扩展功能
文章信息
- 毕贞, 武静, 欧阳志康, 张胜, 黄勇
- BI Zhen, WU Jing, OUYANG Zhikang, ZHANG Sheng, HUANG Yong
- 聚磷生物膜法磷回收研究进展
- Phosphorus recovery by polyphosphate-accumulating organisms biofilm system: a review
- 微生物学通报, 2022, 49(8): 3387-3400
- Microbiology China, 2022, 49(8): 3387-3400
- DOI: 10.13344/j.microbiol.china.211191
-
文章历史
- 收稿日期: 2021-12-15
- 接受日期: 2022-01-16
- 网络首发日期: 2022-02-08
2. 苏州科技大学城市污水资源化利用技术国家地方联合工程实验室, 江苏 苏州 215009
2. National and Local Joint Engineering Laboratory for Municipal Sewage Resource Utilization Technology, Suzhou University of Science and Technology, Suzhou 215009, Jiangsu, China
近年来,磷矿石价格的不断攀升成为推动磷回收技术研发与应用的主要推动力,许多国家已经将磷视为一种重要的战略资源。同时,氮、磷等营养元素排入水体所引发的环境问题日益严重,从污水中去除、回收磷成为了防治水体富营养化、实现污水全面资源化的核心环节之一[1-2]。在这种情势下,从污水中回收磷,发掘“第二磷矿”的理念已经在全球范围内成为研究热点。据测算,城市污水中的磷含量能满足人类15%−20%的磷需求[3],因此从城市污水中回收磷是缓解磷资源短缺与避免水体磷污染的有效途径之一。然而,城市污水中磷酸盐的浓度较低(约5−8 mg/L),不适合直接使用物理或化学等方法回收,现阶段国内外城市污水厂磷回收项目主要基于强化生物除磷(enhance biological phosphorus recovery,EBPR)工艺,以剩余污泥为对象,从污泥浓缩和发酵上清液中回收磷,属于侧流回收技术[4]。因此,欲将污水中含量仅为每升数毫克的磷酸盐富集至磷回收所需要的较高浓度水平,需浓缩大量剩余污泥方能实现,导致磷回收过程中污泥产量大。在该工艺中,活性污泥同时承担脱氮、除磷及有机物降解等多重功能,导致污水中的有机碳未被有效用于生物除磷,而是经其他生物化学过程被转化和消耗,因此,往往需要补充外源有机碳以保证微生物厌氧释磷。据文献[5]计算,EBPR工艺中每去除1 g磷伴随着约120 g有机碳消耗(以COD计)。此外,从富磷污泥中释放出的磷也十分有限(不超过污泥总磷含量的13.4%)[6],若不采用额外的提取措施(如酸浸法等),相当一部分的磷随干污泥外排而流失,致使EBPR系统的磷回收率较低,通常在40%−79%之间[6-7]。针对上述问题,有技术尝试从污泥灰分中进一步回收磷,以提高磷的回收率,但存在设备要求较高、提取液中磷与其他重金属难分离等诸多问题[8]。简而言之,EBPR的侧流磷回收工艺普遍存在有机碳消耗大、磷回收率低、回收工艺复杂、污泥产量大的问题,这增加了城市污水磷回收过程中的碳排放与能源消耗。
生物膜法磷回收的研究始于2013年[9],从原理来看与EBPR法相同,也是在好氧、厌氧交替环境下,利用聚磷生物膜的吸磷和释磷作用,实现磷酸盐的去除与富集并得到高浓度富磷溶液,但操作模式和富集方式明显不同:(1) 生物膜法在好氧条件下将磷酸盐从污水中转移至生物膜而得到去除,在厌氧环境下利用回收液接纳生物膜释放的磷;通过多次循环使用回收液得到浓度不断递增的富磷溶液;(2) 生物膜法无需浓缩剩余污泥,污水中被去除的磷直接进入回收液,可降低磷在回收过程中的损失,简化了回收步骤;(3) 生物膜系统更有利于泥龄较长的聚磷微生物生长,其他短泥龄的微生物被淘汰,因此只需提供满足聚磷微生物生长所需的碳源,可降低系统额外投加碳源的成本。与EBPR法相比,生物膜法具有碳源需求低、磷回收步骤简单、回收效率高、污泥产量低等潜力,与我国目前大力推行的绿色低碳经济发展目标相符。得到的高浓度磷回收液可进一步通过结晶法生成鸟粪石、蓝铁矿等磷产品,用作农业肥料和磷酸盐工业原料,进而实现污水中磷的资源化再利用。尽管生物膜法磷回收工艺起步较晚,目前尚处于实验室研究阶段,但从长远来看,生物膜法回收磷将有望成为实现污水资源化的重要手段之一。本文从聚磷微生物代谢特征、生物膜富集培养和磷回收效果等方面对生物膜法磷回收的研究进展进行梳理与总结,以期为生物膜法磷回收的理论研究和工艺开发提供参考。
1 聚磷生物膜内的主要功能微生物及其代谢特征不论EBPR系统还是生物膜系统,聚磷微生物都是由不同种类微生物组成的复杂生态系统,其中最受关注、研究最多的是聚磷菌(polyphosphate-accumulating organisms,PAOs)和聚糖菌(glycogen-accumulating organisms,GAOs)。这两类微生物在聚磷微生物群落中占比较大,在表观代谢特征上也具有很多相似性,对系统磷去除与回收效率影响显著。随着现代分子生物学技术的快速发展,对PAOs与GAOs的种属鉴定及其代谢特性研究取得了新的进展,部分早期的认识被更新或否定,对上述两类微生物的鉴定及代谢机理至今仍存在争议。
聚磷菌(PAOs)是一类能够从水中超量吸收、储存磷的微生物。早期最具代表性的描述PAOs代谢特征的是Mino模型[10-11]:厌氧环境下,PAOs将储存在胞内的聚磷(Poly-P)水解成可溶性的正磷酸盐(Pi),经磷酸转移蛋白向胞外释放;同时将胞内糖原(Gly)降解为丙酮酸以获取能量;水中的有机碳(以挥发性脂肪酸VFAs为主)通过主动运输进入细胞并合成聚羟基脂肪酸(PHAs)。好氧环境下,PAOs将胞内储存的PHAs分解以补充Gly含量,所产生的能量一部分用于从水中超量摄入Pi并合成Poly-P,另一部分则用于细胞增殖。在很长时期内,符合该上述全部特征的微生物才被认定为PAOs,其中以β-变形菌门红环菌科的Candidatus Accumulibacter (简称Accumulibacter)最具代表性[12]。这类微生物广泛存在于污水厂EBPR系统及实验室规模的生物除磷反应装置中,并一度被认为是对除磷贡献度最大的PAOs。此外,部分来自拟杆菌门、厚壁菌门和疣微菌门等的微生物也被认定为PAOs[13]。
近年来的研究发现,许多具有超量吸磷能力的微生物并不完全符合Mino模型所描述的全部特征,比如Tetrasphaera和Microlunatus能在厌氧条件下分解Poly-P释放Pi、好氧条件下吸收Pi,但它们并不利用VFAs合成PHAs,而是通过发酵代谢以糖和氨基酸为碳源[14],这类微生物被称作发酵型PAOs (fermentation PAOs,FPAOs)。在对丹麦和北美的十余个污水处理厂的研究中发现,由于实际污水中有机碳成分复杂且VFAs含量较低,Tetrasphaera、Dechloromonas和Ca. Obscuribacter等FPAOs的丰度远高于传统的Accumulibacter PAOs[15-17],是EBPR系统中对除磷贡献最大的聚磷微生物。Fernando等[18]首次应用Raman光谱技术证实污水处理厂EBPR系统中的Tetrasphaera FPAOs具有与Accumulibacter PAOs相当的除磷贡献。除了以氧气为电子受体,还有一类PAOs能以NO3–或NO2–等结合态氧为电子受体进行磷循环代谢,将反硝化作用与磷循环耦合,因此被称为反硝化聚磷菌(denitrifying PAOs,DPAOs)[19-20]。因此,凡是能够吸收Pi合成Poly-P并以此为能量物质在厌氧条件下摄取有机物的一类微生物均可被认定为是PAOs[21],其代谢特征被称为聚磷代谢(polyphosphate-accumulating metabolism,PAM)[22]。
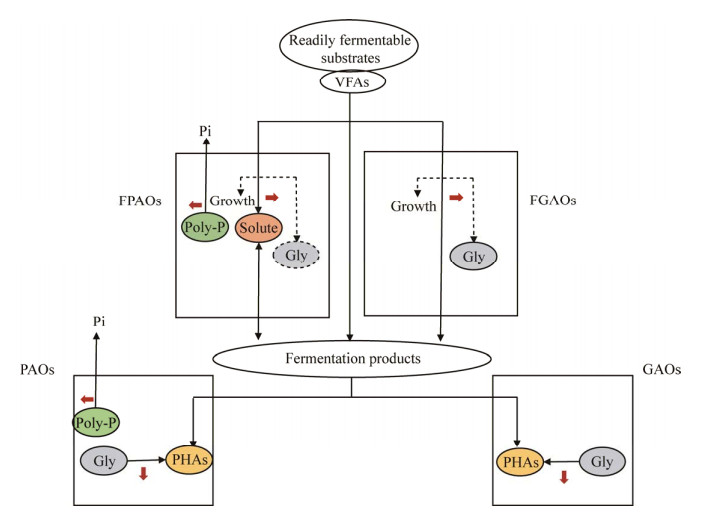
聚糖菌(glycogen-accumulating organisms,GAOs)在代谢特征上与PAOs存在相似之处,但最显著的区别在于GAOs在好氧条件下依赖分解糖原获取能量,因此并不合成聚磷[22],这一代谢特征被称为聚糖代谢(glycogenaccumulating metabolism,GAM)。目前研究最广泛的GAOs有两类,一类来自α变形菌门的Deflfluviicoccus,由cluster Ⅰ−cluster Ⅳ这4个进化分支组成;另一类是来自γ变形菌门的Ca. Competibacter,包括至少7个不同的进化分支[23]。此外,Micropruina[24]、Propionivibrio[25]、Ca. Contendobacter、Kineosphaera等也被鉴定为GAOs。先前的研究认为GAOs与PAOs竞争VFAs但对除磷无贡献,因此一度将系统除磷效果的恶化归因于GAOs的大量增殖[26]。然而新近的研究[27]表明,有些GAOs (如Micropruina)可利用氨基酸等发酵产物合成糖原,因此被称为发酵型GAOs (fermentative GAOs,FGAOs)。由于FGAOs并不与PAOs竞争VFAs,其存在并不会导致系统除磷效果的下降[14]。因此,GAOs的鉴定除了建立在其代谢特征基础上,还需要能够与PAOs竞争VFAs。研究者将上述新发现加入到传统Mino代谢模型中,提出了基于PAOs、FPAOs、GAOs和FGAOs的聚磷微生物群落代谢互作关系模型(图 1),这在很大程度上转变了过去对PAOs和GAOs竞争关系的认知[27]。
|
| 图 1 经典PAOs、GAOs与发酵型PAOs、GAOs之间的代谢互作关系 Figure 1 Proposed interactions between classical and fermentative PAOs and GAOs. |
|
|
长期以来,维持PAOs在聚磷微生物群落中的数量优势、抑制GAOs的过度生长被认为是系统具备良好除磷效果的必要条件,因此提出了以丙酸盐或乙酸盐/丙酸盐混合碳源作为VFAs[28]、温度≤25 ℃[29]、pH > 7.25[30]等控制条件;但近年的研究成果对上述观点提出了质疑。首先,以往研究认为有机碳含量过剩引起的高碳磷比环境会导致GAOs过度生长,对系统除磷不利;但Nielsen等的最新研究认为高碳磷比的影响仅对传统型PAOs与GAOs较显著,对FPAOs与FGAOs的影响尚不明确[27]。再者,在马来西亚[31]和新加坡[16, 32]的污水厂发现,即使温度常年在28−32 ℃之间,PAOs仍表现出竞争优势且系统除磷效果良好,这打破了以往认为生物除磷(磷回收)系统运行温度应低于25 ℃的观点。此外,研究发现某些PAOs (如Dechloromonas)在特定情况下其代谢模式会由PAM转为GAM[22],减少Pi的吸收,在这种情况下,虽然PAOs仍占据数量上的优势,但系统除磷效率显著下降[33-34]。该现象可能受PAOs胞内poly-P含量[35]及其中惰性聚磷(比如与Ca2+结合的聚磷)[36]占比的调控:当poly-P含量过低或惰性聚磷含量较高时,PAOs倾向于依靠糖原降解提供能量,代谢特征由PAM转为GAM。因此,系统除磷效果不仅与PAOs和GAOs的丰度有关,还与这两类微生物的代谢模式、代谢特征等因素有关,目前尚无证据表明GAOs的增殖一定会引发系统除磷效果的恶化[14, 16, 27]。
综上所述,现代分子生物技术的不断进步让人们对聚磷微生物群落结构及PAOs和GAOs的代谢特征有了更加深入、微观的认识;尤其重要的是,发现PAOs细胞内Poly-P、Gly和PHAs这三类能量物质的代谢并不总是同时发生的,这打破了以往对于PAOs代谢特征认识的局限性,引导我们发现新的PAOs。对于系统的磷回收效率而言,不仅取决于PAOs与GAOs的数量,同时与其物种类别、代谢特征等因素有关,而上述因素受碳源种类、碳磷比、温度和pH等诸多环境条件的共同调控。
2 聚磷生物膜富集培养和系统磷回收效能现状 2.1 聚磷生物膜富集培养富集培养吸、释磷能力强的聚磷生物膜是保证生物膜磷回收系统效能的必要条件。研究者们采用了不同的载体用于聚磷生物膜的培养,包括聚酯纤维载体[37]、碳素纤维载体[38]、石英砂[39]、尼龙悬挂载体[40]等。由于碳源、磷负荷、溶解氧等条件不同,所培养出的聚磷生物膜中优势菌差异显著,培养期为60–180 d,详见表 1。此外,反应器构造、曝气强度、搅拌强度、混合状态等水力条件也是影响生物膜吸磷、释磷能力的重要因素。目前,关于聚磷生物膜的富集培养条件尚未达成统一的认识,有待深入研究。
| 载体或填料材质 Carrier ir filler material |
填充比 Filling ratio (%) |
厌氧/好氧时长 Anaerobic aerobic(h/h) |
碳源Carbon source | 磷负荷 Phosphorus load(kg-PO43-(m3·d |
温度 Temperature(℃) |
溶解氧 Dissolved oxygen (mg/L) |
优势菌(属水平) Dominant bacteria (genus level) |
培养期 Cultication period(d) |
参考文献 References |
|||
| 种类 Type |
浓度 Concentration(mg/L) |
投加方式 Dosing mode |
负荷 Load (kg-COD/(m3·d |
|||||||||
| 聚酯纤维 Polyester |
26 | 2/4 | 乙酸钠Sodium acetate | 400 | 间歇 Intermittent |
0.30 | 0.008 | 22±2 | 8 | Pseudomonas | 24 | [37] |
| 碳素纤维 Carbon fiber |
30 | 3/6 | - | 350-400 | 连续 Continuous |
0.75-1.20 | 0.024-0.030 | 25 | 5 | Pseudomonas, Aeromonas, Bacillus |
80 | [38] |
| 尼龙 Nylon |
40 | 6/6 | 乙酸钠 Sodium acetate |
200 | 间歇 Intermittent |
0.60 | 0.030/0.090 | 20 | 4-7 | Acinetobacter, Pseudomonas, Rhodocyclus |
65 | [40] |
| 火山岩、石英砂 Colcanic rock, quartz sand |
85 | 3/5 | - | 180 | 间歇 Intermittent |
0.71 | 0.008 | 20-25 | 2-4 | - | 60 | [41] |
| 尼龙与聚丙烯 Nylon and polypropylene quartz sand |
40 | 6/6 | 乙酸钠 Sodium acetate |
200 | 间歇 Intermittent |
0.60 | 0.030 | 25±2 | 3 | - | 35 | [42] |
| 聚丙烯 Polypropylene |
40 | 4/4 | 乙酸钠 Sodium acetate |
6000 | 间歇 Intermittent |
0.75 | 0.004 | 20-25 | 3 | Zoogloea | 20 | [43] |
| 聚丙烯 Polypropylene |
40 | 3/3 | 乙酸钠 Sodium acetate |
200 | 间歇 Intermittent |
0.80 | 0.042 | - | 4-6 | Accumulibacter, Rhodocyclaceae |
26 | [44] |
| 注: —: 对应参考文献未明确提出 Note: — : The corresponding references are not clearly mentioned. | ||||||||||||
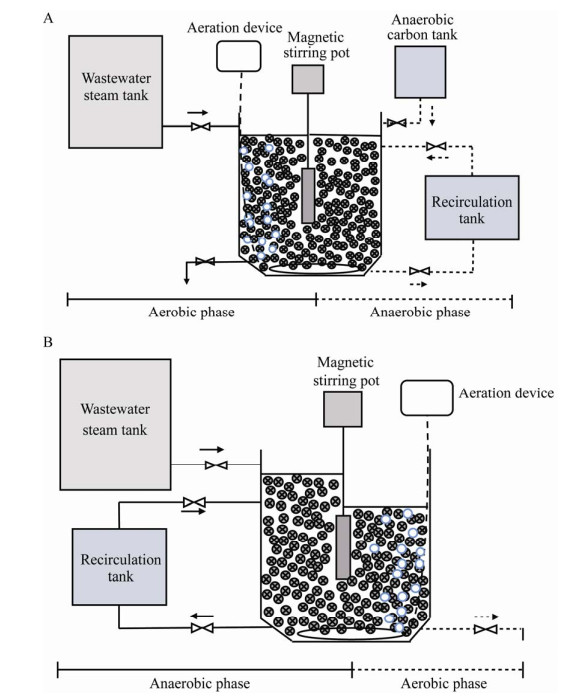
生物膜磷回收工艺的核心目标是获得高浓度磷回收液,现有研究中聚磷生物膜装置大多采用“先好氧-后厌氧”交替运行的模式(以图 2A为例):将污水引入到装有聚磷生物膜的反应器中,经好氧处理将磷从水相转移至生物膜后,将一部分处理后的水排出系统,其余部分收集于回收罐中作为回收液;在厌氧阶段将回收液引入反应器并投加碳源,使生物膜中的磷释放至回收液中,然后将回收液排入磷储罐。在一个回收周期内,生物膜反应器依此反复进行好氧-厌氧交替运行,而回收液被重复使用,直至其中的磷浓度达到预期后被更换,随即开始一个新回收周期。如此能将污水中5−15 mg/L的磷在回收液中富集至100 mg/L以上[9, 37, 39]。如前文所述,由于PAOs对外源有机碳的需求仅体现在厌氧阶段,其在好氧阶段的能量供给依靠胞内的PHAs和Gly;当采用该模式时,污水中的有机物首先在好氧段被其他异养细菌降解利用,而PAOs在厌氧段所需的碳源需额外补充。因此,这种“先好氧-后厌氧”运行模式更适合于处理有机碳被去除或被回收之后的污水。
|
| 图 2 生物膜法磷回收系统的工艺模式 Figure 2 Process modes of biofilm-based Pi recovery system. A:好氧-厌氧模式;B:厌氧-好氧模式 A: Aerobic-anaerobic mode; B: Anaerobic-aerobic mode. |
|
|
近年来,有研究者提出利用城市污水中有机碳进行生物膜法磷回收的设想,并构建与之相匹配的“先厌氧-后好氧”交替运行模式[45] (图 2B):将污水引入到主反应器中,利用污水中的有机碳进行厌氧释磷后,将一部分污水引入回收罐作为回收液,剩余污水经好氧吸磷处理后全部排出;此后按一定比例向主反应器泵入污水和磷回收液,交替进行厌氧、好氧处理。在一个回收周期内,回收液被重复使用,直至其中的磷浓度达到预期要求后被更换,随即开始一个新回收周期。与“先好氧-后厌氧”模式下运行的生物膜磷回收工艺相比,目前该模式下获得的磷回收液浓度为50–60 mg/L,明显偏低。现阶段,生物膜法磷回收相关研究中磷去除、富集效果及实验运行条件详见表 2。
| 磷去除率 Phosphorus removal rate (%) |
回收液磷浓度 Phosphorus concentration of recovery solution (mg/L) |
P/C比 P/C ratio |
模拟废水P浓度 Simulated wastewater P concentration (mg/L) |
好氧阶段 Aerobic stage |
厌氧阶段 Anaerobic stage |
参考文献 References |
|||
| DO (mg/L) |
HRT (h) |
COD浓度 COD concentration (mg/L) |
HRT (h) |
||||||
| 90 | 100 | 0.080 | 8 | 缺氧Anoxia | 2 | 400 | 2 | [9] | |
| 50 | 125 | 0.063 | 5 | 2.0 | 4 | 200 | 4 | [37] | |
| 95 | 91−133 | 0.113 | 10 | 2.0−2.5 | 3 | 800−1 600 | 3 | [39] | |
| 90 | 236 | 0.262 | 16 | 2.0−3.0 | 3 | 900 | 3 | [46] | |
| 60 | 90 | 0.255 | 8 | 缺氧Anoxia | 2 | 370 | 2 | [47] | |
| 95 | 180 | 0.320 | 5 | 6.0 | 3 | 200 | 3 | [48] | |
| 95 | 121 | − | 10 | 6.5 | 4 | 200 | 2 | [49] | |
| 70 | 129 | 0.260 | 16 | 2.0−4.0 | 3 | 1 377 | 3 | [50] | |
| 85 | 115 | 0.090 | 5 | 3.0 | 6 | 200 | 6 | [51] | |
| 85 | 63 | 0.040 | 5 | 3.0 | 6 | 200 | 6 | [52] | |
| 90 | 57 | − | 5 | 2.0−3.0 | 4 | 200 | 4 | [53] | |
| 注:−:对应参考文献未明确提出 Note: − : The corresponding references are not clearly mentioned. |
|||||||||
由表 2可见,尽管多数聚磷生物膜系统可将模拟废水中的低浓度磷回收,并获得浓度超过100 mg/L的富磷回收液,但不容忽视的问题是厌氧阶段有机碳消耗量大,这导致生物膜磷回收系统的P/C比(即每消耗单位质量有机碳所释放的磷,摩尔比)在0.04−0.32之间,远低于EBPR磷回收系统的0.48−0.80[46, 54-55]。碳源投加量大不但与城市污水全面资源化的目标相违背,也未发挥出生物膜法碳源需求低的潜能。与此同时,在追求高浓度磷回收液这一目标的驱使下,部分研究难以兼顾出水磷浓度达标的问题,磷去除率偏低。随后的研究开始关注如何以较低的碳源消耗代价获得高浓度磷回收液。Wong等[47]将好氧时长从4 h延长至10 h,在厌氧条件下投加370 mg/L-COD,得到90 mg/L的磷回收液,系统P/C比从0.07提高到0.26。值得关注的是,Zhang等[48]发现通过提高好氧段溶解氧水平可强化厌氧释磷能力,在厌氧投加200 mg/L-COD条件下,得到浓度为179.5 mg/L的磷回收液且出水磷浓度低于0.5 mg/L,系统P/C比达到0.32,这是目前生物膜法磷回收系统中的最高P/C比。我们认为通过改变溶解氧水平使得生物膜蓄磷量显著提高,是保证生物膜在较低碳源消耗量的条件下获得高浓度磷回收液的关键因素。杨婉静等[49]在厌氧外加200 mg/L-COD的条件下最终富集得到120.95 mg/L的富磷溶液,并提出应重视EPS在生物膜蓄磷水平方面的贡献。
综上所述,通过生物膜法富集回收污水中的磷,其可行性毋庸置疑;获得的回收液中磷浓度普遍大于100 mg/L,这完全满足磷产品再加工的回用标准(50 mg/L),因此,回收液可直接采用化学沉淀法、结晶法等用于鸟粪石、蓝铁矿等磷产品的加工,这也是生物膜法相较活性污泥法进行磷回收的突出优势之一。然而,如何强化系统的磷回收效能并兼顾出水达标排放、是否能够实现利用城市污水有机碳进行磷回收等问题仍是研究热点。
3 聚磷生物膜系统微生物及其代谢机理的研究方法与技术在EBPR系统中,聚磷微生物以悬浮态存在,而在生物膜系统中则以附着态存在。作为一种特殊的细胞聚集体,生物膜生态位中微生物的种群密度、空间分布及物种之间的相互作用关系与EBPR悬浮生态位中显著不同,这有可能使微生物表现出新的特性[56-57]。因此,对生物膜中多聚物成分、含量、物种组成、丰度和代谢特征等开展分析成为生物膜法磷回收研究的重要内容。
(1) 生物膜中多聚物的测定。厌氧/好氧循环条件下聚磷生物膜中PAOs和GAOs胞内聚合物的测定主要针对Poly-P、PHAs和Gly三类物质。Poly-P含量的测定主要有核磁共振法(31P-NMR)[48, 58]和化学染色-荧光原位杂交法(fluorescence in situ hybridization, FISH) [39, 46, 59],后者还可用于计算群落内PAOs细胞数量百分比。PHAs主要由4种单体组成,分别是聚β羟基丁酸酯(PHB)、聚β羟基戊酸酯(PHV)、聚β羟基-2-甲基丁酸酯(PH2MB)和聚β羟基-2-甲基戊酸酯(PH2MV),多数研究采用气相色谱内标法对以上单体物质进行定量分析。早期的多数研究认为PAOs中PHAs主要由PHB组成,其为最易被降解的PHAs单体,因此较高的PHB/PHAs意味着PAOs较高的代谢活性或占据竞争优势[60]。最新研究发现,Tetrasphaera PAOs则以合成PHV为主[61],因此不能简单地以PHAs的组分构成来判定PAOs或GAOs。最常见的Gly定量检测方法为硫酸-苯酚法酸化法(即水解葡萄糖当量法),其准确性得到普遍认可[27, 62],但也有少数研究者质疑该方法的准确性[18]。值得关注的是,近年来越来越多的研究者使用单细胞Raman光谱技术(single cell raman spectroscopy technologies,SCRS)[58-59],该方法可以快速、无损地对微生物群落中的特定物种(如PAOs、GAOs或PAOs的某一亚种)细胞内的poly-P、PHAs和Gly含量进行测定。
(2) 聚磷微生物群落物种组成及功能的多样性分析。FISH技术是发展较早、应用最广泛的PAOs量化技术。该方法利用DAPI荧光染料和特异性核苷酸探针,能有效识别和鉴定PAOs相关种属。在此基础上,结合放射性或稳定同位素标记底物及Raman光谱、MAR-FISH、nano-SIMS、Raman-FISH等技术[63]。由于FISH技术所使用的探针是依据PAOs的16S rRNA基因不同区段序列设计而成,因此对于未知菌种有着局限性[64]。随着16S rRNA基因高通量测序技术的发展,以微生物群落所有基因为对象的宏基因组技术可以更全面地反映微生物群落的遗传多样性和分子生态学信息,同时进行物种多样性和功能多样性分析[65-66]。He等[67]根据Accumulibacter clade IIA的全基因组信息设计引物,在转录水平研究了参与PAOs代谢途径的酶在不同环境因子下的表达差异,阐明了聚磷过程在不同环境因子下的相互关系。需要注意的是,FISH和16S rRNA基因这2种技术的分析结果存在偏差。Rubio-Rincón等[68]分别用这2种技术对同一反应器中PAOs的丰度进行了定量分析,FISH结果显示Accumulibacter PAOs占系统总生物量的61%,而16S rRNA基因技术结果显示该菌只占系统总生物量的2%。Fykse等[69]和Valverde-Pérez等[70]认为造成此类偏差的原因为:16S rRNA基因技术对低丰度微生物的DNA扩增效率远远小于高丰度微生物的DNA扩增效率,尤其是对丰度低于5%的微生物往往存在严重低估;同时,16S rRNA基因技术对DNA提取效率偏差也会影响到检测结果。
4 生物膜法磷回收应用前景展望在污水全面资源化的理念下,城市污水处理将成为资源循环利用的重要载体,以资源和能源回收为目标的城市污水处理厂已在欧洲、北美等地区成为现实。除水资源的循环利用外,有机物、磷等营养盐的回收势必成为未来污水厂的重要任务。伴随着城市污水厂中有机物回收技术(可以转化为能源)取得的进展,有机物回收后的磷去除与回收问题将成为城市污水厂面临的重大问题。在低碳源进水条件下,传统的悬浮生长系统中活性污泥的生长和生物除磷功能将受到严重的制约,侧流回收或富磷污泥/污泥上清液中磷回收也就难以实现,将对现行的EBPR工艺提出严峻的挑战。以资源全回收为发展方向的未来城市污水厂磷去除与回收技术应具备高效低耗、碳排放少、对进水有机物及营养物质需求量少或无、不产生对环境有危害作用的副产物等特征。作为众多磷回收工艺中的一种,生物膜法的研究尚处于研究起步阶段,但因其具有碳源需求低、污泥停留时间长、无需污泥回流、产泥量少、磷回收工艺简单等特征,在运行与投资成本竞争力方面颇具优势与发展潜力。依据现阶段的实验室小试研究,生物膜法可以将污水中每升数毫克的磷富集形成浓度超过200 mg/L的富磷溶液,然而这距离真正实现生物膜法污水主流磷回收的目标相距甚远。就现阶段污水厂普遍采用的同步生物脱氮除磷工艺,由于更侧重于脱氮,往往需采用后置化学除磷作为深度处理,而从化学除磷污泥中进行磷回收存在较大技术难度。因此,目前可考虑用生物膜磷回收技术替代化学除磷,对生物脱氮除磷工艺二级出水进行深度处理和磷回收。
| [1] |
Yuan ZG, Pratt S, Batstone DJ. Phosphorus recovery from wastewater through microbial processes[J]. Current Opinion in Biotechnology, 2012, 23(6): 878-883. DOI:10.1016/j.copbio.2012.08.001 |
| [2] |
Mayer BK, Baker LA, Boyer TH, Drechsel P, Gifford M, Hanjra MA, Parameswaran P, Stoltzfus J, Westerhoff P, Rittmann BE. Total value of phosphorus recovery[J]. Environmental Science & Technology, 2016, 50(13): 6606-6620. |
| [3] |
Mihelcic JR, Fry LM, Shaw R. Global potential of phosphorus recovery from human urine and feces[J]. Chemosphere, 2011, 84(6): 832-839. DOI:10.1016/j.chemosphere.2011.02.046 |
| [4] |
Valverde-Pérez B, Ramin E, Smets BF, Plósz BG. EBP2R-an innovative enhanced biological nutrient recovery activated sludge system to produce growth medium for green microalgae cultivation[J]. Water Research, 2015, 68: 821-830. DOI:10.1016/j.watres.2014.09.027 |
| [5] |
Batstone DJ, Hülsen T, Mehta CM, Keller J. Platforms for energy and nutrient recovery from domestic wastewater: a review[J]. Chemosphere, 2015, 140: 2-11. DOI:10.1016/j.chemosphere.2014.10.021 |
| [6] |
Martí N, Pastor L, Bouzas A, Ferrer J, Seco A. Phosphorus recovery by struvite crystallization in WWTPs: influence of the sludge treatment line operation[J]. Water Research, 2010, 44(7): 2371-2379. DOI:10.1016/j.watres.2009.12.043 |
| [7] |
Melia PM, Cundy AB, Sohi SP, Hooda PS, Busquets R. Trends in the recovery of phosphorus in bioavailable forms from wastewater[J]. Chemosphere, 2017, 186: 381-395. DOI:10.1016/j.chemosphere.2017.07.089 |
| [8] |
Vogel C, Adam C. Heavy metal removal from sewage sludge ash by thermochemical treatment with gaseous hydrochloric acid[J]. Environmental Science & Technology, 2011, 45(17): 7445-7450. |
| [9] |
Wong PY, Cheng KY, Kaksonen AH, Sutton DC, Ginige MP. A novel post denitrification configuration for phosphorus recovery using polyphosphate accumulating organisms[J]. Water Research, 2013, 47(17): 6488-6495. DOI:10.1016/j.watres.2013.08.023 |
| [10] |
Oehmen A, Carvalho G, Lopez-Vazquez CM, Van Loosdrecht MCM, Reis MAM. Incorporating microbial ecology into the metabolic modelling of polyphosphate accumulating organisms and glycogen accumulating organisms[J]. Water Research, 2010, 44(17): 4992-5004. DOI:10.1016/j.watres.2010.06.071 |
| [11] |
Mino T, Van Loosdrecht MCM, Heijnen JJ. Microbiology and biochemistry of the enhanced biological phosphate removal process[J]. Water Research, 1998, 32(11): 3193-3207. DOI:10.1016/S0043-1354(98)00129-8 |
| [12] |
Xu XH, Li X, Li SF, Ran ZL. Research development on the competition between PAO and GAO in enhanced biological phosphorus removal system[J]. Chemical Engineer, 2017, 31(1): 44-48, 43. (in Chinese) 许秀红, 李秀, 李绍峰, 冉治霖. 强化生物除磷系统中聚磷菌和聚糖菌的竞争研究进展[J]. 化学工程师, 2017, 31(1): 44-48, 43. |
| [13] |
Martin HG, Ivanova N, Kunin V, Blackall L, McMahon KD, Hugenholtz P. Genetic blueprints for enhanced biological phosphorus removal (EBPR) based on environmental shotgun sequencing[J]. Proceedings of the Water Environment Federation, 2006, 2006(13): 82-85. DOI:10.2175/193864706783710550 |
| [14] |
Stokholm-Bjerregaard M, McIlroy SJ, Nierychlo M, Karst SM, Albertsen M, Nielsen PH. A critical assessment of the microorganisms proposed to be important to enhanced biological phosphorus removal in full-scale wastewater treatment systems[J]. Frontiers in Microbiology, 2017, 8: 718. DOI:10.3389/fmicb.2017.00718 |
| [15] |
Mielczarek AT, Nguyen HTT, Nielsen JL, Nielsen PH. Population dynamics of bacteria involved in enhanced biological phosphorus removal in Danish wastewater treatment plants[J]. Water Research, 2013, 47(4): 1529-1544. DOI:10.1016/j.watres.2012.12.003 |
| [16] |
Qiu GL, Zuniga-Montanez R, Law Y, Thi SS, Nguyen TQN, Eganathan K, Liu XH, Nielsen PH, Williams RBH, Wuertz S. Polyphosphate-accumulating organisms in full-scale tropical wastewater treatment plants use diverse carbon sources[J]. Water Research, 2019, 149: 496-510. DOI:10.1016/j.watres.2018.11.011 |
| [17] |
Onnis-Hayden A, Srinivasan V, Tooker NB, Li GY, Wang DQ, Barnard JL, Bott C, Dombrowski P, Schauer P, Menniti A, et al. Survey of full-scale sidestream enhanced biological phosphorus removal (S2EBPR) systems and comparison with conventional EBPRs in North America: process stability, kinetics, and microbial populations[J]. Water Environment Research, 2020, 92(3): 403-417. DOI:10.1002/wer.1198 |
| [18] |
Fernando EY, McIlroy SJ, Nierychlo M, Herbst FA, Petriglieri F, Schmid MC, Wagner M, Nielsen JL, Nielsen PH. Resolving the individual contribution of key microbial populations to enhanced biological phosphorus removal with Raman-FISH[J]. The ISME Journal, 2019, 13(8): 1933-1946. DOI:10.1038/s41396-019-0399-7 |
| [19] |
Vieira A, Ribera-Guardia A, Marques R, Barreto Crespo MT, Oehmen A, Carvalho G. The link between the microbial ecology, gene expression, and biokinetics of denitrifying polyphosphate-accumulating systems under different electron acceptor combinations[J]. Applied Microbiology and Biotechnology, 2018, 102(15): 6725-6737. DOI:10.1007/s00253-018-9077-3 |
| [20] |
Marques R, Ribera-Guardia A, Santos J, Carvalho G, Reis MAM, Pijuan MT, Oehmen A. Denitrifying capabilities of Tetrasphaera and their contribution towards nitrous oxide production in enhanced biological phosphorus removal processes[J]. Water Research, 2018, 137: 262-272. DOI:10.1016/j.watres.2018.03.010 |
| [21] |
Seviour RJ, McIlroy S. The microbiology of phosphorus removal in activated sludge processes-the current state of play[J]. Journal of Microbiology: Seoul, Korea, 2008, 46(2): 115-124. |
| [22] |
Acevedo B, Oehmen A, Carvalho G, Seco A, Borrás L, Barat R. Metabolic shift of polyphosphateaccumulating organisms with different levels of polyphosphate storage[J]. Water Research, 2012, 46(6): 1889-1900. DOI:10.1016/j.watres.2012.01.003 |
| [23] |
Saunders AM, Oehmen A, Blackall LL, Yuan Z, Keller J. The effect of GAOs (glycogen accumulating organisms) on anaerobic carbon requirements in full-scale Australian EBPR (enhanced biological phosphorus removal) plants[J]. Water Science and Technology, 2003, 47(11): 37-43. DOI:10.2166/wst.2003.0584 |
| [24] |
Kong YH, Beer M, Seviour RJ, Lindrea KC, Rees GN. Structure and functional analysis of the microbial community in an aerobic: anaerobic sequencing batch reactor (SBR) with no phosphorus removal[J]. Systematic and Applied Microbiology, 2001, 24(4): 597-609. DOI:10.1078/0723-2020-00075 |
| [25] |
Albertsen M, McIlroy SJ, Stokholm-Bjerregaard M, Karst SM, Nielsen PH. "Candidatus Propionivibrio aalborgensis": a novel glycogen accumulating organism abundant in full-scale enhanced biological phosphorus removal plants[J]. Frontiers in Microbiology, 2016, 7: 1033. |
| [26] |
Oehmen A, Lemos PC, Carvalho G, Yuan ZG, Keller J, Blackall LL, Reis MAM. Advances in enhanced biological phosphorus removal: from micro to macro scale[J]. Water Research, 2007, 41(11): 2271-2300. DOI:10.1016/j.watres.2007.02.030 |
| [27] |
Nielsen PH, McIlroy SJ, Albertsen M, Nierychlo M. Re-evaluating the microbiology of the enhanced biological phosphorus removal process[J]. Current Opinion in Biotechnology, 2019, 57: 111-118. DOI:10.1016/j.copbio.2019.03.008 |
| [28] |
Wang YY, Jiang F, Zhang ZX, Xing MY, Lu ZB, Wu M, Yang J, Peng YZ. The long-term effect of carbon source on the competition between polyphosphorus accumulating organisms and glycogen accumulating organism in a continuous plug-flow anaerobic/aerobic (A/O) process[J]. Bioresource Technology, 2010, 101(1): 98-104. DOI:10.1016/j.biortech.2009.07.085 |
| [29] |
Erdal UG, Erdal ZK, Randall CW. The competition between PAOs (phosphorus accumulating organisms) and GAOs (glycogen accumulating organisms) in EBPR (enhanced biological phosphorus removal) systems at different temperatures and the effects on system performance[J]. Water Science and Technology, 2003, 47(11): 1-8. DOI:10.2166/wst.2003.0579 |
| [30] |
Filipe CD, Daigger GT, Grady CP Jr. Stoichiometry and kinetics of acetate uptake under anaerobic conditions by an enriched culture of phosphorus-accumulating organisms at different pHs[J]. Biotechnology and Bioengineering, 2001, 76(1): 32-43. DOI:10.1002/bit.1023 |
| [31] |
Ong YH, Chua ASM, Fukushima T, Ngoh GC, Shoji T, Michinaka A. High-temperature EBPR process: the performance, analysis of PAOs and GAOs and the fine-scale population study of Candidatus "Accumulibacter phosphatis"[J]. Water Research, 2014, 64: 102-112. DOI:10.1016/j.watres.2014.06.038 |
| [32] |
Cokro AA, Law Y, Williams RBH, Cao YS, Nielsen PH, Wuertz S. Non-denitrifying polyphosphate accumulating organisms obviate requirement for anaerobic condition[J]. Water Research, 2017, 111: 393-403. DOI:10.1016/j.watres.2017.01.006 |
| [33] |
Ahn J, Schroeder S, Beer M, McIlroy S, Bayly RC, May JW, Vasiliadis G, Seviour RJ. Ecology of the microbial community removing phosphate from wastewater under continuously aerobic conditions in a sequencing batch reactor[J]. Applied and Environmental Microbiology, 2007, 73(7): 2257-2270. DOI:10.1128/AEM.02080-06 |
| [34] |
McIlroy SJ, Albertsen M, Andresen EK, Saunders AM, Kristiansen R, Stokholm-Bjerregaard M, Nielsen KL, Nielsen PH. 'Candidatus Competibacter'-lineage genomes retrieved from metagenomes reveal functional metabolic diversity[J]. The ISME Journal, 2014, 8(3): 613-624. DOI:10.1038/ismej.2013.162 |
| [35] |
Zhou Y, Pijuan MT, Zeng RJ, Lu HB, Yuan ZG. Could polyphosphate-accumulating organisms (PAOs) be glycogen-accumulating organisms (GAOs)?[J]. Water Research, 2008, 42(10/11): 2361-2368. |
| [36] |
Barat R, Montoya T, Borras L, Ferrer J, Seco A. Calcium phosphate precipitation in a SBR operated for EBPR: interactions with the biological process[J]. Water Science and Technology, 2008, 58(2): 427-433. DOI:10.2166/wst.2008.404 |
| [37] |
Kodera H, Hatamoto M, Abe K, Kindaichi T, Ozaki N, Ohashi A. Phosphate recovery as concentrated solution from treated wastewater by a PAO-enriched biofilm reactor[J]. Water Research, 2013, 47(6): 2025-2032. DOI:10.1016/j.watres.2013.01.027 |
| [38] |
Li J, Xing XH, Wang BZ. Characteristics of phosphorus removal from wastewater by biofilm sequencing batch reactor (SBR)[J]. Biochemical Engineering Journal, 2003, 16(3): 279-285. DOI:10.1016/S1369-703X(03)00071-8 |
| [39] |
Tian Q, Ong SK, Xie XH, Li F, Zhu YB, Wang FR, Yang B. Enhanced phosphorus recovery and biofilm microbial community changes in an alternating anaerobic/aerobic biofilter[J]. Chemosphere, 2016, 144: 1797-1806. DOI:10.1016/j.chemosphere.2015.10.072 |
| [40] |
Meng X, Pan Y, Zhang H, Liao XH, Xu LJ, Feng X, Shan J. Microbial population dynamics during domestication and cultivation of biofilm to remove and enrich phosphate[J]. Environmental Science, 2018, 39(6): 2802-2809. (in Chinese) 孟璇, 潘杨, 章豪, 廖烜弘, 徐林建, 冯鑫, 单捷. 同步去除并富集磷酸盐生物膜驯化过程中微生物种群分析[J]. 环境科学, 2018, 39(6): 2802-2809. |
| [41] |
Wang KW, Zhang C, Qin HX, Huang N, Tian Q. The effect of combined packing on phosphorus removal in the alternating anaerobic/aerobic biofilter (AABF)[J]. Guangdong Chemical Industry, 2015, 42(15): 160-163. (in Chinese) 王康伟, 张成, 秦华星, 黄娜, 田晴. 组合填料厌氧/好氧生物滤池(AABF)除磷效果研究[J]. 广东化工, 2015, 42(15): 160-163. DOI:10.3969/j.issn.1007-1865.2015.15.081 |
| [42] |
Liao XH, Pan Y, Meng X, Xu LJ, Wei PL, Shan J. Acclimation process and performance of phosphorus accumulating biofilm in different filters[J]. Environmental Engineering, 2018, 36(12): 119-124. (in Chinese) 廖烜弘, 潘杨, 孟璇, 徐林建, 魏攀龙, 单捷. 不同填料下聚磷生物膜的驯化过程及性能[J]. 环境工程, 2018, 36(12): 119-124. |
| [43] |
You XY. Rapid culture method andperformance of suspended filler biofilmfacing to phosphate removal andenrichment[D]. Suzhou: Master's Thesis of Suzhou University of Science and Technology, 2019 (in Chinese) 尤星怡. 面向磷酸盐去除与富集的悬浮填料生物膜的快速培养方法及其性能[D]. 苏州: 苏州科技大学硕士学位论文, 2019 |
| [44] |
Zhang H, Gao BX, Pan Y, Feng X. Rapid start-up of phosphorus biofilm and its microbial community characteristics[J]. Acta Scientiae Circumstantiae, 2019, 39(10): 3317-3324. (in Chinese) 章豪, 高碧霄, 潘杨, 冯鑫. 聚磷生物膜的快速启动及微生物特性研究[J]. 环境科学学报, 2019, 39(10): 3317-3324. |
| [45] |
Bi Z, Huang Y, Pan Y, Li X. Method for removing and recovering phosphorus by utilizing organic carbon source in municipal sewage: CN111646573B[P]. 2021-11-23 (in Chinese) 毕贞, 黄勇, 潘杨, 李祥. 一种利用城市污水有机碳源的磷去除与回收方法: CN111646573B[P]. 2021-11-23 |
| [46] |
Tian Q, Zhuang LJ, Ong SK, Wang Q, Wang KW, Xie XH, Zhu YB, Li F. Phosphorus (P) recovery coupled with increasing influent ammonium facilitated intracellular carbon source storage and simultaneous aerobic phosphorus & nitrogen removal[J]. Water Research, 2017, 119: 267-275. DOI:10.1016/j.watres.2017.02.050 |
| [47] |
Wong PY, Cheng KY, Krishna KCB, Kaksonen AH, Sutton DC, Ginige MP. Improvement of carbon usage for phosphorus recovery in EBPR-r and the shift in microbial community[J]. Journal of Environmental Management, 2018, 218: 569-578. DOI:10.1016/j.jenvman.2018.03.130 |
| [48] |
Zhang H, Bi Z, Pan Y, Huang Y, Li DP, Shan J. Enhanced phosphorus storage in suspended biofilm by increasing dissolved oxygen[J]. Science of the Total Environment, 2020, 722: 137876. DOI:10.1016/j.scitotenv.2020.137876 |
| [49] |
Yang WJ, Pan Y, Chen Y, Zhang XY, Ni M. The role of extracellular polymer substances in simultaneous removal/enrichment of phosphate in biofilms system[J]. Acta Scientiae Circumstantiae, 2021, 41(9): 3437-3445. (in Chinese) 杨婉静, 潘杨, 陈越, 张星宇, 倪敏. 胞外聚合物在生物膜同步去除/富集磷酸盐系统中的作用[J]. 环境科学学报, 2021, 41(9): 3437-3445. |
| [50] |
Zhang C, Qin HX, Wang KW, Huang N, Tian Q. Effects of carbon source supplement patterns on phosphorus bio-accumulation and harvesting from an alternating anaerobic/aerobic biofilter[J]. Chinese Journal of Environmental Engineering, 2015, 9(8): 3602-3608. (in Chinese) 张成, 秦华星, 王康伟, 黄娜, 田晴. 补充碳源对交替式厌氧/好氧生物滤池生物蓄磷/回收磷的影响[J]. 环境工程学报, 2015, 9(8): 3602-3608. |
| [51] |
Shan J, Pan Y, Zhang H, Feng X. The anaerobic phosphorus release of biofilm-based phosphorus recovery process[J]. Acta Scientiae Circumstantiae, 2020, 40(8): 2749-2757. (in Chinese) 单捷, 潘杨, 章豪, 冯鑫. 基于生物膜法磷回收工艺厌氧释磷研究[J]. 环境科学学报, 2020, 40(8): 2749-2757. |
| [52] |
You XY, Feng X, Pan Y, Huang Y, Xu LJ. Effects of two phosphate growth environments on simultaneous removal and enrichment of phosphate[J]. Chinese Journal of Environmental Engineering, 2019, 13(10): 2426-2433. (in Chinese) 尤星怡, 冯鑫, 潘杨, 黄勇, 徐林建. 2种磷酸盐生长环境对同步去除与富集磷酸盐工艺的影响[J]. 环境工程学报, 2019, 13(10): 2426-2433. DOI:10.12030/j.cjee.201811193 |
| [53] |
Xu LJ, Pan Y, Zhang H, Feng X, Wei PL, You XY. Effects of aerobic carbon sources on biofilm with simultaneous phosphate removal and enrichment[J]. Environmental Science, 2019, 40(7): 3179-3185. (in Chinese) 徐林建, 潘杨, 章豪, 冯鑫, 魏攀龙, 尤星怡. 好氧段碳源浓度对同步去除和富集磷酸盐生物膜的影响[J]. 环境科学, 2019, 40(7): 3179-3185. |
| [54] |
Filipe CD, Daigger GT, Grady CP Jr. pH as a key factor in the competition between glycogen-accumulating organisms and phosphorus-accumulating organisms[J]. Water Environment Research, 2001, 73(2): 223-232. DOI:10.2175/106143001X139209 |
| [55] |
Smolders GJ, Klop JM, Van Loosdrecht MC, Heijnen JJ. A metabolic model of the biological phosphorus removal process: I. Effect of the sludge retention time[J]. Biotechnology and Bioengineering, 1995, 48(3): 222-233. DOI:10.1002/bit.260480309 |
| [56] |
Røder HL, Olsen NMC, Whiteley M, Burmølle M. Unravelling inter species interactions across heterogeneities in complex biofilm communities[J]. Environmental Microbiology, 2020, 22(1): 5-16. DOI:10.1111/1462-2920.14834 |
| [57] |
Xu RH, Zhang SQ, Meng FG. Large-sized planktonic bioaggregates possess high biofilm formation potentials: bacterial succession and assembly in the biofilm metacommunity[J]. Water Research, 2020, 170: 115307. DOI:10.1016/j.watres.2019.115307 |
| [58] |
Li YY, Cope HA, Rahman SM, Li GY, Nielsen PH, Elfick A, Gu AZ. Toward better understanding of EBPR systems via linking Raman-based phenotypic profiling with phylogenetic diversity[J]. Environmental Science & Technology, 2018, 52(15): 8596-8606. |
| [59] |
Majed N, Gu AZ. Phenotypic dynamics in polyphosphate and glycogen accumulating organisms in response to varying influent C/P ratios in EBPR systems[J]. Science of the Total Environment, 2020, 743: 140603. DOI:10.1016/j.scitotenv.2020.140603 |
| [60] |
Huang HJ, Wang SY, Wang ZW, Guo JH, Peng YZ. Effect of various types of carbon source on biochemical storage and substrate transformation of activated sludge[J]. CIESC Journal, 2010, 61(6): 1510-1515. (in Chinese) 黄惠珺, 王淑莹, 王中玮, 郭建华, 彭永臻. 不同碳源类型对活性污泥PHA贮存及转化的影响[J]. 化工学报, 2010, 61(6): 1510-1515. |
| [61] |
Close K, Marques R, Carvalho VCF, Freitas EB, Reis MAM, Carvalho G, Oehmen A. The storage compounds associated with Tetrasphaera PAO metabolism and the relationship between diversity and P removal[J]. Water Research, 2021, 204: 117621. DOI:10.1016/j.watres.2021.117621 |
| [62] |
Kristiansen R, Nguyen HTT, Saunders AM, Nielsen JL, Wimmer R, Le VQ, McIlroy SJ, Petrovski S, Seviour RJ, Calteau A, et al. A metabolic model for members of the genus Tetrasphaera involved in enhanced biological phosphorus removal[J]. The ISME Journal, 2013, 7(3): 543-554. DOI:10.1038/ismej.2012.136 |
| [63] |
Wagner M. Single-cell ecophysiology of microbes as revealed by Raman microspectroscopy or secondary ion mass spectrometry imaging[J]. Annual Review of Microbiology, 2009, 63: 411-429. DOI:10.1146/annurev.micro.091208.073233 |
| [64] |
Xu Y, Chen YB. Advances in accumulibacter research based on macrogenomics technique[J]. Journal of Microbiology, 2016, 36(2): 104-108. (in Chinese) 徐媛, 陈禹保. 基于宏基因组技术的聚磷菌研究进展[J]. 微生物学杂志, 2016, 36(2): 104-108. DOI:10.3969/j.issn.1005-7021.2016.02.018 |
| [65] |
Sun X, Zhu WJ, Wang L, Wu WX. Review on the main microorganisms and their metabolic mechanisms in enhanced biological phosphorus removal (EBPR) systems[J]. Chinese Journal of Applied Ecology, 2014, 25(3): 892-902. (in Chinese) 孙雪, 朱为静, 王亮, 吴伟祥. 强化生物除磷系统主要微生物及其代谢机理研究进展[J]. 应用生态学报, 2014, 25(3): 892-902. |
| [66] |
Song W. Discovery of novel Accumulibacter clades and the niche differentiation of different Accumulibacter groups[D]. Xiamen: Master's Thesis of Xiamen University, 2018 (in Chinese) 宋威. Accumulibacter新类群的发现及不同类群Accumulibacter的生态位分化研究[D]. 厦门: 厦门大学硕士学位论文, 2018 |
| [67] |
He SM, McMahon KD. 'Candidatus Accumulibacter' gene expression in response to dynamic EBPR conditions[J]. The ISME Journal, 2011, 5(2): 329-340. DOI:10.1038/ismej.2010.127 |
| [68] |
Rubio-Rincón FJ, Welles L, Lopez-Vazquez CM, Abbas B, Van Loosdrecht MCM, Brdjanovic D. Effect of lactate on the microbial community and process performance of an EBPR system[J]. Frontiers in Microbiology, 2019, 10: 125. DOI:10.3389/fmicb.2019.00125 |
| [69] |
Fykse EM, Aarskaug T, Madslien EH, Dybwad M. Microbial community structure in a full-scale anaerobic treatment plant during start-up and first year of operation revealed by high-throughput 16S rRNA gene amplicon sequencing[J]. Bioresource Technology, 2016, 222: 380-387. DOI:10.1016/j.biortech.2016.09.118 |
| [70] |
Valverde-Pérez B, Wágner DS, Lóránt B, Gülay A, Smets BF, Plósz BG. Short-sludge age EBPR processmicrobial and biochemical process characterisation during reactor start-up and operation[J]. Water Research, 2016, 104: 320-329. DOI:10.1016/j.watres.2016.08.026 |
 2022, Vol. 49
2022, Vol. 49