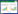扩展功能
文章信息
- 王晶晶, 李秀颖, 宋玉芳, 严俊, 杨毅
- WANG Jingjing, LI Xiuying, SONG Yufang, YAN Jun, YANG Yi
- 环境因子对厌氧微生物脱卤的影响研究进展
- Effects of environmental factors on anaerobic microbial dehalogenation: a review
- 微生物学通报, 2022, 49(10): 4357-4381
- Microbiology China, 2022, 49(10): 4357-4381
- DOI: 10.13344/j.microbiol.china.220121
-
文章历史
- 收稿日期: 2022-01-30
- 接受日期: 2022-04-12
- 网络首发日期: 2022-05-19
有机卤代物在全球范围内的广泛应用(如干洗剂、杀虫剂、化工原料等)及不当释放导致其成为地下环境中普遍存在的一类有机污染物[1-2],其中较为典型的有机卤污染物包括四氯乙烯(perchloroethylene,PCE)、1, 1, 2-三氯乙烷(1, 1, 2-trichloroethane,TCA)、多氯联苯(polychlorobiphenyls,PCBs)、全氟羧酸(perfluorocarboxylic acids,PFCAs)和多溴联苯醚(polybrominated diphenyl ethers,PBDEs)等[3]。大多数有机卤污染物都具有一定的神经毒性和致癌风险,其在地下环境中的持久性稳定存在威胁着生态系统功能、饮用水安全和人类健康[4-5]。因此,地下环境中的有机卤污染是亟待解决的全球性重要环境问题之一,例如双对氯苯基三氯乙烷(dichlorodiphenyltrichloroethane,DDT)等有机氯农药和杀虫剂的广泛使用及历史遗留问题等。绿色、环保、可持续的生物修复技术逐渐成为有机卤污染修复的主要手段之一[6],而在这个过程中起关键作用的是一类被称为有机卤呼吸细菌(organohalide-respiring bacteria,OHRB)的厌氧微生物[7]。除了温度、pH、碳源等这些制约微生物生长的关键因子外[8],有机卤污染物的浓度[9]、共存有机卤污染物的种类和浓度[10],以及环境中电子供受体组成[11]、氧气暴露程度[12]、土壤含水率[13]等也均能决定OHRB的生长和能量转化效率[14],进而影响到有机卤污染物的脱卤效率(图 1)。
有机卤污染场地的原位生物修复工程中,各种各样的因素制约着修复工程的实施效果,因此,有效调控影响OHRB脱卤效率的多种环境因素,可为原位生物修复的成功实施提供科学的理论与技术指导。本文首先简要介绍了OHRB及其还原脱卤原理,然后阐述影响OHRB生长、脱卤功能及效率的关键因子,并对OHRB的未来研究方向进行展望。
1 有机卤呼吸 1.1 有机卤呼吸细菌有机卤呼吸(organohalide respiration,OHR),是在厌氧环境中以有机卤代物作为电子受体、以氢气或甲酸等小分子有机酸作为电子供体,通过还原性脱卤酶(reductive dehalogenase,RDase)裂解卤-碳键的方式获得自身生长代谢所需能量的一种呼吸方式,而具有相应能量代谢功能的细菌被称为OHRB[7, 15-17]。自1982年第一株厌氧脱氯菌株Desulfomonile tiedjei DCB-1[18]被分离纯化以来,多个OHRB在各种自然环境中被发现并分离纯化,而海洋作为一个天然有机卤代物的储存库,是重要的OHRB资源库。例如,变形菌纲(Deltaproteobacteria)中的部分脱氯微生物分离自海洋底泥[19-22]。近年来,有机卤呼吸能量代谢逐渐被证实是治理地下水环境中有机卤代污染物的最有效方法之一,并在全球多个国家的有机卤污染场地得到成功应用[23-28]。
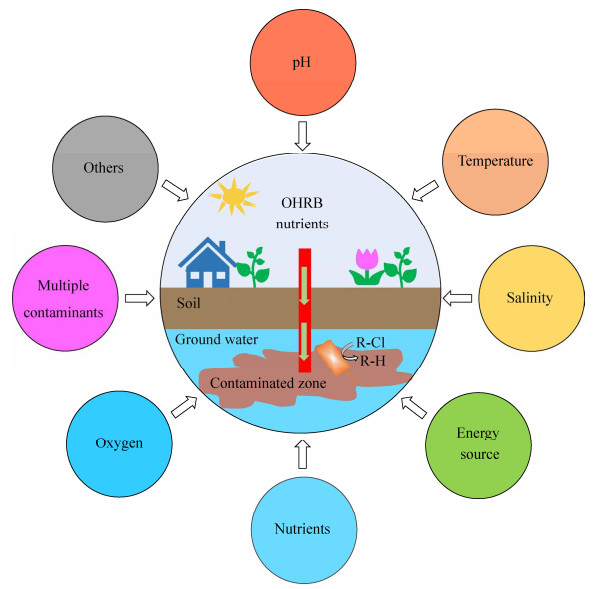
基于16S rRNA基因序列的系统发育树,目前已被发现并分离纯化的OHRB主要来自于厚壁菌门(Firmicutes)、绿弯菌门(Chloroflexi)和变形菌门(Proteobacteria) (图 2)。其中只能以有机卤呼吸的方式获得能量的OHRB称为专性OHRB,而除有机卤呼吸外还有其他能量代谢方式的OHRB称为兼性OHRB[29]。兼性OHRB通常可以依赖多种电子受体进行能量代谢(例如硝酸盐和金属离子等),包括变形菌门中的地杆菌(Geobacter)[30-31]、脱硫单胞菌(Desulfuromonas)[32]、脱硫月牙形菌(Desulfoluna)[33]、厌氧黏杆菌(Anaeromyxobacter)[34]、脱硫念珠菌(Desulfomonile)[18]、脱硫弧菌(Desulfovibrio)[19, 35]、希瓦氏菌(Shewanella)[36]、丛毛单胞菌(Comamonas)[37]、硫化螺旋菌(Sulfurospirillum)[38-40]及厚壁菌门中的脱亚硫酸杆菌(Desulfitobacterium)[41]。专性OHRB主要包括厚壁菌门中能够还原氯代烃、氯苯、氯苯酚及溴苯酚等的脱卤素杆菌(Dehalobacter)[42-45],以及绿弯菌门中主要还原氯代烃、多氯联苯(PCBs)的脱卤拟球菌(Dehalococcoides)[46-49]、脱卤单胞菌(Dehalogenimonas)[50-54]和脱卤微杆菌(Dehalobium chlorocoercia) DF-1菌株[55]。此外,新近研究表明脱卤杆菌能以二氯甲烷(dichloromethane)或者一氯甲烷(chloromethane)为底物进行发酵生长[45, 56]。除此之外,还有一些非OHRB的微生物也能够进行有机卤代污染物的脱卤。例如,能够还原PBDEs的脱溴微生物变化赖氨酸芽孢杆菌(Lysinibacillus varians)[57]和疏水性鞘氨醇菌(Sphingobium hydrophobicum)[58]等,以及能够还原氟乙酸(fluoroacetate)的伯克霍尔德氏菌(Burkholderia)[59]等。除了这些已报道的微生物,还有更多专性OHRB和兼性OHRB陆续被分离纯化,它们将为有机卤污染场地的原位修复提供更丰富的微生物资源。
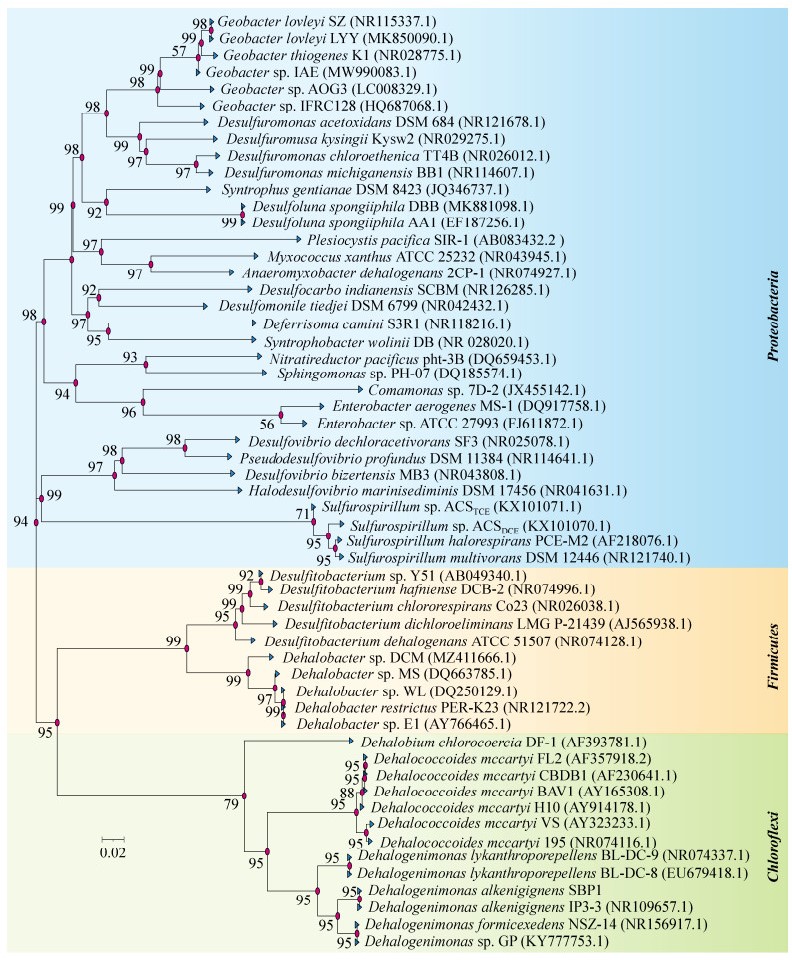
还原脱卤主要通过以下2种反应进行:一种是氢解作用(hydrogenolysis),即碳骨架上的卤素原子被氢原子取代,同时消耗2个电子生成一分子的卤化氢;另一种是二卤消除作用(dihaloelimination),即碳骨架上相邻碳原子的卤素原子被同时移除,碳碳单键转化为碳碳双键,同时消耗2个电子并生成2分子的卤化氢[60]。例如,脱卤素杆菌PER-K23菌株及脱卤拟球菌195菌株对PCE等氯代乙烯的逐步脱氯过程属于氢解作用[61-62],而脱卤单胞菌BL-DC-9菌株将1, 2, 3-三氯丙烷(1, 2, 3-trichloropropane)脱卤为一氯丙烯(allyl chloride)的过程属于二卤消除作用[63] (图 3)。由于芳香族化合物特殊的电子排布,卤代芳香类化合物不会发生二卤消除方式的脱卤,并且氢解作用与二卤消除作用相比其脱掉相同数目卤原子能够产生更多的能量[60, 64]。
有机卤呼吸机理主要是基于模式菌Desulfomonile tiedjei和Dehalobacter restrictus提出的化学渗透假说[14, 18],在这个过程中,氢气等电子供体被氢化酶等氧化,生成质子(H+)和电子,质子通过ATP合成酶促进ATP的合成以供菌体生长,电子被传递给RDase来还原卤代物。在绝大多数的兼性OHRB中电子传递方式为醌依赖型电子传递,而在专性OHRB中电子传递方式为非醌依赖型电子传递[65];也就是说,在兼性OHRB (如硫化螺旋菌)中,电子供体被氢化酶氧化之后,电子将经由电子载体醌传递给下游的RDase,而在专性OHRB (如脱卤拟球菌)中氢化酶和RDase之间无电子载体醌的存在,但其具体电子传递方式仍不清楚[66]。
1.3 有机卤呼吸关键功能酶RDase是一类以钴胺素(例如维生素B12)为辅酶、对氧气等环境因子高度敏感的膜锚定蛋白,是OHRB还原脱卤反应中的关键生物催化剂[66-70]。自从1995年首次在Desulfomonile tiedjei DCB-1菌株中纯化出能够还原脱氯3-氯苯甲酸(3-chlorobenzoate,3-CB)的还原性脱卤酶[71]以来,研究者们对来源于不同OHRB的RDase产生了极大兴趣。然而,由于OHRB生长周期较长且较难获得较高生物量,导致对RDase的生化研究也相对滞后。RDase的分离纯化极其困难,目前常用的办法是应用蓝胶非变性聚丙烯酰胺凝胶电泳(blue native polyacrylamide gel electrophoresis,BN-PAGE)的方法将其从细胞蛋白中分离,对其进行酶促反应及分子量等测定[72-74]。通过电子顺磁共振(electron paramagnetic resonance,EPR)鉴定出RDase具有2个可能参与电子传递的Fe-S簇[75-76]。RDase中的钴胺素是还原脱卤反应的催化中心,通过钴胺素中心原子钴的价态变化来断裂碳-卤键,而铁硫簇在此过程中起传递电子的作用[66]。目前对RDase催化还原脱卤反应的机制还未完全明了,但可以确定的是钴原子为+1价时C−Cl键可发生断裂[76]。
目前至少已有39种分离或纯化的RDase,主要来源于脱卤拟球菌、硫化螺旋菌、脱卤素杆菌和脱亚硫酸杆菌等。例如可还原脱卤氯乙烯的PceA、VcrA和BvcA,可还原脱卤氯代烷烃的DcaA、DcpA和DcrA,可脱卤氯酚类的CprA和DebcprA,以及可还原脱卤氯苯类的CbrA等[66, 77-79]。研究表明,RDase具有较强的底物专一性,导致RDase的功能和序列具有多样性,但目前尚未发现能够进行脱氟反应的RDase[66]。不同来源的RDase也可能具有相同功能,但在序列相似性上具有较大的差异。例如具有二卤消除功能的DcaA和DcpA[17]。大多数RDase对1种或者2种结构类似的底物具有较强的选择性,而一种底物也可以被不同的RDase催化,但是其脱卤速率不会完全相同。例如,PCE和TCE可以被同一种酶PceA催化脱卤,而一氯乙烯(vinyl chloride,VC)则可以被VcrA和BvcA两种酶催化脱卤[66]。Parthasarathy等[80]利用大肠杆菌(Escherichia coli)作为宿主异源表达了脱卤拟球菌VS菌株的VcrA脱辅酶,并进行了体外重建,获得了与VS菌株中活性相当的VcrA,这为未来RDase结构及底物范围等生化性质的研究提供了方法和思路。
2 环境因子对有机卤厌氧脱卤的影响 2.1 pHpH是影响有机卤代物厌氧生物还原脱卤能力及脱卤速率的一个关键因素。一般情况下,OHRB对有机卤代物的还原脱卤需要在中性或接近中性的环境条件下进行。环境pH的改变不仅会影响OHRB对有机卤代物的脱卤速率,而且还影响其还原脱卤的终点。例如,在pH值为7.2时,地杆菌(Geobacter lovleyi) SZ菌株能在2 d内将PCE还原为顺式-1, 2-二氯乙烯(cis-1, 2-dichloroethene,cDCE);而在pH值为6.0时,等量PCE还原为cDCE则需要10 d[81]。再例如,混合菌剂Bio-Dechlor INOCULUM (BDI)在pH为7.2时能够将PCE还原为无毒的乙烯;而在pH值为6.0时,PCE的还原产物主要为cDCE和致癌物VC[82]。除此之外,在OHRB还原PCBs的研究中发现,当pH值为6.0−8.0时容易发生对位脱氯,当pH值为6.0−7.5时则容易发生邻位脱氯[82-83]。
厌氧环境中,微生物的还原脱氯和微生物发酵都会产生大量的酸性物质(表 1),在无足够缓冲能力的条件下可能会导致环境pH值的急剧下降。研究表明,当环境中的pH值小于6.0时,OHRB的脱卤活性会受到显著抑制[84],严重阻碍污染场地中有机卤代物的生物修复[85]。以往通过向有机卤污染场地施加大量碱性物质来调节pH值的方式存在一些缺点,例如施加碱性物料会导致修复成本的大幅增加,并且多余的阳性离子如Na+、K+对环境也会造成影响[86],因此,研究者一直在寻找可在较低pH值条件下仍然具有脱氯活性的OHRB,以期为低pH值环境下有机卤污染生物修复找到一条新途径。研究发现,硫化螺旋菌DSM 12446菌株可以在pH值低于6.0时生长并介导脱氯反应,其在pH值为5.5、6.0和7.2时均能够将PCE在4 d内脱氯为cDCE[87]。Yang等[81]在2017年发现并分离了2株能够在pH值为5.5时还原脱氯PCE的硫化螺旋菌ACSTCE菌株和ACSDCE菌株。2019年,Low等[88]在被有机卤污染的酸性含水层中分离并得到了一株能够耐受低pH值的脱亚硫酸杆菌,并将其命名为Desulfitobacterium sp. AusDCA菌株,该菌株能够通过二卤消除反应将1, 2-二氯乙烷(1, 2-dichloroethane,1, 2-DCA)直接脱氯至无毒的乙烯,脱氯活性和细胞生长的最低pH值为5.0。随着研究的深入,可能会有越来越多的耐受低pH值的OHRB被发现,它们有望为低pH值环境中有机卤污染物的修复消减提供解决方案。
| Reaction types | Chemical equations |
| Reductive dechlorination | C2Cl4+4H2→C2H4+4H++4Cl− |
| C2H4Cl2+2H2→C2H6+2H++2Cl− | |
| Anaerobic fermentation | CH3CHOHCOO−+2H2O→CH3COO−+HCO3−+H++2H2 CH3CH2OH+H2O→CH3COO−+H++2H2O |
| CH3CH2CH2COO−+2H2O→2CH3COO−+2H++2H2 | |
| CH3CH2COO−+3H2O→CH3COO−+HCO3−+H++3H2 | |
| 4CH3OH+2CO2→3CH3COO−+2H2O | |
| 2HCO3−+4H2+H+→CH3COO−+4H2O |
OHRB的适宜生长温度一般为22−38 ℃[89-90],而专性OHRB的适宜生长温度区间则要更窄一些。例如,脱卤拟球菌的适宜生长温度为25−30 ℃[48]。与pH对OHRB的影响类似,温度不仅影响OHRB对有机卤代物的脱卤速率,也会影响脱卤代谢过程及最终脱卤产物的生成。在对混合菌剂KB-1的研究中发现,当温度为10−30 ℃时PCE能够被还原为无毒的乙烯,温度为30 ℃时脱氯速率及细胞生长速度最快;而当温度低于10 ℃或者超过40 ℃时,PCE的脱氯反应停滞于cDCE;当温度超过50 ℃时,脱氯反应及细胞生长则完全停滞[89]。与之相似,在对包含脱卤拟球菌的混合菌剂BDI和OW的研究中发现,当温度为30 ℃时PCE能够被完全还原为乙烯,当温度为35−40 ℃时会有大量VC的积累,当温度超过45 ℃时菌剂会彻底失去脱氯活性[91];但在35 ℃或者40 ℃时能够还原VC的vcrA基因及其他脱卤酶基因的转录均有所上调,这可能是OHRB对温度胁迫的一种响应。在对可厌氧脱卤PCBs的OHRB研究中发现,当温度为20 ℃时容易发生对位氯的脱除,当温度为15 ℃时容易发生邻位氯的脱除[82]。
Kuokka等[92]研究了不同温度条件下芬兰某河流底泥中微生物对1, 2, 3, 4-四氯二苯并呋喃(1, 2, 3, 4-tetrachlorodibenzofuran)的脱卤速率,结果发现当培养条件在21 ℃时其脱卤速率远远大于4 ℃,但是在不同温度条件下起主要还原脱氯作用的脱卤拟球菌的丰度相差无几,这说明在4 ℃或低温条件下以OHRB为主体进行原位生物修复在理论上具有一定的可行性,但修复效率难以达到预期。大多数有机卤污染场地的地下平均温度并非维持在OHRB的最佳生长温度25 ℃,因此,在实际原位修复过程中,需要采取必要的强化措施或手段弥补温度变化对有机卤代物修复效率的不利影响。在一些原位生物修复污染场地的案例中,常通过电阻加热(electrical resistance heating,ERH)等方式增加地下温度,从而联用原位加热处理技术(in situ thermal treatment,ISTT)与原位生物修复技术(in situ bioremediation)[93]来促进微生物还原脱卤过程能够快速启动且彻底进行[90]。
2.3 盐度自然环境中,淡水的盐度一般为0.5‰,江河入海口的盐度在0.5‰−30.0‰之间,海水的平均盐度为35.0‰,盐度过高会导致渗透压升高而引起微生物细胞的质壁分离,破坏细胞膜和酶系统,进而对微生物产生不利影响。例如,5.0‰−20.0‰的盐度会对产甲烷过程及反硝化过程、硫酸盐还原过程等厌氧呼吸过程产生抑制作用[94-95]。盐度还能够显著影响微生物的群落结构[96-97]。Banda等[98]对不同盐度条件下微生物群落结构的研究表明,在盐度低于50.0‰时微生物群落中大部分为细菌,而在盐度高于250.0‰时微生物群落中大部分为古菌。
有机卤代物的生物还原脱卤同样受到盐度的影响。大多数OHRB只能在低盐度条件下生长,而脱卤单胞菌BL-DC-9菌株由于具有多种渗透胁迫响应机制,可以在多种不同的盐度条件下生长,甚至可以在中等盐度条件下存活(20‰质量体积分数的NaCl)[49];而与脱卤单胞菌同一纲的脱卤拟球菌由于基因组中缺乏能够编码进行生物渗透保护机制的基因,导致其不能在多种盐度条件下生长[99]。Li等[100]研究了三氯乙烯(trichloroethene,TCE)在不同盐度(0.45‰−30.00‰)培养条件下的脱卤速率,发现在盐度为10‰的培养条件下荧光假单胞菌(Pseudomonas fluorescens)通过好氧共代谢作用对TCE的脱卤速率最大。在厌氧环境中,OHRB对有机卤代物的还原脱氯及OHRB的丰度均与环境中的盐度有一定关系。Dam等[101]的研究表明,在河口沉积物中多氯二苯并-对-二噁英(polychlorinated dibenzo-p-dioxins,PCDDs)的还原脱氯速率与盐度呈负相关关系,沉积物中可以驱动PCDDs还原脱氯的脱卤拟球菌的丰度也随着盐度和硫酸盐浓度的增加而减少;当沉积物中盐度过大时,体系中几乎检测不到脱卤拟球菌的存在。
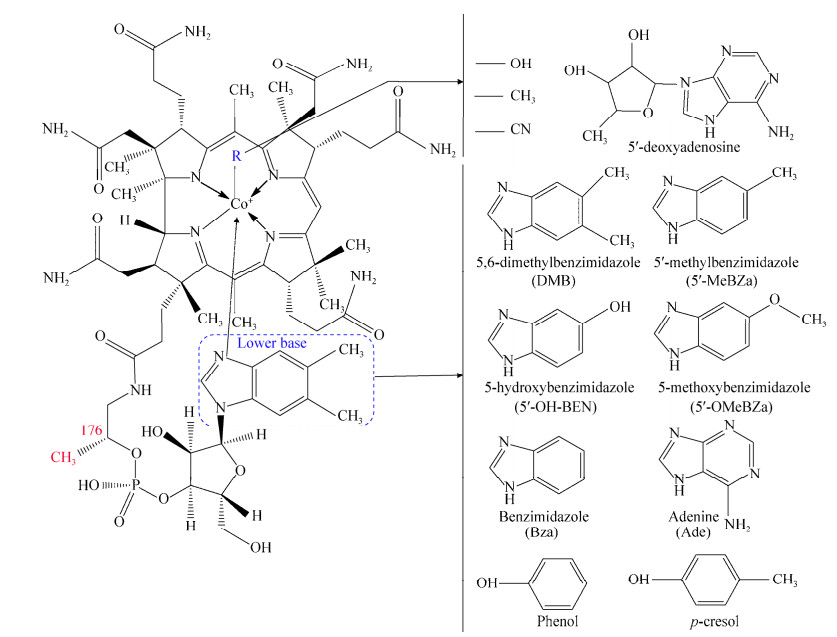
2.4 钴胺素钴胺素是有机卤呼吸能量代谢中关键蛋白还原性脱卤酶的辅酶因子,对于还原脱卤过程必不可少[70]。自然环境中,钴胺素的种类繁多,作为低位配体的苯并咪唑就多达6种,另外还有以苯酚、腺嘌呤等作为低位配体的类型(图 4)[102]。低位配体为5, 6-二甲基苯并咪唑(5, 6-dimethylbenzimidazole,DMB)的钴胺素又被称为维生素B12,这也是人体所必需外源摄取的一种微量营养元素。通常情况下,能够起到辅酶活性的钴胺素其R基为5ʹ-脱氧腺苷(5ʹ-deoxyadenosine)或者甲基。
钴胺素类型影响OHRB的生长及有机卤代物脱卤,不同OHRB能够利用的钴胺素种类有较大差别。Yan等[103]的研究表明,脱卤拟球菌会优先选择以DMB作为低位配体的钴胺素(即维生素B12)进行生长;而以5-甲基苯并咪唑(5-methylbenzimidazole)、5-甲氧基苯并咪唑(5-methoxybenzimidazole)、苯并咪唑(benzimidazole)作为低位配体的钴胺素均能够支持脱卤拟球菌(例如BAV1菌株)对氯代乙烯类进行还原脱氯,然而其脱卤周期区别很大。硫化螺旋菌DSM 12446菌株能够合成去甲基假维生素B12 (norpseudo-B12,即C176位缺少一个甲基且以腺嘌呤作为低位配体的钴胺素)用于PCE到cDCE的还原脱氯[104];而当向DSM 12446菌株培养体系中加入DMB后则会引导其产生维生素B12,进而抑制了DSM 12446菌株的细胞生长和脱氯活性[105]。
并非所有的OHRB都具有从头合成钴胺素的能力。其中一些OHRB如地杆菌SZ菌株可以从头合成钴胺素,因此在缺少外源性钴胺素供给的情况下也可以生长和传代培养[106];还有一些自身不能合成钴胺素的OHRB必须通过摄取其他微生物合成的钴胺素来满足其自身的能量代谢活动[48, 106]。例如,脱卤拟球菌BAV1等菌株需要在外源添加维生素B12的培养条件下方能达到最佳生长速率和最大脱氯速率[48]。Yan等[106]将不能合成钴胺素的脱卤拟球菌及能够合成钴胺素的地杆菌SZ菌株和PCA菌株进行共培养时发现,脱卤拟球菌在与SZ菌株共培养时,PCE能够在较长的周期(100 d左右)内被脱氯为VC,其中PCE至cDCE的脱氯步骤由能够合成钴胺素的SZ菌株所驱动,而cDCE到VC的脱卤则由不能合成钴胺素的脱卤拟球菌所驱动;与SZ菌株不同的是,另一株能够合成钴胺素的地杆菌PCA菌株所产生的钴胺素类型不能支持脱卤拟球菌的生长和脱氯活性。Yan等[107]进一步研究发现,将不能合成钴胺素的脱卤拟球菌与其他能够产生不同类型钴胺素的微生物进行共培养时,还需要额外添加某些低位配体化合物来诱导生成特定类型的钴胺素以支持脱卤拟球菌的生长和脱氯活性;添加DMB能够支持脱卤拟球菌的脱氯活性,这说明脱卤拟球菌具有将不可利用类型钴胺素转化成为自身可利用形式钴胺素的能力。另外,基因组分析也表明,所有的脱卤拟球菌均含有能将DMB和钴胺酰胺(即钴胺素前体)组装为完整钴胺素的基因cobT、cobS、cobC和cobU[108-109]。
在原位生物修复过程中,向有机卤污染场地添加外源维生素B12能够对厌氧还原脱氯产生促进作用[110]。然而,由于维生素B12价格较为昂贵,考虑到原位修复的经济成本,向有机卤污染场地引入能够持续产生钴胺素的微生物,构建合理有效的脱氯微生物群落应该是更佳的生态学解决方案。He等[111]在用脱卤拟球菌195菌株、脱硫脱硫弧菌(Desulfovibrio desulfuricans) ATCC 7757菌株、伍氏醋杆状菌(Acetobacterium woodii) ATCC 29683菌株构建的复合培养体系还原PCE时发现,此复合培养菌群比195菌株单独还原PCE的速率更快,伍氏醋杆状菌ATCC 29683菌株产生的钴胺素能够供给195菌株进行脱氯。这对原位生物修复提供了一种新思路和潜在有效的方法。
2.5 电子供体和碳源OHRB可以利用的电子供体主要可分为氢气和小分子有机酸(甲酸、乙酸、乳酸、丙酮酸)。氢气是OHRB的通用电子供体,并且是大多数专性OHRB的唯一电子供体,例如,脱卤拟球菌[48]、脱卤单胞菌[112-113]、脱卤素杆菌[61]均只能利用氢气作为唯一电子供体进行还原脱氯;而有些兼性OHRB则能够利用乙酸等小分子有机酸作为电子供体[31]。研究表明,在PCE脱卤富集培养体系中添加乙酸的最终脱卤产物为cDCE,而添加乙酸和氢气之后,最终脱卤产物则为乙烯,这说明在不添加氢气时能够脱卤cDCE的专性OHRB无法生长[114]。
除氢气外,专性OHRB脱卤单胞菌也能够利用甲酸作为电子供体[112-113],这是因为其基因组编码了含硒代半胱氨酸的甲酸脱氢酶(formate dehydrogenase),甲酸脱氢酶可以催化甲酸氧化为二氧化碳,并伴随NADH的生成,随后NADH作为直接电子供体参与到下一步的还原反应中[115]。地杆菌、脱硫单胞菌(Desulfomonas)等OHRB还能够利用乙酸作为电子供体支持还原脱氯反应。其中,地杆菌SZ菌株可以利用乙酸同时作为碳源和电子供体来脱卤PCE[31]。有研究报道近期从河流底泥中分离纯化出一株能够将1, 2-DCA以二卤消除的方式直接脱卤为乙烯的地杆菌IAE菌株,该菌株也能够以乙酸为电子供体进行还原脱氯反应[116]。
乳酸、丙酮酸等也是脱卤微生物的重要电子供体,同时也能被微生物用作碳源,在混合培养体系中,可发酵碳源的存在可以为OHRB提供可利用的小分子有机酸(碳源和/或电子供体)及氢气(电子供体)[114]。硫化螺旋菌可以利用氢气、甲酸、丙酮酸等多种电子供体进行还原脱氯,然而不同的电子供体会对其脱氯速率产生一定的影响,在对硫化螺旋菌DSM 12446菌株还原PCE的研究中发现,不同的电子供体对脱卤速率有影响,当用氢气作为电子供体时,PCE可以在2 d内被脱卤为cDCE;而当用丙酮酸作为电子供体时,PCE到cDCE的脱卤则需要6 d[117]。研究还发现,影响OHRB脱氯速率与生长速率的因素除了电子供体的种类之外,还包括电子供体的浓度和所使用的碳源[118]。选择合适的简单碳源(乙酸)可能是OHRB更加适宜的碳源,其可以有效避免因发酵导致的环境pH值快速下降的不利影响[84]。在对还原4-氯苯酚(4-chlorophenol,4-CP)和2, 4-二氯苯酚(2, 4-dichlorophenol,2, 4-DCP)的富集培养体系的研究中发现,补加丙酸盐可以提高氯苯酚的脱卤效率[119];在对序批式反应器(sequencing batch reactor)脱卤2, 4, 6-三氯苯酚(2, 4, 6- trichlorophenol,2, 4, 6-TCP)的研究中发现,适当添加蔗糖会提高2, 4, 6-TCP的脱卤速率,但是过量的外加碳源则会抑制脱氯过程,这主要是由于体系中的发酵菌迅速生长,占据主导地位,与OHRB形成竞争所致[118]。在用厌氧活性污泥脱卤四氯化碳(carbon tetrachloride,CT)的一项研究中发现,葡萄糖作为碳源和电子供体时,CT脱卤速率和细菌的生长速率均大于用乙酸作为电子供体和碳源的处理[120]。在用厌氧底泥富集培养物还原脱卤四氯乙烯时发现,乳酸作为碳源和电子供体时,PCE的脱氯速率要大于以乙酸、甲醇、甲酸作为电子供体和碳源的处理[11]。在用脱氯菌群分别脱卤PCE、TCE和1, 1, 2, 2-四氯乙烷(1, 1, 2, 2-tetrachloroethane)的研究中发现,在添加乙酸和氢气的培养体系中,脱卤拟球菌是主要的OHRB;在添加乳酸的培养体系中,OHRB的生物多样性增加,脱氯速率也更快[121]。
2.6 多种有机卤代物共存污染因为大部分有机卤代物的溶解度较低,在一些污染场地,有机卤污染物均是以重质非水相液体(dense nonaqueous phase liquids,DNAPL)形式存在。研究表明,高浓度有机卤污染物对OHRB的生长会产生抑制效应,进而影响其脱卤速率和脱卤产物[9, 122]。Yang等[123]在用富集菌群Victoria脱卤PCE时发现,当PCE的液相浓度低于1.06 mmol/L时,PCE可以被彻底脱卤为乙烯,但是脱卤周期随着PCE浓度的增大而增加;当PCE的浓度大于2.11 mmol/L (超溶解度,按亨利常数计算浓度)时会发生自抑制(self-inhibition)现象,脱卤产物主要是cDCE,并伴随少量的VC产生或直接停滞在cDCE,PCE不再被彻底脱卤为乙烯。与PCE情况类似的还有TCE,其自抑制浓度一般在1−4 mmol/L之间[123]。Huang等[124]对纯培养微生物密歇根脱硫单胞菌(Desulfuromonas michiganensis) BB1菌株的研究表明,在接种量较低(0.19 mg-protein/L)时,浓度为0.54 mmol/L的PCE可在20 d内被彻底脱卤,而当浓度为0.8 mmol/L时却无明显的脱氯现象发生。类似的情况也发生在其他几株OHRB中,例如,可脱卤PCE的纯培养微生物硫化螺旋菌DSM 12446菌株、地杆菌SZ菌株和脱亚硫酸菌(Desulfitobacterium) Viet1菌株的PCE脱卤过程均会被高于0.54 mmol/L的PCE所抑制[125]。以上研究结果均表明,高浓度有机卤污染物对多数OHRB的生长有抑制效应。然而,Zhao等[126]分离出一株还原氯代乙烯的脱卤拟球菌11G菌株,这株氯代乙烯脱卤菌株能够还原高浓度的氯代乙烯类污染物,利用11G菌株对含5 mmol/L氯代乙烯(2.5 mmol/L TCE,2.5 mmol/L cDCE)的天然水体进行生物强化,实现了完全脱氯,表明11G菌株在高浓度氯代乙烯污染场地的生物修复中具有潜在的应用价值。
除了目标有机卤污染物的浓度限制脱卤微生物的生长及脱卤活性之外,有机卤污染物共存的复合污染体系对目标有机卤代物的微生物脱卤也会产生影响。Duhamel等[127]在用富集菌群KB-1还原PCE时向培养体系中添加氯仿(chloroform,CF)和1, 1, 1-三氯乙烷(1, 1, 1- trichloroethane,1, 1, 1-TCA),结果发现CF和1, 1, 1-TCA均能够对PCE的脱卤产生较明显的抑制作用;尤其是当CF的浓度仅为2.5 μmol/L时,VC脱氯至乙烯的过程就会被严重抑制。在大部分的污染场地,有机卤污染物均是以多种污染物共存形式存在,CF和1, 1, 1-TCA与氯代乙烯类污染物共存的现象十分普遍,这就要求在进行有机卤污染场地的原位修复时需要同时考虑多种污染物共存条件下的修复策略,以及如何避免共存污染物对OHRB产生抑制的作用。然而,并非所有的多种污染物共存均会造成脱卤的抑制。研究表明,PCE可以促进能够还原脱卤PCBs的脱卤拟球菌的分离纯化[128],而且0.05−0.20 mmol/L的PCE能够强化含脱卤拟球菌的富集培养菌液对PCBs的脱卤,并且在PCBs脱卤的同时,PCE也被彻底还原为无毒的乙烯[129]。
2.7 硫酸盐硫酸盐(SO42−)在地下环境中广泛存在,其常见的浓度范围为0.2−30.0 mmol/L。硫酸盐还原过程对脱氯反应及OHRB的生长可能会造成一定的影响,如竞争电子供体、降低脱卤速率等。Kuo等[130]和Baba等[131]研究表明,向PCBs脱氯富集菌群中添加一定浓度的SO42−会对脱氯过程产生一定程度的抑制,这可能是因为SO42−比有机卤更易作为电子受体被硫酸盐还原菌利用。SO42−对脱氯过程的影响与浓度也有关系。El Mamouni等[132]的研究表明,10 mmol/L的SO42−不会对土著微生物还原TCE产生影响,15−20 mmol/L的SO42−会使TCE的脱卤速率减慢。然而,Heimann等[133]和Aulenta等[134]则发现较低浓度(2.5 mmol/L和3.7 mmol/L)的SO42−就会抑制富集培养体系的脱氯过程。Mao等[135]的研究表明,5 mmol/L的SO42−不会对脱卤拟球菌菌株195的生长和脱氯产生抑制作用,而5 mmol/L的S2−则会对菌株195的生长和脱氯产生明显的抑制作用,但向富集培养体系中加入过量乳酸时,加入的SO42−会被还原为S2−,进而会抑制脱氯过程。
Xu等[136]的研究表明,SO42−会对厌氧稻田土富集培养物脱卤五氯苯酚(pentachlorophenol,PCP)产生抑制作用,进一步的微生物群落分析结果表明,在添加SO42−的处理中,能够进行SO42−还原的脱硫弧菌占据一定的丰度,脱硫弧菌可能和OHRB竞争电子供体,进而抑制OHRB对PCP的脱卤,这与Baba等[131]的结果相似。然而,Nelson等[137]在电子供体(H2)充足的条件下也发现了SO42−对PCE脱卤的抑制作用,但这并非由于电子供体的竞争所导致。Zwiernik等[138]的研究表明,向底泥中添加FeSO4能够促进脱氯作用,这可能是因为FeSO4的加入富集了能够进行脱氯过程的硫酸盐还原菌。
SO42−对脱氯微生物的影响比较复杂,简而言之,当体系中含有能够脱卤有机卤的硫酸盐还原菌的情况下,多数存在一个正向促进脱氯的效果;当体系中的硫酸盐还原菌与OHRB相互竞争营养物质时则可能会产生抑制作用。
2.8 硝酸盐硝酸盐(NO3−)是另一种广泛存在的无机污染物,其在地下环境中的浓度为0.05−12.00 mmol/L,而且经常与有机卤污染物在地下环境中复合共存,NO3−对脱氯反应有一定程度的抑制作用[137, 139-140]。一项基于2008−2014年间美国5个污染场地的35口井222次监测数据的分析发现,有机卤代物脱卤潜力与NO3−和亚硝酸盐(NO2−)的浓度呈负相关;同时,脱卤拟球菌的丰度与NO3−和NO2−的浓度都呈负相关[141]。Holliger[142]的研究发现,在脱氯反应体系中加入NO3−可以抑制PCE的脱氯反应,而且抑制程度与NO3−浓度成正比。Yu等[143]的研究发现,低浓度(0.05−1.00 mmol/L)的NO3−对脱卤素杆菌还原脱卤PCP起到促进作用,而高浓度(5−30 mmol/L)的NO3−则对PCP的还原脱氯有抑制作用。
关于NO3−抑制微生物还原脱氯的原因,一种比较广泛的解释为电子竞争。Cao等[144]发现,2, 4, 6-TCP的还原脱氯和NO3−、NO2−的还原反应之间存在电子竞争,当加入足够的电子供体之后,这种抑制效应得到缓解,并且NO3−比NO2−更易与2, 4, 6-TCP竞争电子。Nelson等[137]研究发现,在一个半动态反应器中,8.4 mg/L N-NO3−可以完全抑制PCE的还原脱卤,这种抑制作用在氢气充足的情况下仍会发生,所以电子竞争并非NO3−抑制脱氯的唯一原因,同时该研究也发现,低浓度(13 μmol/L)的N2O会抑制混合培养体系中的脱氯反应。
早在1977年,Blackburn等[145]利用电子脉冲辅助技术发现N2O可以和含+1价钴原子的钴胺素发生反应,生成含+2价钴原子的钴胺素。钴原子价态的改变会导致许多依赖钴胺素的酶蛋白失活[146]。Yin等[147]的研究表明,N2O对地杆菌SZ菌株和脱卤拟球菌BAV1菌株的脱氯过程均存在不同程度的抑制作用,酶促反应动力学研究表明N2O对脱氯微生物来说是一种非竞争性抑制剂,并且对不同脱氯微生物或同一微生物的不同脱氯步骤的Ki值不同。Tas等[148]在NO3−对五氯硝基苯(pentachloronitrobenzene,PCNB)还原脱氯影响的研究中发现,50 mg/L N-NO3−就可以抑制PCNB的还原脱氯过程,认为这可能是因为反硝化作用的中间产物N2O、NO的累积所致。NO2−、N2O和NO均为反硝化过程的中间产物,在富含硝酸盐的水体中均会有不同程度的富集,这可能会影响生态系统中脱氯微生物种群的生长和代谢活性。
2.9 氧气在地下环境及浅水层中,通常存在一定浓度的氧气,而绝大多数的厌氧微生物均有抵御氧气的机制(例如超氧化物歧化酶和过氧化氢酶)[149],它们在短暂接触氧气后并不会丧失代谢活性,而氧气对OHRB的抑制作用是在菌种保藏及在污染场地原位修复过程中均需要考虑的问题。
早在1995年,Natarajan等[150]就研究过氧气暴露对能够脱卤PCB且能产甲烷的厌氧颗粒的影响,该研究发现,氧气暴露会抑制厌氧颗粒的产甲烷能力,在恢复厌氧培养后,经历一段延迟期后会重新产甲烷,而PCB的脱卤反应并未受到氧气暴露的影响,即使氧气暴露时间长达168 h。OHRB有些是兼氧微生物(如硫化螺旋菌),有些则是严格厌氧微生物(如脱卤素杆菌、脱卤拟球菌和脱卤单胞菌等)。在修复工程上,兼性且更耐受氧气的厌氧微生物会使操作更加简单,是工程上的优选。然而污染场地中氯乙烯类污染物的彻底无害化需要严格厌氧的OHRB参与。目前已知cDCE到乙烯的脱氯过程只能由脱卤拟球菌和脱卤单胞菌这2个属中的某些菌株所驱动。由于严格厌氧微生物在氯乙烯类污染场地修复中的关键作用,明确氧气对脱卤拟球菌的影响就显得极为重要。Adrian等[151]认为,所有脱卤拟球菌种群的脱氯活性均会受到氧气的影响,纯培养的脱卤拟球菌在氧气中暴露5 s后即表现出明显的脱氯活性降低。2017年,Liu等[152]研究了氧气对TCE脱卤富集菌群的影响,当体系中严格厌氧时,TCE可以在16 d被彻底脱卤为无毒的乙烯;当体系中溶解氧浓度为7.2 mg/L时,TCE仍然可以脱卤为乙烯,但是其脱卤周期被延长至48 d。Adrian等[151]研究表明,与脱卤拟球菌培养相比,含有脱卤拟球菌的混合培养体系能够对氧气有更强的耐受能力;进一步的研究发现,氧气暴露后有机卤脱卤菌群中的vcrA基因拷贝数不会随着培养时间的增加而增加,而tceA基因则会随着培养时间的增加而增加。Amos等[12]同样在混合培养体系暴露氧气的实验中得到了相同的结论,即不能脱卤VC的FL2菌株相比能够脱卤VC的GT菌株和BAV1菌株对氧气有更强的耐受能力,与Liu等[152]的研究不同的是,在培养基中加入氧气后,TCE只能被还原为VC,而不能继续脱卤为无毒的乙烯,这可能与混合培养体系中非脱氯菌群对氧气的消耗能力也有关系。可见,脱卤VC的脱卤拟球菌对氧气更为敏感,然而VC相比其他氯代乙烯更具毒性和致癌性,如何在原位修复工程中确保VC的彻底脱卤仍然是需要首先考虑解决的问题。
2.10 重金属重金属是世界范围内广泛存在的一类污染物,其中砷(As)、镉(Cd)、铬(Cr)、汞(Hg)、铅(Pb)等对人体毒性较大的几类重金属更是受到广泛关注,有毒重金属(toxic heavy metals,THMs)的环境污染严重威胁人类健康,美国毒物和疾病登记署(Agency for Toxic Substances and Disease Registry,ATSDR) 2019年公布的“优先控制污染物”清单中,排名前五的分别是砷(As)、铅(Pb)、汞(Hg)、VC和PCBs[153-154]。由此可见,重金属和有机卤是目前环境常见的两大类污染物。目前重金属和有机卤的复合污染极其普遍[155-156],但大量研究表明,重金属对微生物尤其是厌氧微生物有明显的抑制作用[157-159],而一些微生物也会在重金属的胁迫下进化出一系列耐受重金属的生理机制[160]。
地下环境中重金属的存在形式主要可分为5种:可交换态、碳酸盐结合态、铁锰氧化物结合态、残渣态和有机结合态;不同形态重金属对微生物的影响较为复杂,研究者们普遍认为能够对微生物产生抑制的主要是可交换态;但是在一定条件下,碳酸盐结合态及其他形态均可以转化为可交换态,进而对微生物的生长及代谢过程产生影响[153, 158]。金属对OHRB有着不同程度的抑制作用[159, 161-162],然而相较于其他厌氧微生物,OHRB可能对重金属有更强的耐受能力[163]。1996年,Pardue等[159]研究了二价镉(Cd2+)对3种底泥富集培养物厌氧脱卤2, 3, 4-三氯苯胺(2, 3, 4-trichloroaniline,2, 3, 4-TCA)的抑制作用,当Cd2+的浓度达到0.2 mg/L时就会完全抑制2, 3, 4-TCA的还原脱氯过程,并且在中性稻田土和洼地硬木土壤中这种抑制效应是连续的,有明显的剂量-效应关系;而在有机质较多的沼泽土中,当Cd2+的浓度达到临界可溶性水平时,2, 3, 4-TCA的还原脱氯突然被完全抑制;但在达到临界可溶性水平之前,几乎无明显的抑制效应,这是由于在不同土壤中镉的存在形式不同所决定的。Kuo等[161]的研究表明,Cd2+、Cu2+、Cr6+、Hg2+对2-氯苯酚(2-chlorophenol,2-CP)和3-CB的厌氧微生物脱卤有不同程度的影响,并且这种影响不能简单归结为“抑制”。当Cd2+、Cu2+和Cr6+在体系中的浓度较低时(Cd2+ < 0.1 mg/L,Cu2+ < 0.01 mg/L,Cr6+ < 0.01 mg/L),对2-CP的脱卤有显著的促进作用,超过此浓度之后则会对2-CP的脱卤产生抑制作用。相比较而言,Hg2+对2-CP厌氧脱卤的影响则恰好相反,当其浓度低于1.0 mg/L时会对脱卤产生抑制,而超过此浓度之后则会促进2-CP的脱卤。Cd2+、Cu2+、Cr6+对2-CP及3-CB脱卤的完全抑制浓度在0.5−5.0 mg/L的范围内波动,表现出非常显著的差异性,比较一致的是添加重金属离子的浓度越高,2-CP和3-CB开始脱卤之前所经历的延迟期就越长。
Lu等[163]分别研究了几种重金属离子(Cd2+、Cu2+、Cr3+、Pb2+)对纯培养(脱卤拟球菌CG1菌株)、共培养(脱卤拟球菌CG1菌株和地杆菌LYY菌株)及利用河流底泥构建的混合培养微生物脱卤PCE和2, 2ʹ, 3, 4, 4ʹ, 5, 5ʹ-七氯联苯[1, 2, 3, 4-tetrachloro-5-(2, 4, 5-trichlorophenyl)benzene,PCB180]的影响,与纯培养和共培养相比,混合培养体系对重金属的耐受能力更强,这可能与非脱氯微生物对重金属的转化有关,而纯培养菌株CG1在脱卤PCE和PCB180时表现出不同的耐受重金属能力;在脱卤PCE时,低浓度的Cr3+、Pb2+(10 mg/L)就表现出明显的抑制作用,但Cd2+、Cu2+的抑制浓度则相对较高(分别为20 mg/L,50 mg/L);与PCE相比,菌株CG1脱卤PCB180时则表现出更强的重金属耐受能力;在对重金属胁迫条件下的脱氯微生态系统进行微生物群落结构分析时发现,Pb2+和Cu2+会促进某些OHRB (如脱卤拟球菌)的富集,这为OHRB的富集和分离工作提供了新的思路。
2.11 流体动力学性质研究表明,脱卤拟球菌在连续流培养状态下,其脱氯速率为序批式反应器培养中的200倍[164]。脱卤素杆菌PER-K23菌株在10 ℃时不能在静置培养条件下生长,却可以在连续流培养条件下生长,而且PCE能够以较快的速度[3.7 μmol/(L·h)]脱卤[165]。地杆菌SZ菌株及硫化螺旋菌DSM 12446菌株等在PCE的液相浓度超过0.54 mmol/L时其脱氯进程会停滞[125],但是在柱实验[166]、污染场地试点[167]等连续流体条件下,PCE在最大溶解浓度时(约1.2 mmol/L)仍然可以被上述OHRB脱卤。Mendoza-Sanchez等[168]在对cDCE脱卤柱实验的研究中发现,在相对较高流动速度(0.54 m/d)的条件下,cDCE能够被彻底脱卤为乙烯,而在0.08 m/d以下的流动条件下脱氯则不能进行。这些研究意味着在地下水环境中,地下水的流动速度关系着碳源等营养物质的补充速度,对OHRB的脱氯和生长具有重要的影响。
2.12 其他因素有机卤污染场地的原位生物修复还受到许多其他环境因子的影响。Doong等[169]的研究表明,在CT脱氯体系中添加0.5 mm直径的玻璃微珠能够使脱氯速率提高2−5倍,并且底物浓度越高,这种优势越明显。Yang等[170]基于非均质含水层的原位脱氯实验表明,土壤渗透性的差异也显著影响有机卤污染物微生物脱氯的程度及速率,低渗层土壤更有利于有机卤污染物的彻底脱卤。Shen等[13]的研究表明,土壤含水率在OHRB介导的原位生物修复中也是影响修复效率的一个重要参数,脱卤速率随着含水率的升高而增大,为保证污染物的有效去除,在将OHRB引入污染场地进行原位修复时,应保证土壤含水率在17%以上。生物膜的形成也对有机卤代物的微生物还原脱氯产生一定的影响,依赖氢气的生物膜反应器已经被广泛应用到脱卤有机卤污染场地的修复过程中,例如1, 1, 1-TCA和CF[171]、PCP[172]、2-CP[173]、4-CP[174]、PCE[175]等的脱卤。研究表明,vcrA基因对于氯代乙烯类污染的彻底无毒化有着十分重要的作用[176],而噬菌体可能与脱卤拟球菌vcrA基因的转移有关,在未来的污染场地原位修复当中,有可能直接向地下注入携带有某种功能基因(例如RDase基因)的噬菌体,而无需注入含OHRB的纯培养液或者混合菌剂[62]。
各种环境因素对OHRB的影响又非单一存在的,而是相互交错。例如,KB-1菌剂在以乳酸作为碳源时,可以在10 ℃时进行PCE的脱氯,而在以丙酸作为碳源时,则最低可在15 ℃时进行PCE的脱氯[89]。另外,基质流动性与温度范围之间也有一定的关系[90, 125, 165]。在进行原位修复时,应该在充分调研污染场地各种地球化学条件的基础上,建立相应的数学模型对原位修复的可行性进行预测,如BioPIC等工具的开发有助于基于各种地球化学生物参数选择合适的修复技术[170]。
3 展望环境因子对有机卤代污染物微生物修复的影响十分复杂,在实施原位生物修复之前,应对污染场地的特征进行调查和评估,详细了解污染物的种类及浓度、可能的脱卤产物以及存在的其他可供微生物利用的电子受体种类及浓度(例如硝酸盐、硫酸盐等)、溶解氢气的浓度、矿物元素、重金属、温度、pH、盐度等。除此之外,对原位微生物群落的检测也极为重要,原位是否存在能够进行关键脱卤行为的微生物是修复方案制定的重要参考依据。在全面了解场地的特征后,综合多方面因素进行修复方案的制定。
鉴于当前全球范围内有机卤污染场地修复的需求,未来应从多方面进行系统性探究,最终达成对污染场地高效彻底修复的目的。
(1) 继续开展RDase分离纯化及生化鉴定。目前研究者们对不同种属OHRB的有机卤呼吸过程中电子传递方式及关键功能酶的作用还缺乏深入的认识。开发OHRB关键功能酶的异源表达及生化特征鉴定是未来研究的一个重点及热点。
(2) 继续开展OHRB的分离纯化。在不同环境条件下富集分离OHRB仍是众多研究者关注的一个研究热点。耐受低pH、低温、复合污染等高抗性、具有应用价值的OHRB菌种仍十分匮乏,分离任务仍然任重道远。
(3) 利用合成微生物组学开发复合菌剂处理复合污染物。在几乎所有的污染场地中,有机卤代物均非单独存在,多种污染物共存现象十分普遍。因此,可以针对不同类型的污染物进行修复菌剂定制来达到同步或分步脱卤的目的,从而提高有机卤代物的原位脱卤速率。
(4) 利用合成生物学的方法与技术增强OHRB抗逆性。在微生物领域,合成生物学的技术与应用已较成熟,然而开发基于OHRB的遗传操作系统,使其具有更强的环境适应性的研究仍然少见。因此,基于模式OHRB开发底盘细胞,开展遗传操作系统研究,对其进行相应的合成生物学改良是解决不良环境因素抑制生物修复的途径之一。
(5) 解析有机卤脱卤菌群中的互作机制。微生物在环境中以不同的相互作用方式存在,在某些非严格厌氧的环境中可以分离到严格厌氧的微生物,这是因为有些能够利用低浓度氧气微生物的存在消耗了微环境中的氧气,可以形成微小的厌氧单元。因此,在调查待修复污染场地的地质化学条件的基础上,将不同作用的微生物进行共培养,构建稳定的微生物菌群,利用微生物生态网络来增强OHRB的抗逆性是另一个重要研究方向。
| [1] |
Stuart M, Lapworth D, Crane E, Hart A. Review of risk from potential emerging contaminants in UK groundwater[J]. Science of the Total Environment, 2012, 416: 1-21. DOI:10.1016/j.scitotenv.2011.11.072 |
| [2] |
Duan XY, Li YX, Li XG, Li MF, Zhang DH. Distributions and sources of polychlorinated biphenyls in the coastal East China Sea sediments[J]. Science of the Total Environment, 2013, 463-464: 894-903. DOI:10.1016/j.scitotenv.2013.06.102 |
| [3] |
Qu RJ, Liu JQ, Li CG, Wang LS, Wang ZY, Wu JC. Experimental and theoretical insights into the photochemical decomposition of environmentally persistent perfluorocarboxylic acids[J]. Water Research, 2016, 104: 34-43. DOI:10.1016/j.watres.2016.07.071 |
| [4] |
Goldman SM, Quinlan PJ, Ross GW, Marras C, Meng C, Bhudhikanok GS, Comyns K, Korell M, Chade AR, Kasten M, et al. Solvent exposures and parkinson disease risk in twins[J]. Annals of Neurology, 2012, 71(6): 776-784. DOI:10.1002/ana.22629 |
| [5] |
Qiu YL, Strid A, Bignert A, Zhu ZL, Zhao JF, Athanasiadou M, Athanassiadis I, Bergman Å. Chlorinated and brominated organic contaminants in fish from Shanghai markets: a case study of human exposure[J]. Chemosphere, 2012, 89(4): 458-466. DOI:10.1016/j.chemosphere.2012.05.099 |
| [6] |
Henry BM. Biostimulation for Anaerobic Bioremediation of Chlorinated Solvents[A]//Stroo HF, WARD CH. In situ Remediation of Chlorinated Solvent Plumes[M]. New York, NY; Springer New York. 2010: 357-423.
|
| [7] |
Suflita JM, Horowitz A, Shelton DR, Tiedje JM. Dehalogenation: a novel pathway for the anaerobic biodegradation of haloaromatic compounds[J]. Science, 1982, 218(4577): 1115-1117. DOI:10.1126/science.218.4577.1115 |
| [8] |
Akimova GP, Sokolova MG. Low temperature impact on protein content and peroxidase activity during pea inoculation with Rhizobium leguminosarum[J]. Journal of Stress Physiology & Biochemistry, 2010, 6(4): 81-89. |
| [9] |
Haest PJ, Springael D, Seuntjens P, Smolders E. Self-inhibition can limit biologically enhanced TCE dissolution from a TCE DNAPL[J]. Chemosphere, 2012, 89(11): 1369-1375. DOI:10.1016/j.chemosphere.2012.05.097 |
| [10] |
Wen LL, Chen JX, Fang JY, Li A, Zhao HP. Effects of 1, 1, 1-trichloroethane and triclocarban on reductive dechlorination of trichloroethene in a TCE-reducing culture[J]. Frontiers in Microbiology, 2017, 8: 1439. DOI:10.3389/fmicb.2017.01439 |
| [11] |
Gao JW, Skeen RS, Hooker BS, Quesenberry RD. Effects of several electron donors on tetrachloroethylene dechlorination in anaerobic soil microcosms[J]. Water Research, 1997, 31(10): 2479-2486. DOI:10.1016/S0043-1354(97)00108-5 |
| [12] |
Amos BK, Ritalahti KM, Cruz-Garcia C, Padilla-Crespo E, Löffler FE. Oxygen effect on Dehalococcoides viability and biomarker quantification[J]. Environmental Science & Technology, 2008, 42(15): 5718-5726. |
| [13] |
Shen R, Yu L, Xu P, Liang ZW, Lu QH, Liang DW, He ZL, Wang SQ. Water content as a primary parameter determines microbial reductive dechlorination activities in soil[J]. Chemosphere, 2021, 267: 129152. DOI:10.1016/j.chemosphere.2020.129152 |
| [14] |
Xu MY, Yu ZQ, Yang YG, Chen XJ, Sun GP, Guo J. Microbial anaerobic respiration and remediation of aquatic sediments contaminated by refractory organic pollutants[J]. Journal of Microbiology, 2017, 37(2): 1-11. (in Chinese) 许玫英, 虞志强, 杨永刚, 陈杏娟, 孙国萍, 郭俊. 微生物厌氧呼吸与有机污染水体沉积物修复[J]. 微生物学杂志, 2017, 37(2): 1-11. DOI:10.3969/j.issn.1005-7021.2017.02.001 |
| [15] |
Leys D, Adrian L, Smidt H. Organohalide respiration: microbes breathing chlorinated molecules[J]. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 2013, 368(1616): 20120316. DOI:10.1098/rstb.2012.0316 |
| [16] |
Richardson RE. Genomic insights into organohalide respiration[J]. Current Opinion in Biotechnology, 2013, 24(3): 498-505. DOI:10.1016/j.copbio.2013.02.014 |
| [17] |
Bommer M, Kunze C, Fesseler J, Schubert T, Diekert G, Dobbek H. Structural basis for organohalide respiration[J]. Science, 2014, 346(6208): 455-458. DOI:10.1126/science.1258118 |
| [18] |
DeWeerd KA, Mandelco L, Tanner RS, Woese CR, Suflita JM. Desulfomonile tiedjei gen. nov. and sp. nov., a novel anaerobic, dehalogenating, sulfate-reducing bacterium[J]. Archives of Microbiology, 1990, 154(1): 23-30. |
| [19] |
Sun B, Cole JR, Sanford RA, Tiedje JM. Isolation and characterization of Desulfovibrio dechloracetivorans sp. nov., a marine dechlorinating bacterium growing by coupling the oxidation of acetate to the reductive dechlorination of 2-chlorophenol[J]. Applied and Environmental Microbiology, 2000, 66(6): 2408-2413. DOI:10.1128/AEM.66.6.2408-2413.2000 |
| [20] |
Ballschmiter K. Pattern and sources of naturally produced organohalogens in the marine environment: biogenic formation of organohalogens[J]. Chemosphere, 2003, 52(2): 313-324. DOI:10.1016/S0045-6535(03)00211-X |
| [21] |
Labbé N, Parent S, Villemur R. Nitratireductor aquibiodomus gen. nov., sp. nov., a novel alpha- proteobacterium from the marine denitrification system of the Montreal Biodome (Canada)[J]. International Journal of Systematic and Evolutionary Microbiology, 2004, 54(Pt 1): 269-273. |
| [22] |
Matturro B, Ubaldi C, Rossetti S. Microbiome dynamics of a polychlorobiphenyl (PCB) historically contaminated marine sediment under conditions promoting reductive dechlorination[J]. Frontiers in Microbiology, 2016, 7: 1502. |
| [23] |
Valo RJ, Häggblom MM, Salkinoja-Salonen MS. Bioremediation of chlorophenol containing simulated ground water by immobilized bacteria[J]. Water Research, 1990, 24(2): 253-258. DOI:10.1016/0043-1354(90)90111-I |
| [24] |
McCarty PL. In situ bioremediation of chlorinated solvents[J]. Current Opinion in Biotechnology, 1993, 4(3): 323-330. DOI:10.1016/0958-1669(93)90103-4 |
| [25] |
Harkness MR, Bracco AA, Brennan MJ, DeWeerd KA, Spivack JL. Use of bioaugmentation to stimulate complete reductive dechlorination of trichloroethene in Dover soil columns[J]. Environmental Science & Technology, 1999, 33(7): 1100-1109. |
| [26] |
Quinlan C, Strevett K, Ketcham M, Grego J. VOC elimination in a compost biofilter using a previously acclimated bacterial inoculum[J]. Journal of the Air & Waste Management Association, 1999, 49(5): 544-553. |
| [27] |
Steffan RJ, Sperry KL, Walsh MT, Vainberg S, Condee CW. Field-scale evaluation of in situ bioaugmentation for remediation of chlorinated solvents in groundwater[J]. Environmental Science & Technology, 1999, 33(16): 2771-2781. |
| [28] |
Cope N, Hughes JB. Biologically-enhanced removal of PCE from NAPL source zones[J]. Environmental Science & Technology, 2001, 35(10): 2014-2021. |
| [29] |
Maphosa F, De Vos WM, Smidt H. Exploiting the ecogenomics toolbox for environmental diagnostics of organohalide-respiring bacteria[J]. Trends in Biotechnology, 2010, 28(6): 308-316. DOI:10.1016/j.tibtech.2010.03.005 |
| [30] |
Lovley DR, Giovannoni SJ, White DC, Champine JE, Phillips EJ, Gorby YA, Goodwin S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals[J]. Archives of Microbiology, 1993, 159(4): 336-344. DOI:10.1007/BF00290916 |
| [31] |
Sung Y, Fletcher KE, Ritalahti KM, Apkarian RP, Ramos-Hernández N, Sanford RA, Mesbah NM, Löffler FE. Geobacter lovleyi sp. nov. strain SZ, a novel metal-reducing and tetrachloroethene-dechlorinating bacterium[J]. Applied and Environmental Microbiology, 2006, 72(4): 2775-2782. DOI:10.1128/AEM.72.4.2775-2782.2006 |
| [32] |
Sung Y, Ritalahti KM, Sanford RA, Urbance JW, Flynn SJ, Tiedje JM, Löffler FE. Characterization of two tetrachloroethene-reducing, acetate-oxidizing anaerobic bacteria and their description as Desulfuromonas michiganensis sp. nov.[J]. Applied and Environmental Microbiology, 2003, 69(5): 2964-2974. DOI:10.1128/AEM.69.5.2964-2974.2003 |
| [33] |
Peng P, Goris T, Lu Y, Nijsse B, Burrichter A, Schleheck D, Koehorst JJ, Liu J, Sipkema D, Sinninghe Damste JS, et al. Organohalide-respiring Desulfoluna species isolated from marine environments[J]. The ISME Journal, 2020, 14(3): 815-827. DOI:10.1038/s41396-019-0573-y |
| [34] |
Strycharz SM, Gannon SM, Boles AR, Franks AE, Nevin KP, Lovley DR. Reductive dechlorination of 2-chlorophenol by Anaeromyxobacter dehalogenans with an electrode serving as the electron donor[J]. Environmental Microbiology Reports, 2010, 2(2): 289-294. DOI:10.1111/j.1758-2229.2009.00118.x |
| [35] |
Boyle AW, Phelps CD, Young LY. Isolation from estuarine sediments of a Desulfovibrio strain which can grow on lactate coupled to the reductive dehalogenation of 2, 4, 6-tribromophenol[J]. Applied and Environmental Microbiology, 1999, 65(3): 1133-1140. DOI:10.1128/AEM.65.3.1133-1140.1999 |
| [36] |
Lohner ST, Spormann AM. Identification of a reductive tetrachloroethene dehalogenase in Shewanella sediminis[J]. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 2013, 368(1616): 20120326. DOI:10.1098/rstb.2012.0326 |
| [37] |
Wang YB, Wu CY, Wang XJ, Zhou SG. The role of humic substances in the anaerobic reductive dechlorination of 2, 4-dichlorophenoxyacetic acid by Comamonas koreensis strain CY01[J]. Journal of Hazardous Materials, 2009, 164(2-3): 941-947. DOI:10.1016/j.jhazmat.2008.08.097 |
| [38] |
Stolz JF, Ellis DJ, Blum JS, Ahmann D, Lovley DR, Oremland RS. Note: Sulfurospirillum barnesii sp. nov. and Sulfurospirillum arsenophilum sp. nov., new members of the Sulfurospirillum clade of the ε-Proteobacteria[J]. International Journal of Systematic Bacteriology, 1999, 49(Pt 3): 1177-1180. |
| [39] |
Luijten MLGC, De Weert J, Smidt H, Boschker HTS, de Vos WM, Schraa G, Stams AJM. Description of Sulfurospirillum halorespirans sp. nov., an anaerobic, tetrachloroethene-respiring bacterium, and transfer of Dehalospirillum multivorans to the genus Sulfurospirillum as Sulfurospirillum multivorans comb. nov.[J]. International Journal of Systematic and Evolutionary Microbiology, 2003, 53(Pt 3): 787-793. |
| [40] |
Schubert T. The organohalide-respiring bacterium Sulfurospirillum multivorans: a natural source for unusual cobamides[J]. World Journal of Microbiology & Biotechnology, 2017, 33(5): 93. |
| [41] |
Fletcher KE, Ritalahti KM, Pennell KD, Takamizawa K, Löffler FE. Resolution of culture Clostridium bifermentans DPH-1 into two populations, a Clostridium sp. and tetrachloroethene-dechlorinating Desulfitobacterium hafniense strain JH1[J]. Applied and Environmental Microbiology, 2008, 74(19): 6141-6143. DOI:10.1128/AEM.00994-08 |
| [42] |
Grostern A, Edwards EA. Characterization of a Dehalobacter coculture that dechlorinates 1, 2-dichloroethane to ethene and identification of the putative reductive dehalogenase gene[J]. Applied and Environmental Microbiology, 2009, 75(9): 2684-2693. DOI:10.1128/AEM.02037-08 |
| [43] |
Nelson JL, Fung JM, Cadillo-Quiroz H, Cheng X, Zinder SH. A role for Dehalobacter spp. in the reductive dehalogenation of dichlorobenzenes and monochlorobenzene[J]. Environmental Science & Technology, 2011, 45(16): 6806-6813. |
| [44] |
Nelson JL, Jiang JD, Zinder SH. Dehalogenation of chlorobenzenes, dichlorotoluenes, and tetrachloroethene by three Dehalobacter spp.[J]. Environmental Science & Technology, 2014, 48(7): 3776-3782. |
| [45] |
Justicia-Leon SD, Ritalahti KM, Mack EE, Löffler FE. Dichloromethane fermentation by a Dehalobacter sp. in an enrichment culture derived from pristine river sediment[J]. Applied and Environmental Microbiology, 2012, 78(4): 1288-1291. DOI:10.1128/AEM.07325-11 |
| [46] |
Maymó-Gatell X, Chien Y, Gossett JM, Zinder SH. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene[J]. Science, 1997, 276(5318): 1568-1571. DOI:10.1126/science.276.5318.1568 |
| [47] |
He JZ, Sung Y, Krajmalnik-Brown R, Ritalahti KM, Löffler FE. Isolation and characterization of Dehalococcoides sp. strain FL2, a trichloroethene (TCE)- and 1, 2-dichloroethene-respiring anaerobe[J]. Environmental Microbiology, 2005, 7(9): 1442-1450. DOI:10.1111/j.1462-2920.2005.00830.x |
| [48] |
Löffler FE, Yan J, Ritalahti KM, Adrian L, Edwards EA, Konstantinidis KT, Müller JA, Fullerton H, Zinder SH, Spormann AM. Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi[J]. International Journal of Systematic and Evolutionary Microbiology, 2013, 63(Pt 2): 625-635. |
| [49] |
LaRoe SL, Fricker AD, Bedard DL. Dehalococcoides mccartyi strain JNA in pure culture extensively dechlorinates Aroclor 1260 according to polychlorinated biphenyl (PCB) dechlorination process N[J]. Environmental Science & Technology, 2014, 48(16): 9187-9196. |
| [50] |
Moe WM, Yan J, Nobre MF, Da Costa MS, Rainey FA. Dehalogenimonas lykanthroporepellens gen. nov., sp. nov., a reductively dehalogenating bacterium isolated from chlorinated solvent-contaminated groundwater[J]. International Journal of Systematic and Evolutionary Microbiology, 2009, 59(Pt 11): 2692-2697. |
| [51] |
Maness AD, Bowman KS, Yan J, Rainey FA, Moe WM. Dehalogenimonas spp. can reductively dehalogenate high concentrations of 1, 2-dichloroethane, 1, 2-dichloropropane, and 1, 1, 2-trichloroethane[J]. AMB Express, 2012, 2(1): 54. DOI:10.1186/2191-0855-2-54 |
| [52] |
Bowman KS, Nobre MF, Da Costa MS, Rainey FA, Moe WM. Dehalogenimonas alkenigignens sp. nov., a chlorinated-alkane-dehalogenating bacterium isolated from groundwater[J]. International Journal of Systematic and Evolutionary Microbiology, 2013, 63(Pt 4): 1492-1498. |
| [53] |
Molenda O, Quaile AT, Edwards EA. Dehalogenimonas sp. strain WBC-2 genome and identification of its trans-dichloroethene reductive dehalogenase, TdrA[J]. Applied and Environmental Microbiology, 2015, 82(1): 40-50. |
| [54] |
Yang Y, Higgins SA, Yan J, Şimşir B, Chourey K, Iyer R, Hettich RL, Baldwin B, Ogles DM, Löffler FE. Grape pomace compost harbors organohalide-respiring Dehalogenimonas species with novel reductive dehalogenase genes[J]. The ISME Journal, 2017, 11(12): 2767-2780. DOI:10.1038/ismej.2017.127 |
| [55] |
May HD, Sowers KR. "Dehalobium chlorocoercia" DF-1—From Discovery to Application[A]//Adrian L, Löffler FE. Organohalide-Respiring Bacteria[M]. Berlin, Heidelberg: Springer Berlin Heidelberg, 2016: 563-586
|
| [56] |
Lee M, Low A, Zemb O, Koenig J, Michaelsen A, Manefield M. Complete chloroform dechlorination by organochlorine respiration and fermentation[J]. Environmental Microbiology, 2012, 14(4): 883-894. DOI:10.1111/j.1462-2920.2011.02656.x |
| [57] |
Zhu CJ, Sun GP, Chen XJ, Guo J, Xu MY. Lysinibacillus varians sp. nov., an endospore-forming bacterium with a filament-to-rod cell cycle[J]. International Journal of Systematic and Evolutionary Microbiology, 2014, 64(Pt 11): 3644-3649. |
| [58] |
Chen XJ, Wang HJ, Xu JJ, Song D, Sun GP, Xu MY. Sphingobium hydrophobicum sp. nov., a hydrophobic bacterium isolated from electronic-waste-contaminated sediment[J]. International Journal of Systematic and Evolutionary Microbiology, 2016, 66(10): 3912-3916. DOI:10.1099/ijsem.0.001287 |
| [59] |
Chan PWY, Yakunin AF, Edwards EA, Pai EF. Mapping the reaction coordinates of enzymatic defluorination[J]. Journal of the American Chemical Society, 2011, 133(19): 7461-7468. DOI:10.1021/ja200277d |
| [60] |
Dolfing J. Energetic Considerations in Organohalide Respiration[A]//Adrian L, Löffler FE. Organohalide- Respiring Bacteria[M]. Berlin, Heidelberg: Springer Berlin Heidelberg, 2016: 31-48
|
| [61] |
Holliger C, Hahn D, Harmsen H, Ludwig W, Schumacher W, Tindall B, Vazquez F, Weiss N, Zehnder AJB. Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetra- and trichloroethene in an anaerobic respiration[J]. Archives of Microbiology, 1998, 169(4): 313-321. DOI:10.1007/s002030050577 |
| [62] |
Löffler FE, Ritalahti KM, Zinder SH. Dehalococcoides and Reductive Dechlorination of Chlorinated Solvents[A]//Stroo HF, Leeson A, Ward CH. Bioaugmentation for Groundwater Remediation[M]. New York: Springer New York, 2013: 39-88
|
| [63] |
Yan J, Rash BA, Rainey FA, Moe WM. Isolation of novel bacteria within the Chloroflexi capable of reductive dechlorination of 1, 2, 3-trichloropropane[J]. Environmental Microbiology, 2009, 11(4): 833-843. DOI:10.1111/j.1462-2920.2008.01804.x |
| [64] |
Dolfing J, Novak I. The Gibbs free energy of formation of halogenated benzenes, benzoates and phenols and their potential role as electron acceptors in anaerobic environments[J]. Biodegradation, 2015, 26: 15-27. DOI:10.1007/s10532-014-9710-5 |
| [65] |
Wang SQ, Qiu L, Liu XW, Xu GF, Siegert M, Lu QH, Juneau P, Yu L, Liang DW, He ZL, et al. Electron transport chains in organohalide-respiring bacteria and bioremediation implications[J]. Biotechnology Advances, 2018, 36(4): 1194-1206. DOI:10.1016/j.biotechadv.2018.03.018 |
| [66] |
Fincker M, Spormann AM. Biochemistry of catabolic reductive dehalogenation[J]. Annual Review of Biochemistry, 2017, 86: 357-386. DOI:10.1146/annurev-biochem-061516-044829 |
| [67] |
Vogel TM. Reductive Dehalogenation[M]//Zehnder AJB. Soil and Groundwater Pollution: Fundamentals, Risk Assessment and legislation. Dordrecht: Springer Netherlands. 1995: 27-30
|
| [68] |
Fetzner S. Bacterial dehalogenation[J]. Applied Microbiology and Biotechnology, 1998, 50(6): 633-657. DOI:10.1007/s002530051346 |
| [69] |
Maillard J, Schumacher W, Vazquez F, Regeard C, Hagen WR, Holliger C. Characterization of the corrinoid iron-sulfur protein tetrachloroethene reductive dehalogenase of Dehalobacter restrictus[J]. Applied and Environmental Microbiology, 2003, 69(8): 4628-4638. DOI:10.1128/AEM.69.8.4628-4638.2003 |
| [70] |
Payne KAP, Quezada CP, Fisher K, Dunstan MS, Collins FA, Sjuts H, Levy C, Hay S, Rigby SEJ, Leys D. Reductive dehalogenase structure suggests a mechanism for B12-dependent dehalogenation[J]. Nature, 2015, 517(7535): 513-516. DOI:10.1038/nature13901 |
| [71] |
Magnuson JK, Romine MF, Burris DR, Kingsley MT. Trichloroethene reductive dehalogenase from Dehalococcoides ethenogenes: sequence of tceA and substrate range characterization[J]. Applied and Environmental Microbiology, 2000, 66(12): 5141-5147. DOI:10.1128/AEM.66.12.5141-5147.2000 |
| [72] |
Padilla-Crespo E, Yan J, Swift C, Wagner DD, Chourey K, Hettich RL, Ritalahti KM, Löffler FE. Identification and environmental distribution of dcpA, which encodes the reductive dehalogenase catalyzing the dichloroelimination of 1, 2-dichloropropane to propene in organohalide-respiring Chloroflexi[J]. Applied and Environmental Microbiology, 2014, 80(3): 808-818. DOI:10.1128/AEM.02927-13 |
| [73] |
Jugder BE, Bohl S, Lebhar H, Healey RD, Manefield M, Marquis CP, Lee M. A bacterial chloroform reductive dehalogenase: purification and biochemical characterization[J]. Microbial Biotechnology, 2017, 10(6): 1640-1648. DOI:10.1111/1751-7915.12745 |
| [74] |
van de Pas BA, Smidt H, Hagen WR, van der Oost J, Schraa G, Stams AJM, de Vos WM. Purification and molecular characterization of ortho-chlorophenol reductive dehalogenase, a key enzyme of halorespiration in Desulfitobacterium dehalogenans[J]. Journal of Biological Chemistry, 1999, 274(29): 20287-20292. DOI:10.1074/jbc.274.29.20287 |
| [75] |
Futagami T, Goto M, Furukawa K. Biochemical and genetic bases of dehalorespiration[J]. The Chemical Record, 2008, 8(1): 1-12. DOI:10.1002/tcr.20134 |
| [76] |
Liao RZ, Chen SL, Siegbahn PEM. Unraveling the mechanism and regioselectivity of the B12-dependent reductive dehalogenase PceA[J]. Chemistry, 2016, 22(35): 12391-12399. DOI:10.1002/chem.201601575 |
| [77] |
Adrian L, Rahnenführer J, Gobom J, Hölscher T. Identification of a chlorobenzene reductive dehalogenase in Dehalococcoides sp. strain CBDB1[J]. Applied and Environmental Microbiology, 2007, 73(23): 7717-7724. DOI:10.1128/AEM.01649-07 |
| [78] |
Ang TF, Maiangwa J, Salleh AB, Normi YM, Leow TC. Dehalogenases: from improved performance to potential microbial dehalogenation applications[J]. Molecules, 2018, 23(5): 1100. DOI:10.3390/molecules23051100 |
| [79] |
Jugder BE, Ertan H, Lee M, Manefield M, Marquis CP. Reductive dehalogenases come of age in biological destruction of organohalides[J]. Trends in Biotechnology, 2015, 33(10): 595-610. DOI:10.1016/j.tibtech.2015.07.004 |
| [80] |
Parthasarathy A, Stich TA, Lohner ST, Lesnefsky A, Britt RD, Spormann AM. Biochemical and EPR- spectroscopic investigation into heterologously expressed vinyl chloride reductive dehalogenase (VcrA) from Dehalococcoides mccartyi strain VS[J]. Journal of the American Chemical Society, 2015, 137(10): 3525-3532. DOI:10.1021/ja511653d |
| [81] |
Yang Y, Cápiro NL, Marcet TF, Yan J, Pennell KD, Löffler FE. Organohalide respiration with chlorinated ethenes under low pH conditions[J]. Environmental Science & Technology, 2017, 51(15): 8579-8588. |
| [82] |
Wu Q, Bedard DL, Wiegel J. Effect of incubation temperature on the route of microbial reductive dechlorination of 2, 3, 4, 6-tetrachlorobiphenyl in polychlorinated biphenyl (PCB)-contaminated and PCB-free freshwater sediments[J]. Applied and Environmental Microbiology, 1997, 63(7): 2836-2843. DOI:10.1128/aem.63.7.2836-2843.1997 |
| [83] |
Wiegel J, Wu QZ. Microbial reductive dehalogenation of polychlorinated biphenyls[J]. FEMS Microbiology Ecology, 2000, 32(1): 1-15. DOI:10.1111/j.1574-6941.2000.tb00693.x |
| [84] |
McCarty PL, Chu MY, Kitanidis PK. Electron donor and pH relationships for biologically enhanced dissolution of chlorinated solvent DNAPL in groundwater[J]. European Journal of Soil Biology, 2007, 43(5-6): 276-282. DOI:10.1016/j.ejsobi.2007.03.004 |
| [85] |
Adamson DT, Lyon DY, Hughes JB. Flux and product distribution during biological treatment of tetrachloroethene dense non-aqueous-phase liquid[J]. Environmental Science & Technology, 2004, 38(7): 2021-2028. |
| [86] |
Hiortdahl KM, Borden RC. Enhanced reductive dechlorination of tetrachloroethene dense nonaqueous phase liquid with EVO and Mg(OH)2[J]. Environmental Science & Technology, 2014, 48(1): 624-631. |
| [87] |
Scholz-Muramatsu H, Neumann A, Meßmer M, Moore E, Diekert G. Isolation and characterization of Dehalospirillum multivorans gen. nov., sp. nov., a tetrachloroethene- utilizing, strictly anaerobic bacterium[J]. Archives of Microbiology, 1995, 163(1): 48-56. DOI:10.1007/BF00262203 |
| [88] |
Low A, Zhao SY, Rogers MJ, Zemb O, Lee M, He JZ, Manefield M. Isolation, characterization and bioaugmentation of an acidotolerant 1, 2-dichloroethane respiring Desulfitobacterium species from a low pH aquifer[J]. FEMS Microbiology Ecology, 2019, 95(5): fiz055. DOI:10.1093/femsec/fiz055 |
| [89] |
Friis AK, Heimann AC, Jakobsen R, Albrechtsen HJ, Cox E, Bjerg PL. Temperature dependence of anaerobic TCE-dechlorination in a highly enriched Dehalococcoides-containing culture[J]. Water Research, 2007, 41(2): 355-364. DOI:10.1016/j.watres.2006.09.026 |
| [90] |
Marcet TF, Cápiro NL, Yang Y, Löffler FE, Pennell KD. Impacts of low-temperature thermal treatment on microbial detoxification of tetrachloroethene under continuous flow conditions[J]. Water Research, 2018, 145: 21-29. DOI:10.1016/j.watres.2018.07.076 |
| [91] |
Fletcher KE, Costanza J, Cruz-Garcia C, Ramaswamy NS, Pennell KD, Löffler FE. Effects of elevated temperature on Dehalococcoides dechlorination performance and DNA and RNA biomarker abundance[J]. Environmental Science & Technology, 2011, 45(2): 712-718. |
| [92] |
Kuokka S, Rantalainen AL, Romantschuk M, Häggblom MM. Effect of temperature on the reductive dechlorination of 1, 2, 3, 4-tetrachlorodibenzofuran in anaerobic PCDD/F-contaminated sediments[J]. Journal of Hazardous Materials, 2014, 274: 72-78. DOI:10.1016/j.jhazmat.2014.03.065 |
| [93] |
Triplett Kingston JL, Dahlen PR, Johnson PC. State-of-the-practice review of in situ thermal technologies[J]. Groundwater Monitoring & Remediation, 2010, 30(4): 64-72. |
| [94] |
Zhou ZJ, Ge L, Huang YF, Liu YQ, Wang SY. Coupled relationships among anammox, denitrification, and dissimilatory nitrate reduction to ammonium along salinity gradients in a Chinese estuarine wetland[J]. Journal of Environmental Sciences, 2021, 106: 39-46. DOI:10.1016/j.jes.2021.01.015 |
| [95] |
Van Den Brand TPH, Roest K, Chen GH, Brdjanovic D, van Loosdrecht MCM. Effects of chemical oxygen demand, nutrients and salinity on sulfate-reducing bacteria[J]. Environmental Engineering Science, 2015, 32(10): 858-864. DOI:10.1089/ees.2014.0307 |
| [96] |
Sørensen KB, Canfield DE, Oren A. Salinity responses of benthic microbial communities in a solar saltern (Eilat, Israel)[J]. Applied and Environmental Microbiology, 2004, 70(3): 1608-1616. DOI:10.1128/AEM.70.3.1608-1616.2004 |
| [97] |
Zhang KP, Shi Y, Cui XQ, Yue P, Li KH, Liu XJ, Tripathi BM, Chu HY. Salinity is a key determinant for soil microbial communities in a desert ecosystem[J]. mSystems, 2019, 4(1): e00225-e00218. |
| [98] |
Banda JF, Zhang Q, Ma LQ, Pei LX, Du ZR, Hao CB, Dong HL. Both pH and salinity shape the microbial communities of the lakes in Badain Jaran Desert, NW China[J]. Science of the Total Environment, 2021, 791: 148108. DOI:10.1016/j.scitotenv.2021.148108 |
| [99] |
Siddaramappa S, Challacombe JF, Delano SF, Green LD, Daligault H, Bruce D, Detter C, Tapia R, Han SS, Goodwin L, et al. Complete genome sequence of Dehalogenimonas lykanthroporepellens type strain (BL-DC-9T) and comparison to "Dehalococcoides" strains[J]. Standards in Genomic Sciences, 2012, 6(2): 251-264. DOI:10.4056/sigs.2806097 |
| [100] |
Li Y, Li B, Wang CP, Fan JZ, Sun HW. Aerobic degradation of trichloroethylene by co-metabolism using phenol and gasoline as growth substrates[J]. International Journal of Molecular Sciences, 2014, 15(5): 9134-9148. DOI:10.3390/ijms15059134 |
| [101] |
Dam HT, Häggblom MM. Impact of estuarine gradients on reductive dechlorination of 1, 2, 3, 4-tetrachlorodibenzo-p-dioxin in river sediment enrichment cultures[J]. Chemosphere, 2017, 168: 1177-1185. DOI:10.1016/j.chemosphere.2016.10.082 |
| [102] |
Allen RH, Stabler SP. Identification and quantitation of cobalamin and cobalamin analogues in human feces[J]. The American Journal of Clinical Nutrition, 2008, 87(5): 1324-1335. DOI:10.1093/ajcn/87.5.1324 |
| [103] |
Yan J, Şimşir B, Farmer AT, Bi M, Yang Y, Campagna SR, Löffler FE. The corrinoid cofactor of reductive dehalogenases affects dechlorination rates and extents in organohalide-respiring Dehalococcoides mccartyi[J]. The ISME Journal, 2016, 10(5): 1092-1101. DOI:10.1038/ismej.2015.197 |
| [104] |
Kräutler B, Fieber W, Ostermann S, Fasching M, Ongania KH, Gruber K, Kratky C, Mikl C, Siebert A, Diekert G. The cofactor of tetrachloroethene reductive dehalogenase of Dehalospirillum multivorans is norpseudo-B12, a new type of a natural corrinoid[J]. Helvetica Chimica Acta, 2003, 86(11): 3698-3716. DOI:10.1002/hlca.200390313 |
| [105] |
Keller S, Ruetz M, Kunze C, Kräutler B, Diekert G, Schubert T. Exogenous 5, 6-dimethylbenzimidazole caused production of a non-functional tetrachloroethene reductive dehalogenase in Sulfurospirillum multivorans[J]. Environmental Microbiology, 2014, 16(11): 3361-3369. DOI:10.1111/1462-2920.12268 |
| [106] |
Yan J, Ritalahti KM, Wagner DD, Löffler FE. Unexpected specificity of interspecies cobamide transfer from Geobacter spp. to organohalide-respiring Dehalococcoides mccartyi strains[J]. Applied and Environmental Microbiology, 2012, 78(18): 6630-6636. DOI:10.1128/AEM.01535-12 |
| [107] |
Yan J, Im J, Yang Y, Löffler FE. Guided cobalamin biosynthesis supports Dehalococcoides mccartyi reductive dechlorination activity[J]. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 2013, 368(1616): 20120320. DOI:10.1098/rstb.2012.0320 |
| [108] |
Scott AI, Roessner CA. Biosynthesis of cobalamin (vitamin B12)[J]. Biochemical Society Transactions, 2002, 30(4): 613-620. DOI:10.1042/bst0300613 |
| [109] |
Schipp CJ, Marco-Urrea E, Kublik A, Seifert J, Adrian L. Organic cofactors in the metabolism of Dehalococcoides mccartyi strains[J]. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 2013, 368(1616): 20120321. DOI:10.1098/rstb.2012.0321 |
| [110] |
Aulenta F, Bianchi A, Majone M, Petrangeli Papini M, Potalivo M, Tandoi V. Assessment of natural or enhanced in situ bioremediation at a chlorinated solvent-contaminated aquifer in Italy: a microcosm study[J]. Environment International, 2005, 31(2): 185-190. DOI:10.1016/j.envint.2004.09.014 |
| [111] |
He JZ, Holmes VF, Lee PKH, Alvarez-Cohen L. Influence of vitamin B12 and cocultures on the growth of Dehalococcoides isolates in defined medium[J]. Applied and Environmental Microbiology, 2007, 73(9): 2847-2853. DOI:10.1128/AEM.02574-06 |
| [112] |
Mukherjee K, Bowman KS, Rainey FA, Siddaramappa S, Challacombe JF, Moe WM. Dehalogenimonas lykanthroporepellens BL-DC-9T simultaneously transcribes many rdhA genes during organohalide respiration with 1, 2-DCA, 1, 2-DCP, and 1, 2, 3-TCP as electron acceptors[J]. FEMS Microbiology Letters, 2014, 354(2): 111-118. DOI:10.1111/1574-6968.12434 |
| [113] |
Lv Y, Li XY, Wang JJ, Jin HJ, Cui YR, Yang Y, Yan J. Isolation and basic characterization of a novel organohaliderespiring bacterium within the genus Dehalogenimonas[J]. Acta Microbiologica Sinica, 2021, 61(4): 1016-1029. (in Chinese) 吕燕, 李秀颖, 王晶晶, 金慧娟, 崔逸儒, 杨毅, 严俊. 一株脱卤单胞菌属有机卤呼吸细菌的分离纯化与基础特征[J]. 微生物学报, 2021, 61(4): 1016-1029. DOI:10.13343/j.cnki.wsxb.20200353 |
| [114] |
Lee IS, Bae JH, McCarty PL. Comparison between acetate and hydrogen as electron donors and implications for the reductive dehalogenation of PCE and TCE[J]. Journal of Contaminant Hydrology, 2007, 94(1-2): 76-85. DOI:10.1016/j.jconhyd.2007.05.003 |
| [115] |
Cui YR, Yang Y, Yan J, Li XY. Advances of using Dehalogenimonas in anaerobic degradation of chlorinated compounds and bioremediation of contaminated sites[J]. Chinese Journal of Biotechnology, 2021, 37(10): 3565-3577. (in Chinese) 崔逸儒, 杨毅, 严俊, 李秀颖. 脱卤单胞菌属在厌氧降解有机氯化物及污染场地修复应用中的研究进展[J]. 生物工程学报, 2021, 37(10): 3565-3577. DOI:10.13345/j.cjb.210209 |
| [116] |
Yan J, Jiang LS, Yang Y, Li XY. A novel organohalide respiration Geobacter and the application[P]: China, CN112111420A. 2020-12-22 (in Chinese) 严俊, 姜丽思, 杨毅, 李秀颖. 一株新型有机卤呼吸地质杆菌及其应用[P]: CN112111420A. 2020-12-22. |
| [117] |
Wang JJ. Inhibition of nitrate reduction on reductive dechlorination of chlorinated compounds[D]. Beijing: Docter's Thesis of University of Chinese Academy of Sciences, 2021 (in Chinese) 王晶晶. 硝酸盐还原对有机氯厌氧生物降解的抑制作用及机理研究[D]. 北京: 中国科学院大学博士学位论文, 2021. |
| [118] |
Wang JG, Sun ZR. Exploring the effects of carbon source level on the degradation of 2, 4, 6-trichlorophenol in the co-metabolism process[J]. Journal of Hazardous Materials, 2020, 392: 122293. DOI:10.1016/j.jhazmat.2020.122293 |
| [119] |
Häggblom MM, Rivera MD, Young LY. Effects of auxiliary carbon sources and electron acceptors on methanogenic degradation of chlorinated phenols[J]. Environmental Toxicology and Chemistry, 1993, 12(8): 1395-1403. DOI:10.1002/etc.5620120808 |
| [120] |
Doong RA, Chen TF, Chang WH. Effects of electron donor and microbial concentration on the enhanced dechlorination of carbon tetrachloride by anaerobic consortia[J]. Applied Microbiology and Biotechnology, 1996, 46(2): 183-186. DOI:10.1007/s002530050802 |
| [121] |
Aulenta F, Pera A, Rossetti S, Petrangeli Papini M, Majone M. Relevance of side reactions in anaerobic reductive dechlorination microcosms amended with different electron donors[J]. Water Research, 2007, 41(1): 27-38. DOI:10.1016/j.watres.2006.09.019 |
| [122] |
Philips J, Haest PJ, Springael D, Smolders E. Inhibition of Geobacter dechlorinators at elevated trichloroethene concentrations is explained by a reduced activity rather than by an enhanced cell decay[J]. Environmental Science & Technology, 2013, 47(3): 1510-1517. |
| [123] |
Yang YR, McCarty PL. Biologically enhanced dissolution of tetrachloroethene DNAPL[J]. Environmental Science & Technology, 2000, 34(14): 2979-2984. |
| [124] |
Huang DY, Becker JG. Dehalorespiration model that incorporates the self-inhibition and biomass inactivation effects of high tetrachloroethene concentrations[J]. Environmental Science & Technology, 2011, 45(3): 1093-1099. |
| [125] |
Amos BK, Christ JA, Abriola LM, Pennell KD, Löffler FE. Experimental evaluation and mathematical modeling of microbially enhanced tetrachloroethene (PCE) dissolution[J]. Environmental Science & Technology, 2007, 41(3): 963-970. |
| [126] |
Zhao SY, He JZ. Reductive dechlorination of high concentrations of chloroethenes by a Dehalococcoides mccartyi strain 11G[J]. FEMS Microbiology Ecology, 2019, 95(1): fiy209. |
| [127] |
Duhamel M, Wehr SD, Yu L, Rizvi H, Seepersad D, Dworatzek S, Cox EE, Edwards EA. Comparison of anaerobic dechlorinating enrichment cultures maintained on tetrachloroethene, trichloroethene, cis-dichloroethene and vinyl chloride[J]. Water Research, 2002, 36(17): 4193-4202. DOI:10.1016/S0043-1354(02)00151-3 |
| [128] |
Wang SQ, Chng KR, Wilm A, Zhao SY, Yang KL, Nagarajan N, He JZ. Genomic characterization of three unique Dehalococcoides that respire on persistent polychlorinated biphenyls[J]. PNAS, 2014, 111(33): 12103-12108. DOI:10.1073/pnas.1404845111 |
| [129] |
Xu GF, Lu QH, Yu L, Wang SQ. Tetrachloroethene primes reductive dechlorination of polychlorinated biphenyls in a river sediment microcosm[J]. Water Research, 2019, 152: 87-95. DOI:10.1016/j.watres.2018.12.061 |
| [130] |
Kuo CE, Liu SM, Liu C. Biodegradation of coplanar polychlorinated biphenyls by anaerobic microorganisms from estuarine sediments[J]. Chemosphere, 1999, 39(9): 1445-1458. DOI:10.1016/S0045-6535(99)00046-6 |
| [131] |
Baba D, Yoshida N, Katayama A. Effects of inhibitors on anaerobic microbial consortium with enhanced dechlorination activity in polychlorinated biphenyl mixture[J]. Journal of Bioscience and Bioengineering, 2007, 104(4): 268-274. DOI:10.1263/jbb.104.268 |
| [132] |
El Mamouni R, Jacquet R, Gerin P, Agathos SN. Influence of electron donors and acceptors on the bioremediation of soil contaminated with trichloroethene and nickel: laboratory- and pilot-scale study[J]. Water Science and Technology: a Journal of the International Association on Water Pollution Research, 2002, 45(10): 49-54. DOI:10.2166/wst.2002.0286 |
| [133] |
Heimann AC, Friis AK, Jakobsen R. Effects of sulfate on anaerobic chloroethene degradation by an enriched culture under transient and steady-state hydrogen supply[J]. Water Research, 2005, 39(15): 3579-3586. DOI:10.1016/j.watres.2005.06.029 |
| [134] |
Aulenta F, Beccari M, Majone M, Papini MP, Tandoi V. Competition for H2 between sulfate reduction and dechlorination in butyrate-fed anaerobic cultures[J]. Process Biochemistry, 2008, 43(2): 161-168. DOI:10.1016/j.procbio.2007.11.006 |
| [135] |
Mao XW, Polasko A, Alvarez-Cohen L. Effects of sulfate reduction on trichloroethene dechlorination by Dehalococcoides-containing microbial communities[J]. Applied and Environmental Microbiology, 2017, 83(8): e03384-e03316. |
| [136] |
Xu Y, Xue LL, Ye Q, Franks AE, Zhu M, Feng X, Xu JM, He Y. Inhibitory effects of sulfate and nitrate reduction on reductive dechlorination of PCP in a flooded paddy soil[J]. Frontiers in Microbiology, 2018, 9: 567. DOI:10.3389/fmicb.2018.00567 |
| [137] |
Nelson DK, Hozalski RM, Clapp LW, Semmens MJ, Novak PJ. Effect of nitrate and sulfate on dechlorination by a mixed hydrogen-fed culture[J]. Bioremediation Journal, 2002, 6(3): 225-236. DOI:10.1080/10889860290777585 |
| [138] |
Zwiernik MJ, Quensen JF, Boyd SA. FeSO4 amendments stimulate extensive anaerobic PCB dechlorination[J]. Environmental Science & Technology, 1998, 32(21): 3360-3365. |
| [139] |
Squillace PJ, Scott JC, Moran MJ, Nolan BT, Kolpin DW. VOCs, pesticides, nitrate, and their mixtures in groundwater used for drinking water in the United States[J]. Environmental Science & Technology, 2002, 36(9): 1923-1930. |
| [140] |
Bennett P, Gandhi D, Warner S, Bussey J. In situ reductive dechlorination of chlorinated ethenes in high nitrate groundwater[J]. Journal of Hazardous Materials, 2007, 149(3): 568-573. DOI:10.1016/j.jhazmat.2007.06.092 |
| [141] |
Lee J, Im J, Kim U, Löffler FE. A data mining approach to predict in situ detoxification potential of chlorinated ethenes[J]. Environmental Science & Technology, 2016, 50(10): 5181-5188. |
| [142] |
Holliger C. The anaerobic microbiology and biotreatment of chlorinated ethenes[J]. Current Opinion in Biotechnology, 1995, 6(3): 347-351. DOI:10.1016/0958-1669(95)80058-1 |
| [143] |
Yu HY, Wang YK, Chen PC, Li FB, Chen MJ, Hu M, Ouyang XG. Effect of nitrate addition on reductive transformation of pentachlorophenol in paddy soil in relation to iron(Ⅲ) reduction[J]. Journal of Environmental Management, 2014, 132: 42-48. |
| [144] |
Cao LF, Sun WH, Zhang YT, Feng SM, Dong JY, Zhang YM, Rittmann BE. Competition for electrons between reductive dechlorination and denitrification[J]. Frontiers of Environmental Science & Engineering, 2017, 11(6): 14. |
| [145] |
Blackburn R, Kyaw M, Swallow AJ. Reaction of cob(Ⅰ)alamin with nitrous oxide and cob(Ⅲ)alamin[J]. Journal of the Chemical Society, Faraday Transactions 1:Physical Chemistry in Condensed Phases, 1977, 73: 250-255. |
| [146] |
Sullivan MJ, Gates AJ, Appia-Ayme C, Rowley G, Richardson DJ. Copper control of bacterial nitrous oxide emission and its impact on vitamin B12-dependent metabolism[J]. PNAS, 2013, 110(49): 19926-19931. DOI:10.1073/pnas.1314529110 |
| [147] |
Yin YC, Yan J, Chen G, Murdoch FK, Pfisterer N, Löffler FE. Nitrous oxide is a potent inhibitor of bacterial reductive dechlorination[J]. Environmental Science & Technology, 2019, 53(2): 692-701. |
| [148] |
Tas DO, Pavlostathis SG. Effect of nitrate reduction on the microbial reductive transformation of pentachloronitrobenzene[J]. Environmental Science & Technology, 2008, 42(9): 3234-3240. |
| [149] |
Kato MT, Field JA, Lettinga G. High tolerance of methanogens in granular sludge to oxygen[J]. Biotechnology and Bioengineering, 1993, 42(11): 1360-1366. DOI:10.1002/bit.260421113 |
| [150] |
Natarajan MR, Wang H, Hickey R, Bhatnagar L. Effect of oxygen and storage conditions on the metabolic activities of polychlorinated biphenyls dechlorinating microbial granules[J]. Applied Microbiology and Biotechnology, 1995, 43(4): 733-738. DOI:10.1007/BF00164781 |
| [151] |
Adrian L, Hansen SK, Fung JM, Görisch H, Zinder SH. Growth of Dehalococcoides strains with chlorophenols as electron acceptors[J]. Environmental Science & Technology, 2007, 41(7): 2318-2323. |
| [152] |
Liu N, Li HJ, Li MY, Ding LZ, Weng CH, Dong CD. Oxygen exposure effects on the dechlorinating activities of a trichloroethene-dechlorination microbial consortium[J]. Bioresource Technology, 2017, 240: 98-105. DOI:10.1016/j.biortech.2017.02.112 |
| [153] |
Rahman Z, Singh VP. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(Ⅵ), mercury (Hg), and lead (Pb)) on the total environment: an overview[J]. Environmental Monitoring and Assessment, 2019, 191(7): 419. DOI:10.1007/s10661-019-7528-7 |
| [154] |
Rahman Z, Singh VP. Bioremediation of toxic heavy metals (THMs) contaminated sites: concepts, applications and challenges[J]. Environmental Science and Pollution Research International, 2020, 27(22): 27563-27581. DOI:10.1007/s11356-020-08903-0 |
| [155] |
Arjoon A, Olaniran AO, Pillay B. Co-contamination of water with chlorinated hydrocarbons and heavy metals: challenges and current bioremediation strategies[J]. International Journal of Environmental Science and Technology, 2013, 10(2): 395-412. DOI:10.1007/s13762-012-0122-y |
| [156] |
Zhang WH, Wu YX, Simonnot MO. Soil contamination due to E-waste disposal and recycling activities: a review with special focus on China[J]. Pedosphere, 2012, 22(4): 434-455. DOI:10.1016/S1002-0160(12)60030-7 |
| [157] |
Kong IC, Hubbard JS, Jones WJ. Metal-induced inhibition of anaerobic metabolism of volatile fatty acids and hydrogen[J]. Applied Microbiology and Biotechnology, 1994, 42(2/3): 396-402. |
| [158] |
Aljerf L. A gateway to metal resistance: bacterial response to heavy metal toxicity in the biological environment[J]. Annals of Advances in Chemistry, 2018, 2: 32-44. |
| [159] |
Pardue JH, Kongara S, Jones JW. Effect of cadmium on reductive dechlorination of trichloroaniline[J]. Environmental Toxicology and Chemistry, 1996, 15(7): 1083-1088. DOI:10.1002/etc.5620150710 |
| [160] |
Prabhakaran P, Ashraf MA, Aqma WS. Microbial stress response to heavy metals in the environment[J]. RSC Advances, 2016, 6(111): 109862-109877. DOI:10.1039/C6RA10966G |
| [161] |
Kuo C, Genthner B. Effect of added heavy metal ions on biotransformation and biodegradation of 2-chlorophenol and 3-chlorobenzoate in anaerobic bacterial consortia[J]. Applied and Environmental Microbiology, 1996, 62(7): 2317-2323. DOI:10.1128/aem.62.7.2317-2323.1996 |
| [162] |
Jeong HY, Hayes KF. Impact of transition metals on reductive dechlorination rate of hexachloroethane by mackinawite[J]. Environmental Science & Technology, 2003, 37(20): 4650-4655. |
| [163] |
Lu QH, Zou XQ, Liu JT, Liang ZW, Shim H, Qiu RL, Wang SQ. Inhibitory effects of metal ions on reductive dechlorination of polychlorinated biphenyls and perchloroethene in distinct organohalide-respiring bacteria[J]. Environment International, 2020, 135: 105373. DOI:10.1016/j.envint.2019.105373 |
| [164] |
Schaefer CE, Condee CW, Vainberg S, Steffan RJ. Bioaugmentation for chlorinated ethenes using Dehalococcoides sp. : comparison between batch and column experiments[J]. Chemosphere, 2009, 75(2): 141-148. DOI:10.1016/j.chemosphere.2008.12.041 |
| [165] |
Holliger C, Schraa G, Stams AJ, Zehnder AJ. A highly purified enrichment culture couples the reductive dechlorination of tetrachloroethene to growth[J]. Applied and Environmental Microbiology, 1993, 59(9): 2991-2997. DOI:10.1128/aem.59.9.2991-2997.1993 |
| [166] |
Isalou M, Sleep BE, Liss SN. Biodegradation of high concentrations of tetrachloroethene in a continuous flow column system[J]. Environmental Science & Technology, 1998, 32(22): 3579-3585. |
| [167] |
Adamson DT, McDade JM, Hughes JB. Inoculation of a DNAPL source zone to initiate reductive dechlorination of PCE[J]. Environmental Science & Technology, 2003, 37(11): 2525-2533. |
| [168] |
Mendoza-Sanchez I, Autenrieth RL, McDonald TJ, Cunningham JA. Biological limitations of dechlorination of cis-dichloroethene during transport in porous media[J]. Environmental Science & Technology, 2018, 52(2): 684-691. |
| [169] |
Doong RA, Chen TF, Wu YW. Anaerobic dechlorination of carbon tetrachloride by free-living and attached bacteria under various electron-donor conditions[J]. Applied Microbiology and Biotechnology, 1997, 47(3): 317-323. DOI:10.1007/s002530050934 |
| [170] |
Yang LR, Hnatko JP, Elsey JL, Christ JA, Pennell KD, Cápiro NL, Abriola LM. Exploration of processes governing microbial reductive dechlorination in a heterogeneous aquifer flow cell[J]. Water Research, 2021, 193: 116842. DOI:10.1016/j.watres.2021.116842 |
| [171] |
Chung J, Rittmann BE. Bio-reductive dechlorination of 1, 1, 1-trichloroethane and chloroform using a hydrogen-based membrane biofilm reactor[J]. Biotechnology and Bioengineering, 2007, 97(1): 52-60. DOI:10.1002/bit.21212 |
| [172] |
Long M, Ilhan ZE, Xia SQ, Zhou C, Rittmann BE. Complete dechlorination and mineralization of pentachlorophenol (PCP) in a hydrogen-based membrane biofilm reactor (MBfR)[J]. Water Research, 2018, 144: 134-144. DOI:10.1016/j.watres.2018.06.071 |
| [173] |
Chang CC, Tseng SK, Chang CC, Ho CM. Degradation of 2-chlorophenol via a hydrogenotrophic biofilm under different reductive conditions[J]. Chemosphere, 2004, 56(10): 989-997. DOI:10.1016/j.chemosphere.2004.04.051 |
| [174] |
Long M, Zeng C, Wang ZC, Xia SQ, Zhou C. Complete dechlorination and mineralization of para-chlorophenol (4-CP) in a hydrogen-based membrane biofilm reactor (MBfR)[J]. Journal of Cleaner Production, 2020, 276: 123257. DOI:10.1016/j.jclepro.2020.123257 |
| [175] |
Fathepure BZ, Tiedje JM. Reductive dechlorination of tetrachloroethylene by a chlorobenzoate-enriched biofilm reactor[J]. Environmental Science & Technology, 1994, 28(4): 746-752. |
| [176] |
Müller JA, Rosner BM, Von Abendroth G, Meshulam-Simon G, McCarty PL, Spormann AM. Molecular identification of the catabolic vinyl chloride reductase from Dehalococcoides sp. strain VS and its environmental distribution[J]. Applied and Environmental Microbiology, 2004, 70(8): 4880-4888. DOI:10.1128/AEM.70.8.4880-4888.2004 |
 2022, Vol. 49
2022, Vol. 49