扩展功能
文章信息
- 韩雪, 高馨竹, 龚伟军, 宋金钊, 赵良洲, 李海燕
- HAN Xue, GAO Xinzhu, GONG Weijun, SONG Jinzhao, ZHAO Liangzhou, LI Haiyan
- 微生物种子包衣的应用与研究进展
- The microbial seed coating and its application
- 微生物学通报, 2023, 50(12): 5534-5547
- Microbiology China, 2023, 50(12): 5534-5547
- DOI: 10.13344/j.microbiol.china.230321
-
文章历史
- 收稿日期: 2023-04-20
- 接受日期: 2023-05-29
- 网络首发日期: 2023-07-10
化肥、杀虫剂和农用化学品的大量使用严重污染空气、土壤和水资源,破坏生态系统的生物多样性,引起全球广泛关注[1]。为应对这些挑战,我国推出精准农业政策,即以先进技术为基础,在提高粮食产量与品质的同时,减少水和农用化学品投入,以降低经济、环境和健康成本,重视土壤及其居民,保持生态系统健康和谐发展[2-4]。
种子包衣是精准农业政策的一项重要技术,它是指利用杀菌剂、杀虫剂、激素、肥料或有益微生物等有效成分包裹在种子表面,使种子外形成具有一定功能和包覆强度的保护层(种衣剂),是现代农业保护种子或幼苗初始发育阶段的常见策略[5]。种衣剂不仅可以为种子提供萌发时所需的营养物质,也可以保护作物免受生物和非生物胁迫的侵害,提高产量和品质[6-7]。
根据功能或用途现可将种衣剂分为物理型种衣剂、化学型种衣剂、生物型种衣剂、特异性种衣剂和综合型种衣剂五类[8]。但物理型种衣剂会减少种子对水分的吸收和氧气的利用,从而延迟种子发芽[9]。化学型种衣剂的使用引起的环境污染对非靶标生物和一些有益生物产生不利影响,使有害生物对农药产生抗性,从而影响生态平衡[10-11]。相较而言,微生物种衣剂具有安全、高效、环保和对人畜无毒的特性,其主要成分是植物有益微生物(plant beneficial microorganisms, PBM)或其分泌物;其可以减少接种剂量且能在特定位置有效释放,从而增强种子活力,进而减少农药和化肥的使用量,减小对环境的影响[12]。此外,现代农业设备可以精准地播种包衣种子,有效控制种植密度,从而实现或接近精准农业,使微生物种子包衣技术具有广阔的应用前景[13-14]。
1 微生物种子包衣植物有益微生物能促进植物生长、提高营养元素的吸收、恢复土壤肥力和增强植物对生物和非生物胁迫的耐受力[15-17]。然而,目前缺乏接种有益微生物的有效方法,在开阔的农田里大面积无针对性地接种此类微生物的成本相对较高[18],而通过种子包衣的方法可以有效接种PBM,促进植物生长。例如,de Gregorio等[19]用纳米纤维固定化细菌包衣大豆种子,提高了大豆的发芽率(98.9%±1.1%),促进大豆生长。Oliveira等[20]发现,在加入100%的Hoagland溶液条件下,利用丛枝菌根真菌(arbuscular mycorrhizal fungi, AMF)制作种子包衣,可使植物茎中钾、硫和锌的含量与未接种的对照相比分别增加到(50.1± 1.54) g/kg、(3.0±0.20) g/kg和(72.3±10.43) mg/kg,而且运用种子包衣技术的植物其根系中AMF的定殖率与常规接种的植物并无显著差异,表明AMF的种子包衣可以有效减少化肥和杀虫剂的使用。因此,有益微生物作为种衣剂在精准农业方面具有良好的应用前景。
1.1 微生物种子包衣配方种子包衣是促进可持续农业的最佳方法之一。微生物种衣剂的配方一般包括3个基本要素:微生物、载体和添加剂[21]。用于微生物种子包衣的载体可以轻松黏附于种子,确保种子萌发和幼苗发育以及其在种子上的存活率和足够的保质期。最常见的微生物包衣载体包括二氧化硅和生物炭[22]。Głodowska等[23]提出,以生物炭作为载体,与假单胞菌(Pseudomonas libanensis)混合后包衣玉米种子,与对照相比,植物鲜重增加2−10 g,株高增加4%−26%。羧甲基纤维素(carboxymethyl cellulose, CMC)和藻酸盐作为常见的微生物种子包衣添加剂,由于成本低和环境安全,常用于商业微生物接种[24]。Zhou等[24]研发的根瘤菌种子包衣包括根瘤菌和钼酸铵,并使用藻酸盐和脱脂牛奶作为黏合剂,当钼酸铵用量为0.2%时,接种根瘤菌菌株ACCC 17631的苜蓿其生长、结瘤和固氮能力达到最大,其中株高最高达42.01 cm,生物量最高达3.04 g/株,最大根瘤数为22.89个/株,最大根瘤重量0.057 g/株。植物有益微生物是种子包衣制作中的关键。Ma等[25]通过种子包衣将AMF异形根孢囊霉(Rhizophagus irregularis) BEG140和BEG140+植物促生菌(plant growth promoting bacteria, PGPB) 假单胞菌(P. libanensis) TR1接种于豇豆种子,其在植株根部的菌根定殖率分别为21.7%和24.2%,TR1能显著增加植物地上部(111%)和总植株干重(101%),以及地上部与根部干重比(83%)。Zhang等[26]发现生物复合种衣剂解淀粉芽孢杆菌(Bacillus amyloliquefaciens) Ba、枯草芽孢杆菌(Bacillus subtilis) Bs wy-1、Bacillus subtilis WXCDD105、荧光假单胞菌(Pseudomonas fluorescens) WXCDD51和贝莱斯芽孢杆菌(Bacillus velezensis) WZ-37处理的番茄种子株高(9.95±0.73) cm、茎径(1.88± 0.15) mm、根长(6.01±0.28) cm、鲜重(2.71±0.08) g和干重(0.172 7±0.01) g均显著高于对照。
1.2 微生物种子包衣贮存期有效的贮存期是种子包衣商业成功的关键因素之一[27]。虽然微生物在种子包衣上存活的最佳条件尚不明确,但人们对影响微生物生存的几个因素如温度、水分、包衣材料和污染物等进行了研究[28]。Georgakopoulos等[29]评估了对甜菜和黄瓜进行生物防治的细菌和真菌拮抗剂,发现其中Pseudomonas adaceae是最有效的生物防治剂,在适宜环境温度下的泥炭配方中可存活2年。据报道,生物炭作为载体可以保持有益微生物的生存和丰度[30-31]。以生物炭为微生物种子包衣的载体,加上慢生型大豆根瘤菌(Bradyrhizobium japonicum)作为接种剂,可以维持较高丰度的细菌种群超4个月,这能确保大豆的高效结瘤[32]。Tripti等[33]研究了含有伯克霍尔德氏菌(Burkholderia sp.)菌株L2和生物炭包衣对番茄种子的影响,结果表明,Burkholderia sp.菌株L2在生物炭中的生存时间延长到240 d,该生物配方不仅能促进种子萌发和植物生长,而且能够增加番茄产量和改善土壤肥力。因此,探索能够提高微生物存活率的配方是开发高效接种剂的关键步骤。
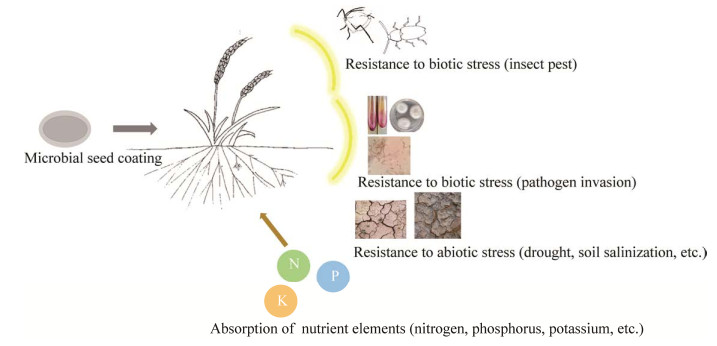
2 微生物种子包衣对作物的益处包衣种子在农作物中应用较多,如小麦[34]和玉米[35]等谷物,以及西红柿、黄瓜、和甜菜等蔬菜[26]。已有研究表明,通过种子包衣将有益微生物应用于作物后可促进种子萌发、植物生长、提高产量和营养价值(表 1,图 1)[36-44]。Hoseini等[36]研究表明,接种哈茨木霉(Trichoderma harzianum) T36的种子包衣后的幼苗出苗率最高(89.44%),与对照相比增加37.2%,芽长和根长分别可达4.17和5.75 cm;Coninck等[38]使用深绿木霉(Trichoderma atroviride)的包衣种子可在低温下储存至少6个月,并且其显著增强了幼苗抗黄色镰刀菌(Fusarium culmorum)病原体感染的能力;Mhada等[43]使用热带根瘤菌(Rhizobium tropici)和海藻糖对种子进行包衣,对照组种子在50 mmol/L盐浓度下的光合活性降低了一半,而R. tropici和海藻糖包衣的种子仍保持其活性,能减轻盐胁迫对植株的负面影响。因此,选择能够赋予植物宿主耐受性的微生物菌株和适当的储存方法以确保微生物的生存、生长和功能来提高作物生产力,可以大大减轻生物与非生物胁迫对植物生态系统功能的负面影响。
| 功能微生物 Functional microbes |
宿主植物 Host plant |
包衣材料 Coating materials |
胁迫类型 Stress |
试验 Experiment |
作用 Function |
参考文献 References |
| 哈茨木霉Trichoderma harzianum | 茴香 Foeniculum vulgare Mill. |
蛭石、高岭土和珍珠岩 Vermiculite, kaolin and perlite |
干旱 Drought |
盆栽 Potting |
促进植物生长(发芽率和组织长度) Promote plant growth (germination rate and tissue length) |
[36] |
| 荧光假单胞菌Pseudomonas fluorescence | 孜然 Cuminum cyminum L. |
蛭石、高岭土和珍珠岩 Vermiculite, kaolin and perlite |
干旱 Drought |
盆栽 Potting |
获得高出苗率,增加植物可溶性蛋白和抗氧化酶活性 Obtain a high emergence rate, increase plant soluble protein and antioxidant enzyme activity |
[37] |
| 深绿木霉Trichoderma atroviride | 玉米 Zea mays L. |
滑石粉、小麦糠和碳酸钙 Talc, wheat bran and calcium carbonate |
枯萎病 Damping-off |
盆栽 Potting |
在低温下延长微生物的保质期,减少幼苗病害的出现 Extend microbial shelf life at low temperatures, reduced the occurrence of seedling diseases |
[38] |
| 绿僵菌 Metarhizium |
玉米 Zea mays L. |
硅藻土和水凝胶 Diatomite and hydrogel |
病虫害 Plant diseases and insect pests |
盆栽 Potting |
改善植物的生长特征,减少病虫害 Improve plant growth characteristics and reduce pests and diseases |
[39] |
| 绿僵菌或球孢白僵菌 Metarhizium or Beauveria bassiana |
玉米 Zea mays L. |
甲基纤维素、黄原胶、菜籽油、膨润土和滑石粉 Methylcellulose, xanthan gum, rapeseed oil, bentonite and talc |
病虫害 Plant diseases and insect pests |
盆栽 Potting |
减少由真菌病原体引起的植物根部腐烂 Reduce plant root decay caused by fungal pathogens |
[40] |
| 枯草芽孢杆菌Bacillus subtilis | 玉米,油菜 Zea mays L., Brassica napus L. |
玉米淀粉、 甲壳素和甘油 Corn starch, chitin and glycerol |
– | 盆栽 Potting |
促进幼苗生长,减少机械磨损期间灰尘颗粒的释放 Promote seedling growth and reduce the release of dust particles during mechanical wear |
[41] |
| 哈茨木霉Trichoderma harzianum | 番茄 Solanum lycopersicum L. |
纤维素和 木薯糊精 Cellulose and cassava dextrin |
病害,盐度和温度 Disease, salinity and temperature |
盆栽 Potting |
促进种子萌发 Promote seed germination |
[42] |
| 热带根瘤菌Rhizobium tropici | 菜豆 Phaseolus vulgaris L. |
海藻糖 Trehalose |
盐度 Salinity |
盆栽 Potting |
增加发芽率,调节形态参数 Increase germination rate and adjust morphological parameters |
[43] |
| 假单胞菌Pseudomonas | 小麦 Triticum aestivum L. |
锌和 阿拉伯树胶 Zinc and arabic gum |
– | 盆栽 Potting |
提高植物产量,胚乳锌含量和增加生物可利用锌 Increase plant yield, endosperm zinc content and enhance bioavailable zinc |
[44] |
| –:未提及 –: Not mentioned. |
||||||
|
| 图 1 微生物种子包衣的作用 Figure 1 The function of microbial seed coating. |
|
|
种子包衣还可改变种子的形态特性如大小、重量、形状和均匀度,提高小粒种子和外形不规则种子的播种性能,有利于机械化精量播种并减少播种特定地点所需的种子数量,以实现精准农业[45]。Ryu等[46]研究表明,用多粘芽孢杆菌(Paenibacillus polymyxa)包衣种子能够增加种子大小,可以帮助更好地精确播种,并保护植物免受各种植物病原体的侵害。Javed等[47]通过用根瘤菌、滑石、氧化钙和膨润土的组合进行种子包衣,有利于机械播种,节约成本,同时也提高了番茄种子的发芽率。
2.2 提高种子质量种子质量是创造最佳作物养分所依赖的重要因素,含有有益微生物的种子包衣可以克服种子萌发的限制,提前开始代谢酶活动和资源调动,从而提高种子质量及作物产量[48]。含有亲水物质的种子包衣通过改善水和空气运动,以及早期和快速完成吸收和活性代谢阶段来提高萌发潜力[49-50]。Wu等[51]发现利用生防菌株产酸克氏杆菌(Klebsiella oxytoca) Rs-5和芽孢杆菌BCL-8 (Bacillus sp. SL-13、SL-14和SL-44)的组合对棉花种子进行包衣,棉花种子在盐碱地中的发芽率提高了11.3%。Singh等[52]研究发现,根瘤菌作为豇豆种子包衣剂可以提高种子质量和作物性能,与未包衣种子相比,在贮藏12个月后其萌发潜力和幼苗活力均有所改善。微生物包衣种子已被广泛接受作为许多作物的标准产品,如小麦、玉米、豇豆、苜蓿、番茄和西葫芦[53]。
2.3 促进植株生长和营养吸收含有有益微生物的种子包衣不仅可用于改善种子发芽、幼苗建立和植物生长,还可以提高作物对营养元素的吸收及增加作物的质量和产量[54]。Rouphael等[55]用AMF [根内根孢囊霉(Rhizophagus intraradices) BEG72和摩西管柄囊霉(Funneliformis mosseae)]以及T. atroviride MUCL 45362对两种洋蓟品种“Romolo”和“Istar”进行种子包衣并种植,结果发现微生物种子包衣提高了植物产量和营养价值,其抗氧化活性、总酚类、咖啡酰奎宁酸和类黄酮等显著提高。Colla等[56]研究发现,通过AMF (Glomus intraradices BEG72和Glomus mossae)和T. atroviride MUCL 45632组合包衣种子能显著改善冬小麦的幼苗生长(芽和根部生物量以及叶数分别增加23%、64%和29%)、产量(根据生长季节而增加8.3%−32.1%)和谷物质量(蛋白质浓度增加6.3%,钾、磷、铁和锌浓度普遍增加)。在盆栽试验中,将T. atroviride以种衣剂的形式接种到土壤中,生菜、甜瓜、胡椒、西红柿和西葫芦的芽干重分别增加167%、56%、115%、68%和58%,在田间条件下生菜芽和根的干重分别增加61%和57%;对于西葫芦,接种两种微生物根内球囊霉(Glomus intraradices)和T. atroviride后其早期产量和总产量分别提高59%和15%[57]。含有微生物的种子包衣在低投入农业中需求量较大,因为其有可能减少化肥的应用并提高食品营养价值。Rocha等[58]发现在未施肥的情况下,接种AMF R. irregularis的种子包衣可使植株的地上部营养浓度如氮、磷、钾和锌的分别增加110%、93%、88%和175%。
2.4 增强种子抗生物胁迫的能力病虫害等生物胁迫严重影响作物生长,降低作物的产量和质量。目前常用的防治方法仍为容易污染环境的化学农药喷施,而通过种子包衣将有益微生物接种到植物中可以有效地拮抗病原菌和害虫,增强作物对病虫害的抵抗能力,从而保护植物的萌发和生长,减少化学农药的使用,从而达到环境友好的目的[59-61]。Kthiri等[62]发现T. harzianum种子包衣可保护植物免受枯萎病的侵害,发病率与对照相比降低约50%;而Wang等[63]则发现B. amyloliquefaciens FS6和噻虫嗪、甲虫灵、氟虫腈的种子包衣对人参灰霉病具有较好的防治作用。Mahmood等[64]发现,在盆栽试验中,微生物种子包衣和杀真菌剂对鹰嘴豆枯萎病的防治效果几乎相同,且T. harzanium和杀真菌剂多菌灵处理种子可将疾病减少率提高到93.75%,比单独施用更有效。Javed等[65]发现,与无包衣处理的种子相比,用Bacillus sp. KS-54包衣水稻种子能有效地抑制水稻种植过程杂草的生长。因此,将微生物包衣应用于农作物上具有可行、经济和环境友好的战略[66]。
2.5 增强种子抗非生物胁迫的能力环境因素,特别是干旱胁迫在萌发阶段可以抑制酶的活性,从而降低作物的萌发和幼苗指数,扰乱植物生命周期[67]。带有微生物制剂的种子包衣可以提高植物的耐旱能力、种子萌发、幼苗指数和生长效率等,是应对干旱胁迫的有效方法之一[68]。Piri等[37]研究发现,在干旱胁迫下,用T. harzianum和P. adaceae对孜然种子进行包衣,不仅可以改善种子的物理特性,还可提高蛋白质含量和抗氧化酶活性,促进其机械化种植。
由不合理灌溉和过量施用农药化肥等引起的土壤盐渍化,也是全球许多农业地区作物生长和生产力的主要限制因素;盐度诱导的应激通常通过非气孔和气孔调节过程降低光合速率,干扰养分摄取,并导致植物的各种代谢紊乱和渗透应激[69-70]。各种研究表明有益微生物的种子包衣在盐胁迫下可以有效地促进植物生长。Shahzad等[71]发现,利用蜡样芽胞杆菌(Bacillus cereus) Y5、Bacillus sp. Y14和B. subtilis Y16等比例混合对小麦种子进行包衣,能够极大地改善气体交换、离子含量和生理生化,与未接种对照相比,株高、穗长、籽粒产量和秸秆产量分别增加14.57%、16.75%、23.77%和22.74%。
此外,炎热和寒冷的极端温度也是作物生产的重要限制因素。Rakesh等[72]发现聚合物薄膜包衣起到温度开关的作用。AMF可以缓解植物的温度应激,其与聚合物薄膜相结合的包衣可以作为减轻植物温度应激的有效工具[73]。重金属胁迫是作物生产的不利因素之一,利用一些可以耐重金属的菌株制成包衣,使作物在重金属胁迫下更好地生长,并且在保证作物可食用性前提下,或可用来修复重金属污染土壤[74]。Singh等[75]研究发现3种耐汞细菌及其复合菌包衣促进芥菜在汞胁迫条件下的生长,同时增强芥菜对汞的积累。
3 常见微生物种子包衣及其应用通过种子接种的PBM可以提高种子发芽率、植物性能及对生物(如病原体和害虫)和非生物(如盐、干旱和重金属)胁迫的耐受性,同时减少农用化学品的使用[76](表 2)。目前,木霉菌、芽孢杆菌等在种子包衣剂制作方面的应用相对广泛。
| 功能微生物 Functional microbes |
宿主植物 Host plant |
作用 Function |
参考文献 References |
| 哈茨木霉 Trichoderma harzianum |
玉米 Zea mays L. |
提高种子的发芽率、发芽速度及根长 Improve the germination rate, germination speed and root length of seeds |
[77] |
| 木霉 Trichoderma |
蓖麻 Ricinus communis L. |
增强抗菌活性和种子品质 Enhance antimicrobial activity and seed quality |
[78] |
| 拟康宁木霉 Trichoderma koningiopsis |
水稻 Oryza sativa L. |
增加叶长和干重 Increase leaf length and dry weight |
[79] |
| 枯草芽孢杆菌 Bacillus subtilis |
棉花 Gossypium hirsutum L. |
增加种子发芽率并促生长 Increase seed germination rate and promote plant growth |
[80] |
| 芽孢杆菌 Bacillus |
小麦 Triticum aestivum L. |
促进植物生长 Promote plant growth |
[81] |
| 蜡样芽胞杆菌 Bacillus cereus |
黄瓜,辣椒 Cucumis sativus L., Capsicum annuum L. |
促进植物生长并具有产IAA功能 Promote plant growth and exhibit IAA production |
[82] |
| 假单胞菌 Pseudomonas libanensis |
豇豆 Vigna unguiculata (L.) Walp. |
提高粮食产量和营养含量 Improve grain yield and nutrient content |
[83] |
| 荧光假单胞菌 Pseudomonas fluorescens |
番茄 Solanum lycopersicum L. |
增加株高、茎径、根长、鲜重和干重 Increase plant height, stem diameter, root length, fresh weight and dry weight |
[26] |
| 绿针假单胞菌 Pseudomonas chlororaphis |
小麦 Triticum aestivum L. |
促进幼苗生长,提高幼苗中防御相关酶过氧化物酶(peroxidase, POD)、超氧化物歧化酶(superoxide dismutase, SOD)、过氧化氢酶(catalase, CAT)、苯丙氨酸解氨酶(phenylalanine ammonia lyase, PAL)和多酚氧化酶(polyphenol oxidase, PPO)的活性 Promote seedling growth and increase the activity of defense related enzymes peroxidase, superoxide dismutase, catalase, phenylalanine ammonia lyase and polyphenol oxidase in seedlings |
[84] |
| 假单胞菌 Pseudomonas |
鹰嘴豆 Cicer arietinum L. |
降低植物疾病发病率,增加植物地上部重量 Reduce the incidence rate of plant diseases and increase the aboveground weight of plants |
[85] |
木霉属(Trichoderma)是根际最常见的腐生真菌物种,主要用于生物农药行业[86]。已有研究发现其具有帮助植物抵御病原菌侵害以及促进宿主植物生长等能力[87-88]。Dogaru等[77]发现加入T. harzianum KUEN 1585的种子包衣可使玉米种子的发芽速度及根长分别提高21.3%和14.9 cm。Chandrika等[78]利用木霉(Trichoderma)与壳聚糖、聚乙二醇制备的共混膜溶液对种子包衣进行优化,增强了抗菌活性和种子品质,但将其应用于生产实践还需要考虑木霉生长条件、包衣制剂等一系列因素。Cortés-Rojas等[79]发现将拟康宁木霉(Trichoderma koningiopsis) Th003采用转鼓法的涂覆工艺制作种子包衣,可使其在8 ℃下保质期延长至15个月,在18 ℃下保持9个月。Swaminathan等[28]发现改变制剂辅料和低温环境可以延长T. atroviridae孢子的存活率,更利于种子包衣储存。因此,从以往研究结果来看,加入木霉菌的种子包衣或许可以大量投入到今后的农业生产中,减少化学农药的使用,降低对土壤及作物污染。
3.2 芽孢杆菌属(Bacillus)种子包衣芽孢杆菌(Bacillus sp.)是研究最广泛的植物根际促生细菌之一,在农业方面有广阔的应用前景。目前芽孢杆菌已成为模式生物,在次级代谢产物产生、孢子形成、生物膜形成、植物根部附着等方面发挥作用[89]。Tu等[80]用B. subtilis SL-13制成的微胶囊化微生物种衣剂(encapsulated microbial seed coating agent, ESCA)处理棉花种子后发芽率提高28.74%,棉苗的株高、根长、全株鲜重和全株干重分别增加52.70%、25.13%、46.47%和33.21%。Ain等[81]利用Bacillus sp. AZ6涂覆尿素制成包衣,与对照相比,小麦株高、根长和分蘖数分别提高24.6%、17.8%和23.3%。Jetiyanon等[82]发现用B. cereus RS87制成的种子包衣,与对照相比,植株株高和根长分别增加约25%和50%,同时菌株RS87可产生大量IAA。因此,从以往研究结果来看,利用芽孢杆菌制作的种子包衣对农作物的生长发育具有促进作用,在农业生产上具有良好的应用价值。
3.3 假单胞菌属(Pseudomonas)种子包衣假单胞菌(Pseudomonas sp.)在环境中广泛分布,其中一些具有生物防治活性、植物生长促进活性、诱导植物系统防御反应等能力[90]。Xu等[84]发现利用Pseudomonas chlororaphis YB-10制成的小麦种子包衣菌落数在107 CFU/mL和108 CFU/mL时,能够显著促进幼苗的生长,同时提高小麦幼苗中防御相关酶过氧化物酶(peroxidase, POD)、超氧化物歧化酶(superoxide dismutase, SOD)、过氧化氢酶(catalase, CAT)、苯丙氨酸解氨酶(phenylalanine ammonia lyase, PAL)和多酚氧化酶(polyphenol oxidase, PPO)的活性,增加植物对病原体的抗性。Hameeda等[85]通过种子包衣方法对鹰嘴豆施用Pseudomonas sp. CDB 35和BWB 21等细菌后,发现植物生物量(干重)增加(18%−30%),在施用CDB 35处理时植物疾病发生率降低47%。因此,假单胞菌属微生物或许可以作为新型种衣剂投入到农业生产中。
4 微生物种子包衣局限性和不一致性大量研究表明微生物种子包衣的接种效率和有效性等同于甚至超过种子浸泡、叶面喷雾、土壤浇灌等其他接种方法[58, 91-92]。相反地,也有研究表明微生物种子包衣的接种效率和有效性不如其他接种方法。Amutha[93]比较了包括种子包衣在内的4种不同接种方法,发现所有方法都可以将Beauveria bassiana接种到棉花中,但叶面喷雾和土壤浇灌的定殖率最高(30.5%)。Müller等[94]在盆栽试验中分别使用2种种子包衣技术和种子浸泡对大丽花接种普城沙雷氏菌(Serratia plymuthica) HRO-C48,结果表明种子浸泡接种HRO-C48对枯萎病抑制效果最明显。Rehman等[44]使用4种不同的方法(土壤浇灌、叶面喷雾、种子浸泡和种子包衣)将Pseudomonas sp. MN12与锌联合使用,以评估对小麦生产力的互作效应,结果表明所有方法均可提高小麦的产量和谷物锌生物强化,但种子浸泡的效果最显著。因此,提高微生物种子包衣的接种效率和有效性也是研究的重点,对微生物种子包衣的应用极其重要。
尽管盆栽试验为微生物接种的益处提供了重要且有用的数据,但还需要在一系列环境中验证田间条件下的微生物效应。现场性能不一致可能是广泛应用有益微生物种子包衣的主要制约因素之一。Shaharoona等[95]研究发现,在盆栽试验中利用产生ACC脱氨酶的假单胞菌Pseudomonas spp.对玉米种子进行包衣可显著提高玉米的生长,然而在田间试验中发现含有ACC脱氨酶的根瘤菌在低水平的肥料下,能更为有效地改善玉米生长和产量。此外,Shaharoona等[96]发现在较低的氮、磷和钾水平下,盆栽和田间试验中产生ACC脱氨酶的P. fluorescens对小麦生长、产量和营养利用效率的影响最为显著。微生物接种方法的有效性也可能因试验规模而异。Kazempour[97]在温室和田间条件下使用不同的接种方法(种子包衣、土壤浇灌和叶面喷雾)评估了P. fluorescens对水稻枯萎病的抑制能力,发现在温室条件下通过种子包衣接种的P. fluorescens更有效,而在田间试验中,通过P. fluorescens种子包衣和叶面喷雾的联合应用获得了最佳效果。因此,明确验证试验的有效性和涵盖该过程所有阶段的微生物应用的结果至关重要。
5 结论与展望种子包衣是推进精准农业的有前途的技术,对作物安全健康的生长具有重要意义,而且满足了日益增长的自动化需求。试验证明,用有益的微生物包衣种子既能提高作物产量和质量,同时降低施用农药的不利影响。此外,种子包衣可以作为杀真菌剂的替代品进行商业应用,因为其对几种根腐病提供了农药同等或更好的生物控制。为更好地了解种子包衣及优化微生物接种剂对作物的应用技术,在农业生产方面大规模地应用,可以从以下几方面进行深入研究:
(1) 关于接种剂配方(例如微生物生存能力、载体成分选择和生产成本)的研究,尽管一些材料(如海藻酸盐)具有提高微生物存活率的能力,但它们也可以阻碍发芽、缩短包衣种子的保质期或增加产品的成本。因此,探索不同包衣材料、作物和微生物的组合配比,以及明确不同微生物种子包衣的作用和配方极为重要。
(2) 微生物种子包衣应用的微生物为植物有益微生物,但有些可能是人畜条件致病菌,因此在投入农业生产前要对其进行一系列风险评估以判断其是否对人畜生存健康造成威胁。
(3) 对更多尚未试验的作物使用微生物种子包衣进行试验,例如中草药、烟草等更多的作物,探究其在种子萌发和植物生长过程中可能发挥的作用,使微生物包衣种子能被更广泛地应用。
(4) 制定微生物种子包衣技术标准,以便于商业化生产和大规模种植。随着研究的深入,有望发现更多的微生物资源,并开发更多的种子包衣新技术,从而为该技术提供更好的前景和潜力。
| [1] |
MA Y. Seed coating with beneficial microorganisms for precision agriculture[J]. Biotechnology Advances, 2019, 37(7): 107423. DOI:10.1016/j.biotechadv.2019.107423 |
| [2] |
GOMIERO T. Soil degradation, land scarcity and food security: reviewing a complex challenge[J]. Sustainability, 2016, 8(3): 281. DOI:10.3390/su8030281 |
| [3] |
REGANOLD JP, WACHTER JM. Organic agriculture in the twenty-first century[J]. Nature Plants, 2016, 2: 15221. DOI:10.1038/nplants.2015.221 |
| [4] |
ZENG DF, FAN Z, TIAN X, WANG WJ, ZHOU MC, LI HC. Preparation and mechanism analysis of an environment-friendly maize seed coating agent[J]. Journal of the Science of Food and Agriculture, 2018, 98(8): 2889-2897. DOI:10.1002/jsfa.8783 |
| [5] |
SKRZYPCZAK D, JARZEMBOWSKI Ł, IZYDORCZYK G, MIKULA K, HOPPE V, MIELKO KA, PUDEŁKO-MALIK N, MŁYNARZ P, CHOJNACKA K, WITEK-KROWIAK A. Hydrogel alginate seed coating as an innovative method for delivering nutrients at the early stages of plant growth[J]. Polymers, 2021, 13(23): 4233. DOI:10.3390/polym13234233 |
| [6] |
CHIN JM, LIM YY, TING ASY. Biopolymers for biopriming of Brassica rapa seeds: a study on coating efficacy, bioagent viability and seed germination[J]. Journal of the Saudi Society of Agricultural Sciences, 2021, 20(3): 198-207. DOI:10.1016/j.jssas.2021.01.006 |
| [7] |
ADAK T, KUMAR J, SHAKIL NA, PANDEY S. Role of nano-range amphiphilic polymers in seed quality enhancement of soybean and imidacloprid retention capacity on seed coatings[J]. Journal of the Science of Food and Agriculture, 2016, 96(13): 4351-4357. DOI:10.1002/jsfa.7643 |
| [8] |
HAN XL. Demonstration and promotion of a whole organic/green plant protection system and examination of biological seed coating agent on corn[D]. Nanjing: Master's Thesis of Nanjing Agricultural University, 2019 (in Chinese). 韩晓丽. 全程有机/绿色植保体系的示范推广及生物种衣剂在玉米上的应用[D]. 南京: 南京农业大学硕士学位论文, 2019. |
| [9] |
XAVIER PB, VIEIRA HD, GUIMARÃES CP. Physiological potential of stylosanthes cv. Campo Grande seeds coated with different materials[J]. Journal of Seed Science, 2015, 37(2): 117-124. DOI:10.1590/2317-1545v37n2145982 |
| [10] |
ZIANI K, URSÚA B, MATÉ JI. Application of bioactive coatings based on chitosan for artichoke seed protection[J]. Crop Protection, 2010, 29(8): 853-859. DOI:10.1016/j.cropro.2010.03.002 |
| [11] |
BEAN TG, GROSS MS, KAROUNA-RENIER NK, HENRY PFP, SCHULTZ SL, HLADIK ML, KUIVILA KM, RATTNER BA. Toxicokinetics of imidacloprid-coated wheat seeds in Japanese quail (Coturnix japonica) and an evaluation of hazard[J]. Environmental Science & Technology, 2019, 53(7): 3888-3897. |
| [12] |
CORTÉS-ROJAS D, BELTRÁN-ACOSTA C, ZAPATA-NARVAEZ Y, CHAPARRO M, GÓMEZ M, CRUZ-BARRERA M. Seed coating as a delivery system for the endophyte Trichoderma koningiopsis Th003 in rice (Oryza sativa)[J]. Applied Microbiology and Biotechnology, 2021, 105(5): 1889-1904. DOI:10.1007/s00253-021-11146-9 |
| [13] |
GARRIDO M, VILELA N. Antagonistic capacity of Trichoderma harzianum compared to rhizotecnia, Nakataea sigmoidea, Sclerotium rolfsii and its effect in native strains of Trichoderma isolated form rice crops[J]. Scientia Agropecuaria, 2019, 10(2): 199-206. DOI:10.17268/sci.agropecu.2019.02.05 |
| [14] |
JAVED T, AFZAL I, SHABBIR R, IKRAM K, ZAHEER MS, FAHEEM M, ALI HH, IQBAL J. Seed coating technology: an innovative and sustainable approach for improving seed quality and crop performance[J]. Journal of the Saudi Society of Agricultural Sciences, 2022, 21(8): 536-545. DOI:10.1016/j.jssas.2022.03.003 |
| [15] |
JIANG YJ, LI QH, MAO WQ, TANG WT, WHITE JF Jr. , LI HY. Endophytic bacterial community of Stellera chamaejasme L. and its role in improving host plants' competitiveness in grasslands[J]. Environmental Microbiology, 2022, 24(8): 3322-3333. DOI:10.1111/1462-2920.15897 |
| [16] |
PARMAR S, SHARMA VK, LI T, TANG WT, LI HY. Fungal seed endophyte FZT214 improves Dysphania ambrosioides Cd tolerance throughout different developmental stages[J]. Frontiers in Microbiology, 2022, 12: 783475. DOI:10.3389/fmicb.2021.783475 |
| [17] |
SILLETTI S, DI STASIO E, van OOSTEN MJ, VENTORINO V, PEPE O, NAPOLITANO M, MARRA R, WOO SL, CIRILLO V, MAGGIO A. Biostimulant activity of Azotobacter chroococcum and Trichoderma harzianum in durum wheat under water and nitrogen deficiency[J]. Agronomy, 2021, 11(2): 380. DOI:10.3390/agronomy11020380 |
| [18] |
O'CALLAGHAN M. Microbial inoculation of seed for improved crop performance: issues and opportunities[J]. Applied Microbiology and Biotechnology, 2016, 100(13): 5729-5746. DOI:10.1007/s00253-016-7590-9 |
| [19] |
de GREGORIO PR, MICHAVILA G, RICCIARDI MULLER L, de SOUZA BORGES C, POMARES MF, SACCOL de SÁ EL, PEREIRA C, VINCENT PA. Beneficial rhizobacteria immobilized in nanofibers for potential application as soybean seed bioinoculants[J]. PLoS One, 2017, 12(5): e0176930. DOI:10.1371/journal.pone.0176930 |
| [20] |
OLIVEIRA RS, ROCHA I, MA Y, VOSÁTKA M, FREITAS H. Seed coating with arbuscular mycorrhizal fungi as an ecotechnologicalapproach for sustainable agricultural production of common wheat (Triticum aestivum L.)[J]. Journal of Toxicology and Environmental Health, Part A, 2016, 79(7): 329-337. DOI:10.1080/15287394.2016.1153448 |
| [21] |
ROCHA I, MA Y, SOUZA-ALONSO P, VOSÁTKA M, FREITAS H, OLIVEIRA RS. Seed coating: a tool for delivering beneficial microbes to agricultural crops[J]. Frontiers in Plant Science, 2019, 10: 1357. DOI:10.3389/fpls.2019.01357 |
| [22] |
ZHANG KK, KHAN Z, YU Q, QU ZJ, LIU JH, LUO T, ZHU KM, BI JG, HU LY, LUO LJ. Biochar coating is a sustainable and economical approach to promote seed coating technology, seed germination, plant performance, and soil health[J]. Plants (Basel, Switzerland), 2022, 11(21): 2864. |
| [23] |
GŁODOWSKA M, HUSK B, SCHWINGHAMER T, SMITH D. Biochar is a growth-promoting alternative to peat moss for the inoculation of corn with a pseudomonad[J]. Agronomy for Sustainable Development, 2016, 36(1): 21. DOI:10.1007/s13593-016-0356-z |
| [24] |
ZHOU JQ, DENG B, ZHANG YJ, COBB AB, ZHANG Z. Molybdate in rhizobial seed-coat formulations improves the production and nodulation of alfalfa[J]. PLoS One, 2017, 12(1): e0170179. DOI:10.1371/journal.pone.0170179 |
| [25] |
MA Y, LÁTR A, ROCHA I, FREITAS H, VOSÁTKA M, OLIVEIRA RS. Delivery of inoculum of Rhizophagus irregularis via seed coating in combination with Pseudomonas libanensis for cowpea production[J]. Agronomy, 2019, 9(1): 33. DOI:10.3390/agronomy9010033 |
| [26] |
ZHANG Y, LI YY, LIANG SB, ZHENG W, CHEN XL, LIU JY, WANG AX. Study on the preparation and effect of tomato seedling disease biocontrol compound seed-coating agent[J]. Life, 2022, 12(6): 849. DOI:10.3390/life12060849 |
| [27] |
PEDRINI S, MERRITT DJ, STEVENS J, DIXON K. Seed coating: science or marketing spin?[J]. Trends in Plant Science, 2017, 22(2): 106-116. DOI:10.1016/j.tplants.2016.11.002 |
| [28] |
SWAMINATHAN J, van KOTEN C, HENDERSON HV, JACKSON TA, WILSON MJ. Formulations for delivering Trichoderma atroviridae spores as seed coatings, effects of temperature and relative humidity on storage stability[J]. Journal of Applied Microbiology, 2016, 120(2): 425-431. DOI:10.1111/jam.13006 |
| [29] |
GEORGAKOPOULOS DG, FIDDAMAN P, LEIFERT C, MALATHRAKIS NE. Biological control of cucumber and sugar beet damping-off caused by Pythium ultimum with bacterial and fungal antagonists[J]. Journal of Applied Microbiology, 2002, 92(6): 1078-1086. DOI:10.1046/j.1365-2672.2002.01658.x |
| [30] |
EGAMBERDIEVA D, RECKLING M, WIRTH S. Biochar-based Bradyrhizobium inoculum improves growth of lupin (Lupinus angustifolius L.) under drought stress[J]. European Journal of Soil Biology, 2017, 78: 38-42. DOI:10.1016/j.ejsobi.2016.11.007 |
| [31] |
EGAMBERDIEVA D, HUA M, RECKLING M, WIRTH S, BELLINGRATH-KIMURA SD. Potential effects of biochar-based microbial inoculants in agriculture[J]. Environmental Sustainability, 2018, 1(1): 19-24. DOI:10.1007/s42398-018-0010-6 |
| [32] |
GŁODOWSKA M, SCHWINGHAMER T, HUSK B, SMITH D. Biochar based inoculants improve soybean growth and nodulation[J]. Agricultural Sciences, 2017, 8(9): 1048-1064. DOI:10.4236/as.2017.89076 |
| [33] |
TRIPTI, KUMAR A, USMANI Z, KUMAR V, ANSHUMALI. Biochar and flyash inoculated with plant growth promoting rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant[J]. Journal of Environmental Management, 2017, 190: 20-27. DOI:10.1016/j.jenvman.2016.11.060 |
| [34] |
BEN-JABEUR M, GRACIA-ROMERO A, LÓPEZ-CRISTOFFANINI C, VICENTE R, KTHIRI Z, KEFAUVER SC, LÓPEZ-CARBONELL M, SERRET MD, ARAUS JL, HAMADA W. The promising MultispeQ device for tracing the effect of seed coating with biostimulants on growth promotion, photosynthetic state and water-nutrient stress tolerance in durum wheat[J]. Euro-Mediterranean Journal for Environmental Integration, 2021, 6(1): 8. DOI:10.1007/s41207-020-00213-8 |
| [35] |
MATTEI V, MOTTA A, SARACCHI M, KUNOVA A, CORTESI P, PIZZATTI C, PASQUALI M. Wheat seed coating with Streptomyces sp. strain DEF39 spores protects against Fusarium head blight[J]. Microorganisms, 2022, 10(8): 1536. DOI:10.3390/microorganisms10081536 |
| [36] |
HOSEINI A, SALEHI A, SAYYED RZ, BALOUCHI H, MORADI A, PIRI R, FAZELI-NASAB B, POCZAI P, ANSARI MJ, AL OBAID S, DATTA R. Efficacy of biological agents and fillers seed coating in improving drought stress in anise[J]. Frontiers in Plant Science, 2022, 13: 955512. DOI:10.3389/fpls.2022.955512 |
| [37] |
PIRI R, MORADI A, BALOUCHI H, SALEHI A. Improvement of cumin (Cuminum cyminum) seed performance under drought stress by seed coating and biopriming[J]. Scientia Horticulturae, 2019, 257: 108667. DOI:10.1016/j.scienta.2019.108667 |
| [38] |
CONINCK E, SCAUFLAIRE J, GOLLIER M, LIÉNARD C, FOUCART G, MANSSENS G, MUNAUT F, LEGRÈVE A. Trichoderma atroviride as a promising biocontrol agent in seed coating for reducing Fusarium damping-off on maize[J]. Journal of Applied Microbiology, 2020, 129(3): 637-651. DOI:10.1111/jam.14641 |
| [39] |
de LIRA AC, MASCARIN MG, DELALIBERA JÚNIOR Í. Microsclerotia production of Metarhizium spp. for dual role as plant biostimulant and control of Spodoptera frugiperda through corn seed coating[J]. Fungal Biology, 2020, 124(8): 689-699. DOI:10.1016/j.funbio.2020.03.011 |
| [40] |
RIVAS-FRANCO F, HAMPTON JG, MORÁN-DIEZ ME, NARCISO J, ROSTÁS M, WESSMAN P, JACKSON TA, GLARE TR. Effect of coating maize seed with entomopathogenic fungi on plant growth and resistance against Fusarium graminearum and Costelytra giveni[J]. Biocontrol Science and Technology, 2019, 29(9): 877-900. DOI:10.1080/09583157.2019.1611736 |
| [41] |
ACCINELLI C, ABBAS HK, SHIER WT. A bioplastic-based seed coating improves seedling growth and reduces production of coated seed dust[J]. Journal of Crop Improvement, 2018, 32(3): 318-330. DOI:10.1080/15427528.2018.1425792 |
| [42] |
MASTOURI F, BJÖRKMAN T, HARMAN GE. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings[J]. Phytopathology, 2010, 100(11): 1213-1221. DOI:10.1094/PHYTO-03-10-0091 |
| [43] |
MHADA M, ZVINAVASHE AT, HAZZOUMI Z, ZEROUAL Y, MARELLI B, KOUISNI L. Bioformulation of silk-based coating to preserve and deliver Rhizobium tropici to Phaseolus vulgaris under saline environments[J]. Frontiers in Plant Science, 2021, 12: 700273. DOI:10.3389/fpls.2021.700273 |
| [44] |
REHMAN A, FAROOQ M, NAVEED M, NAWAZ A, SHAHZAD B. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat[J]. European Journal of Agronomy, 2018, 94: 98-107. DOI:10.1016/j.eja.2018.01.017 |
| [45] |
AFZAL I, JAVED T, AMIRKHANI M, TAYLOR AG. Modern seed technology: seed coating delivery systems for enhancing seed and crop performance[J]. Agriculture, 2020, 10(11): 526. DOI:10.3390/agriculture10110526 |
| [46] |
RYU CM, KIM J, CHOI O, KIM SH, PARK CS. Improvement of biological control capacity of Paenibacillus polymyxa E681 by seed pelleting on sesame[J]. Biological Control, 2006, 39(3): 282-289. DOI:10.1016/j.biocontrol.2006.04.014 |
| [47] |
JAVED T, AFZAL I. Impact of seed pelleting on germination potential, seedling growth and storage of tomato seed[J]. Acta Horticulturae, 2020(1273): 417-424. |
| [48] |
GUBIŠOVÁ M, HUDCOVICOVÁ M, MATUŠINSKÝ P, ONDREIČKOVÁ K, KLČOVÁ L, GUBIŠ J. Superabsorbent polymer seed coating reduces leaching of fungicide but does not alter their effectiveness in suppressing pathogen infestation[J]. Polymers, 2021, 14(1): 76. DOI:10.3390/polym14010076 |
| [49] |
KINTL A, HUŇADY I, VYMYSLICKÝ T, ONDRISKOVÁ V, HAMMERSCHMIEDT T, BRTNICKÝ M, ELBL J. Effect of seed coating and PEG-induced drought on the germination capacity of five clover crops[J]. Plants, 2021, 10(4): 724. DOI:10.3390/plants10040724 |
| [50] |
HUSSAIN M, MEHBOOB N, NAVEED M, SHEHZADI K, AHMAD YASIR T. Optimizing boron seed coating level and boron-tolerant bacteria for improving yield and biofortification of chickpea[J]. Journal of Soil Science and Plant Nutrition, 2020, 20(4): 2471-2478. DOI:10.1007/s42729-020-00313-y |
| [51] |
WU ZS, YAO LX, KALEEM I, LI C. Application efficacy of biological seed coating agent from combination of PGPR on cotton in the field[M]// Advances in Intelligent and Soft Computing. Berlin, Heidelberg: Springer Berlin Heidelberg, 2012: 903-910.
|
| [52] |
SINGH N, THAKUR AK, KAUSHAL R, MEHTA DK, BHARDWAJ RK. Effects of seed pelleting on seed quality of cowpea (Vigna unguiculata L.) during storage[J]. International Journal of Economic Plants, 2018, 5(2): 76-79. DOI:10.23910/IJEP/2018.5.2.0237 |
| [53] |
CARDARELLI M, WOO SL, ROUPHAEL Y, COLLA G. Seed treatments with microorganisms can have a biostimulant effect by influencing germination and seedling growth of crops[J]. Plants (Basel, Switzerland), 2022, 11(3): 259. |
| [54] |
DAMALAS CA, KOUTROUBAS SD, FOTIADIS S. Hydro-priming effects on seed germination and field performance of faba bean in spring sowing[J]. Agriculture, 2019, 9(9): 201. DOI:10.3390/agriculture9090201 |
| [55] |
ROUPHAEL Y, COLLA G, GRAZIANI G, RITIENI A, CARDARELLI M, de PASCALE S. Phenolic composition, antioxidant activity and mineral profile in two seed-propagated artichoke cultivars as affected by microbial inoculants and planting time[J]. Food Chemistry, 2017, 234: 10-19. DOI:10.1016/j.foodchem.2017.04.175 |
| [56] |
COLLA G, ROUPHAEL Y, BONINI P, CARDARELLI M. Coating seeds with endophytic fungi enhances growth, nutrient uptake, yield and grain quality of winter wheat[J]. International Journal of Plant Production, 2015, 9(2): 171-189. |
| [57] |
COLLA G, ROUPHAEL Y, Di MATTIA E, EL-NAKHEL C, CARDARELLI M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops[J]. Journal of the Science of Food and Agriculture, 2015, 95(8): 1706-1715. DOI:10.1002/jsfa.6875 |
| [58] |
ROCHA I, MA Y, CARVALHO MF, MAGALHÃES C, JANOUŠKOVÁ M, VOSÁTKA M, FREITAS H, OLIVEIRA RS. Seed coating with inocula of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria for nutritional enhancement of maize under different fertilisation regimes[J]. Archives of Agronomy and Soil Science, 2019, 65(1): 31-43. DOI:10.1080/03650340.2018.1479061 |
| [59] |
SENE G, THIAO M, SY O, MBAYE MS, SYLLA SN. Seed coating with mycorrhizal fungal spores and LEIFSONIA bacteria: a tool for microbiological fertilization and a seed protection strategy from insect damage[J]. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 2021, 91(4): 909-918. DOI:10.1007/s40011-021-01297-0 |
| [60] |
PALUPI T, RIYANTO F. Seed coating with biological agents to improve the quality of rice seeds contaminated with blast pathogens and increase seedling growth[J]. Biodiversitas Journal of Biological Diversity, 2020, 21(2): 683-688. |
| [61] |
BARNETT SJ, BALLARD RA, FRANCO CMM. Field assessment of microbial inoculants to control Rhizoctonia root rot on wheat[J]. Biological Control, 2019, 132: 152-160. DOI:10.1016/j.biocontrol.2019.02.019 |
| [62] |
KTHIRI Z, JABEUR MB, MACHRAOUI M, GARGOURI S, HIBA K, HAMADA W. Coating seeds with Trichoderma strains promotes plant growth and enhance the systemic resistance against Fusarium crown rot in durum wheat[J]. Egyptian Journal of Biological Pest Control, 2020, 30(1): 1-10. DOI:10.1186/s41938-020-0205-x |
| [63] |
WANG R, WANG CW, ZUO B, LIANG XY, ZHANG DN, LIU RX, YANG LN, LU BH, WANG X, GAO J. A novel biocontrol strain Bacillus amyloliquefaciens FS6 for excellent control of gray mold and seedling diseases of ginseng[J]. Plant Disease, 2021, 105(7): 1926-1935. DOI:10.1094/PDIS-07-20-1593-RE |
| [64] |
MAHMOOD Y, KHAN MA, JAVED N, ARIF MJ. Comparative efficacy of fungicides and biological control agents for the management of chickpea wilt caused by Fusarium oxysporum f. sp. ciceris[J]. Journal of Animal and Plant Sciences, 2015, 25(4): 1063-1071. |
| [65] |
JAVED T, AFZAL I, MAURO RP. Seed coating in direct seeded rice: an innovative and sustainable approach to enhance grain yield and weed management under submerged conditions[J]. Sustainability, 2021, 13(4): 2190. DOI:10.3390/su13042190 |
| [66] |
ELZEIN A, HELLER A, NDAMBI B, MOL MD, KROSCHEL J, CADISCH G. Cytological investigations on colonization of sorghum roots by the mycoherbicide Fusarium oxysporum f. sp. strigae and its implications for Striga control using a seed treatment delivery system[J]. Biological Control, 2010, 53(3): 249-257. DOI:10.1016/j.biocontrol.2010.02.002 |
| [67] |
MAHPARA S, ZAINAB A, ULLAH R, KAUSAR S, BILAL M, LATIF MI, ARIF M, AKHTAR I, AL-HASHIMI A, ELSHIKH MS, ZIVCAK M, ZUAN ATK. The impact of PEG-induced drought stress on seed germination and seedling growth of different bread wheat (Triticum aestivum L.) genotypes[J]. PLoS One, 2022, 17(2): e0262937. DOI:10.1371/journal.pone.0262937 |
| [68] |
SU LQ, LI JG, XUE H, WANG XF. Super absorbent polymer seed coatings promote seed germination and seedling growth of Caragana korshinskii in drought[J]. Journal of Zhejiang University-Science B, 2017, 18(8): 696-706. DOI:10.1631/jzus.B1600350 |
| [69] |
OLJIRA AM, HUSSAIN T, WAGHMODE TR, ZHAO HC, SUN HY, LIU XJ, WANG XZ, LIU BB. Trichoderma enhances net photosynthesis, water use efficiency, and growth of wheat (Triticum aestivum L.) under salt stress[J]. Microorganisms, 2020, 8(10): 1565. DOI:10.3390/microorganisms8101565 |
| [70] |
ZVINAVASHE AT, LIM E, SUN H, MARELLI B. A bioinspired approach to engineer seed microenvironment to boost germination and mitigate soil salinity[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(51): 25555-25561. |
| [71] |
SHAHZAD S, KHAN MY, ZAHIR ZA, ASGHAR HN, CHAUDHRY UK. Comparative effectiveness of different carriers to improve the efficacy of bacterial consortium for enhancing wheat production under salt affected field conditions[J]. Pakistan Journal of Botany, 2017, 49(4): 1523-1530. |
| [72] |
RAKESH P, PRASAD RD, DEVI GU, BHAT BN. Effect of biopolymers and synthetic seed coating polymers on Castor and groundnut seed[J]. International Journal of Pure & Applied Bioscience, 2017, 5(4): 2043-2048. |
| [73] |
BUNN R, LEKBERG Y, ZABINSKI C. Arbuscular mycorrhizal fungi ameliorate temperature stress in thermophilic plants[J]. Ecology, 2009, 90(5): 1378-1388. DOI:10.1890/07-2080.1 |
| [74] |
CHIN JM, LIM YY, TING ASY. Biopriming vegetable seeds with metal-tolerant biocontrol agents to enhance tolerance to biotic and abiotic stress[J]. Biological Control, 2022, 174: 105035. DOI:10.1016/j.biocontrol.2022.105035 |
| [75] |
SINGH S, KUMAR V, GUPTA P, SINGH A. Conjoint application of novel bacterial isolates on dynamic changes in oxidative stress responses of axenic Brassica juncea L. in Hg-stress soils[J]. Journal of Hazardous Materials, 2022, 434: 128854. DOI:10.1016/j.jhazmat.2022.128854 |
| [76] |
PARAVAR A, PIRI R, BALOUCHI H, MA Y. Microbial seed coating: an attractive tool for sustainable agriculture[J]. Biotechnology Reports (Amsterdam, Netherlands), 2023, 37: e00781. |
| [77] |
DOGARU BI, STOLERU V, MIHALACHE G, YONSEL S, POPESCU M-C. Gelatin reinforced with CNCs as nanocomposite matrix for Trichoderma harzianum KUEN 1585 spores in seed coatings[J]. Molecules, 2021, 26(19): 5755. DOI:10.3390/molecules26195755 |
| [78] |
CHANDRIKA KSVP, PRASAD RD, GODBOLE V. Development of chitosan-PEG blended films using Trichoderma: enhancement of antimicrobial activity and seed quality[J]. International Journal of Biological Macromolecules, 2019, 126: 282-290. DOI:10.1016/j.ijbiomac.2018.12.208 |
| [79] |
CORTÉS-ROJAS D, SANTOS-DIAZ A, TORRES-TORRES L, ZAPATA-NARVÁEZ Y, BELTRÁN-ACOSTA C, CRUZ-BARRERA M. Trichoderma koningiopsis survival on coated seeds and effect on plant growth promotion in rice (Oryza sativa)[J]. Current Microbiology, 2023, 80(1): 22. DOI:10.1007/s00284-022-03076-0 |
| [80] |
TU L, HE YH, SHAN CH, WU ZS. Preparation of microencapsulated Bacillus subtilis SL-13 seed coating agents and their effects on the growth of cotton seedlings[J]. BioMed Research International, 2016, 2016: 3251357. |
| [81] |
AIN NU, NAVEED M, HUSSAIN A, MUMTAZ MZ, RAFIQUE M, BASHIR MA, ALAMRI S, SIDDIQUI MH. Impact of coating of urea with Bacillus-augmented zinc oxide on wheat grown under salinity stress[J]. Plants, 2020, 9(10): 1375. DOI:10.3390/plants9101375 |
| [82] |
JETIYANON K, WITTAYA-AREEKUL S, PLIANBANGCHANG P. Film coating of seeds with Bacillus cereus RS87 spores for early plant growth enhancement[J]. Canadian Journal of Microbiology, 2008, 54(10): 861-867. DOI:10.1139/W08-079 |
| [83] |
ROCHA I, SOUZA-ALONSO P, PEREIRA G, MA Y, VOSÁTKA M, FREITAS H, OLIVEIRA RS. Using microbial seed coating for improving cowpea productivity under a low-input agricultural system[J]. Journal of the Science of Food and Agriculture, 2020, 100(3): 1092-1098. DOI:10.1002/jsfa.10117 |
| [84] |
XU W, XU LL, DENG XX, GOODWIN PH, XIA MC, ZHANG J, WANG Q, SUN RH, PAN YM, WU C, YANG LR. Biological control of take-all and growth promotion in wheat by Pseudomonas chlororaphis YB-10[J]. Pathogens (Basel, Switzerland), 2021, 10(7): 903. |
| [85] |
HAMEEDA B, HARINI G, RUPELA OP, KUMAR RAO JVDK, REDDY G. Biological control of chickpea collar rot by Co-inoculation of antagonistic bacteria and compatible rhizobia[J]. Indian Journal of Microbiology, 2010, 50(4): 419-424. DOI:10.1007/s12088-011-0083-8 |
| [86] |
KESWANI C, BISEN K, SINGH V, SARMA BK, SINGH HB. Formulation technology of biocontrol agents: present status and future prospects[M]// Bioformulations: for Sustainable Agriculture. New Delhi: Springer India, 2016: 35-52.
|
| [87] |
MAYO-PRIETO S, MARRA R, VINALE F, RODRÍGUEZ-GONZÁLEZ Á, WOO SL, LORITO M, GUTIÉRREZ S, CASQUERO PA. Effect of Trichoderma velutinum and Rhizoctonia solani on the metabolome of bean plants (Phaseolus vulgaris L.)[J]. International Journal of Molecular Sciences, 2019, 20(3): 549. DOI:10.3390/ijms20030549 |
| [88] |
METWALLY RA. Arbuscular mycorrhizal fungi and Trichoderma viride cooperative effect on biochemical, mineral content, and protein pattern of onion plants[J]. Journal of Basic Microbiology, 2020, 60(8): 712-721. DOI:10.1002/jobm.202000087 |
| [89] |
BLAKE C, CHRISTENSEN MN, KOVÁCS ÁT. Molecular aspects of plant growth promotion and protection by Bacillus subtilis[J]. Molecular Plant-Microbe Interactions: MPMI, 2021, 34(1): 15-25. DOI:10.1094/MPMI-08-20-0225-CR |
| [90] |
PRESTON GM. Plant perceptions of plant growth-promoting Pseudomonas[J]. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 2004, 359(1446): 907-918. DOI:10.1098/rstb.2003.1384 |
| [91] |
AHMED AQ, JAVED N, KHAN SA, ABBAS H, KAMRAN M. Efficacy of rhizospheric organism Rhizobium leguminosarum against Meloidogyne incognita in soybean[J]. Pakistan Journal of Agricultural Sciences, 2016, 53(2): 377-381. DOI:10.21162/PAKJAS/16.1659 |
| [92] |
SCHOINA C, STRINGLIS IA, PANTELIDES IS, TJAMOS SE, PAPLOMATAS EJ. Evaluation of application methods and biocontrol efficacy of Paenibacillus alvei strain K-165, against the cotton black root rot pathogen Thielaviopsis basicola[J]. Biological Control, 2011, 58(1): 68-73. DOI:10.1016/j.biocontrol.2011.04.002 |
| [93] |
AMUTHA M. Establishment of Beauveria bassiana (balsamo) vuillemin as an endophytein cotton[J]. International Journal of Current Microbiology and Applied Sciences, 2017, 6(6): 2506-2513. DOI:10.20546/ijcmas.2017.606.298 |
| [94] |
MÜLLER H, BERG G. Impact of formulation procedures on the effect of the biocontrol agent Serratia plymuthica HRO-C48 on Verticillium wilt in oilseed rape[J]. BioControl, 2008, 53(6): 905-916. DOI:10.1007/s10526-007-9111-3 |
| [95] |
SHAHAROONA B, ARSHAD M, ZAHIR ZA. Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.)[J]. Letters in Applied Microbiology, 2006, 42(2): 155-159. DOI:10.1111/j.1472-765X.2005.01827.x |
| [96] |
SHAHAROONA B, NAVEED M, ARSHAD M, ZAHIR ZA. Fertilizer-dependent efficiency of pseudomonads for improving growth, yield, and nutrient use efficiency of wheat (Triticum aestivum L.)[J]. Applied Microbiology and Biotechnology, 2008, 79(1): 147-155. DOI:10.1007/s00253-008-1419-0 |
| [97] |
KAZEMPOUR MN. Biological control of Rhizoctonia solani, the causal agent of rice sheath blight by antagonistics bacteria in greenhouse and field conditions[J]. Plant Pathology Journal, 2004, 3(2): 88-96. DOI:10.3923/ppj.2004.88.96 |
 2023, Vol. 50
2023, Vol. 50