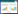扩展功能
文章信息
- 周彪, 甄向凯, 欧阳松应
- ZHOU Biao, ZHEN Xiangkai, OUYANG Songying
- 噬菌体裂解酶应用研究进展
- Research progress in the application of bacteriophage lysin
- 微生物学通报, 2021, 48(9): 3330-3340
- Microbiology China, 2021, 48(9): 3330-3340
- DOI: 10.13344/j.microbiol.china.201085
-
文章历史
- 收稿日期: 2020-11-18
- 接受日期: 2021-04-03
- 网络首发日期: 2021-06-03
2. 福建师范大学南方生物医学研究中心 福建 福州 350117
2. Fujian Normal University, Biomedical Research Center of South, Fuzhou, Fujian 350117, China
裂解酶又称内溶素(Endolysin),是一类由双链DNA噬菌体(dsDNA噬菌体)编码的细菌细胞壁水解酶,在噬菌体感染宿主的后期表达产生,其主要作用是裂解宿主细胞壁释放子代噬菌体[1]。
噬菌体裂解酶种类繁多,大多数革兰氏阳性菌噬菌体裂解酶在结构上存在“双结构域”的特点,主要由催化结构域和结合结构域2个部分组成[2-3] (图 1A)。一般来说,N端为催化结构域(Catalytic Domain,CD),可特异性地切断肽聚糖中的化学键,大多数裂解酶只包含一个催化结构域,少数含有多个催化结构域[4-5]。根据切割肽聚糖的化学键类型,可将裂解酶分为6大类[2] (图 1B):(1) N-乙酰基酰胺酶;(2) N-乙酰-β-D-氨基葡萄糖苷酶;(3) MurNAc-L-丙氨酸酰胺酶;(4)内肽酶;(5) 肽酶;(6) 转糖苷酶。裂解酶的C端为细胞壁结合结构域(Cell Wall Binding Domain,CBD),可特异性地结合肽聚糖上相应的配体[6]。在解析的CBD结构中,它们大多为单体结构,少数为多聚体结构,所在的位置和方向并不保守,大部分位于裂解酶的C端,少数位于N端[7]。由于革兰氏阳性菌没有外膜(Outer Membrane,OM)包被,这使得革兰氏阳性菌噬菌体裂解酶在没有穿孔素(Holin)的协助下,可以从外部裂解细菌的细胞壁。相比之下,大多数革兰氏阴性菌噬菌体裂解酶只有一个CD,没有CBD,由于外膜的存在,革兰氏阴性菌噬菌体裂解酶无法从外部进入肽聚糖层发挥裂解作用,这可能解释了为什么大部分革兰氏阴性菌噬菌体裂解酶是小的单结构域的球状蛋白(分子量在15−20 kD之间)[8-9]。与革兰氏阳性菌噬菌体裂解酶相比,这类裂解酶可能会更好地发挥酶的催化作用(辅助细胞裂解过程中的多个催化反应)[10]。但也有一些特殊情况,如来自绿脓杆菌噬菌体的裂解酶KZ144和EL188就含有CD和CBD这2种结构域[7]。裂解酶的活性和结构特点显示了其良好的抗菌作用,也使其在成为新型抗菌药物方面拥有广阔的前景。
|
| 图 1 裂解酶结构以及对细菌细胞壁肽聚糖的作用位点 Figure 1 The structure of lysin and its action site on bacterial cell wall peptidoglycan 注:A:噬菌体裂解酶的结构简图,其中绿色代表催化结构域,红色代表结合结构域;B:裂解酶作用于细菌细胞壁的作用位点 Note: A: Schematic diagram of the structure of bacteriophage lysin, where the green represents the catalytic domain and the red represents the binding domain; B: Lysin acts on the site of bacterial cell wall |
|
|
1958年,Jacob等[11]首次报道了噬菌体可以编码裂解细菌的蛋白质,即裂解酶,并证实它们在噬菌体感染宿主菌的过程中发挥着重要的作用;1975年,Freimer等[12]首次将裂解酶作为抗菌剂应用于A群链球菌(Group A Streptococcus)后,越来越多的裂解酶被发现和应用。
肺炎链球菌(Streptococcus pneumoniae)是一类高致病性和传染性的院内感染菌,能够引起人体(如中耳炎、鼻窦炎、肺炎、败血症和脑膜炎等)多种疾病。由于肺炎链球菌对抗生素的耐药性增加,由肺炎链球菌引起的细菌性疾病的治疗越来越困难[13]。2008年,Grandgirard等[14]研究表明,纯化的裂解酶Cpl-1在治疗因肺炎链球菌感染引起的败血症和脑膜炎中具有良好的抗菌效果;一次性注射20 mg/kg的Cpl-1可使小鼠脑脊液中肺炎球菌在30 min内迅速减少3个数量级,作用时间持续2 h;向小鼠腹膜中注射200 mg/kg的Cpl-1对脑脊液(Cerebrospinal Fluid,CSF)的抗菌作用则达到2个数量级,持续3 h。因此裂解酶Cpl-1也有望成为治疗肺炎球菌性脑膜炎和菌血症的潜在药物。通过解析的三维结构,裂解酶Cpl-1高效裂解肺炎球菌的作用机理也被合理解释。其是一种胆碱结合蛋白(Choline Binding Protein,CBP)含有一个催化结构域(CD)和一个胆碱结合结构域(Choline Binding Domain,CMB),CD可作用于肽聚糖中的化学键,CMB可以特异性结合细胞壁上的胆碱分子。Cpl-1的CD和CMB在结构上共同构成一个发夹结构,其CMB由6个连续串联重复的胆碱结合基序组成,它们并排排列,形成2个不同的结构区即CI、CII,彼此通过疏水作用靠在一起(图 2)。CD与CMB在结合底物之前相互作用,导致结合域2个结构区之间的角度增大,使裂解酶以正确的方向结合细胞壁上的糖链和胆碱分子,发挥最大的裂解活性,这为对多重耐药性肺炎链球菌的抗菌药物的研发奠定了理论基础[15-17]。

|
| 图 2 裂解酶Cpl-1的晶体结构 Figure 2 Crystal structure of lysin Cpl-1 注:裂解酶Cpl-1整体结构包含N-端的CD域(绿色部分)和C-端CMB (灰色和紫色部分),2个结构域相互作用构成一个发夹结构,其中CMB由6个连续串联重复的胆碱结合基序组成 Note: The overall structure of lysin Cpl-1 contains N-terminal CD domain (green) and C-terminal CMB (gray and purple). The two domains interact to form a hairpin structure, where CMB is composed of six consecutively repeated choline binding motifs |
|
|
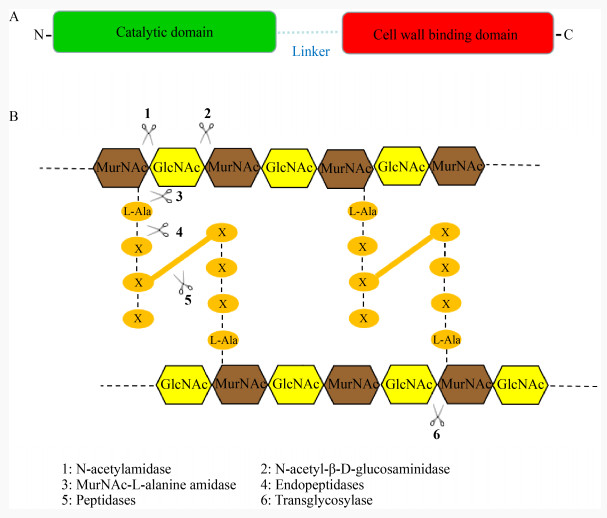
金黄色葡萄球菌(Staphylococcus aureus)是造成院内感染最为严重的一种病原菌,其可以引起皮肤脓肿、伤口感染、髓炎、肺炎、心内膜炎以及中毒性休克综合征等多种疾病[18-20]。2007年,Rashel等[21]发现裂解酶MV-L可以有效清除接种到小鼠鼻腔中的耐甲氧西林金黄色葡萄球菌(Methicillin-Resistant Staphylococcus aureus,MRSA),向小鼠的腹腔注射MV-L (500 U)也能提高小鼠的生存率。2014年,Gu等[22]研究发现裂解酶LysGH15能够高效裂解多种金黄色葡萄球菌,其中包括24株MRSA。研究表明,小鼠在感染金黄色葡萄球菌后,从腹腔注射裂解酶LysGH15 (50 μg)能够对小鼠提供100%的保护率,进一步研究发现LysGH15显著降低小鼠血液中的含菌量以及减少炎症反应的发生[23-24]。为了揭示裂解酶LysGH15高效裂解金黄色葡萄球菌的分子机制,Gu等[22]通过X-Ray晶体衍射法解析了LysGH15的三维结构,裂解酶LysGH15是一种金属离子依赖性肽酶,其包含CHAP、Amidase、SH3b这3个活性域,其中CHAP和Amidase为催化结构域,分别结合一个钙离子和锌离子,SH3b为结合结构域(图 3);钙离子对CHAP的裂解活性至关重要,一旦丢失,CHAP则无法发挥裂解活性;Amidase活性域单独表现不出裂解活性,主要表现为增强CHAP活性的作用,在LysGH15的整体活性中的作用很小;SH3b结合结构域在LysGH15的全长活性中发挥着重要的作用,将SH3b的结合位点突变后,全长LysGH15的裂解活性出现不同程度的下降,这表明CHAP和SH3b对LysGH15的高效裂解活性至关重要。
|
| 图 3 裂解酶LysGH15的晶体结构 Figure 3 Crystal structure of the lysin LysGH15 注:裂解酶LysGH15包含CHAP、Amidase和SH3b这3个活性域,其中CHAP和Amidase分别结合一个Ca2+和Zn2+,CHAP和SH3b对全长LysGH15活性至关重要,而Amidase则主要对CHAP活性起增强作用 Note: Lysin LysGH15 contains three active domains: CHAP, Amidase and SH3b. CHAP and Amidase bind a Ca2+ and Zn2+ respectively. CHAP and SH3b are essential for the activity of full-length LysGH15, while Amidase mainly enhances CHAP activity |
|
|
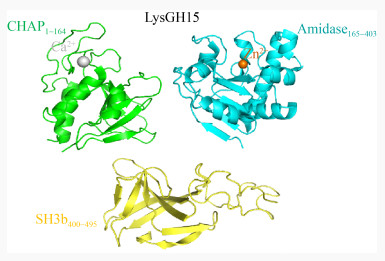
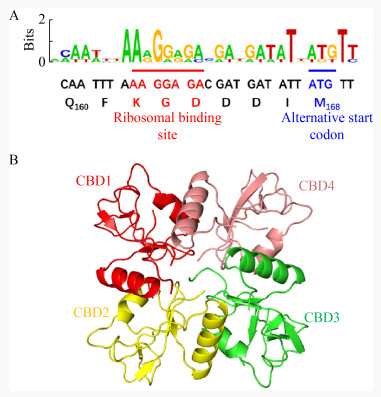
粪肠球菌(Enterococcus faecalis)是一种普遍寄生在人或动物肠道、口腔及生殖道内的革兰氏阳性菌,当其在体内异位寄生时,可引起人的心内膜炎、菌血症、尿道感染及脑膜炎等多种疾病[25-26]。2013年,Zhang等[27]从医院的废水中分离到一株粪肠球菌噬菌体IME-EF1,并表达其裂解酶LysIME-EF1,研究表明LysIME-EF1展现出比亲本噬菌体更宽的裂解谱,能够裂解8株粪肠球菌和1株屎肠球菌(Enterococcus faecium),其中还包括2株万古霉素耐药粪肠球菌(Vancomycin- Resistant Enterococcus faecalis,VREF) V309和V583。2020年,Zhou等[28]解析了LysIME-EF1的三维结构;LysIME-EF1整体结构呈不规则的上下结构,包括一分子全长LysIME-EF1 (绿色和紫红色部分)和三分子额外CBD (彩色部分) (图 4A);全长LysIME-EF1的CHAP结构域构成整体结构上部分,额外的三分子CBD与全长LysIME-EF1的CBD形成环状四聚体构成整体结构的下部分,四分子CBD之间没有任何区别。CBD形成的环状四聚体一面比较平(图 4B右),而另一面则凸出来(图 4B左),LysIME-EF1的CHAP结构域就位于CBD四聚体的凸出来的一面,彼此通过Linker连接,其整体形似“八爪鱼”,由于CBD的环状四聚体结构显著增加了LysIME-EF1结合细菌细胞壁的接触面积,导致其裂解活性比其他粪肠球菌噬菌体裂解酶高;进一步研究发现在LysIME-EF1的168位氨基酸(甲硫氨酸,M)对应的密码子ATG的上游存在一个核糖体结合位点(Ribosomal Binding Site,RBS),这是导致LysIME-EF1额外产生三分子CBD的原因(图 5A)。正是因为这种独特的基因结构促使LysIME-EF1 CBD形成独特的四聚体结构,并赋予其高效裂解粪肠球菌临床株的能力。
|
| 图 4 LysIME-EF1的晶体结构 Figure 4 Crystal structure of LysIME-EF1 注:A:LysIME-EF1的整体结构,其中绿色部分代表全长LysIME-EF1的CHAP结构域;紫色部分为全长LysIME-EF1的CBD,额外的3个CBD为其他颜色;黑色虚线代表linker。B:Lys IME-EF1的4个CBD形成的环状四聚体,一面平整(右),一面凸起(左),N端CHAP结构域位于凸起的CBD四聚体环上 Note: A: The overall structure of LysIME-EF1. The green part represents the CHAP domain of the full length LysIME-EF1 and the purple part is the CBD of the full length LysIME-EF1. The additional three CBDs are in other colors. The black dotted line represents the linker. B: A cyclic tetramer formed by the four CBDs of LysIME-EF1, one side is flat (right) and the other side is convex (left). The N-terminal CHAP domain is located on the convex CBD tetramer ring |
|
|
|
| 图 5 LysIME-EF1的内部翻译替代起始位点的鉴定和裂解酶Lys170 CBD的整体结构[29-30] Figure 5 Identification of the internal translation substitution start site of LysIME-EF1 and the overall structure of Lys170 CBD[29-30] 注:A:内部核糖体结合位点序列用红色下划线标注,起始密码子用蓝线下划线标注;B:CBD1代表全长的Lys170 CBD的结构(红色部分),其他颜色为额外的CBD结构,4个CBD形成四聚体环 Note: The internal ribosomal binding site sequence is underlined in red. The start codon is underlined in blue. B: CBD1 represents the full length Lys170 CBD structure (red) and the other colors are additional CBD structures. The four CBDs form a tetramer ring |
|
|
同样的机制也存在于噬菌体裂解酶Lys170中[29],2021年,Xu等[30]解析了裂解酶Lys170的CBD结构,Lys170 CBD和LysIME-EF1 CBD类似,都是四聚体结构(图 5B)。4个CBD彼此之间依靠氢键和疏水作用维持相互作用,将CBD四聚体化破坏,裂解酶Lys170的裂解活性大幅度降低。同时在Lys170的基因序列中,也存在内部翻译替代起始位点(170 M)和RBS的现象;该系列研究首次从结构和功能角度阐明了粪肠球菌噬菌体的单个基因编码多组分裂解酶的作用机理,对多重耐药性粪肠球菌的抗菌药物开发具有重大意义。
目前在被发现的裂解酶中,结构最为复杂、活性最高的裂解酶是来自A型链球菌噬菌体裂解酶PlyC[31-32]。2001年,Nelson等[33]发现裂解酶PlyC可以在5 s内裂解107 CFU的化脓性链球菌,提前给小鼠口服250 U的PlyC能显著降低细菌的感染率;对于已经感染的小鼠,在口服500 U的PlyC 2 h后,可使小鼠血液含菌量显著降低;PlyC高效裂解细菌的原因归结于其独特的三维结构,其由一分子PlyCA和由8个PlyCB分子组成的环状结构组成,PlyCA和PlyCB分别由2段基因编码(图 6A)。PlyCA含有3个结构域,其中2个分别是负责催化的糖苷水解酶结构域(GyH1–205)和依赖于组氨酸的半胱氨酸酰胺水解酶结构域(CHAP309–465),另外一个则是帮助连接的螺旋对接结构域(Helical Docking Domain 266−288)。PlyCB则是由8个重复的CBD组成环状八聚体结构(图 6B),8个CBD增强了PlyC结合细胞壁的能力,2个催化结构域增强了其催化能力,二者的分工合作奠定了裂解酶PlyC高效裂解活性的结构基础[34]。

|
| 图 6 裂解酶PlyC的整体结构 Figure 6 The overall structure of the lysin PlyC 注:A:裂解酶PlyC整体结构由PlyCA和PlyCB这2个部分以及中间的Linker组成,其中PlyCA包含CHAP和GyH这2个催化活性域,主要发挥PlyC的裂解活性;B:PlyCB是由8个重复单元组成的环状结构,主要发挥结合活性 Note: A: The overall structure of the lysin PlyC is composed of two parts, PlyCA and PlyCB, and a linker in the middle. PlyCA contains two catalytically active domains, CHAP and GyH, which mainly exert the cleavage activity of PlyC. B: PlyCB is composed of eight repeated units and the composed ring structure mainly exerts binding activity |
|
|
裂解酶凭借着对病原菌的高效裂解性以及宿主专一性,使其在增强食品安全性中也有着巨大的潜力[35-37]。研究表明李氏杆菌噬菌体裂解酶HPL118、HPL500和HPLP35在蔬菜、牛奶及其他奶制品中表现出高效的抑菌活性,而且这3种裂解酶对高温不敏感,能够在较高的温度下发挥裂解活性,利用这种特性可以将它们制作成食品添加剂提高食品安全性[38]。
裂解酶的CBD能够特异地结合细菌细胞壁。Kretzer等[39-40]用裂解酶的CBD来取代抗体,通过包覆有重组李斯特氏菌噬菌体裂解酶衍生的CBD分子的顺磁珠,在40 min内可以固定并回收超过90%的李斯特菌(Listeria),而且不受其他微生物存在的影响;相对传统的检测回收方法,其能缩短一半的时间,并且更为灵敏、通用性更高。目前,在此方法的基础上,Yi等[41]开发了一种基于裂解酶CBD的磁性分离和荧光检测技术,用于对真实样品中的金黄色葡萄球菌进行特异性检测和灵敏定量分析,并且通过功能化的磁珠,可将金黄色葡萄球菌样品基质分离处理;检测范围从1.0×102 CFU/mL到1.0×107 CFU/mL不等,最低至78 CFU/mL,整个检测过程在不到50 min的时间内完成;除了普通表皮的葡萄球菌外,其他与普通食源性和医院感染性细菌对金黄色葡萄球菌的检测影响均可忽略不计;此外,该方法在实际应用中的潜力已经通过无菌牛奶和人体血清的检测得以证明。
在农业上,裂解酶可以通过转基因等技术手段在植物细胞进行表达,从而防控植物病原细菌的感染,提高作物产量[42-43]。在动物养殖业,可以使用噬菌体相关的裂解酶来预防和治疗细菌性疾病,从而大大降低动物感染病原菌的风险[44-45]。
3 噬菌体裂解酶的改造与发展裂解酶作为一种潜在的抗菌药物,在治疗细菌感染性疾病中具有巨大的应用前景,但裂解酶在临床上的大规模应用还存在一定的局限性,如裂解范围在不同的菌属之间较窄、在体内裂解活性相对较低、给药方式困难、在体内半衰期较短容易失效或被清除等[46]。目前,研究人员也在积极探索各种方法切实有效地解决这些问题。如将不同来源的噬菌体裂解酶进行融合重组,产生嵌合体裂解酶。嵌合体裂解酶不仅裂解范围更广,而且裂解活性更强、稳定性更高[47-48]。Xu等[49]报道了一种独特的嵌合体裂解酶ClyR,由PlyCAC的CD和PlySs2的CBD组成。该裂解酶对大多数链球菌具有高效裂解活性,同时ClyR对引起龋齿和牛乳腺炎的病原体也具有高效裂解活性,类似的嵌合酶还有PL3、Csl2、PlySK1249、P128等[50-52]。
除嵌合酶外,抗生素和裂解酶联用也是提高抗菌活性、降低细菌耐药性的有效方法之一[53-54]。Schuch等[55-56]将裂解酶CF-301与万古霉素或达托霉素联用后,对因葡萄球菌(Staphylococcus)感染引起的菌血症具有良好的治疗效果,并且在26项独立的菌血症研究中证实了CF-301与抗生素联合使用的优越性。2018年,Letrado等[13]测试了Cpl-711和不同抗生素(阿莫西林、头孢噻肟、左氧氟沙星和万古霉素)的联合使用对几种耐多药肺炎链球菌的杀菌效果。通过小鼠和斑马鱼感染模型证实Cpl-711联合阿莫西林或头孢噻肟对血清型23F多耐药肺炎链球菌临床分离株具有协同杀菌作用,裂解酶SA.100、LysGH15、SAL200在与抗生素联用后也表现出协同效应[23, 57-58]。
裂解酶作为一类小分子蛋白质药物,通常需要注射到血液中发挥抗菌作用,但这种给药方式效率低下并可能引起机体的免疫反应,导致治疗失败,这是限制裂解酶药物发展的重要原因之一[59]。2020年,Wang等[60]将裂解酶Cpl-1和ClyJ-3制成可吸入的气雾剂,重点研究了其抗菌活性、蛋白质结构变化和气溶胶性能;结果表明,雾化后ClyJ-3的生物活性几乎完全丧失,而Cpl-1在使用不同的雾化方式后,其杀菌活性有所降低,但对肺炎链球菌仍然具有裂解作用;该研究结果表明雾化裂解酶进行吸入输送是可行的,但其治疗效果同时取决于蛋白质和雾化器的选择。
裂解酶性质不稳定、体内半衰期短、容易被清除等因素也是限制其发展的一个重要原因。最新的研究表明纳米颗粒可以通过保护蛋白质免于降解,并且能够增加裂解酶稳定性和保质期,从而提高治疗效果[61]。2020年,Kaur等[62]研究表明将藻酸盐-壳聚糖纳米颗粒(Alg-Chi NPs)用作裂解酶LysMR-5的药物递送系统是可行的;在该研究中,载有裂解酶LysMR-5的纳米颗粒是由钙离子诱导的藻酸盐核心与壳聚糖的络合制备而成,抗菌实验和SDS-PAGE电泳结果均显示LysMR-5纳米颗粒的结构完整,以及制备前后生物活性均没有发生改变;同时观察到空白的Alg-Chi NPs对金黄色葡萄球菌也具有直接的抗菌作用,而LysMR-5加载后,纳米颗粒的杀菌活性更高、持续时间更久;细胞和血液相容性研究表明LysMR-5纳米颗粒在体内有很好的生物组织相容性,这使得其引起机体免疫反应的风险大大降低;总体而言,该研究证明了Alg-Chi NPs作为裂解酶LysMR-5和其他治疗性蛋白质在各种生物医学应用中的纳米递送载体的潜力,这种方法不仅降低裂解酶引起机体免疫应答的风险,同时提高了给药效率,即使很小剂量的药物,通过纳米靶向传送也可以发挥很好的治疗效果。
4 展望近年来,由于多重耐药菌的频繁出现及新型抗生素的开发进度缓慢,噬菌体裂解酶成为下一代抗菌药物的潜力巨大[63-65]。从目前的研究来看,裂解酶呈现出具有几种典型特征的新型抗菌剂:(1) 无论在体内或体外都对革兰氏阳性菌具有很好的抗菌效果;(2) 对细菌的裂解活性高,不用考虑其抗生素耐药性问题;(3) 裂解谱较广,相对于亲本噬菌体裂解范围有所增加;(4) 细菌对其产生的耐药性的可能性低;(5) 安全性较高,不会引起较强的免疫抗原性;(6) 容易通过基因工程对其进行改造。
尽管噬菌体裂解酶在某些领域上的应用取得了较大的进步,但在临床中的大规模应用还面临着许多问题,其主要表现在以下几个方面:(1) 裂解酶在体外表达时有些会对表达菌株具有毒性,并且分子量较大、疏水性强,导致蛋白表达时往往以包涵体的形式存在;(2) 对革兰氏阴性菌不敏感,难以自外而内地裂解细胞壁;(3) 裂解酶作为外源蛋白质,进入机体后易受到蛋白酶的降解,而具有较短的半衰期;(4) 裂解酶在治疗过程中的最佳时间和最适剂量难以控制;(5) 裂解酶的长期保存方法、安全的给药方式,以及如何评价裂解酶治疗的效果都不够明确。
面对这些困境,利用分子生物学领域的相关技术方法,从基因水平改造噬菌体裂解酶,提高裂解活性及稳定性、扩大其宿主谱,使其能够特异性裂解某一种属的细菌,甚至不同种属的细菌,是今后裂解酶研究的趋势之一[66],同时更换表达菌株使裂解酶能够在真核生物和细胞水平上表达,从而解决裂解酶对表达菌株具有毒性问题,提高其稳定性也是今后的研究方向之一。其次,筛选和培育能在体内长期循环的噬菌体也是今后的趋势之一,通过连续传代的方法来筛选能在体内长期循环的噬菌体,从而表达其对应的裂解酶,这可有效地延缓机体免疫系统对裂解酶的清除,延长裂解酶在体内存留时间。除此之外,(1) 性质更优裂解酶的筛选与鉴定;(2) 裂解酶在体内的分子动力学以及裂解机制的深入研究;(3) 裂解酶规模化生产工艺的探索;(4) 裂解酶的成药性研究;(5) 推动国家相关部门对裂解酶作为新药申报标准的制定与颁布,这5个方面都是我们下一步需要开展的工作。
| [1] |
Loessner MJ. Bacteriophage endolysins: current state of research and applications[J]. Current Opinion in Microbiology, 2005, 8(4): 480-487. DOI:10.1016/j.mib.2005.06.002 |
| [2] |
Gutiérrez D, Fernández L, Rodríguez A, García P. Are phage lytic proteins the secret weapon to kill Staphylococcus aureus?[J]. mBio, 2018, 9(1): e01923-e01917. |
| [3] |
Roach DR, Donovan DM. Antimicrobial bacteriophage- derived proteins and therapeutic applications[J]. Bacteriophage, 2015, 5(3): e1062590. DOI:10.1080/21597081.2015.1062590 |
| [4] |
Broendum SS, Buckle AM, McGowan S. Catalytic diversity and cell wall binding repeats in the phage-encoded endolysins[J]. Molecular Microbiology, 2018, 110(6): 879-896. DOI:10.1111/mmi.14134 |
| [5] |
Reuter M, Kruger DH. Approaches to optimize therapeutic bacteriophage and bacteriophage-derived products to combat bacterial infections[J]. Virus Genes, 2020, 56(2): 136-149. DOI:10.1007/s11262-020-01735-7 |
| [6] |
Hong S, Son B, Ryu S, Ha NC. Crystal structure of LysB4, an endolysin from Bacillus cereus-targeting bacteriophage B4[J]. Molecules and Cells, 2019, 42(1): 79-86. |
| [7] |
Briers Y, Schmelcher M, Loessner MJ, Hendrix J, Engelborghs Y, Volckaert G, Lavigne R. The high-affinity peptidoglycan binding domain of Pseudomonas phage endolysin KZ144[J]. Biochemical and Biophysical Research Communications, 2009, 383(2): 187-191. DOI:10.1016/j.bbrc.2009.03.161 |
| [8] |
Larpin Y, Oechslin F, Moreillon P, Resch G, Entenza JM, Mancini S. In vitro characterization of PlyE146, a novel phage lysin that targets Gram-negative bacteria[J]. PLoS One, 2018, 13(2): e0192507. DOI:10.1371/journal.pone.0192507 |
| [9] |
Briers Y, Volckaert G, Cornelissen A, Lagaert S, Michiels CW, Hertveldt K, Lavigne R. Muralytic activity and modular structure of the endolysins of Pseudomonas aeruginosa bacteriophages φKZ and EL[J]. Molecular Microbiology, 2007, 65(5): 1334-1344. DOI:10.1111/j.1365-2958.2007.05870.x |
| [10] |
Schmelcher M, Shabarova T, Eugster MR, Eichenseher F, Tchang VS, Banz M, Loessner MJ. Rapid multiplex detection and differentiation of Listeria cells by use of fluorescent phage endolysin cell wall binding domains[J]. Applied and Environmental Microbiology, 2010, 76(17): 5745-5756. DOI:10.1128/AEM.00801-10 |
| [11] |
Jacob F, Fuerst CR. The mechanism of lysis by phage studied with defective lysogenic bacteria[J]. Journal of General Microbiology, 1958, 18(2): 518-526. DOI:10.1099/00221287-18-2-518 |
| [12] |
Freimer EH, Krause RM, McCarty M. Studies of L forms and protoplasts of group A streptococci. I. isolation, growth, and bacteriologic characteristics[J]. Journal of Experimental Medicine, 1959, 110(6): 853-874. DOI:10.1084/jem.110.6.853 |
| [13] |
Letrado P, Corsini B, Díez-Martínez R, Bustamante N, Yuste JE, García P. Bactericidal synergism between antibiotics and phage endolysin Cpl-711 to kill multidrug-resistant pneumococcus[J]. Future Microbiology, 2018, 13: 1215-1223. DOI:10.2217/fmb-2018-0077 |
| [14] |
Grandgirard D, Loeffler JM, Fischetti VA, Leib SL. Phage lytic enzyme Cpl-1 for antibacterial therapy in experimental pneumococcal meningitis[J]. The Journal of Infectious Diseases, 2008, 197(11): 1519-1522. DOI:10.1086/587942 |
| [15] |
Hermoso JA, Monterroso B, Albert A, Galán B, Ahrazem O, Garcı́a P, Martı́nez-Ripoll M, Garcı́a JL, Menéndez M. Structural basis for selective recognition of pneumococcal cell wall by modular endolysin from phage Cp-1[J]. Structure, 2003, 11(10): 1239-1249. DOI:10.1016/j.str.2003.09.005 |
| [16] |
Doehn JM, Fischer K, Reppe K, Gutbier B, Tschernig T, Hocke AC, Fischetti VA, Löffler J, Suttorp N, Hippenstiel S, et al. Delivery of the endolysin Cpl-1 by inhalation rescues mice with fatal pneumococcal pneumonia[J]. The Journal of Antimicrobial Chemotherapy, 2013, 68(9): 2111-2117. DOI:10.1093/jac/dkt131 |
| [17] |
Monterroso B, Albert A, Martínez-Ripoll M, García P, García JL, Menéndez M, Hermoso JA. Crystallization and preliminary X-ray diffraction studies of the complete modular endolysin from Cp-1, a phage infecting Streptococcus pneumoniae[J]. Acta Crystallographica Section D, Biological Crystallography, 2002, 58(Pt9): 1487-1489. |
| [18] |
Cheng Q, Fischetti VA. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci[J]. Applied Microbiology and Biotechnology, 2007, 74(6): 1284-1291. DOI:10.1007/s00253-006-0771-1 |
| [19] |
Patras KA, Wescombe PA, Rösler B, Hale JD, Tagg JR, Doran KS. Streptococcus salivarius K12 limits group B Streptococcus vaginal colonization[J]. Infection and Immunity, 2015, 83(9): 3438-3444. DOI:10.1128/IAI.00409-15 |
| [20] |
Lood R, Raz A, Molina H, Euler CW, Fischetti VA. A highly active and negatively charged Streptococcus pyogenes lysin with a rare D-alanyl-L-alanine endopeptidase activity protects mice against streptococcal bacteremia[J]. Antimicrobial Agents and Chemotherapy, 2014, 58(6): 3073-3084. DOI:10.1128/AAC.00115-14 |
| [21] |
Rashel M, Uchiyama J, Ujihara T, Uehara Y, Kuramoto S, Sugihara S, Yagyu KI, Muraoka A, Sugai M, Hiramatsu K, et al. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage ϕMR11[J]. The Journal of Infectious Diseases, 2007, 196(8): 1237-1247. DOI:10.1086/521305 |
| [22] |
Gu JM, Feng YG, Feng X, Sun CJ, Lei LC, Ding W, Niu FF, Jiao LY, Yang M, Li Y, et al. Structural and biochemical characterization reveals LysGH15 as an unprecedented "EF-hand-like" calcium-binding phage lysin[J]. PLoS Pathogens, 2014, 10(5): e1004109. DOI:10.1371/journal.ppat.1004109 |
| [23] |
Xia FF, Li X, Wang B, Gong PJ, Xiao F, Yang M, Zhang L, Song J, Hu LY, Cheng MJ, et al. Combination therapy of LysGH15 and apigenin as a new strategy for treating pneumonia caused by Staphylococcus aureus[J]. Applied and Environmental Microbiology, 2016, 82(1): 87-94. DOI:10.1128/AEM.02581-15 |
| [24] |
Zhang L, Li D, Li XW, Hu LY, Cheng MJ, Xia FF, Gong PJ, Wang B, Ge JL, Zhang H, et al. LysGH15 kills Staphylococcus aureus without being affected by the humoral immune response or inducing inflammation[J]. Scientific Reports, 2016, 6: 29344. DOI:10.1038/srep29344 |
| [25] |
Barbosa-Ribeiro M, De-Jesus-soares A, Zaia AA, Ferraz CCR, Almeida JFA, Gomes BPFA. Antimicrobial susceptibility and characterization of virulence genes of Enterococcus faecalis isolates from teeth with failure of the endodontic treatment[J]. Journal of Endodontics, 2016, 42(7): 1022-1028. DOI:10.1016/j.joen.2016.03.015 |
| [26] |
Daniel DS, Lee SM, Gan HM, Dykes GA, Rahman S. Genetic diversity of Enterococcus faecalis isolated from environmental, animal and clinical sources in Malaysia[J]. Journal of Infection and Public Health, 2017, 10(5): 617-623. DOI:10.1016/j.jiph.2017.02.006 |
| [27] |
Zhang WH, Mi ZQ, Yin XY, Fan H, An XP, Zhang ZY, Chen JK, Tong YG. Characterization of Enterococcus faecalis phage IME-EF1 and its endolysin[J]. PLoS One, 2013, 8(11): e80435. DOI:10.1371/journal.pone.0080435 |
| [28] |
Zhou B, Zhen XK, Zhou H, Zhao FY, Fan CP, Perčulija V, Tong YG, Mi ZQ, Ouyang SY. Structural and functional insights into a novel two-component endolysin encoded by a single gene in Enterococcus faecalis phage[J]. PLoS Pathogens, 2020, 16(3): e1008394. DOI:10.1371/journal.ppat.1008394 |
| [29] |
Proença D, Velours C, Leandro C, Garcia M, Pimentel M, S o-José C. A two-component, multimeric endolysin encoded by a single gene[J]. Molecular Microbiology, 2015, 95(5): 739-753. DOI:10.1111/mmi.12857 |
| [30] |
Xu XL, Zhang DD, Zhou B, Zhen XK, Ouyang SY. Structural and biochemical analyses of the tetrameric cell binding domain of Lys170 from enterococcal phage F170/08[J]. European Biophysics Journal, 2021, 50(5): 721-729. DOI:10.1007/s00249-021-01511-x |
| [31] |
Shen Y, Köller T, Kreikemeyer B, Nelson DC. Rapid degradation of Streptococcus pyogenes biofilms by PlyC, a bacteriophage-encoded endolysin[J]. Journal of Antimicrobial Chemotherapy, 2013, 68(8): 1818-1824. DOI:10.1093/jac/dkt104 |
| [32] |
Hoopes JT, Stark CJ, Kim HA, Sussman DJ, Donovan DM, Nelson DC. Use of a bacteriophage lysin, PlyC, as an enzyme disinfectant against Streptococcus equi[J]. Applied and Environmental Microbiology, 2009, 75(5): 1388-1394. DOI:10.1128/AEM.02195-08 |
| [33] |
Nelson D, Loomis L, Fischetti VA. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme[J]. Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(7): 4107-4112. DOI:10.1073/pnas.061038398 |
| [34] |
McGowan S, Buckle AM, Mitchell MS, Hoopes JT, Gallagher DT, Heselpoth RD, Shen Y, Reboul CF, Law RHP, Fischetti VA, et al. X-ray crystal structure of the streptococcal specific phage lysin PlyC[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(31): 12752-12757. DOI:10.1073/pnas.1208424109 |
| [35] |
Bai J, Kim YT, Ryu S, Lee JH. Biocontrol and rapid detection of food-borne pathogens using bacteriophages and endolysins[J]. Frontiers in Microbiology, 2016, 7: 474. |
| [36] |
Cho JH, Kwon JG, O'Sullivan DJ, Ryu S, Lee JH. Development of an endolysin enzyme and its cell wall-binding domain protein and their applications for biocontrol and rapid detection of Clostridium perfringens in food[J]. Food Chemistry, 2021, 345: 128562. DOI:10.1016/j.foodchem.2020.128562 |
| [37] |
Jamal M, Bukhari SMAUS, Andleeb S, Ali M, Raza S, Nawaz MA, Hussain T, Rahman SU, Shah SSA. Bacteriophages: an overview of the control strategies against multiple bacterial infections in different fields[J]. Journal of Basic Microbiology, 2019, 59(2): 123-133. DOI:10.1002/jobm.201800412 |
| [38] |
Schmelcher M, Waldherr F, Loessner MJ. Listeria bacteriophage peptidoglycan hydrolases feature high thermoresistance and reveal increased activity after divalent metal cation substitution[J]. Applied Microbiology and Biotechnology, 2012, 93(2): 633-643. DOI:10.1007/s00253-011-3372-6 |
| [39] |
Kretzer J, Schmelcher M, Loessner M. Ultrasensitive and fast diagnostics of viable Listeria cells by CBD magnetic separation combined with A511: detection[J]. Viruses, 2018, 10(11): 626. DOI:10.3390/v10110626 |
| [40] |
Kretzer JW, Lehmann R, Schmelcher M, Banz M, Kim KP, Korn C, Loessner MJ. Use of high-affinity cell wall-binding domains of bacteriophage endolysins for immobilization and separation of bacterial cells[J]. Applied and Environmental Microbiology, 2007, 73(6): 1992-2000. DOI:10.1128/AEM.02402-06 |
| [41] |
Yi ZJ, Wang SH, Meng XY, Wu AQ, Li Q, Song YJ, Zhao RL, Qiao JJ. Lysin cell-binding domain-functionalized magnetic beads for detection of Staphylococcus aureus via inhibition of fluorescence of Amplex Red/hydrogen peroxide assay by intracellular catalase[J]. Analytical and Bioanalytical Chemistry, 2019, 411(27): 7177-7185. DOI:10.1007/s00216-019-02099-0 |
| [42] |
Marin Viegas VS, Ocampo CG, Petruccelli S. Vacuolar deposition of recombinant proteins in plant vegetative organs as a strategy to increase yields[J]. Bioengineered, 2017, 8(3): 203-211. DOI:10.1080/21655979.2016.1222994 |
| [43] |
Islam MR, Son N, Lee J, Lee DW, Sohn EJ, Hwang I. Production of bacteriophage-encoded endolysin, LysP11, in Nicotiana benthamiana and its activity as a potent antimicrobial agent against Erysipelothrix rhusiopathiae[J]. Plant Cell Reports, 2019, 38(12): 1485-1499. DOI:10.1007/s00299-019-02459-1 |
| [44] |
Matamp N, Bhat S. Phage endolysins as potential antimicrobials against multidrug resistant Vibrio alginolyticus and Vibrio parahaemolyticus: current status of research and challenges ahead[J]. Microorganisms, 2019, 7(3): 84. DOI:10.3390/microorganisms7030084 |
| [45] |
Srinivasan R, Chaitanyakumar A, Subramanian P, Mageswari A, Gomathi A, Aswini V, Sankar AM, Ramya M, Gothandam KM. Recombinant engineered phage-derived enzybiotic in Pichia pastoris X-33 as whole cell biocatalyst for effective biocontrol of Vibrio parahaemolyticus in aquaculture[J]. International Journal of Biological Macromolecules, 2020, 154: 1576-1585. DOI:10.1016/j.ijbiomac.2019.11.042 |
| [46] |
Villa TG, Sieiro C. Phage therapy, lysin therapy, and antibiotics: a trio due to come[J]. Antibiotics, 2020, 9(9): 604. DOI:10.3390/antibiotics9090604 |
| [47] |
Oechslin F, Daraspe J, Giddey M, Moreillon P, Resch G. In vitro characterization of PlySK1249, a novel phage lysin, and assessment of its antibacterial activity in a mouse model of Streptococcus agalactiae bacteremia[J]. Antimicrobial Agents and Chemotherapy, 2013, 57(12): 6276-6283. DOI:10.1128/AAC.01701-13 |
| [48] |
Huang L, Luo DH, Gondil VS, Gong YJ, Jia MH, Yan DZ, He J, Hu SC, Yang H, Wei HP. Construction and characterization of a chimeric lysin ClyV with improved bactericidal activity against Streptococcus agalactiae in vitro and in vivo[J]. Applied Microbiology and Biotechnology, 2020, 104(4): 1609-1619. DOI:10.1007/s00253-019-10325-z |
| [49] |
Xu JJ, Yang H, Bi YL, Li WY, Wei HP, Li YH. Activity of the chimeric lysin ClyR against common Gram-positive oral microbes and its anticaries efficacy in rat models[J]. Viruses, 2018, 10(7): 380. DOI:10.3390/v10070380 |
| [50] |
Blázquez B, Fresco-Taboada A, Iglesias-Bexiga M, Menéndez M, García P. PL3 amidase, a tailor-made lysin constructed by domain shuffling with potent killing activity against pneumococci and related species[J]. Frontiers in Microbiology, 2016, 7: 1156. |
| [51] |
Vázquez R, Domenech M, Iglesias-Bexiga M, Menéndez M, García P. Csl2, a novel chimeric bacteriophage lysin to fight infections caused by Streptococcus suis, an emerging zoonotic pathogen[J]. Scientific Reports, 2017, 7: 16506. DOI:10.1038/s41598-017-16736-0 |
| [52] |
Channabasappa S, Chikkamadaiah R, Durgaiah M, Kumar S, Ramesh K, Sreekanthan A, Sriram B. Efficacy of chimeric ectolysin P128 in drug-resistant Staphylococcus aureus bacteraemia in mice[J]. Journal of Antimicrobial Chemotherapy, 2018, 73(12): 3398-3404. |
| [53] |
Septama AW, Panichayupakaranant P. Synergistic effect of artocarpin on antibacterial activity of some antibiotics against methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli[J]. Pharmaceutical Biology, 2016, 54(4): 686-691. DOI:10.3109/13880209.2015.1072566 |
| [54] |
Rao H, Lai PX, Gao Y. Chemical composition, antibacterial activity, and synergistic effects with conventional antibiotics and nitric oxide production inhibitory activity of essential oil from Geophila repens (L.) I.M. johnst[J]. Molecules, 2017, 22(9): 1561. DOI:10.3390/molecules22091561 |
| [55] |
Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, Rotolo JA, Horiuchi Y, Couto DE, Raz A, et al. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia[J]. The Journal of Infectious Diseases, 2014, 209(9): 1469-1478. DOI:10.1093/infdis/jit637 |
| [56] |
Indiani C, Sauve K, Raz A, Abdelhady W, Xiong YQ, Cassino C, Bayer AS, Schuch R. The antistaphylococcal lysin, CF-301, activates key host factors in human blood to potentiate methicillin-resistant Staphylococcus aureus bacteriolysis[J]. Antimicrobial Agents and Chemotherapy, 2019, 63(4): e02291-18. |
| [57] |
Totté JEE, Van Doorn MB, Pasmans SGMA. Successful treatment of chronic Staphylococcus aureus-related dermatoses with the topical endolysin staphefekt SA.100: a report of 3 cases[J]. Case Reports in Dermatology, 2017, 9(2): 19-25. DOI:10.1159/000473872 |
| [58] |
Kim NH, Park WB, Cho JE, Choi YJ, Choi SJ, Jun SY, Kang CK, Song KH, Choe PG, Bang JH, et al. Effects of phage endolysin SAL200 combined with antibiotics on Staphylococcus aureus infection[J]. Antimicrobial Agents and Chemotherapy, 2018, 62(10): e00731-18. |
| [59] |
Gondil VS, Harjai K, Chhibber S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections[J]. International Journal of Antimicrobial Agents, 2020, 55(2): 105844. DOI:10.1016/j.ijantimicag.2019.11.001 |
| [60] |
Wang YC, Khanal D, Chang RYK, Shang XR, Yang H, Britton WJ, Nelson D, Chan HK. Can bacteriophage endolysins be nebulised for inhalation delivery against Streptococcus pneumoniae?[J]. International Journal of Pharmaceutics, 2020, 591: 119982. DOI:10.1016/j.ijpharm.2020.119982 |
| [61] |
Gondil VS, Dube T, Panda JJ, Yennamalli RM, Harjai K, Chhibber S. Comprehensive evaluation of chitosan nanoparticle based phage lysin delivery system; a novel approach to counter S. pneumoniae infections[J]. International Journal of Pharmaceutics, 2020, 573: 118850. DOI:10.1016/j.ijpharm.2019.118850 |
| [62] |
Kaur J, Kour A, Panda JJ, Harjai K, Chhibber S. Exploring endolysin-loaded alginate-chitosan nanoparticles as future remedy for staphylococcal infections[J]. AAPS PharmSciTech, 2020, 21(6): 233. DOI:10.1208/s12249-020-01763-4 |
| [63] |
Sanz-Gaitero M, Van Raaij MJ. Crystallographic structure determination of bacteriophage endolysins[J]. Current Issues in Molecular Biology, 2021, 40: 165-188. |
| [64] |
Golkar Z, Bagasra O, Pace DG. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis[J]. Journal of Infection in Developing Countries, 2014, 8(2): 129-136. DOI:10.3855/jidc.3573 |
| [65] |
Nadeem SF, Gohar UF, Tahir SF, Mukhtar H, Pornpukdeewattana S, Nukthamna P, Moula Ali AM, Bavisetty SCB, Massa S. Antimicrobial resistance: more than 70 years of war between humans and bacteria[J]. Critical Reviews in Microbiology, 2020, 46(5): 578-599. DOI:10.1080/1040841X.2020.1813687 |
| [66] |
Yang H, Yu JP, Wei HP. Engineered bacteriophage lysins as novel anti-infectives[J]. Frontiers in Microbiology, 2014, 5: 542. |
 2021, Vol. 48
2021, Vol. 48