扩展功能
文章信息
- 梁胜男, 柯楚新, 黄鹤, 关嘉琦, 赵丽娜, 李柏良, 霍贵成
- LIANG Shengnan, KE Chuxin, HUANG He, GUAN Jiaqi, ZHAO Lina, LI Bailiang, HUO Guicheng
- 肠道内产丁酸细菌及其产物丁酸生理功能的研究进展
- Butyrate-producing bacteria in the intestinal tract and the physiological function of their metabolite butyrate: a review
- 微生物学通报, 2021, 48(3): 948-959
- Microbiology China, 2021, 48(3): 948-959
- DOI: 10.13344/j.microbiol.china.200466
-
文章历史
- 收稿日期: 2020-05-13
- 接受日期: 2020-06-07
- 网络首发日期: 2020-08-07
2. 黑龙江省疾病预防控制中心 黑龙江 哈尔滨 150030
2. Heilongjiang Provincial Center for Disease Control and Prevention, Harbin, Heilongjiang 150030, China
产丁酸细菌是指能够产生丁酸的一类细菌的总称。产丁酸细菌可以从土壤、粪便中分离,从粪便中分离的菌株安全性较高,在肠道中产丁酸细菌主要来自结肠和盲肠,目前发现的产丁酸细菌主要是厚壁菌门,包括梭菌属、真杆菌属和梭杆菌属等[1]。研究发现,产丁酸细菌对腹泻、结肠炎、糖尿病等疾病有治疗作用。
丁酸(Butyric Acid,Butyrate)是产丁酸细菌的主要代谢物,分子式为C4H8O2,是短链脂肪酸的主要成员之一,在肠道内主要由膳食纤维酵解产生,在体内可以通过脂肪酸氧化为机体供应能量,是肠道上皮细胞的主要供能物质[2]。丁酸与机体健康密切相关,对调节肠道健康、抑制炎症及癌症等病症意义重大。在养殖业中常添加丁酸盐保护动物健康生长,如预防断奶仔猪腹泻、调节鸡肠道菌群并增强其免疫力等[3-4]。丁酸的功能对医疗、食品及养殖业意义重大。本文根据近年来的研究进展,重点阐述了产丁酸细菌的种类及特点、丁酸的合成路径及丁酸在体内的生理功能。
1 合成丁酸的主要细菌 1.1 丁酸梭菌丁酸梭菌(Clostridium butyricum)又称酪酸梭菌、丁酸菌,是普遍存在于土壤、动物及人体粪便中的一类梭状芽胞杆菌,属于梭菌属(Clostridium),是严格厌氧的革兰氏阳性菌,培养后期可变成革兰氏阴性菌,显微镜下可观察到芽胞,细菌单个或成对存在,琼脂平板上呈白色或奶油色不规则菌落,DNA中GC含量为27%−28%,具有运动性[5]。1933年由日本宫入近治博士首次发现并报告[6]。国内已经有许多学者从不同生境中成功分离出C. butyricum[7-9]。
C. butyricum在肠道内产生的淀粉酶可以将碳水化合物降解为低聚糖,促进益生菌对碳源的利用[10],产物丁酸和细菌素可以抑制大肠杆菌、金黄色葡萄球菌等病原菌和食品腐败微生物[11]。这说明C. butyricum不仅可以被用作整肠药品调节肠道菌群,还可以应用在食品加工中抑制食品腐败。鉴于C. butyricum与微生物间的复杂关系,其制剂还常被用于肥料和兽药。断奶仔猪的腹泻导致仔猪成活率低、体质下降、生长缓慢,阻碍了养猪业的发展,在断奶仔猪饲料中添加适量C. butyricum,可以明显增加仔猪日增重比、降低腹泻指数[12]。另外,Wang等[13]研究发现C. butyricum可以有效缓解小鼠结肠炎(Inflammatory Bowel Disease,IBD),显著降低IBD小鼠死亡率。此外,C. butyricum还具有提高免疫力、抗氧化等生理功能[6],研究人员认定其开发前景,相信未来C. butyricum可以广泛应用于医疗、农业、食品等领域。
1.2 普拉梭菌普拉梭菌(Faecalibacterium prausnitzii)属于柔嫩梭菌属(Clostridium leptum),是不产生芽胞、无运动性的严格厌氧的革兰氏阴性菌,其DNA中GC含量为47%−57%。F. prausnitzii在女性、6个月以下婴儿和老年人肠道中丰度较低,在健康人群中占肠道粪便细菌的5%[14],代谢葡萄糖时,除产生大量的丁酸外,还产生少量的乳酸[15]。20世纪70年代,Cato等[16]首次从人粪便中分离得到F. prausnitzii,之后研究人员陆续从牛、猪的粪便中分离得到F. prausnitzii[1]。
癌症的检测,如结肠癌、乳腺癌都可以借助F. prausnitzii,有研究表明患有结肠癌和肠息肉的患者粪便及肠粘膜部分F. prausnitzii含量显著降低[17],F. prausnitzii结合磷酰胆碱也可能成为乳腺癌的检测方法之一,并且F. prausnitzii可以抑制乳腺癌细胞中IL-6的分泌和JAK2/JAK3的磷酸化[18]。F. prausnitzii还可以通过改善Th17/Treg免疫应答,上调TJ蛋白改善肠道屏障,以缓解IBD[19]。李媛媛[20]研究发现F. prausnitzii及其上清液可以通过促进小鼠外周Treg细胞的生成,增加IL-10、TEGE-β1的分泌,抑制小鼠炎症,而且上清液抑制效果高于菌液。另外,研究表明F. prausnitzii产生的胞外囊泡对抗炎因子的促进和促炎因子的抑制也表现出显著的优越性[21-22]。F. prausnitzii与对疾病的指示和缓解作用为医药行业提供了新的发展思路。
1.3 罗斯氏菌属罗斯氏菌属(Roseburia)是严格厌氧的革兰氏染色不定菌属,微弯杆,无芽胞,具有运动性,DNA中GC含量为42.3%。最初Barcenilla从健康人类粪便中分离出菌株L1-82T,后该菌株被归于Roseburia的其中一个种R. intestinalis,并成为该种模式株[23]。
研究发现患有慢性肾病或IBD的患者粪便中R. intestinalis的丰度显著低于健康人[24-25],Jiang提出可以将R. intestinalis作为慢性肾病检测的“微生物标志物”,并且R. intestinalis可以调节抗炎转录信号和转录激活因子的表达,减少结肠中炎性巨噬细胞和Th17细胞的数量,改善IBD[24]。R. intestinalis的鞭毛蛋白可以通过识别TLR5、上调紧密连接蛋白和过表达IL-22、REG3γ修复肠道屏障,改善肠道生态系统,缓解慢性炎症[26]。然而R. intestinalis在医用时发现,灌胃给药的治疗效果并不可观,这可能是因为细菌在消化道中的运动造成了损失,为弥补这一缺陷,Xiao等[27]则利用磁性氧化铁纳米颗粒实现了R. intestinalis在肠道内的靶向传递,这一研究为益生菌对疾病的治疗提出了更多的机会,也迈出了在微生物学中对生物活体无线操纵的第一步。
1.4 真菌属真菌属(Eubacterium)中霍式真杆菌(Eubacterium hallii)和直肠真杆菌(Eubacterium rectale)是人类粪便微生物中比较丰富的物种,可以合成丁酸。
1.4.1 霍式真杆菌E. hallii是一种周身鞭毛运动的兼性厌氧型革兰氏阴性菌。现已从婴儿粪便中分离出一株E. hallii,命名为L2-7T,其DNA中GC含量为38.6%,该菌株能够利用葡萄糖合成丁酸,而且口服可改善小鼠胰岛素敏感性[28]。此外,E. hallii可以利用甘油产生3-羟基丙醛[29],借助该代谢途径转化2-氨基-1-甲基-6-苯基咪唑(4, 5-b)吡啶(2-Amino- 1-Methyl-6-Phenylimidazo[4, 5-b]Pyridine,PhIP),该物质是肉类加工中易产生的致癌物质[30]。在模拟人类结肠微生物发酵的实验中发现,E. hallii的存在使近结肠端PhIP的转化提高了300倍,远结肠端也可提升120倍[30]。为提高食品安全性,肉制品加工时适时适量补充E. hallii或可降低食用时机体对有害物质的吸收,这一发现可能促进肉制品加工行业的发展。
1.4.2 直肠真杆菌E. rectale为厌氧型革兰氏阳性菌,多呈直杆状或稍微弯曲。现已从绵羊和奶牛的瘤胃内容物中分离出3株,其DNA中GC含量在41.4%−42.2%范围内变化,具有运动性[31]。在统计学分析上,E. rectale的丰度与高血压、甲亢等疾病有关,但具体的关联还需要进一步探讨[32-33]。
1.5 丁酸弧菌属丁酸弧菌属(Butyrivibrio)属于瘤胃细菌,被分离于动物瘤胃中,菌体呈弧状,为严格厌氧型革兰氏阴性菌,具有运动性[31],DNA中GC含量为36.0%−41.0%,其模式种为溶纤维丁酸弧菌(Butyrivibrio fibrisolvens)[34-35]。B. fibrisolvens主要以纤维素为底物代谢产生丁酸,是木质纤维素材料解聚多糖降解酶的重要来源,此外,B. fibrisolvens还可以产生乙酸、乳酸等物质[36-37]。
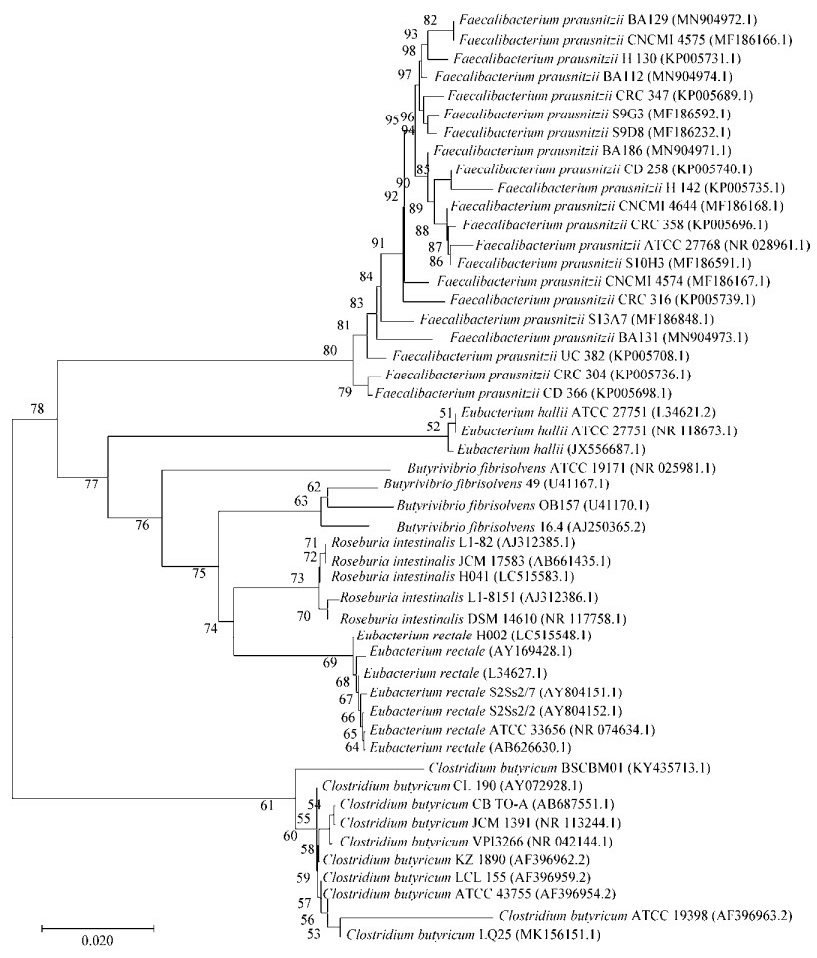
产丁酸细菌种类较多,为更加有效地对比不同产丁酸细菌之间的差异,表 1总结了文中介绍的几种细菌的特点,对部分已分离的菌株进行举例说明,并利用National Center for Biotechnology Information中提供的部分菌株的16S rRNA基因序列绘制系统发育树(图 1),以期能够增强对产丁酸细菌的认识。
| Genus | Species | GC content(mol%) | Characteristics of bacteria | Isolated strain | |||
| Strains | Source | Butyrate production (g/L) | References | ||||
| Clostridium | C. butyricum | 27.0−28.0 | Single or in pairs, spore subterminal, motility, gram-positive bacteria, but can be converted to gram-negative bacteria in late culture | C. butyricum C1-6 | Healthy adult feces | 2.156 | [7] |
| C. butyricum HBUT-01 | River sludge | 4.280 | [8] | ||||
| C. butyricum EL7 | Luzhou-flavor white wine cellar mud | 2.710 | [9] | ||||
| Clostridium leptum | F. prausnitzii | 47.0−57.0 | Rod-shaped, non- flagellate, non- motile, non-spore | F. prausnitzii F31 | Healthy adult feces | 1.535 | [38] |
| Roseburia | R. intestinalis | 42.3 | Slightly curved rod, motility, no spore, variable gram staining | R. intestinalis L1-82T | Healthy adult feces | 1.628 | [39] |
| Eubacterium | E. hallii | 38.2−38.6 | Gram-negative bacteria, motility | E. hallii L2-7T | Infant feces | 2.042 | [28] |
| E. hallii DSM 3353T | Infant feces | 1.989 | [28] | ||||
| E. rectale | 41.4−42.2 | Straight rod or slightly curved, gram-positive, motility | E. rectale CIP 105953T | Healthy adult feces | 2.341 | [40] | |
| Butyrivibrio | B. fibrisolvens | 36.0−41.0 | Arc-shaped, gram-negative bacteria, motility | B. fibrisolvens WH-2 | The source was not given | 0.383 | [41] |
|
| 图 1 基于16S rRNA基因序列的系统发育树 Figure 1 Phylogenetic tree constructed based on 16S rRNA gene sequence Note: 0.020 is the distance scale, which represents the digital scale of the difference between the sequences; the number on the node is bootstrap value, which is used to test the branch credibility of the phylogenetic tree; the sequence in parenthesis is the GenBank sequence number |
|
|
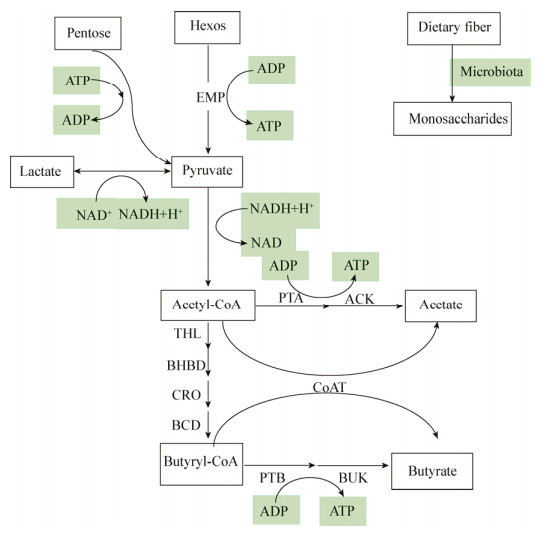
丁酸作为肠上皮细胞的首选能量来源,通过羟甲基戊二酰CoA的β-氧化作用进行能量代谢,为肠粘膜提供约75%的能量,或在肝脏中被分解为酮体和CO2,或进入血液循环,极少部分未被利用的丁酸随着汗液和粪便排出体外[42]。肠道内产丁酸细菌(C. butyricum、F. prausnitzii等)主要以膳食纤维为底物,将其分解为单糖后,经过一系列代谢途径最终合成丁酸(图 2)。糖类物质通过糖代谢途径产生丙酮酸,丙酮酸在丙酮酸脱氢酶系的作用下转化成乙酰CoA,乙酰CoA一方面转化为乙酸,另一方面在一系列酶的作用下转化成丁酰CoA,丁酰CoA在丁酸激酶的作用下生成丁酸;丁酰CoA也可以直接在辅酶A转移酶的作用下直接转化成丁酸,这是合成丁酸的2个途径[14, 43]。
|
| 图 2 丁酸代谢途径 Figure 2 Butyric acid metabolism pathway Note: THL: Acetyl CoA thiolyase; BHBD: β-hydroxybutyryl CoA dehydrogenase; CRO: Crotonase; BCD: Butyryl CoA dehydrogenase; PTA: Acetyl phosphotransferase; AK: Acetokinase; CoAT: CoA-transferase; PTB: Phosphotransferinase; BK: Butyrate kinase |
|
|
合理摄入膳食纤维有利于提高体内产丁酸细菌的丰度和丁酸浓度,还具有降低餐后血糖及胆固醇等功能[44-45],但膳食纤维的过量摄入会影响机体对维生素及其他微量元素的摄入,造成胀气等症状[46]。膳食纤维的理化性质,如溶解性和聚合度等因素决定了胃肠道微生物群合成丁酸的速度和数量[47]。有研究表明,抗性淀粉的补充可以增加肠道中E. rectale的丰度,在培养E. rectale DSM 17629时补充1% 3型抗性淀粉可产生浓度比为1.1:1:1.8 (乙酸: 丙酸: 丁酸)的挥发性脂肪酸[48-49]。殷丹婷[50]分别以抗性淀粉、低聚木糖和菊糖作为发酵底物进行肠道内容物的体外发酵时发现,随着发酵时间的延长,乳酸含量先增加后降低,丁酸含量逐渐上升,其中以低聚木糖和菊糖为底物时产生的丁酸含量较高。
肠道内产丁酸细菌的代谢能力还与其他肠道微生物的存在有关,尤其是乳酸菌和双歧杆菌。Li等[51]研究发现,衰老小鼠模型口服Lactobacillus helveticus KLDS1.8701一段时间后,瑞士乳杆菌处理组小鼠体内丁酸盐的含量是衰老小鼠的2倍。另外,口服Lactobacillus acidophilus KLDS1.0901能够增加肠道内产丁酸细菌Roseburia和Anaerotruncus的丰度,且丁酸含量显著增加[52]。E. hallii不能利用复杂的低聚糖和多糖,但低聚果糖的存在可以增加双歧杆菌的丰度,双歧杆菌产生的乳酸可以被E. hallii利用转化为丁酸盐,不同类型的双歧杆菌对合成丁酸盐的促进作用不同[53-54]。此外,也有学者对E. rectale和Bifidobacterium longum的共生进行研究,在以阿拉伯糖基低聚木糖(Arabinoxylan Oligosaccharides,AXOS)为底物的共培养中,B. longum NCC 2705消耗了AXOS的阿拉伯糖取代基产生乙酸盐,E. rectale ATCC 33656利用乙酸盐产生丁酸盐;而E. rectale ATCC 33656消耗AXOS并释放木糖,又可促进B. longum NCC 2705产生乙酸盐[55]。
3 丁酸的生理功能 3.1 影响肠道健康丁酸作为肠上皮细胞的首选能量来源,其钠盐可以刺激结肠内钠离子和水的吸收,加速肠绒毛增殖,促进肠道发育,提高机体对营养物质的吸收和利用[42]。在养殖业中,给饲料补充一定量的丁酸盐可以促进动物肠道健康。断奶羔羊饲料中添加3 g/kg丁酸钠,连续喂养28 d可以促进羔羊瘤胃乳头、肌层及肠道绒毛的生长,增加肠道绒毛高度[56]。Feng等[3]研究表明,在断奶仔猪饲料中添加丁酸盐喂养一段时间后,仔猪尿液中乳果糖/甘露醇的值低于正常喂养仔猪,即丁酸处理组断奶仔猪肠道通透性降低,腹泻症状得到有效缓解,进一步研究发现丁酸盐作为GPR109A的激动剂,作用于Akt通路调节肠道通透性,以此缓解仔猪腹泻症状。另外,肠道中的丁酸一方面可以促进胃肠肌肉的收缩能力,增强胃肠的蠕动;另一方面还可以促进神经递质的传递,以此来促进胃肠蠕动,缓解便秘问题[57]。
在调节肠道菌群方面,丁酸的亲水、亲脂性使其能够通过革兰氏阳性及革兰氏阴性菌的细胞膜。丁酸在细胞内分解产生H+和RCOO−,H+使酸耐受性差的细菌如沙门氏菌、大肠杆菌等致病菌生长受限,而乳酸菌等益生菌对酸耐受性较强,可以继续存活并繁殖;RCOO–通过破坏DNA结构抑制细菌生长,以此达到调节肠道菌群的目的[42]。关于丁酸对病原菌的抑制,新的研究发现,丁酸的抑菌能力与其作为组蛋白去乙酰化酶(Histone Deacetylase,HDAC)抑制剂和G蛋白偶联受体(G Protein Coupled Receptor,GPCR)的配体有关[58-59]。Xiong等[58]发现丁酸可以通过抑制HDAC活性,提高猪体内巨噬细胞和上皮细胞中抗菌肽的表达,抑制E. coli O157:H7的生长,粪便检测E. coli O157:H7的数量显著减少。Wang等[59]也得出了类似的研究,丁酸钠在机体内可以作为HDAC抑制剂,GPR41/43受体,通过NF-κB途径及MAPKs途径上调牛乳腺上皮细胞内源性抗菌肽的基因表达,抑制了金黄色葡萄球菌的生长。
3.2 抗氧化作用生物体处于应激状态时,体内积累大量未能被消除的活性氧,破坏氧化还原平衡,导致DNA、脂质和蛋白质受到破坏,机体物理屏障受损[60]。丁酸能够提高细胞内谷胱甘肽(一种内源性抗氧化剂清除剂)的水平[61]。2009年,一项首次利用人体研究丁酸盐对于氧化作用影响的实验开展,结果表明丁酸盐处理结肠后,促进了肠粘膜中还原性谷胱甘肽表达通路的进行,还原性谷胱甘肽的含量显著提高[48]。丁酸还可以通过影响酶促抗氧化系统达到抗氧化作用。张卫辉[62]在肉鸡日粮中添加适量丁酸钠,其血浆中超氧化物歧化酶和过氧化氢酶的含量显著高于对照组,并且增强了机体免疫能力。然而丁酸对于机体的抗氧化作用从根本上说是源自对基因和蛋白的调控。Nrf-2是抗氧化反应基因的重要调节因子,肠道内的丁酸通过静脉门转移到肝脏,在肝脏中激活Nrf-2并通过AMPK激活或抑制HDAC诱导Nrf-2在细胞中的表达[40];另外,丁酸还可以抑制Keap1蛋白(Nrf-2的一种胞质抑制因子)的表达,以此维持氧化还原平衡[63]。
3.3 抗炎作用结肠粘膜上皮屏障需要依靠丁酸的氧化来维持,肠道中丁酸含量不足或肠道中硫化物的增加抑制了氧化还原系统对丁酸的作用,都会导致炎症[49]。然而丁酸作为GPCR的配体,是GPR41、GPR43及GPR109A的激动剂[64],可以通过激活肠上皮细胞的GPCR的信号,诱导Treg细胞和T细胞的分化,促进结肠巨噬细胞和树突状细胞的抗炎特性[65]。姚蕾等[66]研究表明丁酸钠可以促进GPR41和GPR43 mRNA的表达,借此发挥抗炎作用。赖衍宗[67]利用丁酸钠对2, 4, 6-三硝基苯磺酸(2, 4, 6-Trinitrobenzenesulfonic Acid Sol,TNBS)诱导的结肠炎大鼠模型进行治疗,结果表明口服丁酸钠可修复TNBS诱导结肠炎大鼠的肠道粘膜,推测该作用与促进肠道粘膜中TGF-β1的表达、调节血浆中IL-8和IL-10的分泌有关。刘京等[68]研究发现,1 mmol/L的丁酸钠可以在TLR4及NF-κB信号通路介导下缓解脂多糖诱导的炎症反应,抗炎因子IL-10的浓度明显增加。吕晓婷[69]研究表明丁酸钠能显著抑制棕榈酸诱导的NF-κB和JNK的磷酸化及TNF-α诱导的IKKα/β的磷酸化,以此抑制骨骼肌炎症。总之,丁酸的抗炎作用主要基于对抗炎因子的促进作用及促炎因子的抑制作用。
3.4 抗癌作用丁酸作为肠上皮细胞的能量来源,为上皮细胞提供能量,促进细胞的增殖和成熟,被发现其促使癌细胞凋亡。该现象产生的原因可能是正常细胞的首选能量来源是丁酸,而癌细胞的首选能量来源是葡萄糖(Warburg效应),因此在癌细胞中可以检测出大量累积的丁酸。丁酸这种矛盾的功能被称为“丁酸悖论”[43, 70]。施灿霞[71]研究表明丁酸钠可能通过促进丙酮酸激酶M2亚型入核,上调Bcl-2的表达而抑制心肌细胞的凋亡;而罗娟等[72]发现丁酸钠通过阻断PI3K/AKT通路促进宫颈癌HeLa细胞凋亡,这再一次证明了“丁酸悖论”的合理性。另外,丁酸还作为HDAC抑制剂提高组蛋白乙酰化水平,使DNA与组蛋白八聚体解离,促进转录因子与DNA的结合,以此抑制肿瘤细胞的生长增殖[73]。体外研究表明,0.5−5.0 mmol的丁酸盐可以刺激吞噬细胞作用,抑制癌细胞生长,促使癌细胞凋亡[74]。王英等[75]研究发现丁酸钠作为HDAC4抑制剂,可以抑制肝癌细胞HepG2的增殖及侵袭能力,促进其凋亡,而且作用效果随丁酸钠浓度的增加而增加。综上所述,丁酸对于癌症的抑制机理有两点,首先基于“Warburg效应”的理论,丁酸不能为癌细胞提供能量;其次是作为HDAC抑制剂发挥作用。
4 结论及展望产丁酸细菌主要从动物及人类粪便中分离,种类主要为梭菌属、柔嫩梭菌属、罗斯式菌属、真菌属及丁酸弧菌属。其中,丁酸梭菌可以促进肠道内益生菌对碳源的利用,抑制有害菌的生长,已经被用于整肠制剂、肥料及兽药中;普拉梭菌和R. intestinalis的丰度可以作为炎症和肾病的检测指标,对炎症的治疗有显著效果;肉制品加工中适量补充霍式真杆菌或可减少加工有害物质对肠道的损坏。合理摄入膳食纤维能够提高肠道内产丁酸细菌的丰度和丁酸的浓度,肠内益生菌(乳酸菌、双歧杆菌)的存在影响产丁酸细菌的丰度及合成丁酸的能力。丁酸在促进肠道发育、调节肠道通透性和肠道菌群平衡、抗氧化、抑制炎症及抗癌等方面有显著意义。
关于产丁酸细菌和丁酸的生理功能研究目前存在以下问题:(1) 由于大多数产丁酸细菌需要严格厌氧的生存环境,这给菌株筛选和工业发酵带来困扰,工业生产中传统的除氧方式在操作上具有一定难度,深层发酵虽然能够达到厌氧的目的,但不利于菌株生长;(2) 虽然大量研究表明肠道内产丁酸细菌的丰度与疾病的发生有关,甚至有研究提出可以将肠道内产丁酸细菌的丰度作为某些病症的检测指标,但二者之间的具体关联还有待研究;(3) 产丁酸细菌合成丁酸前期会产生大量乙酸,能量的消耗导致丁酸产量的降低,如何降低产丁酸细菌的乙酸含量将是未来需要解决的问题;(4) 关于丁酸生理功能的研究还停留在动物实验层面,缺少临床试验的证明。
针对上述问题,本文提出以下解决方案:(1) 分离筛选菌株时严格控制厌氧条件,值得注意的是C. butyricum在培养后期还会改变革兰氏染色特征,因此需要在合适时间进行菌体特征观察,工业生产应注重开发新的除氧方法;(2) 结合医疗统计数据,首先找出产丁酸细菌与疾病的统计学关系,结合产丁酸细菌在体内的代谢通路及产物对机体健康的作用机制,得出产丁酸细菌与病症之间的关系;(3) 从基因层面通过调控丁酸合成通路中的关键酶(丁酸激酶等),根本上提高产丁酸细菌合成丁酸的效率;(4) 大量研究表明丁酸对机体有许多促健康作用,在充分动物实验证据的基础上,结合丁酸对相关疾病的治疗机理,医药行业可对其进行利用研制出治疗药品,进行临床试验的研究。相信随着研究的不断深入,对产丁酸细菌及丁酸的研究会更加透彻,将进一步促进医疗、食品、饲料领域的发展。
| [1] |
Jang YS, Im JA, Choi SY, Lee JI, Lee SY. Metabolic engineering of Clostridium acetobutylicum for butyric acid production with high butyric acid selectivity[J]. Metabolic Engineering, 2014, 23: 165-174. DOI:10.1016/j.ymben.2014.03.004 |
| [2] |
Liu JD, Bayir HO, Cosby DE, Cox NA, Williams SM, Fowler J. Evaluation of encapsulated sodium butyrate on growth performance, energy digestibility, gut development, and Salmonella colonization in broilers[J]. Poultry Science, 2017, 96(10): 3638-3644. DOI:10.3382/ps/pex174 |
| [3] |
Feng WQ, Wu YC, Chen GX, Fu SP, Li B, Huang BX, Wang DL, Wang W, Liu JX. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner[J]. Cellular Physiology and Biochemistry, 2018, 47(4): 1617-1629. DOI:10.1159/000490981 |
| [4] |
Zhang WH. Study on the regulatory effect of sodium butyrate on immune response and antioxidant function in broiler chickens[D]. Nanjing: Doctoral Dissertation of Nanjing Agricultural University, 2011(in Chinese) 张卫辉. 丁酸钠对肉鸡免疫反应和抗氧化功能调控作用研究[D]. 南京: 南京农业大学博士学位论文, 2011 |
| [5] |
Bahl H, Dürre P. Clostridia: Biotechnology and Medical Applications[M]. New York: John Wiley & Sons, 2001.
|
| [6] |
Tang H, Han QP, Zhang PH. Study and application of clostridium butyrates[J]. Feed Review, 2018(3): 8-12, 15. (in Chinese) 唐昊, 韩奇鹏, 张佩华. 丁酸梭菌的研究与应用[J]. 饲料博览, 2018(3): 8-12, 15. DOI:10.3969/j.issn.1001-0084.2018.03.003 |
| [7] |
Gao WW, Shang JC, Zhou X, Fan XP, Li XR, Zhao A, Zhao PH, Zhao L, Meng XC. Isolation and identification of a strain of Clostridium butyricum with high yield of butyric acid and its biological characteristics[J]. Science and Technology of Food Industry, 2020, 41(7): 82-88, 101. (in Chinese) 高文文, 尚佳萃, 周雪, 范小飘, 李欣芮, 赵桉, 赵鹏昊, 赵乐, 孟祥晨. 一株高产丁酸的丁酸梭菌分离鉴定及其生物学性质研究[J]. 食品工业科技, 2020, 41(7): 82-88, 101. |
| [8] |
Xia HL, Chen SS, Chen X, Dai J, Huang YN, Xie T, Li AL, Wang Z. Identification of Clostridium butyricum and optimization of fermentation medium for its growth[J]. Food Science, 2017, 38(8): 56-62. (in Chinese) 夏会丽, 陈思思, 陈雄, 代俊, 黄亚男, 谢婷, 李爱玲, 王志. 丁酸梭菌的鉴定与发酵培养基配方优化[J]. 食品科学, 2017, 38(8): 56-62. |
| [9] |
Li CC. Diversity of clostridia communities and fermentation characteristics of clostridia with high yield of butyric acid in Chinese strong-flavor liquor pit muds[D]. Zhengzhou: Master's Thesis of Zhengzhou University of Light Industry, 2018(in Chinese) 李聪聪. 浓香型白酒窖泥梭菌群落多样性及高产丁酸梭菌发酵特性研究[D]. 郑州: 郑州轻工业学院硕士学位论文, 2018 |
| [10] |
Kong Q. Study on the culture and kinetics models of fermentation by Clostridium butyricum and modulating effect on gastrointestinal microflora in antibiotic-associated diarrhea mice[D]. Hangzhou: Doctoral Dissertation of Zhejiang University, 2006(in Chinese) 孔青. 丁酸梭菌培养与发酵动力学以及调节腹泻小鼠肠道菌群平衡的研究[D]. 杭州: 浙江大学博士学位论文, 2006 |
| [11] |
Fu JJ, Li L, Liu J, Liao T, Tie Y, Wen XP. Research progress in the application of Clostridium butyricum and its metabolites in food processing[J/OL]. Food and Fermentation, 2020, 46(13): 293-298(in Chinese) 附俊杰, 李丽, 刘军, 廖挺, 帖余, 温雪瓶. 丁酸梭菌及其代谢产物在食品加工中的应用[J/OL]. 食品与发酵工业, 2020, 46(13): 293-298 |
| [12] |
Cao GT, Tao F, Hu YH, Li ZM, Zhang Y, Deng B, Zhan XA. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets[J]. Food & Function, 2019, 10(5): 2926-2934. |
| [13] |
Wang Y, Gu Y, Fang K, Mao K, Dou J, Fan H, Zhou C, Wang H. Lactobacillus acidophilus and Clostridium butyricum ameliorate colitis in murine by strengthening the gut barrier function and decreasing inflammatory factors[J]. Beneficial Microbes, 2018, 9(5): 775-787. DOI:10.3920/BM2017.0035 |
| [14] |
Gao WW, Meng XC. A review of recent literature on butyrate-producing bacteria in the human intestine and health-promoting functions of their metabolite butyrate[J]. Food Science, 2019, 40(21): 273-279. (in Chinese) 高文文, 孟祥晨. 人肠道产丁酸细菌及其所产丁酸的促健康作用研究进展[J]. 食品科学, 2019, 40(21): 273-279. |
| [15] |
Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P. Faecalibacterium prausnitzii and human intestinal health[J]. Current Opinion in Microbiology, 2013, 16(3): 255-261. DOI:10.1016/j.mib.2013.06.003 |
| [16] |
Cato EP, Salmon CW, Moore WEC. Fusobacterium prausnitzii (Hauduroy et al.) Moore and Holdeman: emended description and designation of neotype strain[J]. IInternational Journal of Systematic and Evolutionary Microbiology, 1974, 24(2): 225-229. DOI:10.1099/00207713-24-2-225 |
| [17] |
Zhou YL, Yu CG. Quantitative study on the changes of Faecalibacterium prausnitzii in stool and mucous membrane of patients with colon cancer and colon polyp[J]. Acta Universitatis Medicinalis Nanjing (Natural Science), 2017, 37(4): 458-460. (in Chinese) 周仪琳, 于成功. 结肠癌、结肠息肉患者粪便及黏膜中普拉梭菌变化的定量研究[J]. 南京医科大学学报: 自然科学版, 2017, 37(4): 458-460. |
| [18] |
Ma J, Sun LQ, Liu Y, Ren H, Shen YL, Bi F, Zhang T, Wang X. Alter between gut bacteria and blood metabolites and the anti-tumor effects of Faecalibacterium prausnitzii in breast cancer[J]. BMC Microbiology, 2020, 20(1): 82. DOI:10.1186/s12866-020-01739-1 |
| [19] |
Xu HM, Zhou YL, Nie YQ. Study on the relationship and mechanism between Faecalibacterium prausnitzii and inflammatory bowel disease[J]. Guangdong Medical Journal, 2017, 38(2): 318-321. (in Chinese) 徐豪明, 周有连, 聂玉强. 普拉梭菌与炎症性肠病的关系及机制研究[J]. 广东医学, 2017, 38(2): 318-321. DOI:10.3969/j.issn.1001-9448.2017.02.044 |
| [20] |
Li YY. Effects of Faecalibacterium prausnitzii on Treg cells, il-10 and TGF-1 in mice with dextran sulfate sodium-induced ulcerative colitis[D]. Nanjing: Master's Thesis of Nanjing University, 2016(in Chinese) 李媛媛. 普拉梭菌对葡聚糖硫酸钠诱导的溃疡性结肠炎小鼠Treg细胞及IL-10、TGF-β1的影响[D]. 南京: 南京大学硕士学位论文, 2016 |
| [21] |
Rabiei N, Badi SA, Marvasti FE, Sattari TN, Farzam V, Siadat SD. Induction effects of Faecalibacterium prausnitzii and its extracellular vesicles on toll-like receptor signaling pathway gene expression and cytokine level in human intestinal epithelial cells[J]. Cytokine, 2019, 121: 154718. DOI:10.1016/j.cyto.2019.05.005 |
| [22] |
Jafari B, Nejad RAK, Vaziri F, Siadat SD. Evaluation of the effects of extracellular vesicles derived from Faecalibacterium prausnitzii on lung cancer cell line[J]. Biologia, 2019, 74(7): 889-898. DOI:10.2478/s11756-019-00229-8 |
| [23] |
Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, Flint HJ. Phylogenetic relationships of butyrate-producing bacteria from the human gut[J]. Applied and Environmental Microbiology, 2000, 66(4): 1654-1661. DOI:10.1128/AEM.66.4.1654-1661.2000 |
| [24] |
Jiang SH, Xie S, Lv D, Zhang Y, Deng J, Zeng LH, Chen Y. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression[J]. Antonie Van Leeuwenhoek, 2016, 109(10): 1389-1396. DOI:10.1007/s10482-016-0737-y |
| [25] |
Kellermayer R. Roseburia species: Prime candidates for microbial therapeutics in inflammatory bowel disease[J]. Gastroenterology, 2019, 157(4): 1164-1165. DOI:10.1053/j.gastro.2019.05.073 |
| [26] |
Seo B, Jeon K, Moon S, Lee K, Kim WK, Jeong H, Cha KH, Lim MY, Kang W, Kweon MN, et al. Roseburia spp. abundance associates with alcohol consumption in humans and its administration ameliorates alcoholic fatty liver in mice[J]. Cell Host & Microbe, 2020, 27(1): 25-40.E6. DOI:10.1016/S0168-8278(20)30691-7 |
| [27] |
Xiao MW, Shen ZH, Luo WW, Tan B, Meng XR, Wu X, Wu S, Nie K, Tong T, Hong JB, et al. A new colitis therapy strategy via the target colonization of magnetic nanoparticle-internalized Roseburia intestinalis[J]. Biomaterials Science, 2019, 7(10): 4174-4185. DOI:10.1039/C9BM00980A |
| [28] |
Shetty SA, Ritari J, Paulin L, Smidt H, De Vos WM. Complete genome sequence of Eubacterium hallii strain L2-7[J]. Genome Announcements, 2017, 5(43): e01167-17. |
| [29] |
Engels C, Ruscheweyh HJ, Beerenwinkel N, Lacroix C, Schwab C. The common gut microbe Eubacterium hallii also contributes to intestinal propionate formation[J]. Frontiers in Microbiology, 2016, 7: 713. DOI:10.3389/fmicb.2016.00713 |
| [30] |
Fekry MI, Engels C, Zhang JB, Schwab C, Lacroix C, Sturla SJ, Chassard C. The strict anaerobic gut microbe Eubacterium hallii transforms the carcinogenic dietary heterocyclic amine 2-amino-1-methyl-6-phenylimidazo[4, 5-b] pyridine (PhIP)[J]. Environmental Microbiology Reports, 2016, 8(2): 201-209. DOI:10.1111/1758-2229.12369 |
| [31] |
Rosero JA, Killer J, Sechovcová H, Mrázek J, Benada O, Fliegerová K, Havlík J, Kopečný J. Reclassification of Eubacterium rectale (Hauduroy et al. 1937) Prévot 1938 in a new genus Agathobacter gen. nov. as Agathobacter rectalis comb. nov., and description of Agathobacter ruminis sp. nov., isolated from the rumen contents of sheep and cows[J]. International Journal of Systematic and Evolutionary Microbiology, 2016, 66(2): 768-773. DOI:10.1099/ijsem.0.000788 |
| [32] |
Li H, Xie HB, Qi R, Shen MX, Ma YK, Wang HX. Efficacy of regulation of intestinal flora with the Cheqianzi Cuduotang capsules on blood pressure in elderly patients with hypertension[J]. Clinical Journal of Chinese Medicine, 2019, 11(11): 15-19. (in Chinese) 李红, 谢海彬, 齐容, 沈明霞, 马玉坤, 王海霞. 车前子粗多糖胶囊调节肠道菌对老年高血压病患者血压影响的临床研究[J]. 中医临床研究, 2019, 11(11): 15-19. |
| [33] |
Song YC. Changes and function prediction of gut microbiota in patients with hyperthyroidism[D]. Hefei: Master's Thesis of Anhui Medical University, 2019(in Chinese) 宋影春. 甲亢患者的肠道菌群变化与功能预测的研究[D]. 合肥: 安徽医科大学硕士学位论文, 2019 |
| [34] |
Lin CH. Isolation and identification of rumen Butyrivibrio fibrisolvens from Guizhou black goat[D]. Guiyang: Master's Thesis of Guizhou University, 2016(in Chinese) 林聪宏. 贵州黑山羊瘤胃溶纤维丁酸弧菌分离鉴定[D]. 贵阳: 贵州大学硕士学位论文, 2016 |
| [35] |
Liu ZY. Isolation and identification of major cellulolytic bacteria in rumen of sheep and effects of nitrogen sources on their cellulolytic activities[D]. Hohhot: Doctoral Dissertation of Inner Mongolia Agricultural University, 2008(in Chinese) 刘占英. 绵羊瘤胃主要纤维降解细菌的分离鉴定及不同氮源对其纤维降解能力的影响[D]. 呼和浩特: 内蒙古农业大学博士学位论文, 2008 |
| [36] |
Wang F. Effects of LA on CLA deposition and growth-metabolism of Butyrivibrio fibrisolvens in rumen of Guizhou black goats[D]. Guiyang: Master's Thesis of Guizhou University, 2019(in Chinese) 王府. LA对贵州黑山羊CLA的沉积及瘤胃溶纤维丁酸弧菌生长代谢的影响[D]. 贵阳: 贵州大学硕士学位论文, 2019 |
| [37] |
Palevich N, Kelly WJ, Leahy SC, Denman S, Altermann E, Rakonjac J, Attwood GT. Comparative genomics of rumen Butyrivibrio spp. uncovers a continuum of polysaccharide-degrading capabilities[J]. Applied and Environmental Microbiology, 2019, 86(1): e01993-19. |
| [38] |
Fang C, Xu J, Li N, Zhang XJ, Shen J, Zhao YF. Isolation, identification and excellent strain screening of Faecalibacterium prausnitzii in human intestinal tract[J]. Genomics and Applied Biology, 2018, 37(7): 2866-2873. (in Chinese) 方超, 徐佳, 李娜, 张晓君, 申剑, 赵宇峰. 人肠道内Faecalibacterium prausnitzii的分离、鉴定及优良菌株筛选研究[J]. 基因组学与应用生物学, 2018, 37(7): 2866-2873. |
| [39] |
Duncan SH, Hold GL, Barcenilla A, Stewart CS, Flint HJ. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces[J]. International Journal of Systematic and Evolutionary Microbiology, 2002, 52(Pt 5): 1615-1620. DOI:10.1002/cpa.20254 |
| [40] |
Moens F, De Vuyst L. Inulin-type fructan degradation capacity of Clostridium cluster Ⅳ and ⅩⅣa butyrate-producing colon bacteria and their associated metabolic outcomes[J]. Beneficial Microbes, 2017, 8(3): 473-490. DOI:10.3920/BM2016.0142 |
| [41] |
Huang HM. The research on production of biofuel from Butyrivibrio fibrisolvens[D]. Hohhot: Master's Thesis of Inner Mongolia University of Technology, 2013(in Chinese) 黄恒猛. 一株溶纤维丁酸弧菌生产生物燃料的研究[D]. 呼和浩特: 内蒙古工业大学硕士学位论文, 2013 |
| [42] |
Zhang JZ. Effect of dietary sodium butyrate on growth, immunity and intestinal health in rice filed eel[D]. Changsha: Master's Thesis of Hunan Agricultural University, 2015(in Chinese) 张俊智. 饲料中添加丁酸钠对黄鳝生长、免疫及肠道健康的影响[D]. 长沙: 湖南农业大学硕士学位论文, 2015 |
| [43] |
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites[J]. Cell, 2016, 165(6): 1332-1345. DOI:10.1016/j.cell.2016.05.041 |
| [44] |
Hansen TL, Rankins EM, Bobel JM, McKinney M, Hackmann TJ, Warren LK. Postprandial blood glucose and insulin responses of horses to feeds differing in soluble fiber concentration[J]. Journal of Equine Veterinary Science, 2020, 88: 102963. DOI:10.1016/j.jevs.2020.102963 |
| [45] |
Wang CF, Song RZ, Wei SQ, Wang WL, Li F, Tang XZ, Li NY. Modification of insoluble dietary fiber from ginger residue through enzymatic treatments to improve its bioactive properties[J]. LWT, 2020, 125: 109220. DOI:10.1016/j.lwt.2020.109220 |
| [46] |
Huang DY. Enzymatic modification and functional properties of rice bran dietary fiber[D]. Wuxi: Master's Thesis of Jiangnan University, 2014(in Chinese) 黄冬云. 米糠膳食纤维的酶法改性及功能性质研究[D]. 无锡: 江南大学硕士学位论文, 2014 |
| [47] |
Claus R, Günthner D, Letzguß H. Effects of feeding fat-coated butyrate on mucosal morphology and function in the small intestine of the pig[J]. Journal of Animal Physiology and Animal Nutrition, 2007, 91(7/8): 312-318. |
| [48] |
Hamer HM, Jonkers DMAE, Bast A, Vanhoutvin SALW, Fischer MAJG, Kodde A, Troost FJ, Venema K, Brummer RJM. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans[J]. Clinical Nutrition, 2009, 28(1): 88-93. DOI:10.1016/j.clnu.2008.11.002 |
| [49] |
Roediger WEW. The colonic epithelium in ulcerative colitis: an energy-deficiency disease?[J]. The Lancet, 1980, 316(8197): 712-715. DOI:10.1016/S0140-6736(80)91934-0 |
| [50] |
Yin DT. Effects of exogenous lactic acid bacteria on the anti-colon cancer activity of fermentation products of dietary fiber in vitro[D]. Harbin: Master's Thesis of Northeast Agricultural University, 2017(in Chinese) 殷丹婷. 外源乳酸菌对膳食纤维体外发酵产物抗结肠癌活性的影响[D]. 哈尔滨: 东北农业大学硕士学位论文, 2017 |
| [51] |
Li BL, Evivie SE, Lu JJ, Jiao YH, Wang CF, Li ZY, Liu F, Huo GC. Lactobacillus helveticus KLDS1.8701 alleviates D-galactose-induced aging by regulating Nrf-2 and gut microbiota in mice[J]. Food & Function, 2018, 9(12): 6586-6598. DOI:10.1039/C8FO01768A |
| [52] |
Yan FF, Li N, Shi JL, Li HZ, Yue YX, Jiao WS, Wang NN, Song Y, Huo GC, Li BL. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice[J]. Food & Function, 2019, 10(9): 5804-5815. |
| [53] |
Bunesova V, Lacroix C, Schwab C. Mucin cross-feeding of infant Bifidobacteria and Eubacterium hallii[J]. Microbial Ecology, 2018, 75(1): 228-238. DOI:10.1007/s00248-017-1037-4 |
| [54] |
Zhang J, Qin XL, Liu X. A review of the influence of major dietary macronutrients on the gut microbiota[J]. Food Science, 2015, 36(5): 305-309. (in Chinese) 张晶, 覃小丽, 刘雄. 膳食主成分对肠道微生物的影响研究进展[J]. 食品科学, 2015, 36(5): 305-309. |
| [55] |
Rivière A, Gagnon M, Weckx S, Roy D, De Vuyst L. Mutual cross-feeding interactions between Bifidobacterium longum subsp. longum NCC2705 and Eubacterium rectale ATCC 33656 explain the bifidogenic and butyrogenic effects of arabinoxylan oligosaccharides[J]. Applied and Environmental Microbiology, 2015, 81(22): 7767-7781. DOI:10.1128/AEM.02089-15 |
| [56] |
Zuo LJ, Chen X, Wang KX, Jiang N, Zhang AZ. Effects of sodium butyrate on gastrointestinal tract development of weaned lambs[J]. Journal of Animal Nutrition, 2020, 32(4): 1916-1926. (in Chinese) 左丽君, 陈想, 王可鑫, 姜宁, 张爱忠. 丁酸钠对断奶羔羊胃肠道发育的影响[J]. 动物营养学报, 2020, 32(4): 1916-1926. DOI:10.3969/j.issn.1006-267x.2020.04.052 |
| [57] |
Bajka BH, Clarke JM, Topping DL, Cobiac L, Abeywardena MY, Patten GS. Butyrylated starch increases large bowel butyrate levels and lowers colonic smooth muscle contractility in rats[J]. Nutrition Research, 2010, 30(6): 427-434. DOI:10.1016/j.nutres.2010.06.003 |
| [58] |
Xiong HT, Guo BX, Gan ZS, Song DG, Lu ZQ, Yi HB, Wu YM, Wang YZ, Du HH. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition[J]. Scientific Reports, 2016, 6(1): 27070. DOI:10.1038/srep27070 |
| [59] |
Wang X, Zhang MM, Jiang N, Zhang AZ. Sodium phenylbutyrate ameliorates inflammatory response induced by Staphylococcus aureus lipoteichoic acid via suppressing TLR2/NF-κB/NLRP3 pathways in MAC-T cells[J]. Molecules, 2018, 23(12): 3056. DOI:10.3390/molecules23123056 |
| [60] |
Gong FL, Lin X, Wang HQ. Review on the effect of butyrate on intestinal health and the action mechanism[J]. Feed Research, 2020, 43(3): 113-117. (in Chinese) 龚福来, 林雪, 王红权. 丁酸对肠道健康的影响及作用机制的研究进展[J]. 饲料研究, 2020, 43(3): 113-117. |
| [61] |
Ma X, Fan PX, Li LS, Qiao SY, Zhang GL, Li DF. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions[J]. Journal of Animal Science, 2012, 90(S4): 266-268. DOI:10.2527/jas.50965 |
| [62] |
Zhang WH. Study on the regulatory effect of sodium butyrate on immune response and antioxidant function in broiler chickens[D]. Nanjing: Doctoral Dissertation of Nanjing Agricultural University, 2011(in Chinese) 张卫辉. 丁酸钠对肉鸡免疫反应和抗氧化功能调控作用研究[D]. 南京: 南京农业大学博士学位论文, 2011 |
| [63] |
Wang B, Zhu XL, Kim YT, Li J, Huang SY, Saleem S, Li RC, Xu Y, Dore S, Cao WS. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage[J]. Free Radical Biology and Medicine, 2012, 52(5): 928-936. DOI:10.1016/j.freeradbiomed.2011.12.006 |
| [64] |
Huang XY, Guo F, Zeng XC, Ouyang DS. Short-chain fatty acids as signal molecules in intestinal inflammation[J]. Chinese Journal of Clinical Pharmacology and Therapeutics, 2019, 24(11): 1293-1299. (in Chinese) 黄馨仪, 郭飞, 曾祥昌, 欧阳冬生. 短链脂肪酸作为信号分子在肠道炎症中的研究进展[J]. 中国临床药理学与治疗学, 2019, 24(11): 1293-1299. DOI:10.12092/j.issn.1009-2501.2019.11.013 |
| [65] |
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi HD, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis[J]. Immunity, 2014, 40(1): 128-139. DOI:10.1016/j.immuni.2013.12.007 |
| [66] |
Yao L, Sun YQ, Li AL, Wang Y, Li S, Zhang C. Effect of the butyrate on the lymphocyte of β-Lactoglobulin-sensitized mice in vitro[J]. Journal of Chinese Institute of Food Science and Technology, 2017, 17(12): 35-41. (in Chinese) 姚蕾, 孙伊乔, 李艾黎, 王莹, 李爽, 张超. 丁酸钠对β-乳球蛋白过敏小鼠淋巴细胞的体外影响[J]. 中国食品学报, 2017, 17(12): 35-41. |
| [67] |
Lai YZ. Effects of sodium butyrate on colitis mucosal reparation in TNBS-colitis model rats[D]. Nanjing: Master's Thesis of Nanjing University, 2011(in Chinese) 赖衍宗. 丁酸钠对TNBS结肠炎模型大鼠肠粘膜修复的影响[D]. 南京: 南京大学硕士学位论文, 2011 |
| [68] |
Liu J, Dai HY, Ma NN, Wang Y, Huang J, Shen XZ. Sodium butyrate alleviates the lps-induced inflammatory response of bovine embryonic tracheal cells through the TLR4/NF-κB signaling pathway[A]//2019 The 10th Member Representative Conference of Society of Veterinary Surgery and the 24th Academic Seminar Proceedings[C]. Beijing: Chinese Association of Animal Science and Veterinary Medicine, 2019: 303-306(in Chinese) 刘京, 代宏宇, 马娜娜, 汪艳, 黄杰, 沈向真. 丁酸钠通过TLR4/NF-κB信号通路缓解LPS诱导的牛胚气管细胞炎症反应[A]//2019中国畜牧兽医学会兽医外科学分会第十届会员代表大会暨第24次学术研讨会论文集[C]. 北京: 中国畜牧兽医学会, 2019: 303-306 |
| [69] |
Lv XT. Effects of short-chain fatty acids on glucose metabolism and inflammatory signals in skeletal muscle[D]. Tianjin: Master's Thesis of Tianjin Medical University, 2019(in Chinese) 吕晓婷. 短链脂肪酸对骨骼肌糖代谢及炎症信号的作用研究[D]. 天津: 天津医科大学硕士学位论文, 2019 |
| [70] |
Gonçalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport[J]. Current Drug Metabolism, 2013, 14(9): 994-1008. |
| [71] |
Shi CX. The mechanism study of sodium butyrate on myocardial apoptosis based on PKM2 after myocardial infarction in rats[D]. Changchun: Master's Thesis of Jilin University, 2018(in Chinese) 施灿霞. 丁酸钠基于PKM2影响心梗大鼠心肌组织凋亡的机制研究[D]. 长春: 吉林大学硕士学位论文, 2018 |
| [72] |
Luo J, Nan ZF, Luo L, Zhang FL. Effect of histone deacetylase inhibitor sodium butyrate on proliferation and apoptosis in cervical carcinoma HeLa cell[J]. Oncology Progress, 2018, 16(10): 1299-1302. (in Chinese) 罗娟, 南祖峰, 罗玲, 张风莉. 组蛋白脱乙酰酶抑制剂丁酸钠对宫颈癌HeLa细胞增殖与凋亡的影响[J]. 癌症进展, 2018, 16(10): 1299-1302. |
| [73] |
Jiang W. Effects of Gli1 in biological behaviors of lung adenocarcinoma A549 cells treated with sodium butyrate plus docetaxel[D]. Nanning: Doctoral Dissertation of Guangxi Medical University, 2019(in Chinese) 蒋玮. 正丁酸钠联合多西他赛通过抑制Gli1调控肺腺癌A549细胞增殖和凋亡的研究[D]. 南宁: 广西医科大学博士学位论文, 2019 |
| [74] |
Zhang JT, Yi M, Zha LY, Chen SQ, Li ZJ, Li C, Gong MX, Deng H, Chu XW, Chen JH, et al. Sodium butyrate induces endoplasmic reticulum stress and autophagy in colorectal cells: implications for apoptosis[J]. PLoS One, 2016, 11(1): e0147218. |
| [75] |
Wang Y, Wu QB, Shen P, Xie R, Ji GZ, Wang HG. Sodium butyrate regulates the proliferation, apoptosis and invasion of HepG2 cells[J]. Anhui Medical and Pharmaceutical Journal, 2019, 23(8): 1509-1513. (in Chinese) 王英, 吴庆柏, 沈鹏, 谢睿, 季国忠, 王宏刚. 丁酸钠调控HepG2细胞的增殖、凋亡和侵袭[J]. 安徽医药, 2019, 23(8): 1509-1513. |
 2021, Vol. 48
2021, Vol. 48