扩展功能
文章信息
- 薛松, 范殊璇, 史君涵, 傅翔, 邓子新, 马伟
- XUE Song, FAN Shuxuan, SHI Junhan, FU Xiang, DENG Zixin, MA Wei
- 脓肿分枝杆菌MBT结构鉴定与比较演化分析
- Structural identification and phylogenetic analysis of MBT from Mycobacteroides abscessus
- 微生物学通报, 2022, 49(11): 4848-4859
- Microbiology China, 2022, 49(11): 4848-4859
- DOI: 10.13344/j.microbiol.china.220391
-
文章历史
- 收稿日期: 2022-04-17
- 接受日期: 2022-07-06
- 网络首发日期: 2022-07-14
2. 上海交通大学生命科学技术学院, 上海 200240
2. School of Life Sciences and Biotechnology, Shanghai Jiao Tong University, Shanghai 200240, China
脓肿分枝杆菌是非结核条件致病分枝杆菌(non-tuberculosis mycobacteria,NTM),以囊性纤维化、支气管扩张、艾滋病患者及器官移植、老年等免疫低下人群为主要易感群体[1-2],可感染肺部、关节及皮肤等部位[3-4]。在发达国家,临床上脓肿分枝杆菌占快生长非结核分枝杆菌肺部感染的80%且呈逐年上升趋势[5]。由于独特的细胞壁结构与代谢机制[6-7],其能够抵御巨噬细胞和中性粒细胞等免疫细胞攻击,加之天然多耐药[8],导致其治疗周期长、疗效不理想[9]。例如,囊性纤维化患者6个月以上的长周期治疗,治愈率也仅有30%−50%[10]。
铁元素参与细菌的呼吸、DNA合成及自由基清除等重要过程[11],因此与细菌侵染、生长、抵抗巨噬细胞等免疫细胞攻击[12-13]及抗生素耐药[14-15]等密切相关。在生理pH条件下,Fe3+溶解度比细菌生长所需浓度低3−4个数量级[16],在炎症情况下,非特异免疫细胞可以局部产生乳铁蛋白、铁调素等抑制感染部位细菌对铁的获取[17],因此,铁限制是人类等宿主抵御病原菌感染的重要防御手段[18-19]。然而病原菌则通过合成与铁离子亲和性极高的铁载体从宿主转铁蛋白等蛋白中夺取铁[20]。针对结核分枝杆菌的研究发现,铁载体合成缺陷菌株在巨噬细胞中的生长受到显著抑制[21];而铁载体受体蛋白FecB缺失导致其对万古霉素、美罗培南等多种抗生素敏感性增强[22]。
正是由于铁载体的重要功能与作用,目前,有研究将抗生素与病原菌铁载体偶联,以提高抗生素进入菌体效率从而提升菌体内抗生素浓度,即所谓的特洛伊木马策略[23-24],以及针对铁载体合成关键酶开发抑制剂[25]等已成为抗生素研发热点。例如体外实验表明,3-苯基丙烯酸酯类化合物通过竞争性底物抑制结核分枝杆菌铁载体合成途径中的关键酶从而限制其生长[26]。
在缺铁环境中,分枝杆菌主要通过合成疏水的分枝杆菌素(mycobactin,MBT)[27]和亲水的羧基分枝杆菌素(carboxymycobactin,cMBT)高效吸收铁[28]。以结核分枝杆菌为例,细胞外的cMBT螯合铁后,通过HupB将Fe3+转移给位于周质空间中的MBT,Fe-MBT通过铁载体转运蛋白IrtAB将铁元素转运至细胞内,但铁元素从HupB转移到MBT的机制尚不清楚[29]。结核分枝杆菌的MBT生物合成基因簇由mbt-1和mbt-2两部分组成,mbt-1基因簇由mbtA−mbtJ等10个基因构成,主要负责MBT母核的合成[30]。mbt-2基因簇包括mbtK−mbtN等4个基因,负责MBT疏水脂肪酸链的组装[31]。结核、耻垢等菌的MBT相关研究表明,分枝杆菌MBT、cMBT母核结构高度保守(图 1),而母核苯环上R1−R5等位置修饰模式及脂肪酸链长度[29, 31]具有物种特异性。在所有MBT结构已被研究的分枝杆菌中,除海洋分枝杆菌脂肪酸链位于R4外[32],结核、耻垢等其他分枝杆菌脂肪酸链均位于R3[29]。脂肪酸链为MBT固着于细胞膜及IrtAB转运MBT所必需[33]。研究发现,各菌均会合成一系列脂肪酸链长短不一的MBT和cMBT,如结核MBT为C17−20[29, 34],而耻垢为C9−19[29, 35],但其生物学功能与意义尚不清楚。
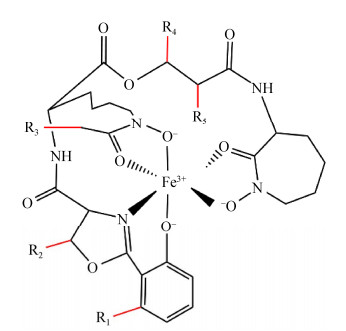
|
| 图 1 分枝杆菌素母核结构与关键修饰基团 Figure 1 Schematic representation of the core structure and key modifications of mycobactins/carboxymycobactin from mycobacteria. |
|
|
针对在水体、人体组织等多种环境中均可生长且具有条件致病性的脓肿分枝杆菌的MBT结构的研究,无疑将对于揭示分枝杆菌铁元素获取机制具有重要意义。
1 材料与方法 1.1 菌株与培养基菌株:M. abscessus ATCC 19977菌株由本实验室保存。
培养基:M. abscessus采用7H9[36]于37 ℃、120 r/min培养2−3 d至指定OD600值,用于后续实验。用于铁载体提取时,采用除铁最低营养培养基[32],经除铁最低营养培养基中传代2次之后,以0.5%比例接种至500 mL除铁最低营养培养基中培养,于37 ℃、120 r/min培养14 d,25 ℃、6 000 r/min离心30 min,分别取上清和沉淀用于cMBT与MBT提取。
1.2 实验方法 1.2.1 铁载体提取M. abscessus铁载体提取参照文献[32]方法进行。
1.2.2 质谱分析MALDI-TOF-MS:将溶于乙腈中的铁载体与HCCA等体积混合,氮气吹干后,进行分析,m/z检测范围为700−1 100。
FT-MS/MS:鞘层气体流速:5 250 kPa,辅助气流速:1 750 kPa,毛细管温度:350 ℃,全扫描模式分辨率:120 000,MS/MS扫描模式分辨率:7 500,喷雾电压:4.0 kV (阳离子)或−3.8 kV (阴离子)。
1.2.3 Fe-cMBT对缺铁脓肿分枝杆菌生长恢复作用分析经缺乏铁元素的最低营养培养基连续培养2代后,按1%接种于按指定浓度添加Fe-cMBT、FeCl3或等体积ddH2O的最低营养培养基,37 ℃、120 r/min培养4 d,每12 h测量OD600值。
1.2.4 系统发育学分析自NCBI (https://www.ncbi.nlm.nih.gov/)与KEGG (https://www.kegg.jp/kegg/kegg2.html)公共数据库下载M. tuberculosis H37Rv、M. smegmatis MC2 155、M. abscessus ATCC 19977、M. marinum M、M. canettii CIPT 140010059、M. bovis AF2122/97、M. avium 2285 (R)、M. fortuitum subsp. fortuitum ATCC 6841等菌株的MBT生物合成蛋白序列,对未注释蛋白采用M. tuberculosis的相关蛋白对目标菌蛋白数据集进行BLASTp,相似度最高、E-value < 10−20的比对结果认作同源蛋白,具体序列accession号如表 1所示。使用MEGA X[37]软件的最大似然方法进行系统发育树构建,通过1 000次自举重复评估节点支持。
| Coding protein | M. abscessus | M. avium | M. canetti | M. marinum |
| MbtA | WP_005088755.1 | EUA36076.1 | WP_014001248.1 | ACC42114.1 |
| MbtB | WP_005110650.1 | EUA36980.1 | WP_014001247.1 | WP_012395305.1 |
| MbtC | WP_005080165.1 | EUA39465.1 | WP_003412278.1 | WP_012395309.1 |
| MbtD | WP_005110643.1 | EUA38842.1 | WP_014001246.1 | WP_012395310.1 |
| MbtE | WP_005110645.1 | EUA38931.1 | WP_041180111.1 | WP_012395307.1 |
| MbtF | WP_005110647.1 | EUA39107.1 | WP_041180276.1 | WP_012395306.1 |
| MbtG | WP_005086054.1 | EUA37716.1 | WP_014001243.1 | WP_041324786.1 |
| MbtH | CAM62331.1 | EUA36332.1 | WP_003412265.1 | WP_011741377.1 |
| MbtI | WP_005096783.1 | EUA39884.1 | WP_014001250.1 | ACC42122.1 |
| MbtJ | WP_005098458.1 | EUA39656.1 | WP_041180112.1 | WP_012395313.1 |
| MbtK | WP_005076691.1 | EUA40352.1 | WP_003406956.1 | WP_012393422.1 |
| MbtM | CAM64737.1 | EUA36117.1 | WP_003406950.1 | WP_237708340.1 |
| MbtN | WP_005082911.1 | EUA39862.1 | WP_003406953.1 | WP_012396082.1 |
| Coding protein | M. smegmatis | M. tuberculosis | M. bovis | M. fortuitum |
| MbtA | ABK70627.1 | WP_003412282.1 | WP_003412282.1 | CRL54347.1 |
| MbtB | WP_011729889.1 | WP_003899299.1 | WP_010950705.1 | CRL56150.1 |
| MbtC | WP_011729888.1 | WP_003412278.1 | WP_003412278.1 | EJZ16316.1 |
| MbtD | ABK75467.1 | WP_003412277.1 | WP_003412277.1 | WP_003879643.1 |
| MbtE | ABK72785.1 | WP_003899297.1 | WP_003899297.1 | WP_051018853.1 |
| MbtF | AFP40852.1 | WP_023637453.1 | WP_031657277.1 | WP_003879641.1 |
| MbtG | WP_011729884.1 | WP_003899296.1 | WP_003899296.1 | WP_003879640.1 |
| MbtH | WP_011729883.1 | WP_003916994.1 | WP_003412265.1 | WP_003884873.1 |
| MbtI | WP_011729893.1 | WP_003412287.1 | WP_003412287.1 | EJZ16323.1 |
| MbtJ | WP_011726795.1 | WP_003899300.1 | WP_003899300.1 | WP_038563859.1 |
| MbtK | WP_011728178.1 | WP_003406956.1 | WP_003406956.1 | WP_003879974.1 |
| MbtM | ABK75882.1 | WP_003406950.1 | WP_003406950.1 | WP_003879972.1 |
| MbtN | ABK70771.1 | WP_003406953.1 | WP_003406953.1 | WP_003881372.1 |
实验结果为3次独立实验平均值,采用GraphPad Prism软件paired t-test模块进行组间差异统计学分析,P < 0.05认定为差异显著。
2 结果与分析 2.1 脓肿分枝杆菌分枝杆菌素的结构从MALDI-TOF-MS谱图(图 2)中可以观察到MBT、Fe-MBT与cMBT、Fe-cMBT的分子离子峰,表明两者均具有与Fe3+的结合能力。MBT的脂肪链长度变化范围为C10−17,其中高丰度同系物链长为C10−13、C15、C17,详见图 2A。从图 2B中可以看到,cMBT脂肪链长度大约从C4−8不等,高丰度组分的链长为C5−8。
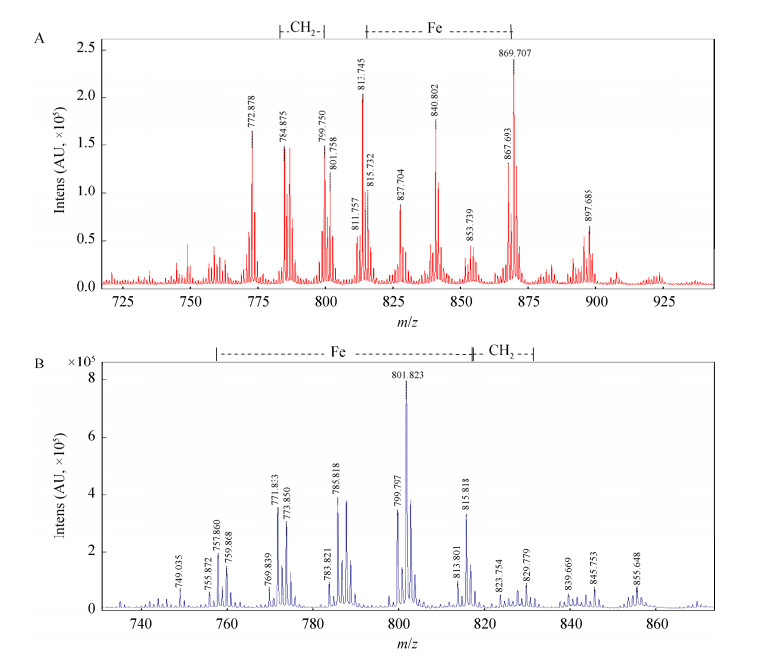
|
| 图 2 Mycobacteroides abscessus MBT与cMBT的MALDI-TOF-MS谱图 Figure 2 MALDI-TOF-MS mass spectrum of MBT and cMBT from Mycobacteroides abscessus. A:MBT谱图;B:cMBT谱图 A: Mass spectrum of MBT; B: Mass spectrum of cMBT. |
|
|
高分辨FT-MS/MS二级质谱谱图分析分别确定了M. abscessus的MBT、cMBT的R1、R2、R3、R4、R5等修饰基团(表 2)。值得注意的是,脓肿分枝杆菌脂肪酸链位于R4位置,即母核的中心酯基团与酰胺基团之间(图 3)。
| Strains | R1 MBT/cMBT |
R2 MBT/cMBT |
R3 MBT/cMBT |
R4 MBT/cMBT |
R5 MBT/cMBT |
Pathogenicity and growth speed | Reference |
| M. abscessus | H | CH3 | CH3 | C10-17/C4-8 or C3-7 | CH3 | Opportunistic Pathogens (RGM) | This Study |
| M. tuberculosis | H | H/H, CH3 | C17-20/C3-9 or C1-9 | CH3 | H | True Pathogens | [29-30] |
| M. smegmatis | H | H/H, CH3 | C9-19/Not available | CH3 | H | Saprophytes (RGM) | [29, 31] |
| M. bovis | H | H/H, CH3 | C17-20/C3-9 or C1-9 | CH3 | H | True Pathogens | [29-30] |
| M. avium | H | CH3 | C11-14, 18/C1-9 or C2-5 | CH2CH3 | CH3 | Opportunistic Pathogens (SGM) | [29-30] |
| M. fortuitum | CH3/Not available | CH3/Not available | C9-17/Not available | CH3/Not available | H/Not available | Opportunistic Pathogens (RGM) | [29] |
| M. marinum | H | CH3 | CH3 | C15-18/C7-9 or C3-6 | CH3 | True Pathogens (SGM) | [32] |
| 注:cMBT的R3或R4末端中有羧基(RCOOH)和甲酯(RCOOCH3)两种形式。RGM:Rapidly-growing mycobacteria;SGM:Slowly-growing mycobacteria。所有的病原性分枝杆菌均为慢生长型 Note: The R3 or R4 terminus of cMBT have two types:carboxyl (RCOOH) and methyl ester (RCOOCH3). RGM: Rapidly-growing mycobacteria; SGM: Slowly-growing mycobacteria. All pathogenic mycobacteria are slowly-growing type. |
|||||||
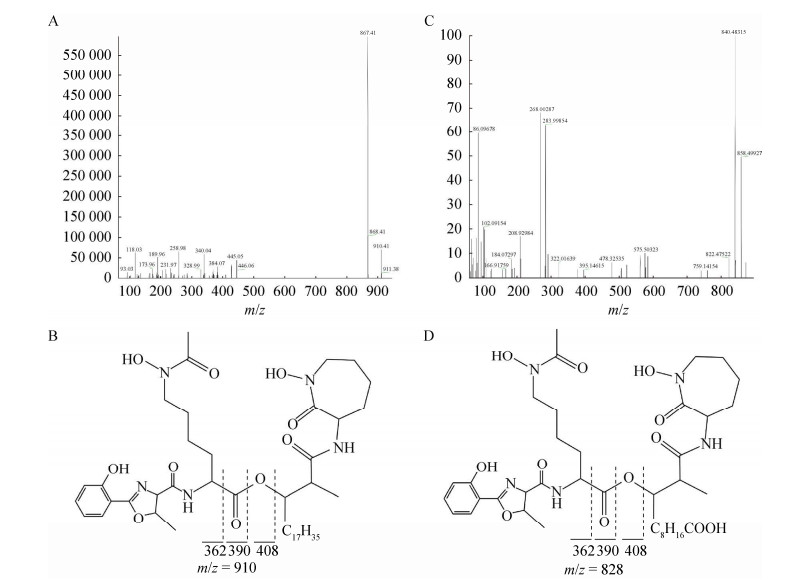
|
| 图 3 Mycobacteroides abscessus MBT和cMBT的FT-MS/MS谱图与推测结构 Figure 3 FT-MS/MS mass spectrum of MBT and cMBT from Mycobacteroides abscessus. A和B:MBT质谱谱图及推测结构;C和D:cMBT质谱谱图与结构 A, B: Mass spectrum and speculated structure of MBT; C, D: Mass spectrum and speculated structure of cMBT. |
|
|
从图 4中可以看到,Fe-cMBT可显著促进M. abscessus生长,培养至72 h,添加1、5和10 μmol/L的Fe-cMBT组OD600值分别比对照组高37.8%、120.7%和178.5%,84 h后分别高10.7%、56.0%和105.2%,96 h后分别高9.4%、61.9%和100.8% (图 4)。
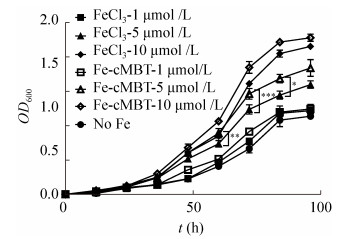
|
| 图 4 Fe-cMBT对缺铁条件下脓肿分枝杆菌生长作用 Figure 4 Effect of Fe-cMBT on the growth of M. abscessus in iron deprivation. *: P < 0.1; **: P < 0.05; ***: P < 0.01. |
|
|
对比cMBT螯合铁与无机铁可以发现,虽然铁离子较为丰富时(如10 μmol/L),Fe-cMBT与FeCl3组无显著差异(结果未展示),但是5 μmol/L铁离子浓度下,培养至60 h时Fe-cMBT组OD600值高于FeCl3组21.1% (P=0.012 4),72 h时高14.7% (P=0.003 9),84 h高16.7% (P=0.052 7),96 h高15.2% (P=0.112 7)。
2.3 分枝杆菌典型菌MBT合成基因簇比较基因组分析 2.3.1 分枝杆菌家族MBT合成基因簇共线性分析从图 5中可以看到,虽然M. smegmatis mbt-1基因簇各成员在基因组中的排列方向与M. tuberculosis、M. canetti和M. bovis等菌相反,M. fortuitum的mbtB与mbtC间插入了2个与MBT合成无关基因及M. avium的mbtE基因的长度比其他分枝杆菌的mbtE长约1 500 bp,但是这些菌mbt-1基因簇成员均按mbtA−mbtH顺序排列。M. abscessus和M. marinum的mbt-1基因簇中仅D-C-E-F-B的排列顺序相同,其他基因排列顺序并不相同;而且M. abscessus的mbt-1由两段构成,中间相隔100多个基因且各包含一个mbtE。
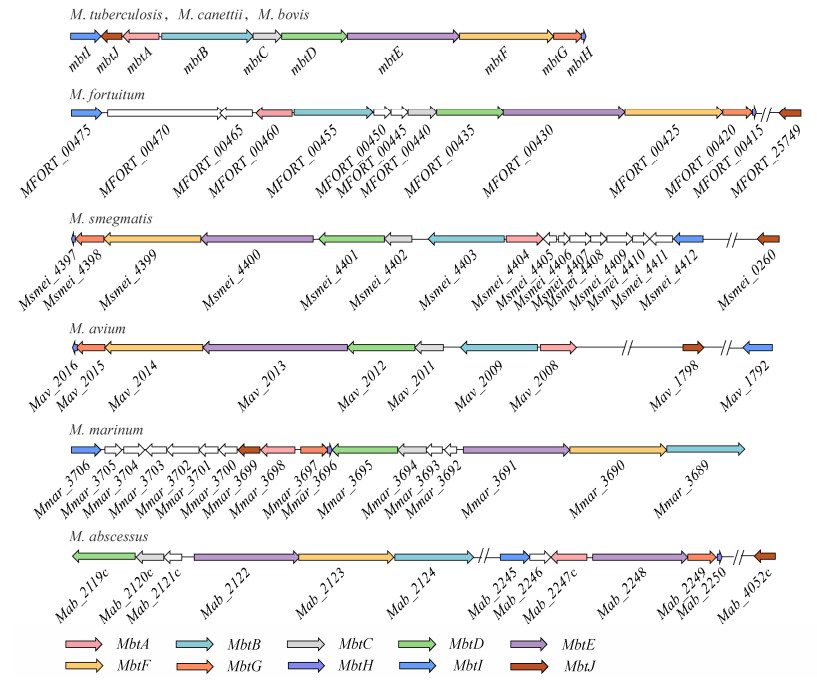
|
| 图 5 分枝杆菌家族不同菌株的mbt-1合成基因簇的排列模式 Figure 5 Alignment patterns of mbt-1 synthesis gene clusters in different strains of the mycobacterium family. 使用不同的颜色标注出了mbtA−mbtJ。从上至下分别为:结核分枝杆菌、偶发分枝杆菌、耻垢分枝杆菌、鸟分枝杆菌、海洋分枝杆菌和脓肿分枝杆菌。卡内蒂分枝杆菌、牛分枝杆菌的基因排列模式与结核分枝杆菌完全相同 The mbtA−mbtJ genes are labeled using different colors from top to bottom: M. tuberculosis, M. fortuitum, M. smegmatis, M. avium, M. marinum, M. abscessus. The gene clusters alignment patterns of M. canetti and M. bovis are same as M. tuberculosis. |
|
|
观察mbt-2相关基因在基因组中的分布可以看出,M. tuberculosis、M. fortuitum、M. smegmatis、M. bovis和M. canetti等菌的mbtK、mbtL、mbtM、mbtN在基因组中连续排列;M. avium的mbtL独立,其余3个基因连续排列;而M. abscessus和M. marinum不仅mbtL缺失,其余3个基因均分散排列(表 3)。
| Strains | mbtK | mbtL | mbtM | mbtN |
| M. abscessus | Mab_3125c | − | Mab_4668c | Mab_1070c |
| M. avium | Mav_2875 | Mav_0029 | Mav_2874 | Mav_2876 |
| M. canetti | Mcan_RS07180 | Mcan_RS07165 | Mcan_RS07170 | Mcan_RS07175 |
| M. marinum | Mmar_1587 | − | Mmar_3272 | Mmar_4532 |
| M. smegmatis | Msmei_2080 | Msmei_2083 | Msmei_2082 | Msmei_2081 |
| M. tuberculosis | Rv1347c | Rv1344 | Rv1345 | Rv1346 |
| M. bovis | BQ2027_RS07055 | BQ2027_RS07040 | BQ2027_RS07045 | BQ2027_RS07050 |
| M. fortuitum | Mfort_06229 | Mfort_06214 | Mfort_06219 | Mfort_06224 |
| 注:−:通过BLASTp比对后无法找到对应的同源基因(E-value > 10−20) Note: −: The corresponding homologous gene cannot be found after BLASTp alignment (E-value > 10−20). |
||||
对脓肿等菌的mbt-1基因簇基因编码的蛋白进行系统发育分析发现,M. tuberculosis与M. canetti、M. bovis最相似,M. fortuitum与M. smegmatis最相似,而M. abscessus与M. marinum最相似且独立成簇(图 6A)。
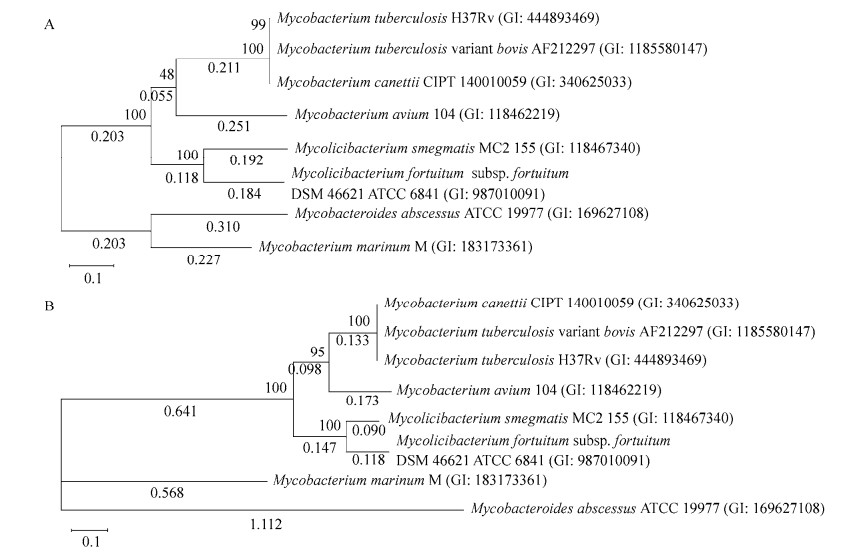
|
| 图 6 分枝杆菌代表菌MBT合成基因编码蛋白系统发育分析 Figure 6 Phylogenetic analysis of proteins encoded by MBT synthesis genes from representative mycobacteria. A:mbt-1系统发育树;B:mbt-2系统发育树。括号中序号为菌株序列的GenBank登录号;分支点处数值为1 000次自举验证可信率(百分比值);系统树枝上数值为其进化距离 A: Phylogenetic tree of mbt-1; B: Phylogenetic tree of mbt-2. The number in brackets is the GenBank accession number of the strain; The value at the branch point is the confidence rate (percentage value) of 1 000 times bootstrap verification; The value on the phylogenetic branch is the evolutionary distance. |
|
|
基于MbtK、MbtM与MbtN的mbt-2系统发育分析发现(M. abscessus与M. marinum无mbtL),mbt-2进化树结构与mbt-1相似,M. abscessus与M. marinum亲缘关系最近(图 6B)。
3 讨论与结论本研究对MBT与cMBT结构鉴定发现,M. abscessus MBT、cMBT母核R1、R2、R3、R5等位置的修饰模式与海洋分枝杆菌相同,脂肪酸链也位于R4位置;但MBT、cMBT脂肪酸链长度分别为C10−17和C4−8,该结构未见报道。Fe-cMBT生物活性分析发现,cMBT以浓度依赖方式促进缺铁M. abscessus生长,而且M. abscessus对cMBT利用效率显著高于无机铁。上述实验结果表明,MBT-cMBT系统是M. abscessus高效吸收环境铁元素的关键系统。
在已有MBT结构报道的分枝杆菌中,M. abscessus MBT母核修饰模式与慢生长型的M. marinum最接近,但脂肪酸链长度及变化范围又与之存在明显差异。对它们进行比较分析可以发现,M. marinum、M. tuberculosis、M. bovis等慢生长型、病原分枝杆菌[30, 38]脂肪酸链长度变化范围仅4个碳,而条件致病菌,无论是慢生长型的M. avium还是快生长型的M. abscessus与M. fortuitum及非致病菌M. smegmatis等条件致病菌的脂肪酸链长度变化范围达C7−11。即致病菌脂肪酸链长度变化范围远小于条件致病菌与环境菌,而且更倾向于合成脂肪酸侧链较长的MBT。我们推测,具有较大变化范围脂肪酸链的MBT或许更有利于细菌从多样、多变环境中获取铁;而长但变化范围小的脂肪酸链或有利于病原分枝杆菌从较为稳定宿主环境,如人体中高效夺取铁元素。
MBT合成基因簇无论是共线性还是系统发育树均为M. abscessus与M. marinum最相似(图 5和图 6),该结果与基于16S rRNA基因序列的亲缘关系分析结果完全不同[39],我们推测两者MBT生物合成基因簇发生过水平转移事件。
M. tuberculosis脂肪酸链位于R3位置,其MBT生物合成研究发现,MbtMN首先将脂肪酸链转移到mbtL编码的酰基载体蛋白上并活化[40],然后由mbtK编码的N-酰基转移酶通过转酰基反应连接到母核的氨基上,最终由MbtG通过酰胺基羟基化等反应生成异羟肟酸[33]。然而M. abscessus与M. marinum的脂肪酸链直接连接在碳链上(R4),这就意味着脂肪酸链是以完全不同的方式添加或母核合成方式与结核等菌不同。同时,脂肪酸链着生于R4位置时,螯合铁的MBT母核与细胞膜距离比R3着生方式更远(图 1)。2种方式对铁元素获取效率是否存在影响值得深入研究。
总而言之,MBT结构解析与功能研究不仅有助于深入阐明分枝杆菌铁获取及致病菌侵染、免疫抵抗、耐药等机制,更能通过揭示MBT在病原分枝杆菌起源过程中的演化规律,为M. abscessus治疗方案与药物研发提供有价值的信息。
| [1] |
Sapriel G, Konjek J, Orgeur M, Bouri L, Frézal L, Roux AL, Dumas E, Brosch R, Bouchier C, Brisse S, et al. Genome-wide mosaicism within Mycobacterium abscessus: evolutionary and epidemiological implications[J]. BMC Genomics, 2016, 17: 118. DOI:10.1186/s12864-016-2448-1 |
| [2] |
Degiacomi G, Sammartino JC, Chiarelli LR, Riabova O, Makarov V, Pasca MR. Mycobacterium abscessus, an emerging and worrisome pathogen among cystic fibrosis patients[J]. International Journal of Molecular Sciences, 2019, 20(23): 5868. DOI:10.3390/ijms20235868 |
| [3] |
De Groote MA, Huitt G. Infections due to rapidly growing Mycobacteria[J]. Clinical Infectious Diseases, 2006, 42(12): 1756-1763. DOI:10.1086/504381 |
| [4] |
Hao HF, Zhang HL. Advances in anti-infective drug therapy for Mycobacterium abscessus pulmonary disease[J]. The Journal of Medical Theory and Practice, 2021, 34(12): 2021-2023, 2017. (in Chinese) 郝红芬, 张宏丽. 脓肿分枝杆菌肺病抗感染药物治疗研究进展[J]. 医学理论与实践, 2021, 34(12): 2021-2023, 2017. DOI:10.19381/j.issn.1001-7585.2021.12.011 |
| [5] |
Varghese G, Shepherd R, Watt P, Bruce JH. Fatal infection with Mycobacterium fortuitum associated with oesophageal achalasia[J]. Thorax, 1988, 43(2): 151-152. DOI:10.1136/thx.43.2.151 |
| [6] |
Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis[J]. Tuberculosis, 2003, 83(1/2/3): 91-97. |
| [7] |
Daher W, Leclercq LD, Viljoen A, Karam J, Dufrêne YF, Guérardel Y, Kremer L. O-methylation of the glycopeptidolipid acyl chain defines surface hydrophobicity of Mycobacterium abscessus and macrophage invasion[J]. ACS Infectious Diseases, 2020, 6(10): 2756-2770. DOI:10.1021/acsinfecdis.0c00490 |
| [8] |
Yam YK, Alvarez N, Go ML, Dick T. Extreme drug tolerance of Mycobacterium abscessus "persisters"[J]. Frontiers in Microbiology, 2020, 11: 359. DOI:10.3389/fmicb.2020.00359 |
| [9] |
Baldwin SL, Larsen SE, Ordway D, Cassell G, Coler RN. The complexities and challenges of preventing and treating nontuberculous mycobacterial diseases[J]. PLoS Neglected Tropical Diseases, 2019, 13(2): e0007083. DOI:10.1371/journal.pntd.0007083 |
| [10] |
Ferro BE, Srivastava S, Deshpande D, Pasipanodya JG, van Soolingen D, Mouton JW, Van Ingen J, Gumbo T. Failure of the amikacin, cefoxitin, and clarithromycin combination regimen for treating pulmonary Mycobacterium abscessus infection[J]. Antimicrobial Agents and Chemotherapy, 2016, 60(10): 6374-6376. DOI:10.1128/AAC.00990-16 |
| [11] |
Herb M, Schramm M. Functions of ROS in macrophages and antimicrobial immunity[J]. Antioxidants: Basel, Switzerland, 2021, 10(2): 313. |
| [12] |
Gokarn K, Pal RB. Activity of siderophores against drug-resistant Gram-positive and Gram-negative bacteria[J]. Infection and Drug Resistance, 2018, 11: 61-75. DOI:10.2147/IDR.S148602 |
| [13] |
Pal R, Hameed S, Fatima Z. Iron deprivation affects drug susceptibilities of Mycobacteria targeting membrane integrity[J]. Journal of Pathogens, 2015, 2015: 938523. |
| [14] |
Pal R, Hameed S, Kumar P, Singh S, Fatima Z. Understanding lipidomic basis of iron limitation induced chemosensitization of drug-resistant Mycobacterium tuberculosis[J]. 3 Biotech, 2019, 9(4): 122. DOI:10.1007/s13205-019-1645-4 |
| [15] |
Li W, Zhang WJ. Advances in the role of trace iron in the pathogenesis of tuberculosis[J]. Chinese Journal of Cellular and Molecular Immunology, 2012, 28(5): 554-556. (in Chinese) 李微, 张万江. 微量铁元素在结核病发病机制中作用的研究进展[J]. 细胞与分子免疫学杂志, 2012, 28(5): 554-556. DOI:10.13423/j.cnki.cjcmi.006422 |
| [16] |
De Serrano LO, Camper AK, Richards AM. An overview of siderophores for iron acquisition in microorganisms living in the extreme[J]. BioMetals, 2016, 29(4): 551-571. DOI:10.1007/s10534-016-9949-x |
| [17] |
Cassat JE, Skaar EP. Iron in infection and immunity[J]. Cell Host & Microbe, 2013, 13(5): 509-519. |
| [18] |
Kochan I. The role of iron in bacterial infections, with special consideration of host-tubercle Bacillus interaction[J]. Current Topics in Microbiology and Immunology, 1973, 60: 1-30. |
| [19] |
Jiang H, Yang QL. Study on the relevance of iron, calcium and hydrogen ions in the immune escape mechanism of Mycobacterium tuberculosis[J]. Progress in Microbiology and Immunology, 2011, 39(1): 62-65. (in Chinese) 姜华, 杨秋林. 铁、钙、氢离子在结核分枝杆菌免疫逃逸机制中的相关研究[J]. 微生物学免疫学进展, 2011, 39(1): 62-65. DOI:10.3969/j.issn.1005-5673.2011.01.015 |
| [20] |
Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria[J]. Annual Review of Microbiology, 2000, 54: 881-941. DOI:10.1146/annurev.micro.54.1.881 |
| [21] |
De Voss JJ, Rutter K, Schroeder BG, Su H, Zhu Y, Barry CE 3rd. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages[J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(3): 1252-1257. DOI:10.1073/pnas.97.3.1252 |
| [22] |
Xu WZ, DeJesus MA, Rücker N, Engelhart CA, Wright MG, Healy C, Lin K, Wang RJ, Park SW, Ioerger TR, et al. Chemical genetic interaction profiling reveals determinants of intrinsic antibiotic resistance in Mycobacterium tuberculosis[J]. Antimicrobial Agents and Chemotherapy, 2017, 61(12): e01334-e01317. |
| [23] |
Miller MJ, Walz AJ, Zhu H, Wu CR, Moraski G, Möllmann U, Tristani EM, Crumbliss AL, Ferdig MT, Checkley L, et al. Design, synthesis, and study of a mycobactin-artemisinin conjugate that has selective and potent activity against tuberculosis and malaria[J]. Journal of the American Chemical Society, 2011, 133(7): 2076-2079. DOI:10.1021/ja109665t |
| [24] |
Zhang L, Liu MF, Cheng AC. Siderophore-antibiotic conjugates: the new antimicrobial agents[J]. Microbiology China, 2016, 43(7): 1598-1604. (in Chinese) 张利, 刘马峰, 程安春. 铁载体-抗生素耦合物: 一种新型的抗菌制剂[J]. 微生物学通报, 2016, 43(7): 1598-1604. |
| [25] |
Patel K, Butala S, Khan T, Suvarna V, Sherje A, Dravyakar B. Mycobacterial siderophore: a review on chemistry and biology of siderophore and its potential as a target for tuberculosis[J]. European Journal of Medicinal Chemistry, 2018, 157: 783-790. DOI:10.1016/j.ejmech.2018.08.030 |
| [26] |
Manos-Turvey A, Cergol KM, Salam NK, Bulloch EMM, Chi G, Pang A, Britton WJ, West NP, Baker EN, Lott JS, et al. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site[J]. Organic & Biomolecular Chemistry, 2012, 10(46): 9223. |
| [27] |
Snow GA. Isolation and structure of mycobactin T, a growth factor from Mycobacterium tuberculosis[J]. The Biochemical Journal, 1965, 97(1): 166-175. DOI:10.1042/bj0970166 |
| [28] |
Gobin J, Moore CH, Reeve JR Jr, Wong DK, Gibson BW, Horwitz MA. Iron acquisition by Mycobacterium tuberculosis: isolation and characterization of a family of iron-binding exochelins[J]. PNAS, 1995, 92(11): 5189-5193. DOI:10.1073/pnas.92.11.5189 |
| [29] |
Sritharan M. Iron homeostasis in Mycobacterium tuberculosis: mechanistic insights into siderophore- mediated iron uptake[J]. Journal of Bacteriology, 2016, 198(18): 2399-2409. DOI:10.1128/JB.00359-16 |
| [30] |
McMahon MD, Rush JS, Thomas MG. Analyses of MbtB, MbtE, and MbtF suggest revisions to the mycobactin biosynthesis pathway in Mycobacterium tuberculosis[J]. Journal of Bacteriology, 2012, 194(11): 2809-2818. DOI:10.1128/JB.00088-12 |
| [31] |
Chao A, Sieminski PJ, Owens CP, Goulding CW. Iron acquisition in Mycobacterium tuberculosis[J]. Chemical Reviews, 2019, 119(2): 1193-1220. DOI:10.1021/acs.chemrev.8b00285 |
| [32] |
Knobloch P, Koliwer-Brandl H, Arnold FM, Hanna N, Gonda I, Adenau S, Personnic N, Barisch C, Seeger MA, Soldati T, et al. Mycobacterium marinum produces distinct mycobactin and carboxymycobactin siderophores to promote growth in broth and phagocytes[J]. Cellular Microbiology, 2020, 22(5): e13163. |
| [33] |
Krithika R, Marathe U, Saxena P, Ansari MZ, Mohanty D, Gokhale RS. A genetic locus required for iron acquisition in Mycobacterium tuberculosis[J]. PNAS, 2006, 103(7): 2069-2074. DOI:10.1073/pnas.0507924103 |
| [34] |
Horwitz LD, Horwitz MA. The exochelins of pathogenic mycobacteria: unique, highly potent, lipid- and water-soluble hexadentate iron chelators with multiple potential therapeutic uses[J]. Antioxidants & Redox Signaling, 2014, 21(16): 2246-2261. |
| [35] |
Ratledge C, Ewing M. The occurrence of carboxymycobactin, the siderophore of pathogenic mycobacteria, as a second extracellular siderophore in Mycobacterium smegmatis
[J]. Microbiology: Reading, England, 1996, 142(Pt 8): 2207-2212. |
| [36] |
Jacobs WR Jr, Kalpana GV, Cirillo JD, Pascopella L, Snapper SB, Udani RA, Jones W, Barletta RG, Bloom BR. Genetic Systems for Mycobacteria[M]//Methods in Enzymology. Amsterdam: Elsevier, 1991: 537-555
|
| [37] |
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms[J]. Molecular Biology and Evolution, 2018, 35(6): 1547-1549. DOI:10.1093/molbev/msy096 |
| [38] |
Johansen MD, Herrmann JL, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus[J]. Nature Reviews Microbiology, 2020, 18(7): 392-407. DOI:10.1038/s41579-020-0331-1 |
| [39] |
Chavadi SS, Stirrett KL, Edupuganti UR, Vergnolle O, Sadhanandan G, Marchiano E, Martin C, Qiu WG, Soll CE, Quadri LEN. Mutational and phylogenetic analyses of the mycobacterial mbt gene cluster[J]. Journal of Bacteriology, 2011, 193(21): 5905-5913. DOI:10.1128/JB.05811-11 |
| [40] |
Shyam M, Shilkar D, Verma H, Dev A, Sinha BN, Brucoli F, Bhakta S, Jayaprakash V. The mycobactin biosynthesis pathway: a prospective therapeutic target in the battle against tuberculosis[J]. Journal of Medicinal Chemistry, 2021, 64(1): 71-100. DOI:10.1021/acs.jmedchem.0c01176 |
 2022, Vol. 49
2022, Vol. 49




