扩展功能
文章信息
- 钱璟, 吴哲元, 郭晓奎, 刘畅
- QIAN Jing, WU Zheyuan, GUO Xiaokui, LIU Chang
- 耐药微生物和抗生素耐药基因与全健康
- Antibiotic-resistant microbes, antibiotic resistance genes and One Health
- 微生物学通报, 2022, 49(10): 4412-4424
- Microbiology China, 2022, 49(10): 4412-4424
- DOI: 10.13344/j.microbiol.china.220177
-
文章历史
- 收稿日期: 2022-02-22
- 接受日期: 2022-04-19
- 网络首发日期: 2022-06-14
2. 上海交通大学医学院免疫学与微生物学系, 上海 200025
2. Department of Immunology and Microbiology, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China
全健康(One Health)是一种在地方、区域和全球3个层面开展工作的跨学科和跨部门协作理念,核心目标在于探索人、动物和环境之间的复杂关系,通过医学、兽医学和环境科学等学科的交叉,以及经济、农业、地理和政策等领域的合作交流,实现个体健康、群体健康和生态健康。抗生素耐药性在人-动物-环境界面的流动与循环对人、动物、环境的健康都产生了极大威胁[1],是适用于全健康理念和模式关注的问题之一。2015年5月召开的世界卫生大会通过了《抗生素耐药性全球行动计划》(Global Action Plan on Antimicrobial Resistance),该计划指出抗生素耐药性不仅对人类常规医疗活动产生重大威胁,也会对人类为应对传染病采取的有效公共卫生措施的可持续性产生负面影响[2-3]。不仅如此,在农业、畜牧业、水产养殖业施用抗生素也对生物产生严重危害[4]。微生物能够通过可移动基因元件(mobile genetic elements,MGE)的水平转移(horizontal gene transfer,HGT)获得抗生素耐药基因(antibiotic resistance genes,ARGs),并进一步造成其在人-动物-环境中的流动传播[5-9]。抗生素耐药菌(antibiotic resistance bacteria,ARB)和耐药基因也被定义为新型环境污染物,对地表水、地下水和土壤产生负面影响,并进一步危害公众健康[10]。由于抗生素耐药菌和耐药基因会形成跨生境和跨物种的传播,因此,从全健康角度综合理解人、动物和环境微生物群之间的联系对于应对这一全球性公共健康挑战至关重要[11]。
本文从全健康视角综述了人、动物、环境中抗生素耐药微生物和抗生素耐药基因的流动和循环过程,并对以“全健康”理念应对抗生素耐药性进行了展望。
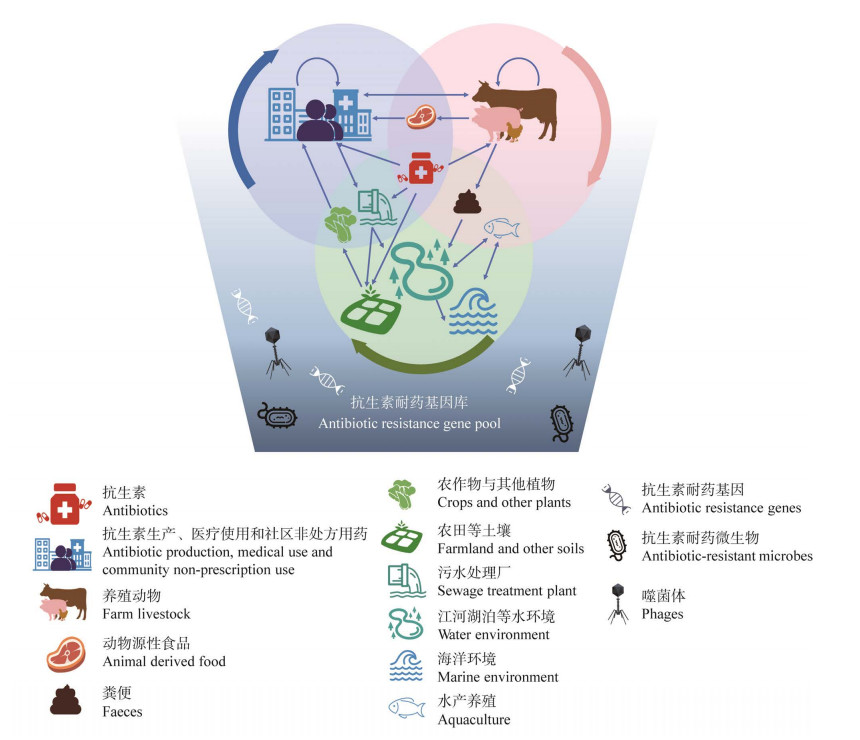
1 抗生素耐药性在人-动物-环境界面的传播人类的生产生活、医疗活动及全球一体化的不断发展均会促进耐药性在人-动物-环境界面的全球传播[12],其中包括了耐药基因和耐药微生物的传播,但耐药基因的传播更隐匿、更多样化,易造成耐药性更广泛的播散(图 1)。
|
| 图 1 抗生素耐药基因在人-动物-环境界面的传播 Figure 1 Transmission of antibiotic resistance genes at the human-animal-environment interfaces. 微生物可以从抗生素耐药基因库中获得耐药基因。抗生素耐药基因可以通过直接或间接的方式在人、动物和环境之间流动、循环 Antibiotic-resistant microbes often acquire resistance genes from the pool. Antibiotic resistance genes can flow and circulate among human, animals and the environment directly or indirectly. |
|
|
在抗生素的生产过程中,具有活性的中间产物和药物本身均可能在生产时排入制药废水而进入自然环境[13-14],或在临床使用之前的运输、销售过程中因意外泄漏排入环境[15],与其他共选择剂共同作用,促进耐药基因的转移和传播。
1.1.2 抗生素的使用医院内抗生素的使用是抗生素耐药性向环境中转移的主要原因[16]。医院排出的废水、废物和医院污泥中均含有高水平的耐药菌和耐药基因[17-18]。微生物暴露于常规城市废水并不会出现耐药性改变,而微生物暴露于医院废水则与磺胺类抗生素耐药性的比例增加有关[19]。
在中低收入国家,抗生素在社区、家庭中的误用和滥用是耐药性产生和传播的重要驱动因素[20]。居民服药后未经转化的抗生素及其代谢产物与尿液、粪便一起排出,进入污水处理厂或自然环境中,从而促进耐药微生物和耐药基因在人和环境中的转移[21]。
1.1.3 微生态制剂的使用近年来,随着对微生物组相关研究的深入,调控微生物组的微生态制剂被广泛应用于食品、药品、养殖畜牧业饲料添加等领域[22-23]。微生态制剂中含有活菌,其携带的耐药基因也存在摄入后引起播散的可能。Liu等对分离自发酵乳制品和医药产品中的41株乳酸菌进行研究发现,大多数菌株对环丙沙星、阿米卡星、甲氧苄啶/磺胺甲恶唑和庆大霉素耐药,并发现携带耐药基因的商用菌株存在[24]。此外,在针对多株益生菌的安全性评价研究中,也发现耐药基因在菌株中的存在,成为其可能的安全风险之一[25-27]。
1.2 动物用抗生素造成不同界面间耐药性的传播 1.2.1 家禽牲畜与抗生素耐药性的传播因具有抗病、促生长的作用,抗生素在畜牧业中广泛使用[23]。动物中持续使用抗生素会促进耐药微生物的出现并加强选择压力[20]。目前,欧洲已经完全禁止抗生素作为生长促进剂给予牲畜,美国也禁止在家禽养殖业中使用喹诺酮类抗生素,但全球范围内仍有很多国家以预防为目的添加抗生素喂饲牲畜[28]。
抗生素会通过牲畜的尿液和粪便从体内排出,通过土壤、地表径流、地下水等转移到环境中并传播[29-30]。粪便堆肥可成为耐药基因交换的场所及在土壤和水环境中转移的媒介,这使得人与家养牲畜和家禽粪便中的耐药菌组成也显著相关[31]。
携带耐药基因的微生物也可通过人与动物的直接接触传播,这一现象不仅发生在动物源性食品加工厂中[32],还在居民与牲畜共用生活区的欠发达地区出现[33]。人类也可通过动物性食物的摄入接触耐药微生物[34]。
1.2.2 水产养殖与抗生素耐药性的传播水产养殖业中抗生素的使用与城市污水的排放共同推动了水环境内抗生素耐药微生物的选择和传播[35-36]。仅在海水养殖场的表层水样中就可检出包括水产养殖业最常用的磺胺增效剂甲氧苄啶在内的11种抗生素[37]。综合养殖场水样中粘菌素抗性基因mcr和替加环素抗性基因tet(X)的总丰度均高于单养淡水养殖场的水样[38]。
在水产养殖场中施用高剂量的抗生素还会对水产品产生毒性作用,并在水产动物肌肉中蓄积,人类可以通过食用水产品而间接摄入抗生素,从而导致肠道菌群获得耐药性[39]。
1.2.3 宠物、野生动物与抗生素耐药性的传播宠物和野生动物也是耐药菌和耐药基因的储存库[40]。在宠物中使用抗生素也有助于耐药微生物从动物向人转移[41-42]。野生鸟类可以通过与人类接触获得多种耐药基因,而且其粪便中的浓度与偶尔喂食抗生素的家禽和牲畜相当[43]。此外,鹿、狐狸等哺乳动物也可作为耐药微生物的宿主和耐药基因传播的潜在媒介[44]。
1.3 环境中抗生素耐药性在不同界面的传播 1.3.1 土壤环境与抗生素耐药性土壤是耐药菌在人、牲畜、农作物之间流动的媒介[45]。研究人员从农业土壤、城市土壤、自然土壤中都分离出多重耐药菌[46]。抗生素可以通过直接施用和动物粪便进入农田土壤环境。在美国,每年有70 t抗生素用于农作物种植[47]。变形杆菌等土壤微生物可从大肠埃希菌中捕获耐药质粒,并促进耐药质粒传播至植物微生物[48]。废水灌溉也促进了耐药微生物和耐药基因向环境中的传播[49]。废水灌溉虽然对土壤微生物群落密度或组成无影响,但废水灌溉田的抗生素耐药基因丰度更高,而且丰度与灌溉强度呈正相关[50]。人类医疗废物的填埋不仅会增加土壤中抗生素耐药基因的丰度和多样性,也会增加土壤中的金属离子浓度,成为抗生素耐药性的协同选择压力[51]。
1.3.2 水环境与抗生素耐药性水环境是微生物最重要的栖息地之一,也是微生物在自然界中迁移的重要媒介,是公认的抗生素耐药基因储存库[52-54]。耐药基因可以在人、动物、家庭饮用水和公共水源中流动[55]。饮用水中就存在着多种抗生素耐药基因及其水平转移现象[56]。城市污水处理厂也是耐药菌和耐药基因交换的场所[57],污水处理无法完全去除耐药基因,氧化还原净水反而会增加抗生素耐药基因的水平转移[58-59]。
1.3.3 空气微粒与抗生素耐药性抗生素耐药基因,尤其是耐碳青霉烯酶基因可以通过医院和社区环境中的PM2.5和PM10传播[60]。家庭[61]、学校、幼儿园[62]等室内场所的空调过滤器灰尘或空气中也存在耐药微生物和耐药基因。抗生素耐药基因还可以吸附在城市扬尘、污水处理厂和垃圾焚烧厂的气溶胶中进入大气,通过气流运动实现全球传播[63]。同时这些气溶胶也可以通过雨雪沉积返回地面,实现抗生素耐药性的循环[64]。
2 全健康视角下的抗生素耐药性传播与控制运用“全健康”的理念和方法整合人、动物和环境中微生物耐药性传播途径特征,增强研究者对抗生素耐药性复杂流行病学的理解,从而调动多学科、多部门协作,遏制抗生素耐药性传播,解决人-动物-环境界面的抗生素耐药性问题[65]。
2015年,世界卫生组织、联合国粮农组织(Food and Agriculture Organization of the United Nations,FAO)和世界动物卫生组织(Office International Des Epizooties,OIE)三方联合发起了《抗微生物药物耐药性全球行动计划》,该计划要求所有国家都通过多部门协调来实施国家行动方案,以确保在人类、动物和环境领域进行全面监测、治理和政策实施。世界卫生组织不断帮助各国建立或完善国家抗生素耐药监测系统,并为国际、区域、国家和组织之间的密切合作提供更全面的标准化抗生素耐药性监测数据[66]。欧盟(European Union,EU)下属的欧洲药品管理局(European Medicines Agency,EMA)、欧洲疾病控制中心(European Centre for Disease Prevention and Control,ECDC)和欧洲食品安全局(European Food Safety Authority,EFSA)等机构在2017年制定《欧盟针对抗生素耐药性的“全健康”行动计划》[67],通过欧洲抗生素消费监测网(European Surveillance of Antimicrobial Consumption Network,ESAC-Net)[68]和欧洲兽用抗生素消费监测项目(European Surveillance of Veterinary Antimicrobial Consumption,ESVAC)[69]对整个欧盟医疗用抗生素和养殖用抗生素的使用进行监管,联合公布各行业抗生素使用数据,并证实了动物中应用抗生素与人体产生耐药基因呈正相关,明确了减少抗生素使用的必要性[70-71]。2020年,瑞典发布了《瑞典2020−2023年抗击抗生素耐药性战略》[72],该计划再次强调了抗生素耐药性在人、动物和环境之间传播的复杂性,该战略既侧重医疗和兽医、农业和环境等多部门之间的合作,又加强EU、OIE、WHO、FAO和经合组织(Organization for Economic Co-operation and Development,OECD)之间的合作[73],具有典型的“全健康”理念特色。虽然国际上已经就使用“全健康”理念解决抗生素耐药性问题达成一致[2],许多国家、地区和国际组织都启动了遏制抗生素耐药性的国家计划,但这一行动需要更多国家,尤其是在医疗领域和养殖动物中使用非处方抗生素缺乏监管的发展中国家参与(表 1)。
| 国家或地区 Country or region |
年份 Year |
负责或发起部门 Responsible or initiating department |
项目或文件 Projects or policies |
参考文献 References |
| 中国 China |
2016 | 中国国家卫健委等14个部门 National Health Commission of the People՚s Republic of China and 14 other departments |
遏制细菌耐药国家行动计划(2016‒2020年) National Action Plan for Containing Antibacterial Resistance (2016–2020) |
[74] |
| 美国 U. S. |
1996 | 美国食品和药物管理局的兽药中心,美国农业部和美国疾病预防控制中心 U.S. Food and Drug Administration Center for Veterinary Medicine (FDA/CVM), U. S. Department of Agriculture (USDA), and U.S. Centers for Disease Control and Prevention (CDC) |
国家抗生素耐药监测系统 National Antimicrobial Resistance Monitoring System (NARMS) |
[75] |
| 2020 | 总统科学技术顾问委员会 President’s Council of Advisors on Science and Technology |
遏制抗生素耐药细菌国家行动计划 National Action Plan for Combating Antibiotic-Resistant Bacteria |
[76] | |
| 加拿大 Canada |
2017 | 加拿大政府和多所学术机构、非政府组织 Government of Canada and multiple academic institutions, non-governmental organizations |
应对抗生素耐药性和抗生素使用:泛加拿大行动框架 Tackling antimicrobial resistance and antimicrobial use: a Pan-Canadian framework for action |
[77] |
| 欧盟 European Union |
2009 | 欧洲食品安全局和欧洲疾病预防和控制中心 European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC) |
欧洲兽用抗生素消费监测项目 European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) |
[68] |
| 2010 | 欧洲疾病预防和控制中心 European Centre for Disease Prevention and Control (ECDC) |
欧洲抗生素耐药性监测网络 European Antimicrobial Resistance Surveillance Network (EARS-Net) |
[78] | |
| 2011 | 欧洲药品管理局和欧洲疾病预防和控制中心 European Medicines Agency (EMA) and European Centre for Disease Prevention and Control (ECDC) |
欧洲抗生素消费监测网 European Surveillance of Antimicrobial Consumption Network (ESAC-Net) |
[69] | |
| 2012 | 欧洲疾病预防和控制中心 European Centre for Disease Prevention and Control (ECDC) |
欧洲食源性和水源性疾病和人畜共患病网络 European Food- and Waterborne Diseases and Zoonoses Network (FWD Net) |
[79] | |
| 2017 | 欧盟委员会 European Commission |
欧盟针对抗生素耐药性的“全健康”行动计划 European One Health Action Plan against antimicrobial resistance |
[67] | |
| 瑞典 Sweden |
2020 | 瑞典卫生和社会事务部 Swedish Ministry of Health and Social Affairs |
瑞典2020‒2023年抗击抗生素耐药性战略 Swedish strategy to combat antibiotic resistance 2020‒2023 |
[72, 80] |
| 荷兰 Netherlands |
2015 | 荷兰卫生、福利和体育部 Ministry of Health, Welfare and Sport of the Netherlands |
医疗领域抗生素耐药性管理协议 Administrative agreements on antibiotic resistance in healthcare |
[81] |
| 比利时 Belgium |
2013 | 比利时抗生素政策协调委员会 Belgian Antibiotic Policy Coordination Commission (BAPCOC) |
2014‒2019年第三个抗击抗生素耐药性的国家战略计划 The third National Strategic Plan to fight against antimicrobial resistance 2014‒2019 |
[82] |
| 英国 United Kingdom |
2013 | 英国卫生部 UK Department of Health |
2013‒2018年英国抗生素耐药性的国家战略 UK 5 Year Antimicrobial Resistance Strategy 2013 to 2018 |
[83] |
| 法国 France |
2011 | 法国劳动、就业和卫生部 French Ministry of Labour, Employment and Health |
法国国家抗生素预警计划(2011‒2016) Plan national d’alerte sur les antibiotiques 2011‒2016 National alert plan on antibiotics 2011‒2016 |
[84] |
| 澳大利亚 Australia |
2015 | 澳大利亚农业部和卫生部 Australian Department of Agriculture and Department of Health |
2015−2019年国家抗生素耐药性战略 National Antimicrobial Resistance Strategy 2015–2019 |
[85] |
| 印度 India |
2012 | 印度疾病预防控制中心 Indian National Centre for Disease Control |
遏制抗生素耐药性国家计划(2012‒2017年十二五规划内 National Programme for the Containment of Antimicrobial Resistance (within the 12th Five Year Plan, 2012‒2017) |
[86] |
| 2012 | 印度医学研究理事会 Indian Council of Medical Research |
抗生素耐药性监测研究网络 Antimicrobial Resistance Surveillance Research Network |
[87] | |
| 2017 | 包括卫生、环境、农业和粮食在内的12个部委 India՚s 12 ministries, including health, environment, agriculture and food departments |
抗生素耐药性国家行动计划 The National Action Plan on Antimicrobial Resistance 关于抗生素耐药性的德里宣言 The Delhi Declaration on AMR |
[88-89] | |
| 越南 Vietnam |
2012 | 越南国家卫生部、越南传染病学会、牛津大学临床研究会和瑞典林雪平大学合作 Collaboration between the Vietnamese Minister of Health, the Vietnamese Infectious Diseases Society, the Oxford University Clinical Research Unit and Linköping University in Sweden |
越南抗生素耐药性控制项目 Viet Nam Resistance (VINARES) Project |
[90] |
| 泰国 Thailand |
1997 | 泰国国家卫生研究院 the National Institutes of Health in Thailand |
泰国国家卫生研究院国家抗生素耐药性监测中心 National AMR Surveillance Center at the National Institutes of Health |
[91] |
| 非洲地区 Africa |
2002 | 美国国际开发署、美国疾病预防控制中心的IDSR团队与世卫组织/非洲区域办事处合作 Collaboration between United States Agency for International Development (USAID), CDC՚s Integrated Disease Surveillance and Response team and the WHO/Africa Regional Office (WHO/AFRO) |
非洲综合疾病监测和应对 AFRO Integrated Disease Surveillance and Response (IDSR) |
[92] |
| 肯尼亚 Kenya |
2017 | 肯尼亚国家抗生素管理咨询委员会 Kenya National Antimicrobial Stewardship Advisory Committee |
预防和控制抗生素耐药性国家政策 National Policy on Prevention and Containment of AMR |
[93] |
| 南非 South Africa |
2015 | 南非国家卫生部 South African National Department of Health |
抗生素耐药性-国家战略框架(2014‒2024)和抗生素耐药性战略框架实施计划(2014‒2019) Antimicrobial resistance. National strategy framework 2014–2024. And implementation plan for the antimicrobial resistance strategy framework in South Africa, 2014–2019 |
[94] |
| 拉丁美洲地区 Latin America |
1996 | 泛美卫生组织/世界卫生组织美洲地区办事处及其成员国 Pan American Health Organization/World Health Organization Regional Office for the Americas (PAHO/AMRO) and its member countries |
拉丁美洲抗生素耐药性监测网络 Red Latinoamericana de Vigilancia a las Resistencias Antimicrobianas (ReLAVRA) Latin American Network for Antimicrobial Resistance Surveillance |
[95] |
2016年,中国国家卫健委等14个部门联合制定《遏制细菌耐药国家行动计划(2016−2020年)》,该计划不仅完善了临床和养殖业的抗生素应用和细菌耐药的监测网络,还加强了对水、土壤等环境中抗生素污染的监测,实现农林畜牧业和水产养殖业的联防联动[74]。2021年中国细菌耐药监测网(China Antimicrobial Surveillance Network,CHINET)的监测结果显示,在甲氧西林耐药的细菌中,金黄色葡萄球菌检出率由2016年的38.4%下降至30.0%,而检出率处于高位的表皮葡萄球菌未发现针对万古霉素、去甲万古霉素和替加环素的耐药菌株,其他凝固酶阴性的葡萄球菌(检出率为77.7%)中也未发现针对万古霉素和替加环素的耐药菌株;铜绿假单胞菌、鲍曼不动杆菌和耐碳青霉烯肺炎克雷伯菌对硫酸粘菌素仍高度敏感。目前,我国各地区的医院、养殖场的耐药监测水平参差不齐,影响监测网络中的数据质量,而且各个监测网之间存在“信息孤岛”现象。因此,“全健康”理念下战略部署和整合现有平台来监管“人-动物-环境”中的耐药性显得尤为重要。
3 总结与展望各种人类活动促进抗生素耐药性在人-动物-环境界面的流动与循环,“全健康”理念是解决这一复杂而又隐匿传播的有效策略。目前,将“全健康”政策应用于遏制抗生素耐药性的行动仍然处于全球倡议、区域合作的起步阶段,“全健康”治理的碎片化问题仍然突出。
我们倡导更多的国家和地区应用“全健康”方法控制抗生素耐药性传播,通过减少在食用动物养殖中使用人类医疗中发挥重要作用的抗生素,禁止以促进养殖动物生长为目的长期在饲料中使用抗生素,在人群中通过预防感染以减少过度使用抗生素,加强个人卫生和饮用水等卫生设施,加强环境污物治理来阻止抗生素耐药微生物的快速传播,并通过改进监测、药物管理、感染控制、医疗卫生、畜牧业和寻找抗微生物药物的替代品等多部门协同合作来应对抗生素耐药性的全球挑战,促进人类健康、动物健康和生态环境协调发展。
| [1] |
Zhang ZY, Zhang Q, Wang TZ, Xu NH, Lu T, Hong WJ, Penuelas J, Gillings M, Wang MX, Gao WW, et al. Assessment of global health risk of antibiotic resistance genes[J]. Nature Communications, 2022, 13: 1553. DOI:10.1038/s41467-022-29283-8 |
| [2] |
World Health Organization. Global action plan on antimicrobial resistance[J]. Microbe: the News Magazine of the American Society for Microbiology, 2015, 10(9): 354-355. DOI:10.1128/microbe.10.354.1 |
| [3] |
Rawson TM, Ming D, Ahmad R, Moore LSP, Holmes AH. Antimicrobial use, drug-resistant infections and COVID-19[J]. Nature Reviews Microbiology, 2020, 18(8): 409-410. DOI:10.1038/s41579-020-0395-y |
| [4] |
Silbergeld EK, Graham J, Price LB. Industrial food animal production, antimicrobial resistance, and human health[J]. Annual Review of Public Health, 2008, 29: 151-169. DOI:10.1146/annurev.publhealth.29.020907.090904 |
| [5] |
Spratt BG. Resistance to antibiotics mediated by target alterations[J]. Science, 1994, 264(5157): 388-393. DOI:10.1126/science.8153626 |
| [6] |
Peterson E, Kaur P. Antibiotic resistance mechanisms in bacteria: relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens[J]. Frontiers in Microbiology, 2018, 9: 2928. DOI:10.3389/fmicb.2018.02928 |
| [7] |
Brañas P, Villa J, Viedma E, Mingorance J, Orellana MA, Chaves F. Molecular epidemiology of carbapenemase-producing Klebsiella pneumoniae in a hospital in Madrid: successful establishment of an OXA-48 ST11 clone[J]. International Journal of Antimicrobial Agents, 2015, 46(1): 111-116. DOI:10.1016/j.ijantimicag.2015.02.019 |
| [8] |
Forsberg KJ, Reyes A, Wang B, Selleck EM, Sommer MOA, Dantas G. The shared antibiotic resistome of soil bacteria and human pathogens[J]. Science, 2012, 337(6098): 1107-1111. DOI:10.1126/science.1220761 |
| [9] |
Stoesser N, Sheppard AE, Pankhurst L, De Maio N, Moore CE, Sebra R, Turner P, Anson LW, Kasarskis A, Batty EM, et al. Evolutionary history of the global emergence of the Escherichia coli epidemic clone ST131[J]. mBio, 2016, 7(2): e02162. |
| [10] |
Zarei-Baygi A, Harb M, Wang P, Stadler LB, Smith AL. Evaluating antibiotic resistance gene correlations with antibiotic exposure conditions in anaerobic membrane bioreactors[J]. Environmental Science & Technology, 2019, 53(7): 3599-3609. |
| [11] |
MacKenzie JS, Jeggo M. The one health approach-why is it so important?[J]. Tropical Medicine and Infectious Disease, 2019, 4(2): 88. DOI:10.3390/tropicalmed4020088 |
| [12] |
Cabello FC, Godfrey HP, Buschmann AH, Dölz HJ. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance[J]. The Lancet Infectious Diseases, 2016, 16(7): e127-e133. DOI:10.1016/S1473-3099(16)00100-6 |
| [13] |
Thai PK, Ky LX, Binh VN, Nhung PH, Nhan PT, Hieu NQ, Dang NTT, Tam NKB, Anh NTK. Occurrence of antibiotic residues and antibiotic-resistant bacteria in effluents of pharmaceutical manufacturers and other sources around Hanoi, Vietnam[J]. Science of the Total Environment, 2018, 645: 393-400. DOI:10.1016/j.scitotenv.2018.07.126 |
| [14] |
Bielen A, Šimatović A, Kosić-Vukšić J, Senta I, Ahel M, Babić S, Jurina T, González Plaza JJ, Milaković M, Udiković-Kolić N. Negative environmental impacts of antibiotic-contaminated effluents from pharmaceutical industries[J]. Water Research, 2017, 126: 79-87. DOI:10.1016/j.watres.2017.09.019 |
| [15] |
Kookana RS, Williams M, Boxall ABA, Larsson DGJ, Gaw S, Choi K, Yamamoto H, Thatikonda S, Zhu YG, Carriquiriborde P. Potential ecological footprints of active pharmaceutical ingredients: an examination of risk factors in low-, middle- and high-income countries[J]. Philosophical Transactions of the Royal Society B: Biological Sciences, 2014, 369(1656): 20130586. DOI:10.1098/rstb.2013.0586 |
| [16] |
Hocquet D, Muller A, Bertrand X. What happens in hospitals does not stay in hospitals: antibiotic-resistant bacteria in hospital wastewater systems[J]. Journal of Hospital Infection, 2016, 93(4): 395-402. DOI:10.1016/j.jhin.2016.01.010 |
| [17] |
Lamba M, Graham DW, Ahammad SZ. Hospital wastewater releases of carbapenem-resistance pathogens and genes in urban India[J]. Environmental Science & Technology, 2017, 51(23): 13906-13912. |
| [18] |
Rodriguez-Mozaz S, Chamorro S, Marti E, Huerta B, Gros M, Sànchez-Melsió A, Borrego CM, Barceló D, Balcázar JL. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river[J]. Water Research, 2015, 69: 234-242. DOI:10.1016/j.watres.2014.11.021 |
| [19] |
Hutinel M, Fick J, Larsson DGJ, Flach CF. Investigating the effects of municipal and hospital wastewaters on horizontal gene transfer[J]. Environmental Pollution, 2021, 276: 116733. DOI:10.1016/j.envpol.2021.116733 |
| [20] |
Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, et al. Antibiotic resistance—the need for global solutions[J]. The Lancet Infectious Diseases, 2013, 13(12): 1057-1098. DOI:10.1016/S1473-3099(13)70318-9 |
| [21] |
Michael CA, Dominey-Howes D, Labbate M. The antimicrobial resistance crisis: causes, consequences, and management[J]. Frontiers in Public Health, 2014, 2: 145. |
| [22] |
Wen CX. Microecological preparation combined with mesalazine in the treatment of ulcerative colitis: effect observation on 34 cases[J]. Chinese Journal of Coloproctology, 2021, 41(10): 31-32. (in Chinese) 温晨旭. 微生态制剂联合美沙拉嗪治疗溃疡性结肠炎34例疗效观察[J]. 中国肛肠病杂志, 2021, 41(10): 31-32. |
| [23] |
Zhang W, Zuo FR, Qiu Q, Zhang CJ, Wang QJ, Zhou Y. Effect of compound probiotics on laying performance, egg quality, serum parameters and intestinal morphology of laying hens[J]. Feed Research, 2022, 45(2): 40-45. (in Chinese) 张伟, 左方瑞, 邱权, 张成杰, 王启军, 周樱. 复合微生态制剂对蛋鸡产蛋性能、蛋品质、血清生化指标和肠道形态的影响[J]. 饲料研究, 2022, 45(2): 40-45. DOI:10.13557/j.cnki.issn1002-2813.2022.02.009 |
| [24] |
Liu C, Zhang ZY, Dong K, Yuan JP, Guo XK. Antibiotic resistance of probiotic strains of lactic acid bacteria isolated from marketed foods and drugs[J]. Biomed Environ Sci, 2009, 22(5): 401-412. DOI:10.1016/S0895-3988(10)60018-9 |
| [25] |
Fu XM, Lyu L, Wang Y, Zhang Y, Guo XK, Chen Q, Liu C. Safety assessment and probiotic characteristics of Enterococcus lactis JDM1[J]. Microbial Pathogenesis, 2022, 163: 105380. DOI:10.1016/j.micpath.2021.105380 |
| [26] |
Wei YX, Zhang ZY, Liu C, Malakar PK, Guo XK. Safety assessment of Bifidobacterium longum JDM301 based on complete genome sequences[J]. World Journal of Gastroenterology, 2012, 18(5): 479-488. DOI:10.3748/wjg.v18.i5.479 |
| [27] |
Zhang ZY, Liu C, Zhu YZ, Wei YX, Tian F, Zhao GP, Guo XK. Safety assessment of Lactobacillus plantarum JDM1 based on the complete genome[J]. International Journal of Food Microbiology, 2012, 153(1/2): 166-170. |
| [28] |
Cantas L, Shah SQA, Cavaco LM, Manaia CM, Walsh F, Popowska M, Garelick H, Bürgmann H, Sørum H. A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota[J]. Frontiers in Microbiology, 2013, 4: 96. |
| [29] |
Xie WY, Shen Q, Zhao FJ. Antibiotics and antibiotic resistance from animal manures to soil: a review[J]. European Journal of Soil Science, 2018, 69(1): 181-195. DOI:10.1111/ejss.12494 |
| [30] |
Bartlett JG, Gilbert DN, Spellberg B. Seven ways to preserve the miracle of antibiotics[J]. Clinical Infectious Diseases, 2013, 56(10): 1445-1450. DOI:10.1093/cid/cit070 |
| [31] |
Zhang X, Ma CJ, Zhang W, Li W, Yu JL, Xue D, Wu XL, Deng GC. Shifts in microbial community, pathogenicity-related genes and antibiotic resistance genes during dairy manure piled up[J]. Microbial Biotechnology, 2020, 13(4): 1039-1053. DOI:10.1111/1751-7915.13551 |
| [32] |
Shanta IS, Hasnat MA, Zeidner N, Gurley ES, Azziz-Baumgartner E, Sharker MAY, Hossain K, Khan SU, Haider N, Bhuyan AA, et al. Raising backyard poultry in rural Bangladesh: financial and nutritional benefits, but persistent risky practices[J]. Transboundary and Emerging Diseases, 2017, 64(5): 1454-1464. DOI:10.1111/tbed.12536 |
| [33] |
Roess AA, Winch PJ, Akhter A, Afroz D, Ali NA, Shah R, Begum N, Seraji HR, El Arifeen S, Darmstadt GL, et al. Household animal and human medicine use and animal husbandry practices in rural Bangladesh: risk factors for emerging zoonotic disease and antibiotic resistance[J]. Zoonoses and Public Health, 2015, 62(7): 569-578. DOI:10.1111/zph.12186 |
| [34] |
Engberg J, Aarestrup FM, Taylor DE, Gerner-Smidt P, Nachamkin I. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates[J]. Emerging Infectious Diseases, 2001, 7(1): 24-34. DOI:10.3201/eid0701.010104 |
| [35] |
Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Teillant A, Laxminarayan R. Global trends in antimicrobial use in food animals[J]. PNAS, 2015, 112(18): 5649-5654. DOI:10.1073/pnas.1503141112 |
| [36] |
Schar D, Sommanustweechai A, Laxminarayan R, Tangcharoensathien V. Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: optimizing use and addressing antimicrobial resistance[J]. PLoS Medicine, 2018, 15(3): e1002521. DOI:10.1371/journal.pmed.1002521 |
| [37] |
Han QF, Zhao S, Zhang XR, Wang XL, Song C, Wang SG. Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, north China[J]. Environment International, 2020, 138: 105551. DOI:10.1016/j.envint.2020.105551 |
| [38] |
Xu CY, Lv ZQ, Shen YB, Liu DJ, Fu YL, Zhou L, Liu WW, Chen K, Ye HL, Xia X, et al. Metagenomic insights into differences in environmental resistome profiles between integrated and monoculture aquaculture farms in China[J]. Environment International, 2020, 144: 106005. DOI:10.1016/j.envint.2020.106005 |
| [39] |
Heuer OE, Kruse H, Grave K, Collignon P, Karunasagar I, Angulo FJ. Human health consequences of use of antimicrobial agents in aquaculture[J]. Clinical Infectious Diseases, 2009, 49(8): 1248-1253. DOI:10.1086/605667 |
| [40] |
Hudson CR, Quist C, Lee MD, Keyes K, Dodson SV, Morales C, Sanchez S, White DG, Maurer JJ. Genetic relatedness of Salmonella isolates from nondomestic birds in southeastern United States[J]. Journal of Clinical Microbiology, 2000, 38(5): 1860-1865. DOI:10.1128/JCM.38.5.1860-1865.2000 |
| [41] |
Scott Weese J. Antimicrobial resistance in companion animals[J]. Animal Health Research Reviews, 2008, 9(2): 169-176. DOI:10.1017/S1466252308001485 |
| [42] |
Joosten P, Ceccarelli D, Odent E, Sarrazin S, Graveland H, Van Gompel L, Battisti A, Caprioli A, Franco A, Wagenaar JA, et al. Antimicrobial usage and resistance in companion animals: a cross-sectional study in three European countries[J]. Antibiotics: Basel, Switzerland, 2020, 9(2): 87. |
| [43] |
Zhao HR, Sun RN, Yu PF, Alvarez PJJ. High levels of antibiotic resistance genes and opportunistic pathogenic bacteria indicators in urban wild bird feces[J]. Environmental Pollution, 2020, 266: 115200. DOI:10.1016/j.envpol.2020.115200 |
| [44] |
Carroll D, Wang J, Fanning S, McMahon BJ. Antimicrobial resistance in wildlife: implications for public health[J]. Zoonoses and Public Health, 2015, 62(7): 534-542. DOI:10.1111/zph.12182 |
| [45] |
Tyrrell C, Burgess C, Brennan F, Walsh F. Antibiotic resistance in grass and soil[J]. Biochem Soc Trans, 2019. |
| [46] |
Walsh F, Duffy B. The culturable soil antibiotic resistome: a community of multi-drug resistant bacteria[J]. PLoS One, 2013, 8(6): e65567. DOI:10.1371/journal.pone.0065567 |
| [47] |
Hollis A, Ahmed Z. Preserving antibiotics, rationally[J]. The New England Journal of Medicine, 2013, 369(26): 2474-2476. DOI:10.1056/NEJMp1311479 |
| [48] |
Xu H, Chen ZY, Huang RY, Cui YX, Li Q, Zhao YH, Wang XL, Mao DQ, Luo Y, Ren HQ. Antibiotic resistance gene-carrying plasmid spreads into the plant endophytic bacteria using soil bacteria as carriers[J]. Environmental Science & Technology, 2021, 55(15): 10462-10470. |
| [49] |
Munir M, Wong K, Xagoraraki I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan[J]. Water Research, 2011, 45(2): 681-693. DOI:10.1016/j.watres.2010.08.033 |
| [50] |
Kampouris ID, Agrawal S, Orschler L, Cacace D, Kunze S, Berendonk TU, Klümper U. Antibiotic resistance gene load and irrigation intensity determine the impact of wastewater irrigation on antimicrobial resistance in the soil microbiome[J]. Water Research, 2021, 193: 116818. DOI:10.1016/j.watres.2021.116818 |
| [51] |
Chi T, Zhang AG, Zhang XF, Li AD, Zhang HH, Zhao ZQ. Characteristics of the antibiotic resistance genes in the soil of medical waste disposal sites[J]. Science of the Total Environment, 2020, 730: 139042. DOI:10.1016/j.scitotenv.2020.139042 |
| [52] |
Baquero F, Martínez JL, Cantón R. Antibiotics and antibiotic resistance in water environments[J]. Current Opinion in Biotechnology, 2008, 19(3): 260-265. DOI:10.1016/j.copbio.2008.05.006 |
| [53] |
Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy MC, Michael I, Fatta-Kassinos D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review[J]. Science of the Total Environment, 2013, 447: 345-360. DOI:10.1016/j.scitotenv.2013.01.032 |
| [54] |
Zhang XX, Zhang T, Fang HHP. Antibiotic resistance genes in water environment[J]. Applied Microbiology and Biotechnology, 2009, 82(3): 397-414. DOI:10.1007/s00253-008-1829-z |
| [55] |
Purohit MR, Chandran S, Shah H, Diwan V, Tamhankar AJ, Stålsby Lundborg C. Antibiotic resistance in an Indian rural community: a 'one-health' observational study on commensal coliform from humans, animals, and water[J]. International Journal of Environmental Research and Public Health, 2017, 14(4): 386. DOI:10.3390/ijerph14040386 |
| [56] |
Ma LP, Li B, Jiang XT, Wang YL, Xia Y, Li AD, Zhang T. Catalogue of antibiotic resistome and host-tracking in drinking water deciphered by a large scale survey[J]. Microbiome, 2017, 5(1): 154. DOI:10.1186/s40168-017-0369-0 |
| [57] |
Karkman A, Do TT, Walsh F, Virta MPJ. Antibiotic-resistance genes in waste water[J]. Trends in Microbiology, 2018, 26(3): 220-228. DOI:10.1016/j.tim.2017.09.005 |
| [58] |
Jong MC, Harwood CR, Blackburn A, Snape JR, Graham DW. Impact of redox conditions on antibiotic resistance conjugative gene transfer frequency and plasmid fate in wastewater ecosystems[J]. Environmental Science & Technology, 2020, 54(23): 14984-14993. |
| [59] |
Neudorf KD, Huang YN, Ragush CM, Yost CK, Jamieson RC, Truelstrup Hansen L. Antibiotic resistance genes in municipal wastewater treatment systems and receiving waters in Arctic Canada[J]. Science of the Total Environment, 2017, 598: 1085-1094. DOI:10.1016/j.scitotenv.2017.04.151 |
| [60] |
He P, Wu Y, Huang WZ, Wu XW, Lv JY, Liu PD, Bu L, Bai ZJ, Chen SY, Feng WR, et al. Characteristics of and variation in airborne ARGs among urban hospitals and adjacent urban and suburban communities: a metagenomic approach[J]. Environment International, 2020, 139: 105625. DOI:10.1016/j.envint.2020.105625 |
| [61] |
Gandara A, Mota LC, Flores C, Perez HR, Green CF, Gibbs SG. Isolation of Staphylococcus aureus and antibiotic-resistant Staphylococcus aureus from residential indoor bioaerosols[J]. Environmental Health Perspectives, 2006, 114(12): 1859-1864. DOI:10.1289/ehp.9585 |
| [62] |
Li N, Chai YM, Ying GG, Jones KC, Deng WJ. Airborne antibiotic resistance genes in Hong Kong kindergartens[J]. Environmental Pollution, 2020, 260: 114009. DOI:10.1016/j.envpol.2020.114009 |
| [63] |
Kellogg CA, Griffin DW. Aerobiology and the global transport of desert dust[J]. Trends in Ecology & Evolution, 2006, 21(11): 638-644. |
| [64] |
Green JK, Konings AG, Alemohammad SH, Berry J, Entekhabi D, Kolassa J, Lee JE, Gentine P. Regionally strong feedbacks between the atmosphere and terrestrial biosphere[J]. Nature Geoscience, 2017, 10(6): 410-414. DOI:10.1038/ngeo2957 |
| [65] |
World Health Organization. AMR and One Health[EB/OL]. [2022-02-16]. https://www.who.int/southeastasia/outbreaks-and-emergencies/health-emergency-information-risk-assessment/antimicrobial-resistance-one-health
|
| [66] |
Tornimbene B, Eremin S, Escher M, Griskeviciene J, Manglani S, Pessoa-Silva CL. WHO Global Antimicrobial Resistance Surveillance System early implementation 2016-17[J]. The Lancet Infectious Diseases, 2018, 18(3): 241-242. DOI:10.1016/S1473-3099(18)30060-4 |
| [67] |
European Commission. A European One Health Action Plan against Antimicrobial Resistance[EB/OL]. [2022-02-16]. https://health.ec.europa.eu/system/files/2020-01/amr_2017_action-plan_0.pdf
|
| [68] |
European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2014[J]. EFSA Journal, 2016, 14(2): 4380. |
| [69] |
EMA. Sales of veterinary antimicrobial agents in 31 European countries in 2017[EB/OL]. [2022-02-16]. https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2017_en.pdf
|
| [70] |
European Centre for Disease Prevention and Control, European Food Safety Authority, European Medicines Agency. ECDC/EFSA/EMA first joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals[J]. EFSA Journal, 2015, 13(1): 4006. DOI:10.2903/j.efsa.2015.4006 |
| [71] |
European Centre for Disease Prevention and Control (ECDC), (efsa) EFSA, (ema) EMA. ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals: Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report[J]. EFSA Journal European Food Safety Authority, 2017, 15(7): e04872. |
| [72] |
Ministry of Health and Social Affairs. Swedish strategy to combat antibiotic resistance 2020–2023[EB/OL]. [2022-02-16]. https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/swedish-strategy-to-combat-antimicrobial-resistance-2020‒2023.pdf?sfvrsn=dacbb55f_1&download=true
|
| [73] |
Eriksen J, Björkman I, Röing M, Essack SY, Stålsby Lundborg C. Exploring the one health perspective in Sweden՚s policies for containing antibiotic resistance[J]. Antibiotics: Basel, Switzerland, 2021, 10(5): 526. |
| [74] |
Government of the People՚s Republic of China. National Action Plan to Combat Antibiotic Resistance (2016‒2020)[EB/OL]. [2022-02-16]. http://www.gov.cn/xinwen/2016-08/25/content_5102348.htm 中华人民共和国中央政府. 《遏制细菌耐药国家行动计划(2016‒2020年)》[EB/OL]. [2022-02-16]. http://www.gov.cn/xinwen/2016-08/25/content_5102348.htm. |
| [75] |
Karp BE, Tate H, Plumblee JR, Dessai U, Whichard JM, Thacker EL, Hale KR, Wilson W, Friedman CR, Griffin PM, et al. National antimicrobial resistance monitoring system: two decades of advancing public health through integrated surveillance of antimicrobial resistance[J]. Foodborne Pathogens and Disease, 2017, 14(10): 545-557. DOI:10.1089/fpd.2017.2283 |
| [76] |
The White House, Washington, DC. National action plan for combating antibiotic-resistant bacteria[EB/OL]. [2022-02-16]. https://www.hhs.gov/sites/default/files/carb-national-action-plan-2020-2025.pdf#:~:text=The%20National%20Action%20Plan%20for%20Combating%20Antibiotic-Resistant%20Bacteria,Americans%20by%20changing%20the%20course%20of%20antibiotic%20resistance.
|
| [77] |
Government of Canada, Minister of Health. Tackling Antimicrobial Resistance and Antimicrobial Use: a Pan-Canadian Framework for Action[EB/OL]. [2022-02-16]. https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/canada-communicable-disease-report-ccdr/monthly-issue/2017-43/ccdr-volume-43-11-november-2-2017/ccdr-43-11-phac-eng.pdf#:~:text=Tackling%20Antimicrobial%20Resistance%20and%20Antimicrobial%20Use%3A%20A%20Pan-Canadian,collaborative%20action%20on%20AMR%20and%20antimicrobial%20use%20%28AMU%29.
|
| [78] |
European Centre for Disease Prevention and Control Stockholm. Surveillance of antimicrobial resistance in Europe 2018[EB/OL]. [2022-02-16]. https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf
|
| [79] |
ECDC. EU protocol for harmonised monitoring of antimicrobial resistance in human Salmonella and Campylobacter isolates[EB/OL]. [2022-02-16]. https://www.ecdc.europa.eu/sites/default/files/documents/antimicrobial-resistance-Salmonella-Campylobacter-harmonised-monitoring.pdf
|
| [80] |
Government Offices of Sweden. Swedish strategy to combat antibiotic resistance 2016[EB/OL]. [2022-02-16]. https://www.government.se/49bbcf/contentassets/168838e186de455ca7fe868bee92d209/swedish-strategy-to-combat-antibiotic-resistance.pdf
|
| [81] |
Government of the Netherlands. Administrative agreements on antibiotic resistance in healthcare[EB/OL]. [2022-02-16]. https://www.government.nl/topics/antibiotic-resistance/documents/leaflets/2015/09/22/tackling-antibiotic-resistance
|
| [82] |
Belgian Antibiotic Policy Coordination Commission (BAPCOC). Note de politique pour la législature 2014‒2019. [Policy paper for the 2014‒2019 term] [EB/OL]. [2022-02-16]. https://organesdeconcertation.sante.belgique.be/sites/default/files/documents/belgische_commissie_voor_de_coordinatie_van_het_antibioticabeleid-fr/19100224_fr.pdf
|
| [83] |
UK Department of Health. UK 5 Year Antimicrobial Resistance Strategy 2013 to 2018[EB/OL]. [2022-02-16]. https://www.gov.uk/government/publications/uk-5-year-antimicrobial-resistance-strategy-2013-to-2018
|
| [84] |
Ministère Du Travail, De l'Emploi et de la Santé[Ministry of Labour, Employment and Health]. Plan national d'alerte sur les antibiotiques 2011‒2016. [National alert plan on antibiotics 2011‒2016] [EB/OL]. [2022-02-16]. https://solidarites-sante.gouv.fr/IMG/pdf/plan_antibiotiques_2011-2016_DEFINITIF.pdf
|
| [85] |
Department of Agriculture and Department of Health. National Antimicrobial Resistance Strategy 2015–2019[EB/OL]. [2022-02-16]. https://www.awe.gov.au/agriculture-land/animal/health/amr/antimicrobial-resistance-strategy
|
| [86] |
India. Directorate General of Health Services. National policy for containment of antimicrobial resistance[EB/OL]. [2022-02-16]. http://www.ncdc.gov.in/ab_policy.pdf
|
| [87] |
Indian Council of Medical Research. Indian Council of Medical Research: annual report 2012-13[EB/OL]. [2022-02-16]. http://www.icmr.nic.in/annual/2012-13/english/annual.htm
|
| [88] |
Government of India. Delhi Declaration on AMR[EB/OL]. [2022-02-16]. http://www.searo.who.int/entity/india/topics/antimicrobial_resistance/delhi_dec_amr.pdf
|
| [89] |
MoHFW, Government of India. Government of India National Action Plan on Antimicrobial Resistance (NAP-AMR)[EB/OL]. [2022-02-16]. https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/national-action-plan-on-amr-(india).pdf?sfvrsn=9f396e90_1&download=true
|
| [90] |
Wertheim HFL, Chandna A, Vu PD, Pham CV, Nguyen PDT, Lam YM, Nguyen CV, Larsson M, Rydell U, Nilsson LE, et al. Providing impetus, tools, and guidance to strengthen national capacity for antimicrobial stewardship in Vietnam[J]. PLoS Medicine, 2013, 10(5): e1001429. DOI:10.1371/journal.pmed.1001429 |
| [91] |
The National Institutes of Health, Thailand. The National AMR Surveillance Center at the National Institutes of Health[EB/OL]. [2022-02-16]. http://narst.dmsc.moph.go.th/
|
| [92] |
Grundmann H, Klugman KP, Walsh T, Ramon-Pardo P, Sigauque B, Khan W, Laxminarayan R, Heddini A, Stelling J. A framework for global surveillance of antibiotic resistance[J]. Drug Resistance Updates, 2011, 14(2): 79-87. DOI:10.1016/j.drup.2011.02.007 |
| [93] |
Cox JA, Vlieghe E, Mendelson M, Wertheim H, Ndegwa L, Villegas MV, Gould I, Hara GL. Antibiotic stewardship in low- and middle-income countries: the same but different?[J]. Clinical Microbiology and Infection, 2017, 23(11): 812-818. DOI:10.1016/j.cmi.2017.07.010 |
| [94] |
National Department of Health, South Africa. Antimicrobial resistance. National strategy framework 2018–24. And implementation plan for the antimicrobial resistance strategy framework in South Africa, 2014–19[EB/OL]. [2022-02-16]. https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/south-africa-antimicrobial-resistance-national-action-plan-2018---2024.pdf?sfvrsn=533118b0_1&download=true
|
| [95] |
PAHO/AMRO. Red Latinoamericana y del Caribe de Vigilancia de la Resistencia a los Antimicrobianos -ReLAVRA[EB/OL]. [2022-02-16]. https://www.paho.org/es/temas/resistencia-antimicrobianos/red-latinoamericana-caribe-vigilancia-resistencia-antimicrobianos
|
 2022, Vol. 49
2022, Vol. 49