扩展功能
文章信息
- 黄国鑫, 王婧怡, 郑伊琳, 李庆南, 苏冀彦, 萧文鸾
- HUANG Guoxin, WANG Jingyi, ZHENG Yilin, LI Qingnan, SU Jiyan, HSIAO W.L. Wendy
- 硫酸盐还原菌与肠道疾病相关性的研究进展
- The association between sulfate-reducing bacteria and gut-related diseases: a review
- 微生物学通报, 2022, 49(7): 2789-2804
- Microbiology China, 2022, 49(7): 2789-2804
- DOI: 10.13344/j.microbiol.china.210979
-
文章历史
- 收稿日期: 2021-10-20
- 接受日期: 2022-01-20
- 网络首发日期: 2022-03-12
2. 汕头市中心医院药学部, 广东 汕头 515000;
3. 南方医科大学附属佛山妇幼保健院, 广东 佛山 528000;
4. 珠海澳科大科技研究院, 广东 珠海 519000
2. Department of Pharmacy, Shantou Central Hospital, Shantou 515000, Guangdong, China;
3. Foshan Women and Children Hospital, Southern Medical University, Foshan 528000, Guangdong, China;
4. Zhuhai MUST Science and Technology Research Institute, Zhuhai 519000, Guangdong, China
人体肠道内定殖着数量庞大的微生物,主要包括细菌、真菌与病毒,被统称为“肠道菌群”。人体中肠道微生物总量约为0.2 kg,数量约与人体细胞总数一致,但其基因组总量却是人体基因组总量的130倍[1-2]。肠道菌群通过与宿主肠道细胞、肠道免疫系统长期的相互作用,形成了“肠道菌群-宿主”共生关系,对宿主的健康具有重要影响。近年来,随着多组学技术的日益发展,肠道菌群组成结构及其生理功能得到进一步的阐述。现有研究表明,失调的肠道菌群结构与多种疾病的发生发展有着密切联系,特别是肠道疾病,如肠易激综合征(irritable bowel syndrome,IBS)、克罗恩病(Crohn’s disease,CD)、溃疡性结肠炎(ulcerative colitis,UC)和结直肠癌(colorectal cancer,CRC)[3-6]。
硫酸盐还原菌(sulfate-reducing bacteria,SRB)是一种厌氧的原核生物,也是世界上最古老的细菌之一,普遍存在于自然界各个环境中,如海水、水底沉积物、油井、天然气井、土壤和火山灰等。自然环境中SRB具有腐蚀金属和净化污水的作用,同时,SRB是唯一依赖无机硫盐来保存能量的微生物,它们具有异化硫酸盐的作用,可以将硫酸盐作为呼吸的终端电子受体,在产生硫化氢(H2S)的同时为自身生长繁殖提供能量;此外,SRB也可将亚硫酸盐、硫代硫酸盐等作为电子受体进而氧化肠道内有机物,产生能量以供自身生长繁殖需要[7]。自然界中现已被鉴定的SRB约有200种分布于60个属,主要包括变形菌纲的脱硫弧菌属(Desulfovibrio)、脱硫微菌属(Desulfomicrobium)、脱硫杆菌属(Desulfobacter)和脱硫叶菌属(Desulfobulbus);同时也包括脱硫肠状菌属(Desulfotomaculum)、古菌硫细菌属(Archaeoglobus)和热脱硫弧菌属(Thermodesulfovibrio)等[8]。定殖于宿主肠道中的SRB主要包括脱硫弧菌属和脱硫微菌属。在正常的生理条件下,定殖在肠道内的SRB维持在一定的丰度,通过代谢产生适量的H2S以维持宿主正常的生理需求[9]。H2S和一氧化氮(NO)是机体生理平衡的两类重要的气体传递介质和信号传导分子,具有多种生理作用,机体内H2S和NO的失衡会导致多种疾病的发生发展[10]。现有临床与临床前研究显示,在溃疡性结肠炎、克罗恩病及结直肠癌等肠道疾病下,粪便样本中往往被检测到高丰度的SRB[11-13],提示SRB与肠道疾病的发生发展具有密切的联系。然而,目前SRB并未同具核梭杆菌属(Fusobacteria)和幽门螺旋杆菌属(Helicobacteria)等胃肠道致病菌一样得到足够的重视。
近年来,针对SRB与肠道疾病相关性的研究进展综述少见报道。同时,对于SRB引起的肠道疾病的致病机制研究,特别是SRB对宿主免疫系统的影响,以及SRB代谢产物的活性研究仍有待进一步深入探讨。因此,本综述对现有SRB与肠道疾病相关性及其致病机制进行归纳总结,以期增强本领域科研工作者对SRB的关注。此外,本综述也对SRB后续可能的研究方向进行探讨,以深化对肠道疾病发生发展过程中SRB作用的认识,为本领域研究提供一定的依据和启示,也为SRB相关肠道疾病的治疗提供依据。
1 宿主肠道内SRB的分布与生理特征SRB作为肠道菌群的重要组成成员,在多种动物肠道内均可定殖,其中脱硫弧菌属是肠道内最主要的成员。报道显示该菌属在结肠SRB所占的比例约为65%[14]。研究也显示在小鼠1周龄时,其肠道内并未检测到SRB的存在,但在2−8周龄时可以检测到大量SRB的定殖[15]。在人体肠道内,脱硫弧菌属的相对丰度也随着年龄的变化而发生改变,研究发现老年人肠道内该菌属的相对丰度显著高于青少年[16]。一项针对23−53岁健康中、青年志愿者的研究显示,他们的粪便中均检测到SRB的定殖;虽然肠道内定殖的SRB在种属上存在明显的个体差异,但差异与年龄无关[17]。另外,在肠道的不同位置,SRB的分布种类也各不相同。脱硫肠状菌属主要分布于宿主的胃和小肠位置,而脱硫弧菌属则主要分布于肠道的末端位置,研究认为可能是由于胃肠道不同位置的pH值对SRB的定殖造成了影响[18]。此外,研究也显示,饮食结构也对SRB的生长有着重要影响,如单糖的摄入会显着促进Desulfovibrio piger的生长[19],而高脂的摄入则显著促进另一类SRB——沃氏嗜胆菌(Bilophila wadsworthia)的增殖[20]。
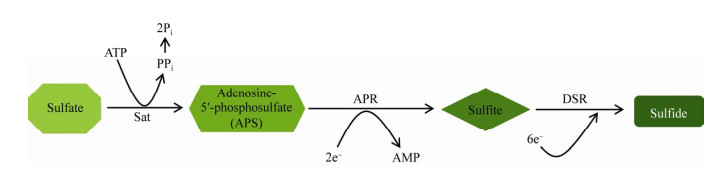
SRB通常无法直接将淀粉、纤维素、蛋白质等有机物质直接降解,而是需要其他的微生物将这些物质进行降解,并将其代谢产物作为底物进行利用以获取生长所需能量。肠道内SRB以有机物降解产生的氢气、乙醇、短链脂肪酸(short-chain fatty acids,SCFAs)和氨基酸等作为底物,将硫化物还原为H2S,同时获取该生化反应过程释放的能量作为自身生长所需的能量[14]。此过程中需要腺苷酰硫酸盐还原酶(adenosine-5′-phosphosulfate reductase,APR)和异化型亚硫酸盐还原酶(dissimilatory sulfate reductase,DSR)的参与;其中DSR可以催化亚硫酸盐还原为硫化物,是整个还原过程的关键环节[21-23],apr基因所编码的APR可以将腺苷酰硫酸盐还原为硫酸盐或者亚硫酸盐,为H2S的生成提供电子受体(图 1)。dsrAB基因编码的DSR可以催化亚硫酸盐的电子转移到硫化物上,该酶包括α和β两个亚基,其中dsrA为结合亚基,而dsrB为催化亚基;apr和dsrAB已被证明了是SRB的保守基因序列,常被用于SRB的标记与其定量研究[24-25]。
2 SRB与肠道疾病的相关性及其分布特征 2.1 SRB与炎症性肠病(inflammatory bowel disease,IBD)炎症性肠病是一种慢性复发性的肠道炎症性疾病,临床表型包括克罗恩病(Crohn’s disease,CD)和溃疡性结肠炎(ulcerative colitis,UC),其临床特征是肠道炎症、组织损伤、腹痛、持续性腹泻、体重减轻及直肠出血等[26]。目前IBD的发病机制尚不明确。一般认为,遗传、免疫失衡、药物、环境、压力应激等多个因素都与IBD的发生发展息息相关。近年来,随着肠道菌群的研究日益深化,累积的研究显示,肠道菌群组成结构的变化也是IBD发生发展的一个重要诱因[6, 27-28]。
如表 1所示,SRB与IBD的发生发展存在着紧密的联系。Loubinoux等在一项包括健康志愿者、IBD和其他消化道疾病患者的研究中发现,24%的健康志愿者、68%的IBD患者及34%的其他消化道疾病患者的粪便中均检测到SRB的存在,主要包括Desulfovibrio piger、D. desulfuricans和D. fairfieldensis,但D. piger的含量在IBD患者中显著高于其他消化道疾病患者和健康志愿者[11]。近期一项关于CD患者及其代谢产物相关性研究的结果显示,CD患者经自身造血干细胞移植治疗后,分别将治疗后CD缓解与复发患者的粪便样本转移到Il-10−/−小鼠,通过菌群-代谢相关性分析显示,Desulfovibrio、Escherichia和Shigella的丰度与小鼠炎症的发展呈正相关;而将患者粪便菌群移植到小鼠后的相关代谢组学分析结果则显示,该炎症小鼠肠道内明显出现硫代谢的失调,与临床CD患者的研究结果相一致,而Desulfovibrio正是分解硫基氨基酸并产生H2S的主要细菌之一[29]。
| Disease | The associated SRB | Data resource | Pathogenesis | References |
| IBD_CD | Desulfovibrio sp. | Il-10−/− mice model | The overgrowth of SRB induced dysbiosis of GM composition; immune dysfunction; abnormal sulfur metabolism | [29] |
| D. piger, D. desulfuricans, D. fairfieldensis |
Clinical data analysis | − | [11] | |
| IBD_UC | D. desulfuricans, D. vulgaris | Clinical data analysis | − | [30] |
| Desulfovibrio spp. | Clinical data analysis | − | [31] | |
| Bilophila wadsworthia, D. indonesiensis | Il-10–/– mice model TNBS-induced colitis mice model |
The overgrowth of SRB induced dysbiosis of GM composition; immune dysfunction; high concentration of H2S | [32] | |
| Desulfovibrio, Desulfomicrobium | Clinical data analysis | High concentration of H2S | [33] | |
| IBS | Desulfovibrio spp. | Clinical data analysis | − | [34-36] |
| Desulfovibrio spp. | Patients fecal transplanted rat | GM composition alteration destruction of the intestinal mucosa | [37] | |
| CLD | Desulfovibrio spp. | Clinical data analysis | − | [38-39] |
| Desulfovibrionaceae | Clinical data analysis | − | [40] | |
| CRC | D. vietnamensis, D. longreachensis, B. wadsworthia |
Clinical data analysis | − | [41] |
| D. desulfuricans | Clinical retrospective analysis | − | [42] | |
| Desulfovibrio spp., D. piger | Apcmin/+ mice model | GM composition alteration; destruction of the intestinal mucosa | [43-45] | |
| Desulfovibrio spp. | 1, 2-dimethylhydrazine induced CRC rat | GM composition alteration; destruction of the intestinal mucosa | [46] | |
| Desulfovibrio spp. | AOM/DSS induced CRC mice | GM composition alteration; abnormal DNA methylation | [47] | |
| 注:IBD:炎症性肠病;CD:克罗恩病;UC:溃疡性结肠炎;IBS:肠易激综合征;CLD:乳糜泻;CRC:结直肠癌;TNBS:2, 4, 6-三硝基苯磺酸;AOM:氧化偶氮甲烷;DSS:葡聚糖硫酸钠;GM:肠道菌群;−:不适用 Note: IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis; IBS: Irritable bowel syndrome; CLD: Celiac disease; CRC: Colorectal cancer; TNBS: 2, 4, 6-trinitro-benzenesulfonic acid; AOM: Azoxymethane; DSS: Dextran sodium sulfate; GM: Gut microbiota; −: Not applicable. |
||||
Rowan等收集1967−2008年间113篇与SRB相关的文献,对SRB呼吸、硫化氢代谢、黏液凝胶层组成及其与溃疡性结肠炎的相关性进行了分析,证明了SRB是导致UC发生的一个重要环境因素[48]。该团队后续研究从UC病人肠道内发现2株与UC相关的菌种D. desulfuricans和D. vulgaris,其在肠道黏液层的丰度显著高于健康志愿者[30]。另外也有报道,与处于非急性期的UC患者相比较,急性期的UC患者肠道内的SRB显著升高[49]。陈坤关于UC患者治疗前后及健康志愿者的肠道菌群定量比较中发现,UC患者治疗前SRB的丰度显著高于治疗后和健康人;而治疗后UC患者肠道内SRB的数量与健康人相比无统计学差异[31]。在基础研究部分,相关的实验证明高脂饮食会导致Il-10−/−小鼠肠道内沃氏嗜胆菌的富集,进而诱导严重结肠炎的出现[20];另一研究将Desulfovibrio indonesiensis和来自肠炎患者的SRB分别移植到小鼠肠道后,发现小鼠的肠道正常生理结构均发生了变化,而来自肠炎患者的SRB对肠道结构影响更加明显;该研究同时观察到,D. indonesiensis可以显著加重三硝基苯磺酸诱导的小鼠肠炎[32]。近期一项基于健康志愿者与UC病人的肠道菌群组成结构分析显示,UC患者与健康者粪便中均检测出SRB,但UC患者SRB的相对丰度显著高于健康志愿者;健康志愿者脱硫弧菌属: 脱硫微菌属约为93:7,而在UC患者中,这一比例约为99:1;该研究对其代谢产物的分析则显示,UC患者粪便中的H2S浓度更高,而SRB产生的乙酸盐与H2S在导致肠炎上具有协同作用[33]。
2.2 SRB与肠易激综合征(irritable bowel syndrome,IBS)肠易激综合征是一种常见的肠道功能失调疾病,目前其具体发病原因尚未清晰。IBS在临床上可以分为3种亚型,即腹泻型IBS、便秘型IBS和混合型IBS[50-51]。早期关于IBS的相关研究中,采用腹部X光片照射发现IBS患者肠道气体的体积明显高于正常对照组[52]。其他研究也显示,IBS患者肠道内氢气的量大约是健康者的2倍,而通过饮食的调整可以减少肠道气体产生并减轻IBS的症状,这可能与肠道内耗氢菌的丰度改变有关,其丰度与活性可能是IBS发病的一个重要因素[34];近期的一项报道发现腹泻型IBS患者呼出的H2S比健康志愿者显著增多,表明H2S是IBS关键诱导因子,并认为H2S可以作为一种腹泻型IBS非侵入性的检测指标[53]。Chassard等后续研究显示,与健康人相比,IBS患者肠道内SRB的水平明显高于健康志愿者,而且随着SRB的定殖,耗氢菌的丰度也相对增加;进一步研究证明,与正常人相比,便秘型IBS患者肠道内SRB的量是正常人的10–100倍[35],但在腹泻型IBS粪便菌群中并未发现可能导致IBS的具核梭杆菌和艰难梭菌的富集[36]。另外,一项关于IBS患者和健康志愿者的粪便菌群移植研究发现,IBS患者粪便菌群移植的大鼠肠道内SRB含量显著高于接受健康志愿者粪便菌群移植的大鼠[37]。
2.3 SRB与乳糜泻(celiac disease,CLD)乳糜泻是一种常见的慢性自身免疫性肠道疾病,主要是由于麸质(gluten)的摄入造成肠道免疫失稳而引起。目前CLD并无比较有效的治疗手段,关键是避免含有麸质食物的摄入。CLD的发生常伴有肠道粘膜和肠道生理屏障的破坏、肠道上皮细胞通透性增强及肠道菌群结构发生变化[38, 54]。目前一些报道也认为SRB与CLD的进展存在一定的相关性。Collado等采用荧光原位杂交的方法测定了相同年龄段的正常孩童与CLD孩童的粪便菌群结构,结果显示包括SRB在内的多个肠道菌群菌属在CLD患者的粪便中显著增加[38]。针对CLD孩童患者十二指肠肠道菌群结构的研究也显示,CLD患者活检样本肠道菌群中SRB的丰度高于正常孩童[39]。近期一项比较CLD患者和多重自身免疫(poly-autoimmunity) CLD患者肠道菌群结构差异的研究显示,SRB与多重自身免疫CLD患者的疾病进程正相关,并且认为SRB可以作为判断多重自身免疫CLD患者的一个潜在重要指标[40]。
2.4 SRB与结直肠癌(colorectal cancer,CRC)结直肠癌也是一类常见的肠道恶性疾病,在多种癌症中其发病率和致死率都位居前五。近10年来的统计资料显示,我国CRC的发病率逐年增加,而且其发病人群在国内外的研究资料均显示具有年轻化的特点[55-56]。由于早期CRC的临床症状并不明显,患者往往错失最佳的治疗时间。目前,提倡定期进行肠镜检查以预防或早期介入CRC的进展。然而在CRC的致病机制研究中,除了遗传基因突变外,肠道菌群结构的改变也是CRC发病率显著提高的重要诱因,已发现具核梭杆菌、幽门螺旋杆菌及产肠毒素脆弱拟杆菌(enterotoxigenic Bacteroides fragilis)等均与CRC的发生发展息息相关[57-59]。现有相关研究认为SRB也是一类重要的促进CRC进展的微生物。Yachida等发现与健康志愿者相比,II期和III期CRC患者的粪便样本内SRB丰度显著增加[41]。近期一项基于临床病例的回顾性分析则表明,唾液中D. desulfuricans的水平可以作为预测CRC进展的一个指标[42]。在自发性结直肠癌(Apcmin/+)小鼠肠道内,未产生息肉前SRB的含量与健康小鼠相等;但是随着Apcmin/+小鼠肠道内息肉的形成,肠道内SRB的丰度也随着息肉数量的增加而增加;同时,实验发现当通过药物干预减少息肉数量后,肠道内SRB的相对丰度及调控H2S生成的dsrA基因所编码酶的表达也随之减少甚至消失[43-45]。一项基于1, 2-二甲基肼诱导CRC大鼠肠道菌群结构变化的研究也显示,与正常大鼠相比,CRC大鼠肠道内Desulfovibrio的相对丰度显著提高[46]。在AOM/DSS诱导CRC小鼠肠道内也检测到Desulfovibrio相对丰度的显著提高,而使用黑树莓花青素进行预防性给药后,可降低Desulfovibrio相对丰度的同时减缓CRC的进展[47]。另外一些研究认为,SRB促进CRC进展的主要原因是SRB的代谢终产物H2S可以破坏DNA结构、激活炎症与癌症信号通路、干扰肠道细胞内丁酸氧化并破坏结肠上皮屏障功能,并能诱导粘膜过度增殖、分化、凋亡和恶性癌变等[33, 60-62]。
3 SRB的致病机制研究进展 3.1 SRB过度生长破坏肠道共生菌群的平衡肠道共生菌维持在一定比例以保持宿主肠道微生态的平衡,这对于维持宿主的健康十分重要。然而,由于年龄、饮食、免疫失调及药物等的影响,常导致某些肠道菌群,特别是潜在致病菌的过度生长,进而破坏肠道微环境的稳定,引起或促进疾病的进程。SRB与甲烷菌都是肠道内氢气利用菌,能够利用肠道内发酵产生的氢气而维持肠道微环境的稳定,保证机体健康。报道显示SRB与甲烷菌对肠道内氢气利用有明显的竞争关系[63]。在肠道内甲烷菌大量生长的环境下,SRB水平较低甚至检测不到;而临床志愿者增加摄入含有硫酸盐的饮食后,粪便中产甲烷菌数量减少而SRB数量显著增加[64-65]。因此,当SRB过度生长时会在一定程度上抑制包括甲烷菌在内的其他共生菌群的生长。另一方面,由于乙酸是SRB代谢过程中主要的电子供体之一,同时SRB的最终产物H2S会影响结肠细胞对丁酸的摄取,在一定程度上也必然影响肠道内短链脂肪酸生成菌的生长和代谢。相关的研究均显示,在SRB过度生长的肠道疾病患者中,与SCFAs生成相关的肠菌,包括双歧杆菌属、柔嫩梭菌(Faecalibacterium prausnitzii)、丁酸梭菌(Clostridium butyricum)、Butyricicoccus pullicaecorum和Eubacterium rectale等菌的相对丰度均显著降低[66-70]。此外,由于共生肠道菌群的生长需要合适的pH环境,宿主的结直肠一般维持在弱酸性到中性,然而肠道环境的酸化可在肠道相关疾病中被检测到[71-72],这在一定程度上可能也与菌群结构平衡的破坏及其代谢产物累积相关。
3.2 SRB自身产生内毒素脂多糖(lipopolysaccharides,LPS)SRB属于革兰氏阴性菌,其细菌外壁上有脂质与多糖构成的脂多糖物质。LPS是一种内毒素,当细菌死亡后,LPS从细菌外壁释放,并与宿主细胞表面受体相结合,通过信号通路的传导影响宿主细胞功能,进而导致或加重肠道的炎症进程。Wei等对SRB分离纯化的LPS进行化学结构解析,结果显示,脱硫弧菌科(Desulfovibrionaceae)产生的LPS及其变异体与肠杆菌科(Enterobacteriaceae)有相似的脂质结构,而且均可通过单核细胞刺激炎症细胞因子的释放,说明来源于SRB的LPS同样具有显著的促炎活性[73]。Weglarz等将从D. desulfuricans、大肠杆菌(Escherichia coli)和沙门氏菌(Salmonella minnesota)中分离得到的LPS处理人外周血单核细胞后,发现来自D. desulfuricans的LPS诱导细胞产生致炎因子的作用最强[74]。LPS也可以和先天性免疫细胞表面的Toll-like受体4结合,导致免疫表型发生广泛的转录和翻译重编程,进而引起肠道内促炎细胞因子的释放[75]。也有报道显示,LPS激活的血清补体(complements)能引起内皮细胞的损伤,并诱导内皮细胞表达大量黏附分子进而引发细胞凋亡[76]。由于肠道固有层存在许多微血管,LPS也可通过该途径对肠道正常生理结构造成破坏。然而对儿童持续性CD的血清研究中也发现,LPS与CD的发展呈正相关;而且在三硝基苯磺酸(trinitrobenzene sulfonic acid,TNBS)诱导的持续性肠炎小鼠模型中也发现血清中LPS结合蛋白的浓度显著增加,证明了LPS信号通路在肠道炎症中的重要作用[77]。另一项临床研究也显示,与正常志愿者相比,LPS水平在CD病人中显著增加;随着疾病严重程度的增加,LPS可以比正常水平高出2–6倍,因此有研究认为可将LPS作为判断疾病发生发展的一个生物学指标[78]。
3.3 SRB代谢产物H2S破坏肠壁上皮屏障的正常功能SRB通过自身硫酸盐还原酶的作用获取H2S生成过程中释放的能量,并将其作为自身生长繁殖的能量来源。研究显示,代谢过程中所产生的H2S在小鼠肠道的浓度为0.2−1.0 mmol/L,在人体肠道内为3−4 mmol/L[79];在正常生理浓度下,H2S可以上调环氧合酶2的表达,并下调前列腺素2的表达,同时抑制与NF-κB炎症信号通路相关的趋化因子和细胞因子的表达[9, 80]。也有研究报道显示,低浓度的H2S可以干扰中性粒细胞在组织损伤条件下的聚集和浸润,降低细胞凋亡的发生,表现出抗炎的活性[81-82]。
高浓度的H2S聚集在肠道内会对肠道的正常生理结构造成严重的破坏,同时也被认为是SRB导致一系列肠道疾病发生的重要原因。研究认为,SRB过度生长代谢过程中产生的硫化物可以影响肠上皮细胞粘液分子间的二硫键,促进粘液的溶解,驱动粘蛋白变性,进而破坏肠道上皮膜的正常生理屏障功能,导致潜在的致病菌侵袭肠道上皮细胞;研究显示,在UC患者的标本中发现83%的患者结肠黏膜有细菌侵袭的现象,而CD患者中56%的回肠标本和25%的结肠标本存在细菌感染的现象,但在健康对照组的肠道组织中则未发现细菌侵袭的现象[83-84]。此外,一项针对大鼠结肠细胞线粒体的研究显示,在H2S的供体硫氢化钠(NaHS)的作用下,大鼠结肠细胞线粒体的呼吸作用会被显著抑制,同时iNOS及IL-6等炎症相关因子的表达上调,进而导致结肠细胞炎症信号通路的激活[85];另一报道也显示,结肠细胞线粒体功能的改变会破坏肠道上皮屏障的完整性[86]。
短链脂肪酸特别是丁酸在维持肠道健康方面具有重要的作用。一方面,丁酸可以作为肠道上皮细胞的能量来源被摄取并促进上皮细胞的正常更新;另一方面,丁酸可以通过调节肠道免疫系统稳态抑制肠道相关炎症与癌症通路的激活,进而保证肠道上皮细胞生理功能的完整[67, 69]。然而研究显示,H2S通过抑制酰基CoA脱氢酶(acyl-CoA dehydrogenase)的活性,使脂肪酸β-氧化无法继续进行,进而抑制小鼠结肠细胞的丁酸盐摄入[87-88]。深圳华大基因公司对345例中国人样本进行深度测序后发现,肠道硫酸盐还原菌代谢过程中产生的H2S气体能抑制丁酸盐的氧化,进而对肠上皮细胞造成毒害作用[89-90]。Hulin等的体外研究结果也显示H2S能抑制丁酸盐的氧化,进而对肠上皮细胞造成毒害作用[91]。此外,也有研究证明H2S可以激活p38-MAPK和NF-κB信号通路,进而加速炎症和癌症的进程[84, 92]。
3.4 SRB代谢产物H2S导致DNA损伤及细胞凋亡近年来,研究表明H2S、NO和CO均为人体中重要的气体传递介质和信号传导分子[93-94]。H2S在组织中的浓度约为30−100 μmol/L,然而研究也指出,当体内H2S浓度过高时会对细胞和器官造成不同程度的损伤,特别是导致细胞凋亡和DNA的破坏;体外实验表明,当DNA修复受到抑制时,利用Comet assay检测硫化物浓度为250 μmol/L时会导致显著的DNA损伤;这些数据表明,H2S可能导致基因组不稳定或结直肠癌相关基因的累积突变[95]。另外,也有研究表明,H2S在较低的浓度下也能诱导DNA损伤反应,如将Ca9-22牙龈鳞状细胞在含5 ng/mL H2S的条件下培养24 h后会显著减低DNA的合成,减少视网膜母细胞瘤蛋白磷酸化,同时激活p21蛋白表达[96]。此外,以50 ng/mL H2S处理口腔角质细胞干细胞24 h和48 h后,可以显著增加细胞凋亡及其相关凋亡因子如caspase 3、caspase 9、CHK2、Bcl-2和SIRT3的蛋白水平[97]。其他研究结果也表明,H2S浓度为1 µmol/L时即有破坏DNA双链结构的作用[60, 62]。
Coutinho等将D. indonesiensis与人源上皮细胞进行共培养,发现D. indonesiensis会引起HCT8人源上皮细胞的凋亡,而且当大肠杆菌存在时D. indonesiensis诱导上皮细胞凋亡的作用被显著增强;同时,来源于UC病人的SRB也会引起上皮细胞凋亡,但来源于健康志愿者的SRB则无明显的细胞凋亡现象发生[98]。细胞凋亡现象的发生可能与SRB产生的H2S有关。Sakuma等用GYY4137 (一种缓慢释放的H2S供体)探究H2S对Caco-2细胞的影响,发现H2S可以使Caco-2细胞阻滞在S-G2/M期并诱导细胞凋亡和坏死[99]。Baskar等也发现,H2S导致细胞周期阻滞在G1期,并诱导p53引起细胞凋亡[62]。
4 总结与展望肠道作为宿主体内最主要的菌群栖息地,肠道菌群对于宿主健康的维持无疑具有重要的作用。现有的大量研究结果均表明,宿主心血管系统、生殖系统、神经系统、内分泌系统及消化系统等正常生理功能的发挥与以肠道菌群为中心的肠道微环境稳态密不可分,而包括胃肠道疾病的多种疾病则往往伴随着肠道微生态的紊乱,尤其是IBD、IBS、CLD和CRC的发生发展更与菌群结构及其代谢功能的失衡息息相关。
SRB作为共生肠道菌群的组成成员之一,在维持肠道微环境的平衡过程中有其不可或缺的作用,但在不同肠道疾病中均检测到SRB的过度增长。以往的研究表明,SRB是肠道内一种潜在的致病菌,在肠道疾病进程中具有重要作用。因此,对于SRB的生理病理作用及其相关代谢产物进行更深入的研究,可以为包括CRC在内的多种肠道疾病的发生发展机制和预防治疗提供有意义的科学证据。
目前对于SRB的致病机制研究大多聚焦在其代谢产物H2S。现已证明H2S可以破坏肠道正常生理屏障作用,从而导致肠道上皮细胞更多地暴露在潜在致病菌中,活化与肠道炎症和癌症相关的信号通路,以及抑制肠道上皮细胞摄取SCFAs的能力从而诱导上皮细胞的凋亡等。虽然部分报道显示,H2S在宿主心血管系统和生殖、呼吸系统健康中具有重要意义,而且在一定浓度范围内也有抑制肠道炎症并可促进结肠上皮细胞线粒体维持正常生理功能的作用[82, 100-101];同时宿主自身也具有将H2S通过甲基化的形式转换为无毒性硫代硫酸盐的能力[102]。因此,部分学者对H2S在肠道中的毒性作用持一定的相反态度。我们认同,适量浓度的H2S对于宿主是有益的,而过度SRB的生长及高浓度H2S的累积可引起肠道菌群结构改变,进而导致肠道内环境的失衡,使肠道上皮细胞更多暴露于致病因子中,同时宿主对H2S的正常运输和解毒作用也受到严重的干扰。此外,H2S作为一种气体性传递介质,过量的生成和累积也会对包括肠道在内的多种组织器官造成破坏。
根据现有的报道,SRB实际上对甲烷菌、乳酸菌和SCFAs生成菌的生长均具有明显的抑制作用;反之,甲烷菌和乳酸菌的生长也在一定程度上会抑制SRB的生长,乳酸菌已被认为是可以有效减缓肠道炎症进程的肠道益生菌[103]。以往许多关于SRB致病机制的研究多集中在探讨其代谢终产物H2S对宿主的毒性作用而引起疾病的发生发展上,忽略了SRB的过度生长对于整个肠道菌群结构的改变,以及在肠道菌群结构发生改变后宿主肠道内免疫稳态与代谢产物发生的变化。因此,后续研究工作应该重视肠道内与SRB存在竞争与互生关系的菌群的生长变化,对于探讨与肠道疾病相关的标志性菌群的变化具有一定的科学意义,也可为SRB失衡引起的疾病的治疗提供重要信息。此外,SRB是否与幽门螺旋杆菌和具核梭杆菌一样通过特异性的结合位点直接激活肠道上皮的炎症与癌症相关通路,甚至对上皮细胞的基因突变产生影响,还需要更进一步的研究与探讨。
最后,针对SRB的致病机理,我们希望能够综合现有的数据与结果,为寻找治疗与SRB相关肠道疾病的方案提供一定的依据,如IBD、IBS和CRC等。传统的治疗方案主要包括运用抗炎、抗癌和免疫疗法进行干预,如柳氮磺胺吡啶、地塞米松、硫代嘌呤、环孢素A及环磷酰胺等。近年来,肠道微生态的研究为上述疾病的预防与治疗开拓了新思路与新手段。相关的研究显示,采取粪便移植或通过特定菌群移植是一类治疗肠道微生态失衡的新型潜在疗法。如利用双歧杆菌、乳酸菌,柔嫩梭菌和丁酸梭菌等,可以有效治疗或辅助治疗上述肠道疾病[104-106]。一项荟萃分析(Meta-analysis)也显示,通过提高肠道内乳酸菌的含量可以有效抑制SRB的生长进而减缓IBD的进程[103]。此外,运用具有益生元作用,包括来源于中草药的提取物等用于治疗SRB引起的肠道疾病也有相关的报道。在与SRB有关的小鼠模型研究中发现,使用源于中草药的灵芝多糖、绞股蓝总皂苷及其有效单体人参皂苷Rb3和Rd等,可以有效抑制肠道内SRB的生长,进而达到抑制CRC进程的作用[43-45],这些发现也为SRB引发的肠道疾病提供了另一种可行的治疗方案。另外,有研究也指出硫化物可以经化学作用破坏肠道黏膜屏障[84],上述研究一方面阐明H2S导致肠道疾病的途径,另一方面也延伸出新的治疗思路,即通过强化或保护肠道黏膜屏障作为治疗IBD等相关肠道疾病的另一选择。上述相关研究成果表明进一步深化了SRB及H2S致病机理研究的重要性及迫切性。
| [1] |
Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans[J]. Cell, 2016, 164(3): 337-340. DOI:10.1016/j.cell.2016.01.013 |
| [2] |
Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body[J]. PLoS Biology, 2016, 14(8): e1002533. DOI:10.1371/journal.pbio.1002533 |
| [3] |
Rubinstein MR, Baik JE, Lagana SM, Han RP, Raab WJ, Sahoo D, Dalerba P, Wang TC, Han YW. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator annexin A1[J]. EMBO Reports, 2019, 20(4): e47638. |
| [4] |
Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer[J]. Nature Reviews Immunology, 2021, 21(10): 653-667. DOI:10.1038/s41577-021-00534-x |
| [5] |
Zhang T, Ji XH, Lu GC, Zhang FM. The potential of Akkermansia muciniphila in inflammatory bowel disease[J]. Applied Microbiology and Biotechnology, 2021, 105(14/15): 5785-5794. |
| [6] |
Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, et al. The gut microbiota and host health: a new clinical frontier[J]. Gut, 2016, 65(2): 330-339. DOI:10.1136/gutjnl-2015-309990 |
| [7] |
Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed[J]. Physiological Reviews, 2012, 92(2): 791-896. DOI:10.1152/physrev.00017.2011 |
| [8] |
Barton LL, Fauque GD. Biochemistry, physiology and biotechnology of sulfate-reducing bacteria[J]. Advances in Applied Microbiology, 2009, 68: 41-98. |
| [9] |
Blachier F, De Sá Resende A, Da Silva Fogaça Leite G, Vasques Da Costa A, Lancha AH Jr. Colon epithelial cells luminal environment and physiopathological consequences: impact of nutrition and exercise[J]. Nutrire, 2018, 43: 2. DOI:10.1186/s41110-018-0061-6 |
| [10] |
Kolluru GK, Shen XG, Kevil CG. A tale of two gases: NO and H2S, foes or friends for life?[J]. Redox Biology, 2013, 1(1): 313-318. DOI:10.1016/j.redox.2013.05.001 |
| [11] |
Loubinoux J, Bronowicki JP, Pereira IAC, Mougenel JL, Le Faou AE. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases[J]. FEMS Microbiology Ecology, 2002, 40(2): 107-112. DOI:10.1111/j.1574-6941.2002.tb00942.x |
| [12] |
Scanlan PD, Shanahan F, Marchesi JR. Culture-independent analysis of desulfovibrios in the human distal colon of healthy, colorectal cancer and polypectomized individuals[J]. FEMS Microbiology Ecology, 2009, 69(2): 213-221. DOI:10.1111/j.1574-6941.2009.00709.x |
| [13] |
Pitcher MC, Beatty ER, Cummings JH. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis[J]. Gut, 2000, 46(1): 64-72. DOI:10.1136/gut.46.1.64 |
| [14] |
Willis CL, Cummings JH, Neale G, Gibson GR. Nutritional aspects of dissimilatory sulfate reduction in the human large intestine[J]. Current Microbiology, 1997, 35(5): 294-298. DOI:10.1007/s002849900257 |
| [15] |
Deplancke B, Hristova KR, Oakley HA, McCracken VJ, Aminov R, Mackie RI, Gaskins HR. Molecular ecological analysis of the succession and diversity of sulfate-reducing bacteria in the mouse gastrointestinal tract[J]. Applied and Environmental Microbiology, 2000, 66(5): 2166-2174. DOI:10.1128/AEM.66.5.2166-2174.2000 |
| [16] |
Fite A, MacFarlane GT, Cummings JH, Hopkins MJ, Kong SC, Furrie E, MacFarlane S. Identification and quantitation of mucosal and faecal desulfovibrios using real time polymerase chain reaction[J]. Gut, 2004, 53(4): 523-529. DOI:10.1136/gut.2003.031245 |
| [17] |
Feng Z, Li M, Long WM, Pang XY. Sulfate reducing bacteria and butyrate producing bacteria in gut of healthy adults show gender differences[J]. Genomics and Applied Biology, 2015, 34(9): 1842-1847. (in Chinese) 冯舟, 李敏, 龙文敏, 庞小燕. 健康成年人肠道硫酸盐还原菌和丁酸盐产生菌的性别差异[J]. 基因组学与应用生物学, 2015, 34(9): 1842-1847. |
| [18] |
Kengen SW, De Bok FA, Van Loo ND, Dijkema C, Stams AJ, De Vos WM. Evidence for the operation of a novel Embden-Meyerhof pathway that involves ADP-dependent kinases during sugar fermentation by Pyrococcus furiosus[J]. Journal of Biological Chemistry, 1994, 269(26): 17537-17541. DOI:10.1016/S0021-9258(17)32474-2 |
| [19] |
Rey FE, Gonzalez MD, Cheng JY, Wu M, Ahern PP, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium[J]. PNAS, 2013, 110(33): 13582-13587. DOI:10.1073/pnas.1312524110 |
| [20] |
Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice[J]. Nature, 2012, 487(7405): 104-108. DOI:10.1038/nature11225 |
| [21] |
Bradley AS, Leavitt WD, Johnston DT. Revisiting the dissimilatory sulfate reduction pathway[J]. Geobiology, 2011, 9(5): 446-457. DOI:10.1111/j.1472-4669.2011.00292.x |
| [22] |
Geets J, Borremans B, Diels L, Springael D, Vangronsveld J, Van Der Lelie D, Vanbroekhoven K. DsrB gene-based DGGE for community and diversity surveys of sulfate-reducing bacteria[J]. Journal of Microbiological Methods, 2006, 66(2): 194-205. DOI:10.1016/j.mimet.2005.11.002 |
| [23] |
Klein M, Friedrich M, Roger AJ, Hugenholtz P, Fishbain S, Abicht H, Blackall LL, Stahl DA, Wagner M. Multiple lateral transfers of dissimilatory sulfite reductase genes between major lineages of sulfate-reducing prokaryotes[J]. Journal of Bacteriology, 2001, 183(20): 6028-6035. DOI:10.1128/JB.183.20.6028-6035.2001 |
| [24] |
Meyer B, Kuever J. Molecular analysis of the diversity of sulfate-reducing and sulfur-oxidizing prokaryotes in the environment, using aprA as functional marker gene[J]. Applied and Environmental Microbiology, 2007, 73(23): 7664-7679. DOI:10.1128/AEM.01272-07 |
| [25] |
Dar SA, Yao L, Van Dongen U, Kuenen JG, Muyzer G. Analysis of diversity and activity of sulfate-reducing bacterial communities in sulfidogenic bioreactors using 16S rRNA and dsrB genes as molecular markers[J]. Applied and Environmental Microbiology, 2007, 73(2): 594-604. DOI:10.1128/AEM.01875-06 |
| [26] |
Hall AB, Yassour M, Sauk J, Garner A, Jiang X, Arthur T, Lagoudas GK, Vatanen T, Fornelos N, Wilson R, et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients[J]. Genome Medicine, 2017, 9(1): 103. DOI:10.1186/s13073-017-0490-5 |
| [27] |
Khan I, Ullah N, Zha LJ, Bai YR, Khan A, Zhao T, Che TJ, Zhang CJ. Alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD treatment targeting the gut microbiome[J]. Pathogens, 2019, 8(3): 126. DOI:10.3390/pathogens8030126 |
| [28] |
Sun MM, Wu W, Liu ZJ, Cong YZ. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases[J]. Journal of Gastroenterology, 2017, 52(1): 1-8. DOI:10.1007/s00535-016-1242-9 |
| [29] |
Metwaly A, Dunkel A, Waldschmitt N, Raj ACD, Lagkouvardos I, Corraliza AM, Mayorgas A, Martinez-Medina M, Reiter S, Schloter M, et al. Integrated microbiota and metabolite profiles link Crohn's disease to sulfur metabolism[J]. Nature Communications, 2020, 11: 4322. DOI:10.1038/s41467-020-17956-1 |
| [30] |
Rowan F, Docherty NG, Murphy M, Murphy TB, Coffey JC, O'Connell PR. Bacterial colonization of colonic crypt mucous gel and disease activity in ulcerative colitis[J]. Annals of Surgery, 2010, 252(5): 869-875. DOI:10.1097/SLA.0b013e3181fdc54c |
| [31] |
Chen K. Analysis of sulfate-reducing bacteria in the gut of ulcerative colitis patients[D]. Shanghai: Master's Thesis of Shanghai Jiao Tong University, 2014 (in Chinese) 陈坤. 溃疡性结肠炎患者肠道硫酸盐还原菌的检测[D]. 上海: 上海交通大学硕士学位论文, 2014 |
| [32] |
Figliuolo VR, Dos Santos LM, Abalo A, Nanini H, Santos A, Brittes NM, Bernardazzi C, De Souza HSP, Vieira LQ, Coutinho-Silva R, et al. Sulfate-reducing bacteria stimulate gut immune responses and contribute to inflammation in experimental colitis[J]. Life Sciences, 2017, 189: 29-38. DOI:10.1016/j.lfs.2017.09.014 |
| [33] |
Kushkevych I, Dordević D, Vítězová M. Possible synergy effect of hydrogen sulfide and acetate produced by sulfate-reducing bacteria on inflammatory bowel disease development[J]. Journal of Advanced Research, 2021, 27: 71-78. DOI:10.1016/j.jare.2020.03.007 |
| [34] |
King T, Elia M, Hunter J. Abnormal colonic fermentation in irritable bowel syndrome[J]. The Lancet, 1998, 352(9135): 1187-1189. DOI:10.1016/S0140-6736(98)02146-1 |
| [35] |
Chassard C, Dapoigny MC, Scott K, Del'Homme C, Dubray C, Eschalier A, Flint HJ, Bernalier-Donadille A. The intestinal microbiota of irritable bowel syndrome patients is characterized by functional dysbiosis[J]. Gastroenterology, 2009, 136(5): A-214. |
| [36] |
Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR[J]. The American Journal of Gastroenterology, 2005, 100(2): 373-382. DOI:10.1111/j.1572-0241.2005.40312.x |
| [37] |
Crouzet L, Gaultier E, Del'Homme C, Cartier C, Delmas E, Dapoigny M, Fioramonti J, Bernalier- Donadille A. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota[J]. Neurogastroenterology and Motility: the Official Journal of the European Gastrointestinal Motility Society, 2013, 25(4): e272-e282. DOI:10.1111/nmo.12103 |
| [38] |
Collado M, Calabuig M, Sanz Y. Differences between the fecal microbiota of coeliac infants and healthy controls[J]. Current Issues in Intestinal Microbiology, 2007, 8: 9-14. |
| [39] |
Nadal I, Donant E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease[J]. Journal of Medical Microbiology, 2007, 56(Pt 12): 1669-1674. |
| [40] |
Bibbò S, Abbondio M, Sau R, Tanca A, Pira G, Errigo A, Manetti R, Pes GM, Dore MP, Uzzau S. Fecal microbiota signatures in celiac disease patients with poly-autoimmunity[J]. Frontiers in Cellular and Infection Microbiology, 2020, 10: 349. DOI:10.3389/fcimb.2020.00349 |
| [41] |
Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, Watanabe H, Masuda K, Nishimoto Y, Kubo M, et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer[J]. Nature Medicine, 2019, 25(6): 968-976. DOI:10.1038/s41591-019-0458-7 |
| [42] |
Wang Y, Zhang Y, Wang Z, Tang J, Cao DX, Qian Y, Xie YH, Chen HY, Chen YX, Chen ZF, et al. A clinical nomogram incorporating salivary Desulfovibrio desulfuricans level and oral hygiene index for predicting colorectal cancer[J]. Annals of Translational Medicine, 2021, 9(9): 754. DOI:10.21037/atm-20-8168 |
| [43] |
Chen L, Brar MS, Leung FCC, Hsiao WL. Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate-reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in Apcmin/+ mice[J]. Oncotarget, 2016, 7(21): 31226-31242. DOI:10.18632/oncotarget.8886 |
| [44] |
Khan I, Huang GX, Li XA, Liao WL, Leong WK, Xia WR, Bian XQ, Wu JL, Hsiao WLW. Mushroom polysaccharides and Jiaogulan saponins exert cancer preventive effects by shaping the gut microbiota and microenvironment in Apcmin/+ mice[J]. Pharmacological Research, 2019, 148: 104448. DOI:10.1016/j.phrs.2019.104448 |
| [45] |
Huang G, Khan I, Li X, Chen L, Leong W, Ho LT, Hsiao WLW. Ginsenosides Rb3 and Rd reduce polyps formation while reinstate the dysbiotic gut microbiota and the intestinal microenvironment in Apcmin/+ mice[J]. Scientific Reports, 2017, 7: 12552. DOI:10.1038/s41598-017-12644-5 |
| [46] |
Zhu QC, Jin ZM, Wu W, Gao RY, Guo BM, Gao ZG, Yang YZ, Qin HL. Analysis of the intestinal lumen microbiota in an animal model of colorectal cancer[J]. PLoS One, 2014, 9(6): e90849. |
| [47] |
Chen LL, Jiang BW, Zhong CG, Guo J, Zhang LH, Mu T, Zhang QH, Bi XL. Chemoprevention of colorectal cancer by black raspberry anthocyanins involved the modulation of gut microbiota and SFRP2 demethylation[J]. Carcinogenesis, 2018, 39(3): 471-481. DOI:10.1093/carcin/bgy009 |
| [48] |
Rowan FE, Docherty NG, Coffey JC, O'Connell PR. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis[J]. British Journal of Surgery, 2009, 96(2): 151-158. DOI:10.1002/bjs.6454 |
| [49] |
Zinkevich V, Beech IB. Screening of sulfate-reducing bacteria in colonoscopy samples from healthy and colitic human gut mucosa[J]. FEMS Microbiology Ecology, 2000, 34(2): 147-155. DOI:10.1111/j.1574-6941.2000.tb00764.x |
| [50] |
Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain[J]. Gut, 1999, 45(Suppl 2): II43-II47. |
| [51] |
Yu HB, Li AP, Dai L. Status of treatment for different subtypes of irritable bowel syndrome[J]. Journal of Gastroenterology and Hepatology, 2014, 6(23): 609-617. (in Chinese) 于洪波, 李爱萍, 戴林. 肠易激综合征的治疗现状及研究进展[J]. 胃肠病学和肝脏病学杂志, 2014, 6(23): 609-617. |
| [52] |
Koide A, Yamaguchi T, Odaka T, Koyama H, Tsuyuguchi T, Kitahara H, Ohto M, Saisho H. Quantitative analysis of bowel gas using plain abdominal radiograph in patients with irritable bowel syndrome[J]. The American Journal of Gastroenterology, 2000, 95(7): 1735-1741. DOI:10.1111/j.1572-0241.2000.02189.x |
| [53] |
Banik GD, De A, Som S, Jana S, Daschakraborty SB, Chaudhuri S, Pradhan M. Hydrogen sulphide in exhaled breath: a potential biomarker for small intestinal bacterial overgrowth in IBS[J]. Journal of Breath Research, 2016, 10(2): 026010. DOI:10.1088/1752-7155/10/2/026010 |
| [54] |
De Sousa Moraes LF, Grzeskowiak LM, De Sales Teixeira TF, Gouveia Peluzio M. Intestinal microbiota and probiotics in celiac disease[J]. Clinical Microbiology Reviews, 2014, 27(3): 482-489. DOI:10.1128/CMR.00106-13 |
| [55] |
Burnett-Hartman AN, Lee JK, Demb J, Gupta S. An update on the epidemiology, molecular characterization, diagnosis, and screening strategies for early-onset colorectal cancer[J]. Gastroenterology, 2021, 160(4): 1041-1049. DOI:10.1053/j.gastro.2020.12.068 |
| [56] |
Hur J, Otegbeye E, Joh HK, Nimptsch K, Ng K, Ogino S, Meyerhardt JA, Chan AT, Willett WC, Wu KN, et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women[J]. Gut, 2021, 70(12): 2330-2336. DOI:10.1136/gutjnl-2020-323450 |
| [57] |
Sobhani I, Bergsten E, Couffin S, Amiot A, Nebbad B, Barau C, De'Angelis N, Rabot S, Canoui-Poitrine F, Mestivier D, et al. Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures[J]. PNAS, 2019, 116(48): 24285-24295. DOI:10.1073/pnas.1912129116 |
| [58] |
Gagnière J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E, Bringer MA, Pezet D, Bonnet M. Gut microbiota imbalance and colorectal cancer[J]. World Journal of Gastroenterology, 2016, 22(2): 501-518. DOI:10.3748/wjg.v22.i2.501 |
| [59] |
Cover TL, Blaser MJ. Helicobacter pylori in health and disease[J]. Gastroenterology, 2009, 136(6): 1863-1873. DOI:10.1053/j.gastro.2009.01.073 |
| [60] |
Attene-Ramos MS, Wagner ED, Gaskins HR, Plewa MJ. Hydrogen sulfide induces direct radical-associated DNA damage[J]. Molecular Cancer Research, 2007, 5(5): 455-459. DOI:10.1158/1541-7786.MCR-06-0439 |
| [61] |
Wu DD, Si WR, Wang MJ, Lyu SY, Ji AL, Li YZ. Hydrogen sulfide in cancer: friend or foe?[J]. Nitric Oxide, 2015, 50: 38-45. DOI:10.1016/j.niox.2015.08.004 |
| [62] |
Baskar R, Li L, Moore PK. Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells[J]. The FASEB Journal, 2007, 21(1): 247-255. DOI:10.1096/fj.06-6255com |
| [63] |
Gibson GR, Cummings JH, MacFarlane GT. Competition for hydrogen between sulphate-reducing bacteria and methanogenic bacteria from the human large intestine[J]. The Journal of Applied Bacteriology, 1988, 65(3): 241-247. DOI:10.1111/j.1365-2672.1988.tb01891.x |
| [64] |
Thauer RK. Anaerobic oxidation of methane with sulfate: on the reversibility of the reactions that are catalyzed by enzymes also involved in methanogenesis from CO2[J]. Current Opinion in Microbiology, 2011, 14(3): 292-299. DOI:10.1016/j.mib.2011.03.003 |
| [65] |
Christl SU, Gibson GR, Cummings JH. Role of dietary sulphate in the regulation of methanogenesis in the human large intestine[J]. Gut, 1992, 33(9): 1234-1238. DOI:10.1136/gut.33.9.1234 |
| [66] |
Fite A, MacFarlane S, Furrie E, Bahrami B, Cummings JH, Steinke DT, MacFarlane GT. Longitudinal analyses of gut mucosal microbiotas in ulcerative colitis in relation to patient age and disease severity and duration[J]. Journal of Clinical Microbiology, 2013, 51(3): 849-856. DOI:10.1128/JCM.02574-12 |
| [67] |
Kumari R, Ahuja V, Paul J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of north India[J]. World Journal of Gastroenterology, 2013, 19(22): 3404-3414. DOI:10.3748/wjg.v19.i22.3404 |
| [68] |
Kabeerdoss J, Sankaran V, Pugazhendhi S, Ramakrishna BS. Clostridium leptum group bacteria abundance and diversity in the fecal microbiota of patients with inflammatory bowel disease: a case-control study in India[J]. BMC Gastroenterology, 2013, 13: 20. DOI:10.1186/1471-230X-13-20 |
| [69] |
Varela E, Manichanh C, Gallart M, Torrejón A, Borruel N, Casellas F, Guarner F, Antolin M. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis[J]. Alimentary Pharmacology & Therapeutics, 2013, 38(2): 151-161. |
| [70] |
Swidsinski A, Loening-Baucke V, Vaneechoutte M, Doerffel Y. Active Crohn's disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora[J]. Inflammatory Bowel Diseases, 2008, 14(2): 147-161. DOI:10.1002/ibd.20330 |
| [71] |
Nugent SG, Kumar D, Rampton DS, Evans DF. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs[J]. Gut, 2001, 48(4): 571-577. DOI:10.1136/gut.48.4.571 |
| [72] |
Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry[J]. Cancer Research, 2009, 69(11): 4918-4925. DOI:10.1158/0008-5472.CAN-08-4806 |
| [73] |
Wei ZS, Augusto LA, Zhao LP, Caroff M. Desulfovibrio desulfuricans isolates from the gut of a single individual: structural and biological lipid A characterization[J]. FEBS Letters, 2015, 589(1): 165-171. DOI:10.1016/j.febslet.2014.11.042 |
| [74] |
Weglarz L, Parfiniewicz B, Mertas A, Kondera-Anasz Z, Jaworska-Kik M, Dzierzewicz Z, Światkowska L. Effect of endotoxins isolated from Desulfovibrio desulfuricans soil and intestinal strain on the secretion of TNF-α by human mononuclear cells[J]. Polish Journal of Environmental Studies, 2006, 15(4): 615-622. |
| [75] |
Frosali S, Pagliari D, Gambassi G, Landolfi R, Pandolfi F, Cianci R. How the intricate interaction among toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology[J]. Journal of Immunology Research, 2015, 2015: 489821. |
| [76] |
Shen LX, Li M, Sun QY, Shi JS. Adhesion molecule release and apoptosis of endothelial cell induced by lipopolysaccharide-activated complement[J]. Chinese Pharmacological Bulletin, 2011, 27(9): 1245-1249. (in Chinese) 沈良贤, 李敏, 孙黔云, 石京山. 脂多糖激活补体诱导内皮细胞释放黏附分子和凋亡[J]. 中国药理学通报, 2011, 27(9): 1245-1249. DOI:10.3969/j.issn.1001-1978.2011.09.015 |
| [77] |
Pasternak BA, D'Mello S, Jurickova II, Han XN, Willson T, Flick L, Petiniot L, Uozumi N, Divanovic S, Traurnicht A, et al. Lipopolysaccharide exposure is linked to activation of the acute phase response and growth failure in pediatric Crohn's disease and murine colitis[J]. Inflammatory Bowel Diseases, 2010, 16(5): 856-869. DOI:10.1002/ibd.21132 |
| [78] |
Magro DO, Kotze PG, Martinez CAR, Camargo MG, Guadagnini D, Calixto AR, Vasques ACJ, Ayrizono MLS, Geloneze B, Pareja JC, et al. Changes in serum levels of lipopolysaccharides and CD26 in patients with Crohn's disease[J]. Intestinal Research, 2017, 15(3): 352-357. DOI:10.5217/ir.2017.15.3.352 |
| [79] |
Rose P, Moore PK, Ming SH, Nam OC, Armstrong JS, Whiteman M. Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis[J]. World Journal of Gastroenterology, 2005, 11(26): 3990-3997. DOI:10.3748/wjg.v11.i26.3990 |
| [80] |
Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, Jeon SB, Jeon WK, Chae HJ, Chung HT. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-κB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide[J]. Free Radical Biology and Medicine, 2006, 41(1): 106-119. DOI:10.1016/j.freeradbiomed.2006.03.021 |
| [81] |
Wallace JL, Ferraz JGP, Muscara MN. Hydrogen sulfide: an endogenous mediator of resolution of inflammation and injury[J]. Antioxidants & Redox Signaling, 2012, 17(1): 58-67. |
| [82] |
Zanardo RCO, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation[J]. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 2006, 20(12): 2118-2120. DOI:10.1096/fj.06-6270fje |
| [83] |
Ijssennagger N, Van Der Meer R, Van Mil SWC. Sulfide as a mucus barrier-breaker in inflammatory bowel disease?[J]. Trends in Molecular Medicine, 2016, 22(3): 190-199. DOI:10.1016/j.molmed.2016.01.002 |
| [84] |
Ijssennagger N, Belzer C, Hooiveld GJ, Dekker J, Van Mil SWC, Müller M, Kleerebezem M, Van Der Meer R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon[J]. PNAS, 2015, 112(32): 10038-10043. DOI:10.1073/pnas.1507645112 |
| [85] |
Beaumont M, Andriamihaja M, Lan A, Khodorova N, Audebert M, Blouin JM, Grauso M, Lancha L, Benetti PH, Benamouzig R, et al. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: the adaptive response[J]. Free Radical Biology and Medicine, 2016, 93: 155-164. DOI:10.1016/j.freeradbiomed.2016.01.028 |
| [86] |
Wang A, Keita ÅV, Phan V, McKay CM, Schoultz I, Lee J, Murphy MP, Fernando M, Ronaghan N, Balce D, et al. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis[J]. The American Journal of Pathology, 2014, 184(9): 2516-2527. DOI:10.1016/j.ajpath.2014.05.019 |
| [87] |
Babidge W, Millard S, Roediger W. Sulfides impair short chain fatty acid beta-oxidation at acyl-CoA dehydrogenase level in colonocytes: implications for ulcerative colitis[J]. Molecular and Cellular Biochemistry, 1998, 181(1/2): 117-124. DOI:10.1023/A:1006838231432 |
| [88] |
Roediger WE, Duncan A, Kapaniris O, Millard S. Sulphide impairment of substrate oxidation in rat colonocytes: a biochemical basis for ulcerative colitis?[J]. Clinical Science: London, England: 1979, 1993, 85(5): 623-627. |
| [89] |
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes[J]. Nature, 2012, 490(7418): 55-60. DOI:10.1038/nature11450 |
| [90] |
Huycke MM, Gaskins HR. Commensal bacteria, redox stress, and colorectal cancer: mechanisms and models[J]. Experimental Biology and Medicine: Maywood, N J, 2004, 229(7): 586-597. DOI:10.1177/153537020422900702 |
| [91] |
Hulin SJ, Singh S, Chapman MAS, Allan A, Langman MJS, Eggo MC. Sulphide-induced energy deficiency in colonic cells is prevented by glucose but not by butyrate[J]. Alimentary Pharmacology & Therapeutics, 2002, 16(2): 325-331. |
| [92] |
Lee S, Park JM, Jeong M, Han YM, Go EJ, Ko WJ, Cho JY, Kwon CI, Hahm KB. Korean red ginseng ameliorated experimental pancreatitis through the inhibition of hydrogen sulfide in mice[J]. Pancreatology, 2016, 16(3): 326-336. DOI:10.1016/j.pan.2016.02.012 |
| [93] |
Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter?[J]. FASEB Journal, 2002, 16(13): 1792-1798. DOI:10.1096/fj.02-0211hyp |
| [94] |
Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver[J]. Gastroenterology, 2006, 131(1): 259-271. DOI:10.1053/j.gastro.2006.02.033 |
| [95] |
Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values[J]. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 2008, 295(5): R1479-R1485. DOI:10.1152/ajpregu.90566.2008 |
| [96] |
Takeuchi H, Setoguchi T, Machigashira M, Kanbara K, Izumi Y. Hydrogen sulfide inhibits cell proliferation and induces cell cycle arrest via an elevated p21 Cip1 level in Ca9-22 cells[J]. Journal of Periodontal Research, 2008, 43(1): 90-95. |
| [97] |
Calenic B, Yaegaki K, Ishkitiev N, Kumazawa Y, Imai T, Tanaka T. p53-pathway activity and apoptosis in hydrogen sulfide-exposed stem cells separated from human gingival epithelium[J]. Journal of Periodontal Research, 2013, 48(3): 322-330. DOI:10.1111/jre.12011 |
| [98] |
Coutinho CMLM, Coutinho-Silva R, Zinkevich V, Pearce CB, Ojcius DM, Beech I. Sulphate-reducing bacteria from ulcerative colitis patients induce apoptosis of gastrointestinal epithelial cells[J]. Microbial Pathogenesis, 2017, 112: 126-134. DOI:10.1016/j.micpath.2017.09.054 |
| [99] |
Sakuma S, Minamino S, Takase M, Ishiyama Y, Hosokura H, Kohda T, Ikeda Y, Fujimoto Y. Hydrogen sulfide donor GYY4137 suppresses proliferation of human colorectal cancer Caco-2 cells by inducing both cell cycle arrest and cell death[J]. Heliyon, 2019, 5(8): e02244. DOI:10.1016/j.heliyon.2019.e02244 |
| [100] |
Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao XY, Scalia R, Kiss L, Szabo C, et al. Hydrogen sulfide attenuates myocardial ischemia- reperfusion injury by preservation of mitochondrial function[J]. PNAS, 2007, 104(39): 15560-15565. DOI:10.1073/pnas.0705891104 |
| [101] |
Esechie A, Kiss L, Olah G, Horváth EM, Hawkins H, Szabo C, Traber DL. Protective effect of hydrogen sulfide in a murine model of acute lung injury induced by combined burn and smoke inhalation[J]. Clinical Science, 2008, 115(3): 91-97. DOI:10.1042/CS20080021 |
| [102] |
Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa[J]. The Journal of Clinical Investigation, 1999, 104(8): 1107-1114. DOI:10.1172/JCI7712 |
| [103] |
Dordević D, Jančíková S, Vítězová M, Kushkevych I. Hydrogen sulfide toxicity in the gut environment: meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes[J]. Journal of Advanced Research, 2021, 27: 55-69. DOI:10.1016/j.jare.2020.03.003 |
| [104] |
Chen DF, Jin DC, Huang SM, Wu JY, Xu MQ, Liu TY, Dong WX, Liu X, Wang SN, Zhong WL, et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota[J]. Cancer Letters, 2020, 469: 456-467. DOI:10.1016/j.canlet.2019.11.019 |
| [105] |
Hansen R, Russell RK, Reiff C, Louis P, McIntosh F, Berry SH, Mukhopadhya I, Bisset WM, Barclay AR, Bishop J, et al. Microbiota of de-novo pediatric IBD: increased Faecalibacterium prausnitzii and reduced bacterial diversity in Crohn's but not in ulcerative colitis[J]. The American Journal of Gastroenterology, 2012, 107(12): 1913-1922. DOI:10.1038/ajg.2012.335 |
| [106] |
Luerce TD, Gomes-Santos AC, Rocha CS, Moreira TG, Cruz DN, Lemos L, Sousa AL, Pereira VB, De Azevedo M, Moraes K, et al. Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis[J]. Gut Pathogens, 2014, 6: 33. DOI:10.1186/1757-4749-6-33 |
 2022, Vol. 49
2022, Vol. 49