| 硝磺草酮 Mesotrione |
Bacillus sp. 3B6 | 1 700 | 100 (10 h) | [37] |
| Bacillus sp. Mes11 | 34 | 100 (50 h) | [38] | |
| Bacillus pumilus HZ-2 | 200 | 100 (5 d) | [39] | |
| Pantoea ananatis | 13.6 | 100 (24 h) | [40] | |
| Escherichia coli DH5α | 136 | 100 (3 h) | [41] | |
| Bradyrhizobium sp. SR1 | 35 | 100 (20 d) | [35] |
Romdhane等[35]发现了一株能够降解2种三酮类除草剂的慢生根瘤菌(Bradyrhizobium sp. SR1),在48 h内能够将35 mg/L的磺草酮完全降解,并且检测到一种代谢物2-氯-4-甲磺酰基苯甲酸(2-chloro-4-methylsulfonyl benzoic acid, CMBA);该菌株完全降解相同浓度的硝磺草酮需要20 d,同时在降解液中检测到了2种常见产物,即2-氨基-4-甲磺酰基苯甲酸(2-amino-4- methylsulfonylbenzoic acid, AMBA)和2-硝基-4-甲磺酰基苯甲酸(4-methylsulfonyl-2- nitrobenzoic acid, MNBA),并且随着时间的延长,可以观察到MNBA向AMBA的1:1定量转化。Calvayrac等[36]分离筛选出8株耐受磺草酮的菌株,其中一株假单胞菌(Pseudomonas sp. 1OP)能够以磺草酮为唯一碳源,以(NH4)2SO4为氮源,在30 d内将34 mg/L的磺草酮降解80%以上,并且生成主要代谢产物CMBA,其3种主要中间产物的结构式见图 1。
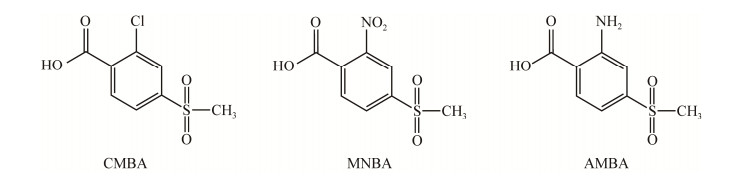
|
| 图 1 三种主要中间产物的结构式 Figure 1 Structural formulas of three main intermediates. |
|
|
Durand等[37]于2006年从云水里首次分离出了一株能够降解硝磺草酮的菌株芽孢杆菌(Bacillus sp.) 3B6,其能够在10 h内将1 700 mg/L的硝磺草酮完全降解,生成2种代谢产物AMBA和MNBA。Batisson等[38]发现未灭菌的土壤40 d内对4.5 mg/kg硝磺草酮的降解率达50%,从土壤中分离出耐受硝磺草酮的菌株有芽孢杆菌(Bacillus sp.) Mes11和节杆菌(Arthrobacter sp.),仅芽孢杆菌能够快速降解硝磺草酮,24 h内能完全降解340 mg/L的硝磺草酮,产物AMBA不能被降解,在培养基中积累。韩海涛等[39]以广东省惠州市第七污水处理厂厌氧污泥池中的活性污泥为原料,筛选出硝磺草酮降解菌株芽孢杆菌(Bacillus sp.) HZ-2,通过对其16S rRNA基因测序分析,鉴定为Bacillus pumilus;该菌株能耐受和降解高浓度硝磺草酮,在适宜的条件下5 d内可将200 mg/L的硝磺草酮完全降解,当硝磺草酮的初始浓度提高至400 mg/L时,菌株HZ-2仍能良好生长,降解率仍可达到98.29%。周亮成[42]在实验室的硝磺草酮降解菌株Bacillus sp. HZ-2前期培养基础上,添加惰性载体和多种助剂研发出能够降解硝磺草酮的HZ-2菌剂;在田间试验中,用该菌剂处理14 d后,对3 898 g/hm2的硝磺草酮的降解率达到48%,一定程度上减少了硝磺草酮的残留。Pileggi等[40]从水环境中筛选出一株高效降解硝磺草酮的菠萝泛菌(Pantoea ananatis),该菌株能够在24 h内将13.6 mg/L的硝磺草酮完全降解,通过2次液相色谱-质谱联用仪(liquid chromatograph mass spectrometer/ mass spectrometer, LC-MS/MS)分析检测了硝磺草酮在菌株作用下可能产生的降解中间体。Olchanheski等[41]研究发现大肠杆菌(Escherichia coli DH5α)在3 h内对10倍推荐剂量(136 mg/L)硝磺草酮的降解率可达100%。
还有研究报道,土壤中的环磺酮和双环草酮的降解有微生物的参与[43-44],但降解环磺酮、双环草酮的微生物尚未见报道[33]。
2.3 三酮类除草剂生物降解相关的酶降解三酮类除草剂微生物资源的不断丰富,促进了对其降解分子机制的研究。Carles等[45]通过分析硝磺草酮高效降解菌株Bacillus sp. Mes11的蛋白组学,揭示了9个可能降解硝磺草酮的硝基还原酶(nitroreductase, NR),分子量在21.9–29.3 kDa之间,通过外源表达确定了NfsAfrp家族中的2种硝基还原酶基因(NfrA1和NfrA2/YcnD)参与了硝磺草酮生物转化,辅因子分别是NADPH和NADPH/NADH,最适pH值和温度为6.0–6.5和23–25 ℃,并且证实该NRs对其他硝基化合物,如除草剂地乐西、杀虫剂对硫磷等也有一定的降解作用。有学者基于以上2种硝基还原酶和相对应的辅因子研发了监测硝磺草酮生物传感器,响应值(电流大小)与硝磺草酮浓度成正比[46]。
在E. coli DH5α降解硝磺草酮速率最快的30 min内,谷胱甘肽-S-转移酶(glutathione-S- transferase, GST)活性显著上升[41]。Prione等[47]研究发现,P. anantis在硝磺草酮开始降解(17.5 h)和降解完成时,GST的活性最大,说明这两种菌中可能都有一个GST参与硝磺草酮的降解,但是还需要进一步验证其降解效率。此外,已有报道表明植物中存在有硝磺草酮降解能力的基因/酶[48-51],如细胞色素P450、单加氧酶、糖基化酶等,这对挖掘降解微生物中降解硝磺草酮的酶有一定的指导意义。
目前尚未见其他三酮类除草剂降解相关酶的报道。
2.4 关于2种三酮类除草剂的微生物降解途径三酮类除草剂最早在1993年上市,至今仅二十余年,环境中能够适应和降解该类除草剂的微生物资源较少,因此,关于菌株降解硝磺草酮的机理研究报道相对更少。硝磺草酮的微生物降解主要通过环己二酮和苯磺酰基之间的β-二酮键断裂及硝基的还原实现,目前公认的降解途径大致分为2个途径[4, 52-53]:(1) 硝磺草酮→M2 (游离或环化的羟胺类物质)→AMBA,这是硝磺草酮降解的主要途径。在组成型和氧不敏感的硝基还原酶功能下,先产生具有毒性和诱变性的中间体M2,在氧气存在时通过β-二酮键的断裂能定量转化为AMBA,并通过添加内标物证实了戊二酸是硝磺草酮有效的生物转化物,迅速被消耗,戊二酸源于环己二酮部分的氧化裂解。(2) 硝磺草酮→MNBA→AMBA,这被认为是一条次要的降解途径。多次研究发现MNBA很难被检测到,推测是生成后立即转化为下一个物质。Durand等对MNBA的生物转化反应证明了MNBA的消散伴随着AMBA的定量积累[52-53]。Pantoea ananatis降解硝磺草酮有不同的降解途径,产生了不同于AMBA和MNBA的降解中间产物,主要通过去硝基化(m/z=292)、去甲基化、环己二酮开环(m/z=334、m/z=288)等步骤完成对硝磺草酮的降解[40]。水稻中也检测到类似的物质[48],推测认为是硝磺草酮在Pantoea anantis和水稻中存在相同的代谢途径(图 2)。
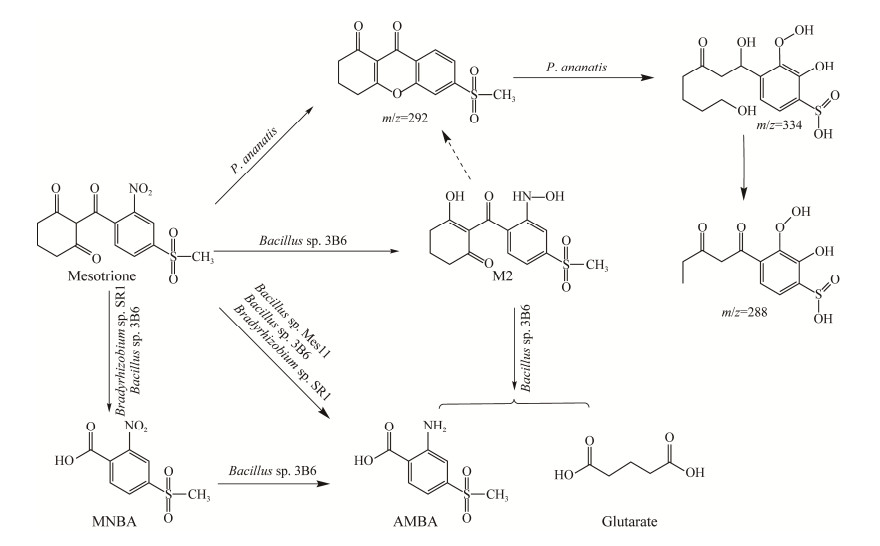
|
| 图 2 硝磺草酮微生物降解途径 Figure 2 Degradation pathway of mesotrione by microorganisms. |
|
|
磺草酮主要的降解产物为CMBA和1, 3-环己二酮(1, 3-cyclohexanedione, CHD),也有学者提出了一种新的中间代谢产物羟基磺草酮(hydroxy-sulcotrione)[35],特殊情况下会转化为苯庚酸衍生物[30, 54]。但是在已经报道的2株磺草酮降解菌的降解产物中并未检测到CHD,可能是和硝磺草酮一样在pH > 7.0时β-二酮键断裂后开环成5-氧代乙酸,最终生成戊二酸(图 3)[52]。
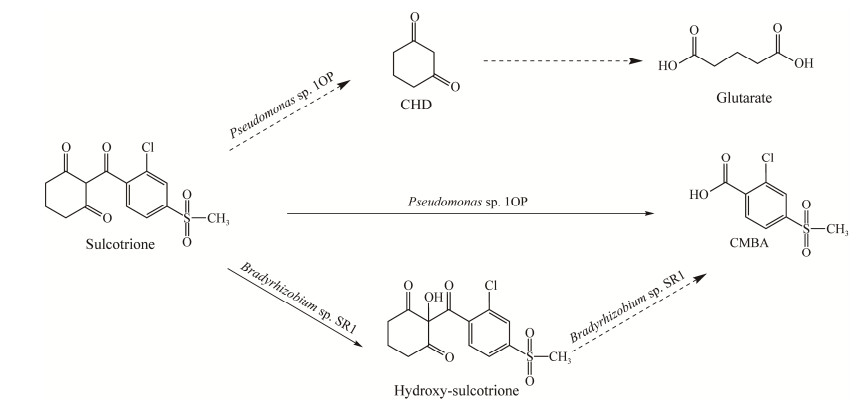
|
| 图 3 磺草酮的微生物降解途径 Figure 3 Degradation pathway of sulcotrione by microorganisms. |
|
|
三酮类除草剂具有除草效果好、杀草谱广、靶标性强等特征,可以在芽前、苗后使用,是替代阿特拉津等三氮苯类除草剂的重要品种之一[55],但是因其易残留,从而会对生态环境造成一定的危害。微生物降解除草剂技术因其绿色、安全、高效等特点,成为备受关注的消除除草剂残留的重要手段之一。
当前能够降解三酮类除草剂的微生物资源报道较少,仅有Bacillus、Pseudomonas、Pantoea等少数几类菌属,亟待在传统分离菌株的基础上尝试开发更多样的分离培养技术[56-60],加强对新降解资源的挖掘、鉴定、评价与利用[61-64],筛选更多潜在的降解菌株。同时,这些已报道的降解菌株主要是聚焦在对除草剂原药的降解上,而针对毒性比原药分子稍强的中间降解产物(如AMBA、CMBA)的研究尚未开展,导致中间产物积累、除草剂不能完全降解将成为一大隐患。然而合成微生物群落(synthetic microbial community, SMC)的出现有望解决这一问题,SMC定义为按照功能导向性的需求将已知的2个或多个遗传背景清晰的微生物菌株在自定义的培养环境中共培养构建的微生物群落[65-66],因其对环境有更好的适应性,使微生物彼此配合发挥更大的作用[67]。SMC应用于污染物降解的研究已有报道,Yu等[68]在研究双酚A的微生物群落降解效果时发现,假单胞菌(Pseudomonas sp.)能够加快鞘氨醇单胞菌(Sphingomonas sp.)对双酚A的完全矿化速度,Pusillimonas sp.也有类似于Pseudomonas sp.的中间产物4-羟基苯甲酸酯(4-hydroxybenzoate, 4-HBZ)转运蛋白,同样能够促进双酚A的快速矿化。Li等[69]利用改造过的E. coli BL21及分离得到的Ochrobactrum sp. LL-1这2种菌对甲基对硫磷(MP)进行了完全矿化。几种关键菌株降解复杂有机物时协同作用,最终将残留较强的污染物完全降解,既降低了微生物群落复杂性,又保留了较好的降解效果,非常有应用前景。该项研究有望应用在三酮类除草剂的完全矿化上,以已知降解中间产物为底物筛选合适的降解菌株,与降解母体分子的菌株组合共同矿化除草剂。
| [1] |
WEEDSCIENCE. Current status of the international herbicide-resistant weed database[DB/OL]. http://www.weedscience.org/Home.aspx.
|
| [2] |
XIN J, XU XB, WANG L, ZHU YR, WANG Y. Study on the resistance of ALS inhibitor herbicides[J]. Journal of Anhui Agricultural Sciences, 2019, 47(4): 18-21. (in Chinese) 辛洁, 徐小博, 王磊, 朱晔荣, 王勇. ALS抑制剂类除草剂的抗性研究概述[J]. 安徽农业科学, 2019, 47(4): 18-21. |
| [3] |
BEAUDEGNIES R, EDMUNDS AJF, FRASER TEM, HALL RG, HAWKES TR, MITCHELL G, SCHAETZER J, WENDEBORN S, WIBLEY J. Herbicidal 4-hydroxyphenylpyruvate dioxygenase inhibitors: a review of the triketone chemistry story from a syngenta perspective[J]. Bioorganic & Medicinal Chemistry, 2009, 17(12): 4134-4152. |
| [4] |
CARLES L, JOLY M, JOLY P. Mesotrione herbicide: efficiency, effects, and fate in the environment after 15 years of agricultural use[J]. Clean-Soil, Air, Water, 2017, 45(9): 1700011. DOI:10.1002/clen.201700011 |
| [5] |
XU L. The risk of resistance is low, and the market is gradually increasing every year-brief analysis of HPPD inhibitor herbicides[J]. Pesticide News, 2020(4): 48-49. (in Chinese) 徐磊. 抗性风险低, 市场逐渐年增长——HPPD抑制剂类除草剂简析[J]. 农药快讯, 2020(4): 48-49. |
| [6] |
HUA NZ. Triketone herbicide and its application[J]. World Pesticides, 2015, 37(6): 7-13. (in Chinese) 华乃震. 三酮类除草剂产品及其应用[J]. 世界农药, 2015, 37(6): 7-13. |
| [7] |
ZHANG YB. Research and development of bicyclomesotrione herbicide[J]. World Pesticides, 2006, 28(2): 9-14. (in Chinese) 张一宾. 除草剂双环磺草酮的研究开发[J]. 世界农药, 2006, 28(2): 9-14. |
| [8] |
MITCHELL G, BARTLETT DW, FRASER TE, HAWKES TR, HOLT DC, TOWNSON JK, WICHERT RA. Mesotrione: a new selective herbicide for use in maize[J]. Pest Management Science, 2001, 57(2): 120-128. DOI:10.1002/1526-4998(200102)57:2<120::AID-PS254>3.0.CO;2-E |
| [9] |
WILLIAMS MM, PATAKY JK. Factors affecting differential sensitivity of sweet corn to HPPD-inhibiting herbicides[J]. Weed Science, 2010, 58(3): 289-294. DOI:10.1614/WS-D-09-00058.1 |
| [10] |
DYSON JS, BEULKE S, BROWN CD, LANE MCG. Adsorption and degradation of the weak acid mesotrione in soil and environmental fate implications[J]. Journal of Environmental Quality, 2002, 31(2): 613-618. DOI:10.2134/jeq2002.6130 |
| [11] |
KONG DY, SHI LL, SHAN ZJ, WU XW, WANG F, GAO SX. Adsorption and leaching behavior of herbicide mesotrione in soils[J]. China Environmental Science, 2008, 28(8): 753-757. (in Chinese) 孔德洋, 石利利, 单正军, 吴星卫, 王菲, 高士祥. 除草剂甲基磺草酮在土壤中的吸附及淋溶特性[J]. 中国环境科学, 2008, 28(8): 753-757. |
| [12] |
HE M, JIA CH, PING H. Research progress on environmental activities of the triketone herbicide mesotrione[J]. Agrochemicals, 2012, 51(6): 402-404, 423. (in Chinese) 贺敏, 贾春虹, 平华. 三酮类除草剂硝磺草酮的环境行为研究进展[J]. 农药, 2012, 51(6): 402-404, 423. |
| [13] |
KAYE JP, HART SC. Competition for nitrogen between plants and soil microorganisms[J]. Trends in Ecology & Evolution, 1997, 12(4): 139-143. |
| [14] |
RICHARDSON AE, LYNCH JP, RYAN PR, DELHAIZE E, SMITH FA, SMITH SE, HARVEY PR, RYAN MH, VENEKLAAS EJ, LAMBERS H, OBERSON A, CULVENOR RA, SIMPSON RJ. Plant and microbial strategies to improve the phosphorus efficiency of agriculture[J]. Plant and Soil, 2011, 349(1/2): 121-156. |
| [15] |
ROMDHANE S, DEVERS-LAMRANI M, BEGUET J, BERTRAND C, CALVAYRAC C, SALVIA MV, JRAD AB, DAYAN FE, SPOR A, BARTHELMEBS L, MARTIN-LAURENT F. Assessment of the ecotoxicological impact of natural and synthetic β-triketone herbicides on the diversity and activity of the soil bacterial community using omic approaches[J]. The Science of the Total Environment, 2019, 651(Pt 1): 241-249.
|
| [16] |
DU ZK, ZHU YY, ZHU LS, ZHANG J, LI B, WANG JH, WANG J, ZHANG C, CHENG C. Effects of the herbicide mesotrione on soil enzyme activity and microbial communities[J]. Ecotoxicology and Environmental Safety, 2018, 164: 571-578. DOI:10.1016/j.ecoenv.2018.08.075 |
| [17] |
SUN YB, WANG RL, XU YM, LIANG XF, WANG L, ZHANG GL. Ecological effects of herbicide mesotrione on soil microbial communities[J]. China Environmental Science, 2016, 36(1): 190-196. (in Chinese) 孙约兵, 王润珑, 徐应明, 梁学峰, 王林, 张贵龙. 除草剂硝磺草酮对土壤微生物生态效应研究[J]. 中国环境科学, 2016, 36(1): 190-196. DOI:10.3969/j.issn.1000-6923.2016.01.031 |
| [18] |
XIE ZH, CAI YF, CHEN G, HUANG JF, YU HW. Induction of micronuclei and nuclear anomalies in erythrocytes of Misgurnus anguillicaudatus by herbicide acetochlor[J]. Fisheries Science, 2004, 23(6): 17-19. (in Chinese) 谢志浩, 蔡亚非, 陈国, 黄剑锋, 余红卫. 四种除草剂对泥鳅红细胞微核及核异常诱导[J]. 水产科学, 2004, 23(6): 17-19. |
| [19] |
STA C, LEDOIGT G, FERJANI E, GOUPIL P. Exposure of Vicia faba to sulcotrione pesticide induced genotoxicity[J]. Pesticide Biochemistry and Physiology, 2012, 103(1): 9-14. DOI:10.1016/j.pestbp.2012.02.002 |
| [20] |
STA C, GOUJON E, FERJANI E, LEDOIGT G. Toxicity of sulcotrione and grape marc on Vicia faba cells[J]. Journal of Agricultural and Food Chemistry, 2014, 62(49): 11777-11785. DOI:10.1021/jf503323t |
| [21] |
FELIX J, DOOHAN DJ, BRUINS D. Differential vegetable crop responses to mesotrione soil residues a year after application[J]. Crop Protection, 2007, 26(9): 1395-1403. DOI:10.1016/j.cropro.2006.11.013 |
| [22] |
SOLTANI N, SIKKEMA PH, ROBINSON DE. Response of four market classes of dry bean to mesotrione soil residues[J]. Crop Protection, 2007, 26(11): 1655-1659. DOI:10.1016/j.cropro.2007.01.004 |
| [23] |
GOUJON E, STA C, TRIVELLA A, GOUPIL P, RICHARD C, LEDOIGT G. Genotoxicity of sulcotrione pesticide and photoproducts on Allium cepa root meristem[J]. Pesticide Biochemistry and Physiology, 2014, 113: 47-54. DOI:10.1016/j.pestbp.2014.06.002 |
| [24] |
GOUJON E, RICHARD C, GOUPIL P, LEDOIGT G. Cytotoxicity on Allium cepa of the two main sulcotrione photoproducts, xanthene-1, 9-dione-3, 4-dihydro-6-methylsulphonyl and 2-chloro-4- mesylbenzoic acid[J]. Pesticide Biochemistry and Physiology, 2015, 124: 37-42. DOI:10.1016/j.pestbp.2015.04.001 |
| [25] |
WU NX, JIN Y, JIN F, TAN YF, TAO H, ZHENG MY, CHEN RP, LIU KC, GAO M. Effects of sulcotrione [2-(2-chloro-4-mesylbenzoyl)-cyclohexane-1, 3-dione] on enzymes involved in tyrosine catabolism and the extent of the resulting tyrosinemia and its relationship with corneal lesions in rats[J]. Pesticide Biochemistry and Physiology, 2011, 99(2): 162-169. DOI:10.1016/j.pestbp.2010.11.013 |
| [26] |
PIANCINI LDS, GUILOSKI IC, de ASSIS HCS, CESTARI MM. Mesotrione herbicide promotes biochemical changes and DNA damage in two fish species[J]. Toxicology Reports, 2015, 2: 1157-1163. DOI:10.1016/j.toxrep.2015.08.007 |
| [27] |
NI Y, LAI JH, WAN JB, CHEN LS. Photosynthetic responses and accumulation of mesotrione in two freshwater algae[J]. Environmental Science Processes & Impacts, 2014, 16(10): 2288-2294. |
| [28] |
ZHANG FW, YAO XF, SUN SA, WANG LP, LIU WT, JIANG XY, WANG JX. Effects of mesotrione on oxidative stress, subcellular structure, and membrane integrity in Chlorella vulgaris[J]. Chemosphere, 2020, 247: 125668. DOI:10.1016/j.chemosphere.2019.125668 |
| [29] |
BONNET JL, BONNEMOY F, DUSSER M, BOHATIER J. Toxicity assessment of the herbicides sulcotrione and mesotrione toward two reference environmental microorganisms: Tetrahymena pyriformis and Vibrio fischeri[J]. Archives of Environmental Contamination and Toxicology, 2008, 55(4): 576-583. DOI:10.1007/s00244-008-9145-2 |
| [30] |
CHAABANE H, VULLIET E, CALVAYRAC C, COSTE CM, COOPER JF. Behaviour of sulcotrione and mesotrione in two soils[J]. Pest Management Science, 2008, 64(1): 86-93. DOI:10.1002/ps.1456 |
| [31] |
SU WC, HAO HD, WU RH, XU HL, XUE F, LU CT. Degradation of mesotrione affected by environmental conditions[J]. Bulletin of Environmental Contamination and Toxicology, 2017, 98(2): 212-217. DOI:10.1007/s00128-016-1970-9 |
| [32] |
HALLE AT, RICHARD C. Simulated solar light irradiation of mesotrione in natural waters[J]. Environmental Science & Technology, 2006, 40(12): 3842-3847. |
| [33] |
DUMAS E, GIRAUDO M, GOUJON E, HALMA M, KNHILI E, STAUFFERT M, BATISSON I, BESSE-HOGGAN P, BOHATIER J, BOUCHARD P, CELLE-JEANTON H, GOMES MC, DELBAC F, FORANO C, GOUPIL P, GUIX N, HUSSON P, LEDOIGT G, MALLET C, MOUSTY C, PRÉVOT V, RICHARD C, SARRAUTE S. Fate and ecotoxicological impact of new generation herbicides from the triketone family: an overview to assess the environmental risks[J]. Journal of Hazardous Materials, 2017, 325: 136-156. DOI:10.1016/j.jhazmat.2016.11.059 |
| [34] |
WANG KM. Progress on the organic pollutants biodegradation[J]. Journal of Qingyuan Polytechnic, 2008, 1(2): 71-74. (in Chinese) 王可美. 有机污染物的生物降解研究进展[J]. 清远职业技术学院学报, 2008, 1(2): 71-74. |
| [35] |
ROMDHANE S, DEVERS-LAMRANI M, MARTIN-LAURENT F, CALVAYRAC C, ROCABOY-FAQUET E, RIBOUL D, COOPER JF, BARTHELMEBS L. Isolation and characterization of Bradyrhizobium sp. SR1 degrading two β-triketone herbicides[J]. Environmental Science and Pollution Research International, 2016, 23(5): 4138-4148. DOI:10.1007/s11356-015-4544-1 |
| [36] |
CALVAYRAC C, MARTIN-LAURENT F, FAVEAUX A, PICAULT N, PANAUD O, COSTE CM, CHAABANE H, COOPER JF. Isolation and characterisation of a bacterial strain degrading the herbicide sulcotrione from an agricultural soil[J]. Pest Management Science, 2012, 68(3): 340-347. DOI:10.1002/ps.2263 |
| [37] |
DURAND S, AMATO P, SANCELME M, DELORT AM, COMBOURIEU B, BESSE-HOGGAN P. First isolation and characterization of a bacterial strain that biotransforms the herbicide mesotrione[J]. Letters in Applied Microbiology, 2006, 43(2): 222-228. DOI:10.1111/j.1472-765X.2006.01923.x |
| [38] |
BATISSON I, CROUZET O, BESSE-HOGGAN P, SANCELME M, MANGOT JF, MALLET C, BOHATIER J. Isolation and characterization of mesotrione-degrading Bacillus sp. from soil[J]. Environmental Pollution, 2009, 157(4): 1195-1201. DOI:10.1016/j.envpol.2008.12.009 |
| [39] |
HAN HT, LIU J, GAO YF, ZHANG JY, ZHONG GH. Isolation, identification and characterization of a mesotrione-degrading bacterial strain[J]. Journal of Huazhong Agricultural University, 2013, 32(3): 62-66. (in Chinese) 韩海涛, 刘婕, 高云飞, 张建英, 钟国华. 硝磺草酮降解菌的分离鉴定及其降解特性[J]. 华中农业大学学报, 2013, 32(3): 62-66. |
| [40] |
PILEGGI M, PILEGGI SAV, OLCHANHESKI LR, da SILVA PAG, MUNOZ GONZALEZ AM, KOSKINEN WC, BARBER B, SADOWSKY MJ. Isolation of mesotrione-degrading bacteria from aquatic environments in Brazil[J]. Chemosphere, 2012, 86(11): 1127-1132. DOI:10.1016/j.chemosphere.2011.12.041 |
| [41] |
OLCHANHESKI LR, DOURADO MN, BELTRAME FL, ZIELINSKI AAF, DEMIATE IM, PILEGGI SAV, AZEVEDO RA, SADOWSKY MJ, PILEGGI M. Mechanisms of tolerance and high degradation capacity of the herbicide mesotrione by Escherichia coli strain DH5-α[J]. PLoS One, 2014, 9(6): e99960. DOI:10.1371/journal.pone.0099960 |
| [42] |
ZHOU LC. Preparation and application of two pesticide degrading bacteria preparations[D]. Guangzhou: Master's Thesis of South China Agricultural University, 2018 (in Chinese). 周亮成. 两种农药降解菌制剂的制备及应用[D]. 广州: 华南农业大学硕士学位论文, 2018. |
| [43] |
RANI N, DUHAN A, TOMAR D. Ultimate fate of herbicide tembotrione and its metabolite TCMBA in soil[J]. Ecotoxicology and Environmental Safety, 2020, 203: 111023. DOI:10.1016/j.ecoenv.2020.111023 |
| [44] |
WILLIAMS KL, GLADFELDER JJ, QUIGLEY LL, BALL DB, TJEERDEMA RS. Dissipation of the herbicide benzobicyclon hydrolysate in a model California rice field soil[J]. Journal of Agricultural and Food Chemistry, 2017, 65(42): 9200-9207. DOI:10.1021/acs.jafc.7b03679 |
| [45] |
CARLES L, BESSE-HOGGAN P, JOLY M, VIGOUROUX A, MORÉRA S, BATISSON I. Functional and structural characterization of two Bacillus megaterium nitroreductases biotransforming the herbicide mesotrione[J]. The Biochemical Journal, 2016, 473(10): 1443-1453. DOI:10.1042/BJ20151366 |
| [46] |
HDIOUECH S, BRUNA F, BATISSON I, BESSE-HOGGAN P, PREVOT V, MOUSTY C. Amperometric detection of the herbicide mesotrione based on competitive reactions at nitroreductase@layered double hydroxide bioelectrode[J]. Journal of Electroanalytical Chemistry, 2019, 835: 324-328. DOI:10.1016/j.jelechem.2019.01.054 |
| [47] |
PRIONE LP, OLCHANHESKI LR, TULLIO LD, SANTO BCE, RECHE PM, MARTINS PF, CARVALHO G, DEMIATE IM, PILEGGI SAV, DOURADO MN, PRESTES RA, SADOWSKY MJ, AZEVEDO RA, PILEGGI M. GST activity and membrane lipid saturation prevents mesotrione-induced cellular damage in Pantoea ananatis[J]. AMB Express, 2016, 6(1): 70. DOI:10.1186/s13568-016-0240-x |
| [48] |
CHEN ZJ, LV Y, ZHAI XY, YANG H. Comprehensive analyses of degradative enzymes associated with mesotrione-degraded process in rice for declining environmental risks[J]. The Science of the Total Environment, 2021, 758: 143618. DOI:10.1016/j.scitotenv.2020.143618 |
| [49] |
PANG S, DUAN LS, LIU ZQ, SONG XY, LI XF, WANG CJ. Co-induction of a glutathione-S-transferase, a glutathione transporter and an ABC transporter in maize by xenobiotics[J]. PLoS One, 2012, 7(7): e40712. |
| [50] |
DIMAANO NG, IWAKAMI S. Cytochrome P450-mediated herbicide metabolism in plants: current understanding and prospects[J]. Pest Management Science, 2021, 77(1): 22-32. |
| [51] |
KREUZ K, TOMMASINI R, MARTINOIA E. Old enzymes for a new job (herbicide detoxification in plants)[J]. Plant Physiology, 1996, 111(2): 349-353. |
| [52] |
DURAND S, LéGERET B, MARTIN AS, SANCELME M, DELORT AM, BESSE-HOGGAN P, COMBOURIEU B. Biotransformation of the triketone herbicide mesotrione by a Bacillus strain. Metabolite profiling using liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry[J]. Rapid Communications in Mass Spectrometry: RCM, 2006, 20(17): 2603-2613. |
| [53] |
DURAND S, SANCELME M, BESSE-HOGGAN P, COMBOURIEU B. Biodegradation pathway of mesotrione: complementarities of NMR, LC-NMR and LC-MS for qualitative and quantitative metabolic profiling[J]. Chemosphere, 2010, 81(3): 372-380. |
| [54] |
ROUCHAUD J, NEUS O, BULCKE R, COOLS K, EELEN H. Sulcotrione soil metabolism in summer corn crops[J]. Bulletin of Environmental Contamination and Toxicology, 1998, 61(5): 669-676. |
| [55] |
ZHANG YB. Herbicides as HPPD inhibitors and their market development[J]. Modern Agrochemicals, 2013, 12(5): 5-8. (in Chinese) 张一宾. HPPD抑制剂类除草剂及其市场开发进展[J]. 现代农药, 2013, 12(5): 5-8. |
| [56] |
YASUMOTO-HIROSE M, NISHIJIMA M, NGIRCHECHOL MK, KANOH K, SHIZURI Y, MIKI W. Isolation of marine bacteria by in situ culture on media-supplemented polyurethane foam[J]. Marine Biotechnology (New York, N Y), 2006, 8(3): 227-237. |
| [57] |
PENG LL, WANG Q, XIN MX. Advanced in uncultivable microorganisms in nature[J]. Journal of Microbiology, 2011, 31(2): 75-79. (in Chinese) 彭伶俐, 王琴, 辛明秀. 自然界中不可培养微生物的研究进展[J]. 微生物学杂志, 2011, 31(2): 75-79. |
| [58] |
LI XD, QU JH, ZHANG LJ, LI HF. Progresses on the influence factors of culturability and cultivation strategies of environmental microorganisms[J]. Life Science Research, 2017, 21(2): 154-158. 李晓丹, 屈建航, 张璐洁, 李海峰. 环境微生物可培养性影响因素及培养方法研究进展[J]. 生命科学研究, 2017, 21(2): 154-158. |
| [59] |
NICHOLS D, CAHOON N, TRAKHTENBERG EM, PHAM L, MEHTA A, BELANGER A, KANIGAN T, LEWIS K, EPSTEIN SS. Use of ichip for high-throughput in situ cultivation of "uncultivable" microbial species[J]. Applied and Environmental Microbiology, 2010, 76(8): 2445-2450. |
| [60] |
JANSSEN PH, YATES PS, GRINTON BE, TAYLOR PM, SAIT M. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions acidobacteria, actinobacteria, proteobacteria, and verrucomicrobia[J]. Applied and Environmental Microbiology, 2002, 68(5): 2391-2396. |
| [61] |
YAO L, JIA XJ, ZHAO JD, CAO Q, XIE XT, YU LL, HE J, TAO Q. Degradation of the herbicide dicamba by two sphingomonads via different O-demethylation mechanisms[J]. International Biodeterioration & Biodegradation, 2015, 104: 324-332. |
| [62] |
SØRENSEN SR, JUHLER RK, AAMAND J. Degradation and mineralisation of diuron by Sphingomonas sp. SRS2 and its potential for remediating at a realistic µg L(-1) diuron concentration[J]. Pest Management Science, 2013, 69(11): 1239-1244. |
| [63] |
FERNANDES AFT, BRAZ VS, BAUERMEISTER A, PASCHOAL JAR, LOPE NP, STEHLING EG. Degradation of atrazine by Pseudomonas sp. and Achromobacter sp. isolated from Brazilian agricultural soil[J]. International Biodeterioration & Biodegradation, 2018, 130: 17-22. |
| [64] |
DUC HD, THUY NTD, TRUC HTT, NHU NTH, OANH NT. Degradation of butachlor and propanil by Pseudomonas sp. strain But2 and Acinetobacter baumannii strain DT[J]. FEMS Microbiology Letters, 2020, 367(18): fnaa151. |
| [65] |
DENG T, GUAN XT, WU B, HE ZL. Application of mathematical model in the construction of synthetic microbial community[J]. Chinese Journal of Applied and Environmental Biology, 2020, 26(4): 809-819. (in Chinese) 邓婷, 关晓彤, 吴波, 贺志理. 数学模型在合成微生物群落构建中的应用[J]. 应用与环境生物学报, 2020, 26(4): 809-819. |
| [66] |
CHEN MX, WEI Z, TIAN L, TAN Y, HUANG JD, DAI L. Construction and application of synthetic microbial community[J]. Chinese Science Bulletin, 2021, 66(3): 273-283. (in Chinese) 陈沫先, 韦中, 田亮, 谭扬, 黄建东, 戴磊. 合成微生物群落的构建与应用[J]. 科学通报, 2021, 66(3): 273-283. |
| [67] |
ZHANG XL, WANG JR, LI QZ, FU CX, WANG C, XU N. Research progress of synthetic microbial community and its application in fermented food[J]. China Brewing, 2021, 40(3): 17-21. (in Chinese) 张小龙, 王嘉瑞, 李青卓, 付彩霞, 汪超, 徐宁. 合成微生物群落及在发酵食品中应用研究进展[J]. 中国酿造, 2021, 40(3): 17-21. |
| [68] |
YU K, YI S, LI B, GUO F, PENG XX, WANG ZP, WU Y, ALVAREZ-COHEN L, ZHANG T. An integrated meta-omics approach reveals substrates involved in synergistic interactions in a bisphenol A (BPA)-degrading microbial community[J]. Microbiome, 2019, 7(1): 16. |
| [69] |
LI L, YANG C, LAN WS, XIE S, QIAO CL, LIU JX. Removal of methyl parathion from artificial off-gas using a bioreactor containing a constructed microbial consortium[J]. Environmental Science & Technology, 2008, 42(6): 2136-2141. |
 2023, Vol. 50
2023, Vol. 50




