扩展功能
文章信息
- 王静, 王士金, 王威, 魏伟
- WANG Jing, WANG Shijin, WANG Wei, WEI Wei
- 肠道病毒D68受体研究进展
- Enterovirus D68 receptors: a review
- 微生物学通报, 2022, 49(8): 3500-3507
- Microbiology China, 2022, 49(8): 3500-3507
- DOI: 10.13344/j.microbiol.china.211183
-
文章历史
- 收稿日期: 2021-12-13
- 接受日期: 2022-03-14
- 网络首发日期: 2022-04-12
2. 吉林大学艾滋病与病毒研究所, 吉林 长春 130021
2. Institute of Virology and AIDS Research, Jilin University, Changchun 130021, Jilin, China
肠道病毒D68 (Enterovirus D68,EV-D68)属于小核糖核酸病毒科肠道病毒属,脊髓灰质炎病毒(Poliovirus)、埃可病毒(Echovirus)、鼻病毒(Rhinovirus)和手足口病毒(hand-foot-and-mouth virus)等高致病性人类病毒均属于肠道病毒家族。肠道病毒大多依赖粪-口消化道途径进行传播,也有少部分能够通过呼吸道等方式扩散传播。其中,肠道病毒D68型是近年在全球范围内周期性暴发的重要呼吸道传播病毒,引起患者严重的呼吸系统疾病,与急性缓驰性脊髓炎等神经系统症状也有密切关联,是当前公共卫生安全的重大隐患之一。病毒识别暴露于细胞表面的受体引发病毒侵入与感染,是病毒宿主范围的关键决定因素并支配着宿主对病毒的易感性。本文综述了EV-D68的病毒受体鉴定与功能特性相关的研究进展,以期为EV-D68病毒阻断病毒入侵药物研发提供参考信息。
1 EV-D68的生物学特性EV-D68于1962年首次从美国加利福尼亚州儿童鼻咽拭子中分离[1]。不同于其他肠道病毒的粪-口传播途径,EV-D68病毒则依赖呼吸道途径进行传播,其感染后主要引起呼吸道系统症状,近期研究发现EV-D68病毒感染与急性缓驰性脊髓炎有着密切关联[2]。EV-D68病毒颗粒为直径约30 nm的二十面体无包膜病毒,其基因组为一条长约7.4 kb的单股正链RNA,含有单一开放阅读框编码病毒蛋白前体,经蛋白酶加工产生结构蛋白(VP1、VP2、VP3和VP4)与非结构蛋白(2A、2B、2C、3A、3B、3C和3D)[3]。EV-D68颗粒由60个结构蛋白形成的基础原件排列构成,其中VP1、VP2和VP3暴露于颗粒表面,VP4隐藏于内侧,在五角星形的二十面体顶点上存在一个凹陷结构域(canyon)[3]。其中,canyon结构域在病毒与细胞受体相互作用中发挥了至关重要的作用[4-6]。
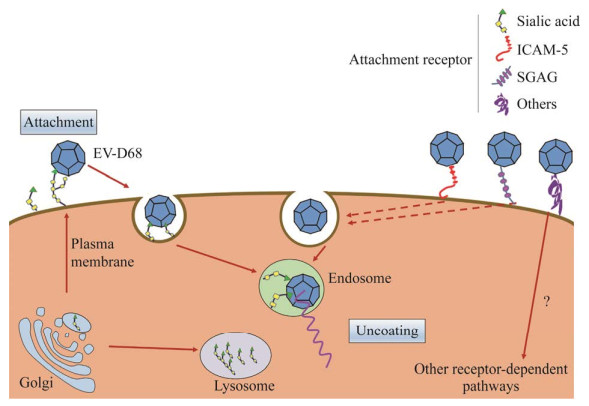
2 EV-D68病毒的复制周期肠道病毒感染起始于病毒表面配体与细胞表面受体的相互作用,黏附到宿主细胞表面,在入侵受体介导下通过内吞作用进入细胞内[7-8]。伴随着内吞囊泡酸化,受体进一步诱发病毒颗粒解体,使病毒RNA释放到细胞质(图 1)。肠道病毒感染的同时会诱导细胞内膜重排,形成病毒复制细胞器(replication organelles),为病毒基因组合成与转运提供良好的微环境,躲避了细胞内免疫系统的识别[9-11]。病毒RNA随后被包裹进入VP0、VP1和VP3组成的衣壳内形成前病毒粒子,随后,VP0在蛋白酶作用下被切割为VP2和VP4产生成熟的病毒颗粒,成熟的病毒颗粒通过细胞裂解或胞吐过程离开细胞开始下一轮感染[12-13]。综上所述,病毒受体是EV-D68病毒顺利感染的先决条件,决定着病毒感染宿主和个体的易感性。本文就EV-D68现已发现的3种主要受体进行综述。表 1为3种受体的发现时间、所涉及的基因、相关机制及通路的总结。
|
| 图 1 EV-D68入侵模式图 Figure 1 EV-D68 attachment and entry. |
|
|
| 项目 Item |
唾液酸 Sialic acid (SIA) |
硫酸化糖胺聚糖 Sulfated glycosaminoglycan (SGAG) |
细胞间隙黏附素5 Intercellular adhesion molecule 5 (ICAM-5) |
| 发现时间 Discovery time |
2015 | 2019 | 2016 |
| 合成基因 Synthetic gene |
生物合成(GNE和NANS),激活(CMAS),转运(SLC35A1和SLC35A2)[14];转移(B4GALT1),连接(ST3GAL4和ST6GAL1)[15] Involved in biosynthesis (GNE and NANS), activation (CMAS), transport (SLC35A1 and SLC35A2)[14]; shift (B4GALT1), linkage (ST3GAL4 and ST6GAL1)[15] |
糖胺聚糖核心四糖(B3GAT3、FAM20B、B3GALT6、B4GALT7、UXS1和XYLT2),硫酸乙酰肝素EXT1、EXT2和EXTL3),UDP-葡萄糖醛酸酯(UGP2和UGDH),参与硫酸化(SLC35B2和NDST1)[16] Involved in the synthesis of glycosaminoglycan core tetrasaccharide (B3GAT3, FAM20B, B3GALT6, B4GALT7, UXS1, XYLT2), heparan sulfate (EXT1, EXT2, EXTL3), UDP-glucuronate (UGP2, UGDH), involved in sulfidation (SLC35B2, NDST1)[16] |
ICAM-5基因(人的编码基因位于19号染色体p13.2的80 kb区域[17-18];鼠的编码基因位于9号染色体30 kb基因簇区域内[19]) ICAM-5 gene (human encoding gene is located in the 80 kb region of p13.2 on chromosome 19[17-18]; encoding gene of mouse is located in the 30 kb gene cluster region of chromosome 9[19]) |
| 相关机制 Related mechanism |
致病菌入侵宿主细胞受体,帮助致病菌逃逸机体免疫系统识别,细胞间识别与突触形成,肿瘤细胞转化[20] Invasion of host cell receptors by pathogenic bacteria, helping pathogens escape the body’s immune system recognition, intercellular recognition and synapse formation, tumor cell transformation[20] |
细胞黏附[21-22];细胞分化、迁移,细胞信号转导[22];参与肿瘤发生及发展[23] Cell adhesion[21-22]; cell differentiation and migration, cell signal transduction[22]; involved in tumorigenesis and tumor progession[23] |
肠道病毒EV-D68受体[24];神经发育疾病,神经元树突棘成熟和突出发育的障碍,端脑神经元回路的形成和维持[19];影响脊椎分化[25] Enterovirus D68 receptor[24]; neurodevelopmental diseases, retardation of maturation and protrusion of dendritic spines[19]; formation and maintenance of telencephalic neural circuits; affecting spinal differentiation[25] |
| 下游通路 Downstream pathways |
Nrf2/ARE信号通路[26];Rho/ROCK-JNK/ERK信号通路[27];BDNF/TrkB信号通路[28];MAPK/ERK信号通 路[29];PI3K/Akt信号通路,NF-κB 信号通路[30-31] Nrf2/ARE signal pathway[26]; Rho/ROCK-JNK/ERK signal pathway[27]; BDNF/TrkB[28]; MAPK/ERK signal pathway[29]; PI3K/Akt signal pathway, NF-κB signal pathway[30-31] |
cGAS-CDNs-STING信号通路[32];TBK1-IRF3-IFN信号通路,NF-κB信号通路[33] cGAS-CDNs-STING signal pathway[32]; TBK1-IRF3-IFN signal pathway, NF-κB signal pathway[33] |
ERM/PI3K/Akt信号通路[34];Hippo信号通路[35] ERM/PI3K/Akt signal pathway[34]; Hippo signal pathway[35] |
唾液酸,又名N-乙酰基神经氨酸,广泛存在于高等脊椎动物细胞膜外侧面。唾液酸作为一种高度多样化单糖,常存在于N-、O-糖蛋白和鞘糖脂的末端[36-37]。唾液酸的正常生理功能主要表现在促进细胞与细胞间黏附、细胞信号传导及个体发育。唾液酸常存在于黏膜表面复杂的黏液层中,在外源病原体感染后作为诱骗受体捕获病原体,阻碍病毒迁移至靶器官组织[38]。然而,定位于细胞膜表面的唾液酸分子可以被多种病毒特异性识别,作为病毒受体介导病毒颗粒的黏附与入侵。流感病毒、呼肠孤病毒、轮状病毒、博卡病毒、腺病毒等多种病毒均能识别唾液酸分子进而黏附到宿主细胞表面[39-40]。Uncapher等首次发现了唾液酸是一个鼻病毒的受体,参与介导病毒进入细胞过程[41],神经氨酸酶处理移除细胞膜表面唾液酸分子,能够显著阻断EV-D68病毒与细胞间黏附和病毒感染。Liu等发现唾液酸作为EV-D68的受体发挥作用,体外实验发现SAα-2, 6和SAα-2, 3在体外可以引起病毒颗粒脱壳,说明唾液酸在EV-D68病毒脱壳过程中也发挥作用[42]。Baggen等通过全基因组功能基因筛选,证实参与唾液酸合成与转运相关基因SLC35A1、GNE和NANS对EV-D68病毒感染至关重要,发现EV-D68特异性结合SAα-2, 6和SAα-2, 3分子,进一步佐证了唾液酸是一个EV-D68病毒的功能性受体[15]。然而近期有研究发现,相较于1962年分离的原始株Fermon,EV-D68流行株对唾液酸的依赖性显著下降,提示EV-D68病毒还存在非唾液酸化的病毒受体[15, 24]。
3.2 硫酸化糖胺聚糖(sulfated glycosaminoglycan,SGAG)硫酸化糖胺聚糖是一类带有负电荷、结构复杂的线性多糖,由高尔基体中的糖胺聚糖(glycosaminoglycan,GAG)经过异构化、硫酸化修饰产生,在体内通常以蛋白聚糖(proteoglycan,PG)形式存在。基于核心二糖结构,GAG可分为肝素/硫酸乙酰肝素(heparinsulfate,HS)、硫酸软骨素/硫酸皮肤素、透明质酸及硫酸角质素4个亚家族。SGAG广泛分布于细胞外基质、胞内膜泡与细胞膜上,位于细胞膜的SGAG在多种病毒(如虫媒病毒、疱疹病毒、乳突瘤病毒等)的感染过程中发挥着不可或缺的作用[43-44]。硫酸乙酰肝素蛋白多糖(heparan sulfate proteoglycan,HSPG)由无支链带负电荷的硫酸乙酰肝素链与蛋白多糖共价连接构成。在单纯疱疹、登革热等病毒中,带负电荷的硫酸化HS链能够与病毒衣壳蛋白表面的碱性残基相互作用,促进病毒颗粒在宿主细胞表面的富集,增强病毒的入侵机率[21, 45-46]。Shukla等报道HSPG在介导单纯疱疹病毒入侵后还参与到病毒颗粒的内化、细胞内转运和释放的过程[46]。2019年,Baggen等的研究发现EV-D68可以利用SGAG入侵宿主细胞,SGAG诱导EV-D68病毒衣壳蛋白重排并刺激病毒粒子基因组的释放;体外可溶性肝素可竞争性结合并阻断病毒颗粒与宿主细胞结合,有效抑制了EV-D68流行分离株(EV-D68-947)病毒的感染;唾液酸依赖的EV-D68的Fermon毒株则不受SGAG的影响[16]。进一步提示,在EV-D68病毒传播过程中,病毒受体选择偏好性会发生演进,进而可能引起病毒组织嗜性的改变。同时也为EV-D68病毒的异位感染提供了重要的理论支持[42, 47]。
3.3 细胞间隙黏附素5 (intercellular adhesion molecule 5,ICAM-5)细胞间隙黏附素5又称为端脑素(telencephalin),是一个免疫球蛋白超家族成员(immunoglobulin superfamily,Ig-SF),在脑端神经元中特异性表达。人ICAM-5含有2个疏水片段,分别为N端的信号肽与C端的跨膜区。全长蛋白分子共含有15个N-连接糖基化位点,其胞外部分存在9个Ig样结构域[18, 48]。同属于细胞黏附分子家族的ICAM-1蛋白作为鼻病毒、柯萨奇病毒A21和柯萨奇病毒A24的受体已被广泛研究[49-50]。2016年,有研究通过对EV-D68病毒的允许细胞系(293T细胞)与非允许细胞系(Vero细胞)进行了比较分析,发现ICAM-5蛋白是EV-D68入侵的功能性受体;ICAM-5表达水平决定着EV-D68病毒的感染力;运用ICAM-5可溶性重组蛋白片段不仅能够中和病毒与细胞膜ICAM-5结合,还能在体外诱导EV-D68病毒提前脱壳,起到抗病毒的保护效果[24]。同时,研究还发现β-环糊精(β-cyclodextrin)能够通过驱散ICAM-5蛋白在细胞膜脂筏结构域的聚集,从而阻断EV-D68病毒感染[51],进一步证实了EV-D68病毒与ICAM-5互作是一个重要抗病毒作用靶标。
目前有越来越多的EV-D68病毒感染患者被报道出现了神经症状,数据统计显示北美地区EV-D68病毒的暴发流行程度与当年急性缓驰性脊髓炎患病人数呈正相关。端脑神经元特异性表达病毒受体ICAM-5进一步支持EV-D68病毒具有侵染神经系统的能力。
4 EV-D68病毒受体研究展望EV-D68病毒的全球性流行与特效疫苗药物的匮乏,激励着EV-D68病毒分子致病机理的深入研究。受体作为病毒感染的先决条件,是分子病毒学的研究重点。唾液酸、硫酸化糖胺聚糖SGAG和细胞间隙黏附素ICAM-5已被证明是EV-D68病毒受体,对了解EV-D68病毒入侵细胞生理过程具有较重要的科学意义,同时也为阻断病毒感染提供了重要的作用靶标。唾液酸酶处理、ICAM-5胞外重组蛋白、ICAM-5抗体等处理均能有效抑制病毒的感染与复制。
基于EV-D68受体在病毒感染中的关键作用,许多研究提供了重要的解决思路。Sun等建立的动物模型中,在EV-D68感染小鼠后肌肉、脊髓出现严重的坏死且肺泡壁增厚,表现出强烈的组织嗜性[52]。Hixon等的动物模型研究表明EV-D68感染在神经系统的致病性[53],提示可以利用转基因动物模型结合抗病毒药物和疫苗研发,检测抗病毒药物及疫苗在动物体内的保护作用。Imamura等通过中和(neutralization,NT)和血凝抑制(hemagglutinationin hibition,HI)试验揭示了EV-D68的唾液酸受体结合特性[54-55],靶向唾液酸受体的作用部位,以阻断病毒与受体的结合,防止病毒入侵。最近有研究证明,针对EV-D68病毒样颗粒制备出的单克隆抗体8F12在ICR小鼠中可以有效抑制EV-D68病毒的复制;冷冻电镜结果显示8F12通过与病毒的峡谷区(canyon)结合,进而在空间上阻碍病毒与宿主细胞表面唾液酸受体的结合,通过干扰病毒的吸附而产生高效的抗病毒作用[56]。提示利用单克隆抗体技术靶向结合病毒配体可抑制病毒入侵。因此,建立完善的EV-D68受体研究方案已成为探究病毒感染、传播及进化并加快抗病毒药物和疫苗研发的关键所在。
鉴于病毒受体在病毒感染与致病中的重要作用,靶向病毒-受体相互作用研究已取得一定的研究进展,但仍存有重要的科学问题亟待深入研究,考虑到ICAM-5蛋白神经细胞特异性表达的局限性,尚存在其他潜在的EV-D68病毒受体有待进一步鉴定研究。此外,目前已知的多个EV-D68病毒受体在病毒体内感染中的作用仍不清楚,病毒受体-宿主细胞作用机制相关研究的缺失严重阻碍了特异性药物的研发。目前靶向EV-D68受体的单克隆抗体制剂在实验室阶段已经取得了初步疗效[56],但受限于EV-D68受体转基因动物模型缺乏,新型药物的前期临床效果评价严重受阻,期望通过基因编辑技术建立EV-D68受体转基因动物模型及疫苗药物效果评价体系,助力抗病毒药物研发与疫苗保护效果的评价。总而言之,EV-D68受体功能研究的不断完善必将推进EV-D68病毒治疗技术的应用转化与推广。
| [1] |
Sun J, Hu XY, Yu XF. Current understanding of human Enterovirus D68[J]. Viruses, 2019, 11(6): 490. DOI:10.3390/v11060490 |
| [2] |
Greninger AL, Naccache SN, Messacar K, Clayton A, Yu GX, Somasekar S, Federman S, Stryke D, Anderson C, Yagi S, et al. A novel outbreak Enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012-14): a retrospective cohort study[J]. The Lancet Infectious Diseases, 2015, 15(6): 671-682. DOI:10.1016/S1473-3099(15)70093-9 |
| [3] |
Liu Y, Sheng J, Fokine A, Meng G, Shin WH, Long F, Kuhn RJ, Kihara D, Rossmann MG. Structure and inhibition of EV-D68, a virus that causes respiratory illness in children[J]. Science, 2015, 347(6217): 71-74. DOI:10.1126/science.1261962 |
| [4] |
Rossmann MG, Arnold E, Erickson JW, Frankenberger EA, Griffith JP, Hecht HJ, Johnson JE, Kamer G, Luo M, Mosser AG, et al. Structure of a human common cold virus and functional relationship to other Picornaviruses[J]. Nature, 1985, 317(6033): 145-153. DOI:10.1038/317145a0 |
| [5] |
Zhao Y, Zhou D, Ni T, Karia D, Kotecha A, Wang X, Rao Z, Jones EY, Fry EE, Ren J, et al. Hand-foot-and-mouth disease virus receptor KREMEN1 binds the canyon of coxsackie virus A10[J]. Nature Communications, 2020, 11: 38. DOI:10.1038/s41467-019-13936-2 |
| [6] |
Rossmann MG. The canyon hypothesis: hiding the host cell receptor attachment site on a viral surface from immune surveillance[J]. Journal of Biological Chemistry, 1989, 264(25): 14587-14590. DOI:10.1016/S0021-9258(18)63732-9 |
| [7] |
Marsh M, Helenius A. Virus entry: open sesame[J]. Cell, 2006, 124(4): 729-740. DOI:10.1016/j.cell.2006.02.007 |
| [8] |
Brown DM, Hixon AM, Oldfield LM, Zhang Y, Novotny M, Wang W, Das SR, Shabman RS, Tyler KL, Scheuermann RH. Contemporary circulating Enterovirus D68 strains have acquired the capacity for viral entry and replication in human neuronal cells[J]. mBio, 2018, 9(5): e01954-e01918. |
| [9] |
Belov GA, Nair V, Hansen BT, Hoyt FH, Fischer ER, Ehrenfeld E. Complex dynamic development of Poliovirus membranous replication complexes[J]. Journal of Virology, 2012, 86(1): 302-312. DOI:10.1128/JVI.05937-11 |
| [10] |
Limpens RWAL, Van Der Schaar HM, Kumar D, Koster AJ, Snijder EJ, Van Kuppeveld FJM, Bárcena M. The transformation of Enterovirus replication structures: a three-dimensional study of single- and double-membrane compartments[J]. mBio, 2011, 2(5): e00166-e00111. |
| [11] |
Laufman O, Perrino J, Andino R. Viral generated inter-organelle contacts redirect lipid flux for genome replication[J]. Cell, 2019, 178(2): 275-289. DOI:10.1016/j.cell.2019.05.030 |
| [12] |
Goodfellow I. The genome-linked protein VPg of vertebrate viruses—a multifaceted protein[J]. Current Opinion in Virology, 2011, 1(5): 355-362. DOI:10.1016/j.coviro.2011.09.003 |
| [13] |
Dai WL, Zhang C, Zhang XY, Xiong P, Liu QW, Gong ST, Geng LL, Zhou DM, Huang Z. A virus-like particle vaccine confers protection against Enterovirus D68 lethal challenge in mice[J]. Vaccine, 2018, 36(5): 653-659. DOI:10.1016/j.vaccine.2017.12.057 |
| [14] |
Ng BG, Asteggiano CG, Kircher M, Buckingham KJ, Raymond K, Nickerson DA, Shendure J, Bamshad MJ, University of Washington Center for Mendelian Genomics, Ensslen M, et al. Encephalopathy caused by novel mutations in the CMP-sialic acid transporter, SLC35A1[J]. American Journal of Medical Genetics Part A, 2017, 173(11): 2906-2911. DOI:10.1002/ajmg.a.38412 |
| [15] |
Baggen J, Thibaut HJ, Staring J, Jae LT, Liu Y, Guo HB, Slager JJ, De Bruin JW, Van Vliet ALW, Blomen VA, et al. Enterovirus D68 receptor requirements unveiled by haploid genetics[J]. PNAS, 2016, 113(5): 1399-1404. DOI:10.1073/pnas.1524498113 |
| [16] |
Baggen J, Liu Y, Lyoo H, Van Vliet ALW, Wahedi M, De Bruin JW, Roberts RW, Overduin P, Meijer A, Rossmann MG, et al. Bypassing pan-Enterovirus host factor PLA2G16[J]. Nature Communications, 2019, 10: 3171. DOI:10.1038/s41467-019-11256-z |
| [17] |
Mizuno T, Yoshihara Y, Inazawa J, Kagamiyama H, Mori K. cDNA cloning and chromosomal localization of the human telencephalin and its distinctive interaction with lymphocyte function-associated antigen-1[J]. The Journal of Biological Chemistry, 1997, 272(2): 1156-1163. DOI:10.1074/jbc.272.2.1156 |
| [18] |
Kilgannon P, Turner T, Meyer J, Wisdom W, Gallatin WM. Mapping of the ICAM-5 (telencephalin) gene, a neuronal member of the ICAM family, to a location between ICAM-1 and ICAM-3 on human chromosome 19p13.2[J]. Genomics, 1998, 54(2): 328-330. DOI:10.1006/geno.1998.5565 |
| [19] |
Yang HP. Structure, expression, and function of ICAM-5[J]. Comparative and Functional Genomics, 2012, 2012: 368938. |
| [20] |
Wang FL, Cui SX, Xie YY, Tian GH, Tang W, Wang YS. Detection of linked sialic acid residues in human gastric adenocarcinoma and its significance[J]. Chinese Journal of Clinical Oncology, 2008, 35(24): 1403-1406. (in Chinese) 王风玲, 崔淑香, 解砚英, 田桂红, 唐伟, 王元书. 胃癌细胞表面α-2, 3唾液酸糖链结构的检测及意义[J]. 中国肿瘤临床, 2008, 35(24): 1403-1406. DOI:10.3969/j.issn.1000-8179.2008.24.008 |
| [21] |
Kamhi E, Joo EJ, Dordick JS, Linhardt RJ. Glycosaminoglycans in infectious disease[J]. Biological Reviews, 2013, 88(4): 928-943. DOI:10.1111/brv.12034 |
| [22] |
Lin XH. Functions of heparan sulfate proteoglycans in cell signaling during development[J]. Development: Cambridge, England, 2004, 131(24): 6009-6021. DOI:10.1242/dev.01522 |
| [23] |
Raskov H, Orhan A, Salanti A, Gaggar S, Gögenur I. Natural killer cells in cancer and cancer immunotherapy[J]. Cancer Letters, 2021, 520: 233-242. DOI:10.1016/j.canlet.2021.07.032 |
| [24] |
Wei W, Guo HR, Chang JL, Yu YZ, Liu GC, Zhang NN, Willard SH, Zheng S, Yu XF. ICAM-5/telencephalin is a functional entry receptor for Enterovirus D68[J]. Cell Host & Microbe, 2016, 20(5): 631-641. |
| [25] |
Raemaekers T, Peric A, Baatsen P, Sannerud R, Declerck I, Baert V, Michiels C, Annaert W. ARF6-mediated endosomal transport of telencephalin affects dendritic filopodia-to-spine maturation[J]. The EMBO Journal, 2012, 31(15): 3252-3269. DOI:10.1038/emboj.2012.182 |
| [26] |
Psefteli PM, Kitscha P, Vizcay G, Fleck R, Chapple SJ, Mann GE, Fowler M, Siow RC. Glycocalyx sialic acids regulate Nrf2-mediated signaling by fluid shear stress in human endothelial cells[J]. Redox Biology, 2021, 38: 101816. DOI:10.1016/j.redox.2020.101816 |
| [27] |
Zhang C, Chen JY, Liu YH, Xu DY. Sialic acid metabolism as a potential therapeutic target of atherosclerosis[J]. Lipids in Health and Disease, 2019, 18(1): 173. DOI:10.1186/s12944-019-1113-5 |
| [28] |
Chen Y, Zheng ZQ, Zhu X, Shi YJ, Tian DD, Zhao FJ, Liu N, Hüppi PS, Troy FA, Wang B. Lactoferrin promotes early neurodevelopment and cognition in postnatal piglets by upregulating the BDNF signaling pathway and polysialylation[J]. Molecular Neurobiology, 2015, 52(1): 256-269. DOI:10.1007/s12035-014-8856-9 |
| [29] |
Li CZ, Xie XH, Liu ZJ, Yang JH, Zuo DM, Xu SM. Neu5Ac induces human dental pulp stem cell osteo-/odontoblastic differentiation by enhancing MAPK/ERK pathway activation[J]. Stem Cells International, 2021, 2021: 5560872. |
| [30] |
Abdulkhalek S, Amith SR, Franchuk SL, Jayanth P, Guo M, Finlay T, Gilmour A, Guzzo C, Gee K, Beyaert R, et al. Neu1 sialidase and matrix metalloproteinase-9 cross-talk is essential for toll-like receptor activation and cellular signaling[J]. The Journal of Biological Chemistry, 2011, 286(42): 36532-36549. DOI:10.1074/jbc.M111.237578 |
| [31] |
Yu X, Wu Q, Wang LP, Zhao YJ, Zhang QQ, Meng QT, Pawan, Wang SJ. Silencing of ST6GalNAc I suppresses the proliferation, migration and invasion of hepatocarcinoma cells through PI3K/AKT/NF-κB pathway[J]. Tumor Biology, 2016, 37(9): 12213-12221. DOI:10.1007/s13277-016-5086-y |
| [32] |
Motwani M, Pesiridis S, Fitzgerald KA. DNA sensing by the cGAS-STING pathway in health and disease[J]. Nature Reviews Genetics, 2019, 20(11): 657-674. DOI:10.1038/s41576-019-0151-1 |
| [33] |
Fang R, Wang CG, Jiang QF, Lv MZ, Gao PF, Yu XY, Mu P, Zhang R, Bi S, Feng JM, et al. NEMO-IKKβ are essential for IRF3 and NF-κB activation in the cGAS-STING pathway[J]. The Journal of Immunology, 2017, 199(9): 3222-3233. DOI:10.4049/jimmunol.1700699 |
| [34] |
Maruya SI, Myers JN, Weber RS, Rosenthal DI, Lotan R, El-Naggar AK. ICAM-5 (telencephalin) gene expression in head and neck squamous carcinoma tumorigenesis and perineural invasion![J]. Oral Oncology, 2005, 41(6): 580-588. DOI:10.1016/j.oraloncology.2005.01.002 |
| [35] |
Jiang YM, Pin L, Shi WQ, Huang Q, Wang LL, Liu HS. SAA1 regulates pro-labour mediators in term labour by activating YAP pathway[J]. Molecular and Cellular Biochemistry, 2021, 476(7): 2791-2801. DOI:10.1007/s11010-021-04125-1 |
| [36] |
Russell CJ, Webster RG. The genesis of a pandemic influenza virus[J]. Cell, 2005, 123(3): 368-371. DOI:10.1016/j.cell.2005.10.019 |
| [37] |
Soares CO, Grosso AS, Ereño-Orbea J, Coelho H, Marcelo F. Molecular recognition insights of sialic acid glycans by distinct receptors unveiled by NMR and molecular modeling[J]. Frontiers in Molecular Biosciences, 2021, 8: 727847. DOI:10.3389/fmolb.2021.727847 |
| [38] |
Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways[J]. The Journal of Clinical Investigation, 2002, 109(5): 571-577. DOI:10.1172/JCI0215217 |
| [39] |
Guo HB, Rabouw H, Slomp A, Dai ML, Van Der Vegt F, Van Lent JWM, McBride R, Paulson JC, De Groot RJ, Van Kuppeveld FJM, et al. Kinetic analysis of the influenza A virus HA/NA balance reveals contribution of NA to virus-receptor binding and NA-dependent rolling on receptor-containing surfaces[J]. PLoS Pathogens, 2018, 14(8): e1007233. DOI:10.1371/journal.ppat.1007233 |
| [40] |
Maginnis MS. Virus-receptor interactions: the key to cellular invasion[J]. Journal of Molecular Biology, 2018, 430(17): 2590-2611. DOI:10.1016/j.jmb.2018.06.024 |
| [41] |
Uncapher CR, Dewitt CM, Colonno RJ. The major and minor group receptor families contain all but one human rhinovirus serotype[J]. Virology, 1991, 180(2): 814-817. DOI:10.1016/0042-6822(91)90098-V |
| [42] |
Liu Y, Sheng J, Baggen J, Meng G, Xiao C, Thibaut HJ, Van Kuppeveld FJM, Rossmann MG. Sialic acid-dependent cell entry of human Enterovirus D68[J]. Nature Communications, 2015, 6: 8865. DOI:10.1038/ncomms9865 |
| [43] |
Ströh LJ, Stehle T. Glycan engagement by viruses: receptor switches and specificity[J]. Annual Review of Virology, 2014, 1(1): 285-306. DOI:10.1146/annurev-virology-031413-085417 |
| [44] |
García B, Merayo-Lloves J, Martin C, Alcalde I, Quirós LM, Vazquez F. Surface proteoglycans as mediators in bacterial pathogens infections[J]. Frontiers in Microbiology, 2016, 7: 220. |
| [45] |
Cagno V, Tseligka ED, Jones ST, Tapparel C. Heparan sulfate proteoglycans and viral attachment: true receptors or adaptation bias?[J]. Viruses, 2019, 11(7): 596. DOI:10.3390/v11070596 |
| [46] |
Shukla D, Liu J, Blaiklock P, Shworak NW, Bai XM, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. A novel role for 3-O-sulfated heparan sulfate in Herpes simplex virus 1 entry[J]. Cell, 1999, 99(1): 13-22. DOI:10.1016/S0092-8674(00)80058-6 |
| [47] |
Smura T, Ylipaasto P, Klemola P, Kaijalainen S, Kyllönen L, Sordi V, Piemonti L, Roivainen M. Cellular tropism of human Enterovirus D species serotypes EV-94, EV-70, and EV-68 in vitro: implications for pathogenesis[J]. Journal of Medical Virology, 2010, 82(11): 1940-1949. DOI:10.1002/jmv.21894 |
| [48] |
Birkner K, Loos J, Gollan R, Steffen F, Wasser B, Ruck T, Meuth SG, Zipp F, Bittner S. Neuronal ICAM-5 plays a neuroprotective role in progressive neurodegeneration[J]. Frontiers in Neurology, 2019, 10: 205. DOI:10.3389/fneur.2019.00205 |
| [49] |
Royston L, Tapparel C. Rhinoviruses and respiratory Enteroviruses: not as simple as ABC[J]. Viruses, 2016, 8(1): 16. DOI:10.3390/v8010016 |
| [50] |
Shafren DR, Dorahy DJ, Greive SJ, Burns GF, Barry RD. Mouse cells expressing human intercellular adhesion molecule-1 are susceptible to infection by coxsackievirus A21[J]. Journal of Virology, 1997, 71(1): 785-789. DOI:10.1128/jvi.71.1.785-789.1997 |
| [51] |
Jiang YH, Liu SN, Shen SY, Guo HR, Huang HL, Wei W. Methyl-β-cyclodextrin inhibits EV-D68 virus entry by perturbing the accumulation of virus particles and ICAM-5 in lipid rafts[J]. Antiviral Research, 2020, 176: 104752. DOI:10.1016/j.antiviral.2020.104752 |
| [52] |
Sun SY, Bian LL, Gao F, Du RX, Hu YL, Fu Y, Su Y, Wu X, Mao QY, Liang ZL. A neonatal mouse model of Enterovirus D68 infection induces both interstitial pneumonia and acute flaccid myelitis[J]. Antiviral Research, 2019, 161: 108-115. DOI:10.1016/j.antiviral.2018.11.013 |
| [53] |
Hixon AM, Yu GX, Leser JS, Yagi S, Clarke P, Chiu CY, Tyler KL. A mouse model of paralytic myelitis caused by Enterovirus D68[J]. PLoS Pathogens, 2017, 13(2): e1006199. DOI:10.1371/journal.ppat.1006199 |
| [54] |
Imamura T, Okamoto M, Nakakita SI, Suzuki A, Saito M, Tamaki R, Lupisan S, Roy CN, Hiramatsu H, Sugawara KE, et al. Antigenic and receptor binding properties of Enterovirus 68[J]. Journal of Virology, 2014, 88(5): 2374-2384. DOI:10.1128/JVI.03070-13 |
| [55] |
Imamura T, Okamoto M, Oshitani H. Receptor-binding assays of Enterovirus D68[J]. Methods in Molecular Biology: Clifton, N J, 2020, 2132: 629-639. |
| [56] |
Zhang C, Xu C, Dai W, Wang Y, Liu Z, Zhang X, Wang X, Wang H, Gong S, Cong Y, et al. Functional and structural characterization of a two-MAb cocktail for delayed treatment of Enterovirus D68 infections[J]. Nature Communications, 2021, 12: 2904. DOI:10.1038/s41467-021-23199-5 |
 2022, Vol. 49
2022, Vol. 49