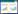扩展功能
文章信息
- 肖锟, 刘聪洋, 王仁女, 董伟, 潘涛
- XIAO Kun, LIU Congyang, WANG Rennü, DONG Wei, PAN Tao
- 表面活性剂影响微生物降解多环芳烃的研究进展
- Effect of surfactants on microbial degradation of polycyclic aromatic hydrocarbons : a review
- 微生物学通报, 2021, 48(2): 582-595
- Microbiology China, 2021, 48(2): 582-595
- DOI: 10.13344/j.microbiol.china.200229
-
文章历史
- 收稿日期: 2020-03-13
- 接受日期: 2020-07-16
- 网络首发日期: 2020-09-24
2. 江西理工大学资源与环境工程学院 江西 赣州 341000
2. School of Resource and Environmental Engineering, Jiangxi University of Science and Technology, Ganzhou, Jiangxi 341000, China
多环芳烃(Polycyclic Aromatic Hydrocarbons,PAHs)是煤、石油、有机高分子化合物等不完全燃烧的产物[1]。PAHs具有较强的生物毒性,存在致癌、致畸和致突变等危害,而且自然降解率低[2]。研究人员在处理环境中的PAHs污染时,常用的去除手段包括物理、化学和生物法。其中,生物法具有无额外污染、经济效益高等特点而受到广泛关注[3]。PAHs的生物降解主要是利用微生物的解毒能力,将PAHs彻底矿化为CO2和水。微生物功能的发挥在很大程度上依赖水环境[4],但强疏水性的PAHs广泛存在于土壤颗粒和有机相中,在水中的溶解度很低,极大地影响微生物降解效率[5]。表面活性剂由于能够增溶PAHs而提高其表观溶解度,已被广泛用于PAHs的生物降解研究[6]。
研究发现,表面活性剂不仅能够增加PAHs的表观溶解度,而且能提高PAHs的微生物降解效率[7-8]。Li等总结了微生物降解PAHs的步骤,解释了PAHs在表面活性剂存在下的传质强化机制[9]。然而添加表面活性剂并不总是能够促进PAHs的降解[10]。大量有关表面活性剂抑制PAHs降解的报道让其实际应用前景十分暗淡[11-13]。对于这些看似冲突性的研究成果,本文进行了机理性的概括和阐述。
在众多表面活性剂中,非离子表面活性剂较为特殊,其水溶液在高于浊点温度或在添加物存在的条件下会形成浊点系统(Cloud Point System,CPS)。有研究发现,CPS在促进微生物降解PAHs方面同样具有效果[14]。Pan等对CPS增强PAHs降解进行了研究[15],并在PAHs增溶和表面活性剂毒性方面将CPS与传统胶束系统进行了对比[16]。在Pan等研究的基础上,本文对CPS中PAHs的生物降解进行了归纳总结,并对表面活性剂影响PAHs微生物降解的研究前景进行了展望。
1 PAHs生物降解中常用的表面活性剂表面活性剂是一类同时具有疏水和亲水基团的两亲性物质[17],这种特殊结构令表面活性剂能够提高疏水性有机化合物的表观溶解度[18-19]。根据来源,PAHs生物降解中的常用表面活性剂可分为化学表面活性剂和生物表面活性剂两大类,如表 1所示。
| 类型 Types |
名称 Names |
PAHs处理结果 Results of PAHs treatment |
效果 Effects |
参考文献 References |
| 化学表面活性剂 Chemical surfactants |
Triton X-100 | 萘的溶解度增加100倍;菲、蒽的溶解度增加1 000倍 The solubility increased by 100 times for naphthalene and 1 000 times for phenanthrene and anthracene |
↑ | [20] |
| 高分子量PAHs的降解增加了7.59倍 The degradation of PAHs with high molecular weight increased by 7.59 times |
↑ | [21] | ||
| PAHs的去除率达83% The removal rate of PAHs was 83% | ↑ | [22] | ||
| 芘的降解受到抑制The degradation of pyrene was inhibited | ↓ | [23] | ||
| 延缓了菲的生物降解The biodegradation of phenanthrene was delayed | ↓ | [24] | ||
| Tween-80 | 苯并[a]芘降解率57.2% The degradation rate of benzo[a]pyrene was 57.2% | ↑ | [25] | |
| 320 mg/L的芴降解率达100% The degradation rate of fluorene was 100% at 320 mg/L | ↑ | [8] | ||
| 菲的去除率达99.9% The removal rate of phenanthrene was 99.9% | ↑ | [26] | ||
| 混合PAHs去除率达92.6% The removal rate of mixed PAHs was 92.6% | ↑ | [27] | ||
| 高浓度吐温-80抑制了菲和芘的降解 High concentration Tween-80 inhibited the degradation of phenanthrene and pyrene |
↓ | [11] | ||
| Tween-20 | 蒽的降解速率提高了1倍The degradation rate of anthracene was doubled | ↑ | [28] | |
| 菲和芘的降解率分别为99.03%和97.39% The degradation rates of phenanthrene and pyrene were 99.03% and 97.39%, respectively |
↑ | [11] | ||
| 对菲的矿化产生了负面影响A negative impact on phenanthrene mineralization | ↓ | [29] | ||
| SDS | 蒽的去除率提高了1倍The removal rate of anthracene was doubled | ↑ | [28] | |
| 不影响萘的生物降解The biodegradation of naphthalene was not affected | − | [30] | ||
| 生物表面活性剂 Biosurfactants |
鼠李糖脂Rhamnolipid | 菲去除量达到12.24 mg/kg The removal rate of phenanthrene was 12.24 mg/kg |
↑ | [7] |
| PAHs降解率提高了4.2倍The degradation rate of PAHs was increased by 4.2 times | ↑ | [31] | ||
| 菲的降解率达99.5% The degradation rate of phenanthrene was 99.5% | ↑ | [32] | ||
| 蒽的降解率为52% The degradation rate of anthracene was 52% | ↑ | [33] | ||
| 菲的降解率由73.6%降至23.4% The degradation rate of phenanthrene decreased from 73.6% to 23.4% |
↓ | [34] | ||
| 抑制了PAHs的矿化The mineralization of PAHs was inhibited | ↓ | [35] | ||
| 不影响荧蒽的生物降解No effect on the biodegradation of fluoranthene | − | [36] | ||
| 皂角苷Saponin | 芘的降解率提高了2.1倍The degradation rate of pyrene was increased by 2.1 times | ↑ | [37] | |
| 萘、苊、菲和芘的表观溶解度分别提高了1.78、6.05、35.58、155.55倍 The apparent solubility of naphthalene, acenaphthene, phenanthrene and pyrene increased by 1.78, 6.05, 35.58 and 155.55 times, respectively |
↑ | [38] | ||
| 土壤中菲、芘洗脱效率可达84.1%和81.4% The elution efficiency of phenanthrene and pyrene in soil was 84.1% and 81.4% PAHs的降解没有增强The degradation of PAHs was not enhanced |
↑ − |
[39] [40] |
||
| 槐糖脂Sophorolipid糖脂类Glycolipid | 菲的降解率提高了1.5倍 The degradation rate of phenanthrene was increased by 1.5 times 蒽的降解率达到了82.9% The degradation rate of anthracene was 82.9% |
↑ ↑ |
[41] [42] |
|
| 脂肽类Lipopeptide | 高分子量PAHs去除率显著提升 The removal rate of PAHs with high molecular weight was significantly improved 菲、芴和萘的去除率无明显变化 The removal rates of phenanthrene, fluorene and naphthalene did not change significantly |
↑ − |
[43] [43] |
|
| 三萜皂苷Triterpenoid saponin | PAHs的降解率提高到78.7% The degradation rate of PAHs increased to 78.7% |
↑ | [44] | |
| 注:↑:促进;↓:抑制;-:无作用 Note: ↑: Promoting; ↓: Inhibiting; -: No effect |
||||
近年来,大量研究人员利用化学表面活性剂提高PAHs溶解度并促进其降解[45]。Bautista等研究证明非离子表面活性剂吐温-80和Triton X-100使萘在水中的溶解度增加了约100倍,而菲和蒽甚至提高到了1 000倍[20]。这大幅增加了PAHs在环境水相中的生物可及性。对于粘附在土壤颗粒上的PAHs,提高其表观溶解度对于生物降解十分必要。Wang等研究了吐温-80对模拟污染土壤中PAHs生物降解的影响[25]。与无添加对照相比,5 g/kg的吐温-80将苯并[a]芘的降解率提高了15.5%,达到57.2%[25]。吐温-80对PAHs生物降解的增强能力在Reddy等的后续研究中得到进一步证明,他们发现1.5% (体积比)吐温-80的添加使320 mg/L的芴在24 h内降解率几乎达到100%[8]。
在某些情况下,当有意混合不同类型的表面活性剂时,会观察到它们之间的协同作用,例如,向阴离子表面活性剂溶液中添加非离子表面活性剂可显著降低临界胶束浓度(Critical Micelle Concentration,CMC)并增大胶束体积,强化增溶效果[45]。因此,在实际应用中,使用混合表面活性剂促进PAHs降解也是一种选择[6]。Zhao等利用十二烷基硫酸钠(Sodium Dodecyl Sulfate,SDS)与吐温-80组成的阴-非离子混合表面活性剂具有更低的CMC,并大幅提高了菲的表观溶解度和降解效率[46]。这说明利用混合表面活性剂提高PAHs的生物利用度是有效的。
1.2 生物表面活性剂生物表面活性剂具有和化学表面活性剂类似的结构,在增加PAHs的表观溶解度和提高其生物降解效率方面显示出极大的潜力[47]。近年来,生物表面活性剂由于其低毒和高生物降解性而受到广泛关注[48]。
在PAHs增溶生物降解中常用的生物表面活性剂有鼠李糖脂、槐糖脂、肽脂和皂角苷等,而其中使用最多、研究最广泛的是鼠李糖脂[49]。截止到2020年7月,我们在Web of Science以“Biosurfactant”作为关键词检索到关于生物表面活性剂的英文文献共4 812篇,其中有关“Rhamnolipid”的文献有1 357篇,占28%;同样,在中国知网中以“生物表面活性剂”作为关键词检索到中文期刊论文共1 525篇,结果中有关“鼠李糖脂”的文献有168篇,占11%。许多研究表明了鼠李糖脂对土壤中PAHs污染具有良好的去除效果。在An等的研究中,添加50 mg/L鼠李糖脂可使植物和沟渠土壤中菲的去除量分别从为3.37 mg/kg和7.98 mg/kg提高到8.76 mg/kg和12.24 mg/kg,而且随着土壤中鼠李糖脂含量的增加,菲的去除量也随之上升[7]。Wang等也发现,在PAHs污染土壤中添加鼠李糖脂,60 d后PAHs的降解率提高了4.2倍[31]。与化学表面活性剂相比,鼠李糖脂在促进PAHs微生物降解方面的效果更加显著。Pei等对比了吐温-80和鼠李糖脂对鞘氨醇单胞菌GF2B降解菲的影响,结果显示前者抑制菲的降解(33.5%),而后者却促进了菲的降解(99.5%)[32]。以上研究表明,鼠李糖脂能有效地促进土壤中PAHs的去除,适用于此类污染土壤的修复。
除鼠李糖脂之外,其他生物表面活性剂在促进PAHs生物降解方面的研究相对有限。皂角苷是一种在植物中提取的生物表面活性剂,当其浓度超过CMC时,菲、芘和苯并[a]芘的表观溶解度都显著增加[37]。Zhou等对比了皂角苷与化学表面活性剂对菲的增溶作用,结果表明,皂角苷对菲的摩尔增溶率约为非离子表面活性剂的3-6倍[38]。另外,Bezza等从石油污染土壤中分离得到一株铜绿假单胞菌,该菌株能产脂肽类生物表面活性剂,与水溶液相比,当脂肽类生物表面活性剂浓度为400 mg/L时,菲、荧蒽和芘的去除率分别增加了19、33和45倍[43]。
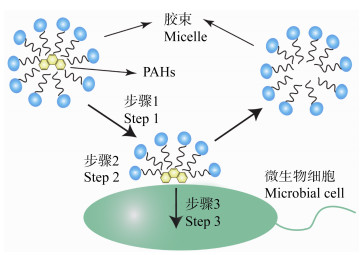
2 表面活性剂影响PAHs生物降解的原理 2.1 PAHs的表观增溶如图 1所示,由于同时含有亲水基团和疏水基团,表面活性剂在低于CMC时容易分配到水相与气相或水相与有机相的相界面处,从而改变溶液的表面张力或界面能[50]。在土壤-水系统中,表面活性剂的亲水基团伸向水相,而疏水基团更倾向于与土壤或疏水性物质结合,在结合之后,由于表面活性剂亲水基团对疏水性物质的排斥作用,从而促使PAHs从土壤中解吸[51]。
|
| 图 1 表面活性剂增溶PAHs Figure 1 Surfactant solubilization of PAHs |
|
|
如果添加的表面活性剂浓度达到CMC,单分子开始聚合,形成10-200个分子的稳定聚集体,也称为胶束[52]。通过胶束与土壤颗粒的竞争,PAHs会被封装进入胶束的疏水核心,脱离土壤的吸附,达到表观增溶的效果。在实际土壤修复过程中,往往环境本身就存在表面活性剂共存的情况。混合表面活性剂相较于单一表面活性剂,常常表现出更小的CMC,对土壤中PAHs具有协同增溶作用,可以有效降低PAHs污染场地的修复成本[53]。
2.2 PAHs的传质强化在表面活性剂的胶束水溶液中,水相中PAHs能够直接被微生物捕获而降解,胶束相中的PAHs分子通过扩散到水相然后再被微生物利用;同时,PAHs分子通过半胶束直接向细胞传质,如图 2所示,胶束中的PAHs向细胞的传质由3个步骤组成[9]。步骤1:胶束增溶的PAHs被运送到微生物细胞附近。步骤2:胶束在细胞周围形成半胶束层。有研究人员提出这一现象可以用于描述PAHs的生物降解[54-56]和溶解[57]。步骤3:PAHs从半胶束中转移到细胞内。在混合充分的情况下,步骤1通常与胶束中PAHs的含量有关,步骤2和3控制胶束相PAHs的生物降解,此过程受胶束中PAHs与细胞表面吸附力的影响。
综上可知,表面活性剂可以通过增溶提高PAHs的表观溶解度,以达到增强微生物降解的目的。由于土壤中的PAHs大多数以土壤吸附态、非水相结合态的形式存在,微生物降解PAHs会受到限制[5]。当表面活性剂加入后,PAHs会聚集在其胶束内核中形成表观溶解。表面活性剂胶束内核中的PAHs具有更好的流动性,这种传质的强化有利于微生物降解[16]。Bezza等向杂酚油污染土壤中添加生物表面活性剂后,土壤中持久性PAHs的解吸能力显著提高,其降解率也提高了1.5倍[58]。
2.3 细胞表面性质的变化表面活性剂可以作用于细胞表面,增加细胞表面的疏水性(Cell Surface Hydrophobicity,CSH)。CSH是调节细胞、疏水基质和各种固体表面相互作用的重要参数之一。一般来说,具有高CSH的微生物对碳氢化合物有强烈的吸附作用[59]。CSH的增加使PAHs更容易在细菌表面附着,有利于胶束中的PAHs向微生物细胞内部转移[60]。Zhang等发现吐温-80导致菌株CSH增加,从而促进了芘在细胞表面的吸附,增强了降解[61]。然而Zhao等的研究中,鼠李糖脂却显著降低了高疏水性菌株bum的CSH,最终抑制了降解[62]。这可能和表面活性剂吸附于细胞表面的方向性有关[63]。
对于相对亲水的菌株PYR-1,表面活性剂的亲水基团吸附于细胞表面,疏水尾部暴露在环境中,表现为菌株CSH增加[61]。然而对于高疏水性菌株bum,表面活性剂只能将疏水基团吸附于细胞表面,并将亲水端暴露在水相中,从而降低了CSH[62]。2种情况下,表面活性剂和细胞之间的吸附力并不相同,前者通常来源于表面活性剂和细胞表面极性结构之间的相互作用,如氢键、偶极、静电或短程力[64],而后者主要表现为表面活性剂疏水尾与细胞表面的非极性结构(如脂质、蛋白质)之间的范德华力和疏水性作用力[65]。表面活性剂吸附于细胞表面方向性的不同总是会将亲水性菌株变为疏水性菌株,或疏水性菌株变为亲水性菌株[66]。例如,鼠李糖脂处理后的低疏水性微生物群落和高疏水性微生物群落,它们的CSH分别表现为增加和降低[67]。在Ahimou等的研究中,脂肽的吸附同样降低了2株疏水性枯草杆菌的CSH[68]。
对于某些细菌来说,表面活性剂会使细胞外膜丢失脂多糖,这可能是改变CSH的另一种机制[69]。在Al-Tahhan等的研究中,随着鼠李糖脂的添加,铜绿假单胞菌细胞外膜的脂多糖被释放,CSH增加[69]。Chen等利用鼠李糖脂诱导铜绿假单胞菌细胞表面释放脂多糖后,CSH升高并增强了疏水性碳氢化合物在细胞表面的积累[70]。
在污染土壤的实际修复过程中,微生物在土壤颗粒间的流动性也会影响降解效率。表面活性剂会通过改变微生物细胞的表面性质[71],进而增强微生物在土壤中的转移,强化生物降解[72]。鼠李糖脂可促进石英砂中细菌的解吸,随着鼠李糖脂生物表面活性剂浓度的增加,细菌与石英砂之间的相互作用减弱,从而减少了细菌在石英砂中滞留,增强了细菌的流动性[73]。
2.4 表面活性剂对微生物细胞膜的影响微生物细胞膜主要参与酶转运蛋白的合成与运输,以及营养物质的跨膜转运等[74]。微生物细胞膜由磷脂双分子层组成,具有两亲性,结构上类似于表面活性剂。由于这种化学结构的相似,表面活性剂会黏附在微生物细胞膜上,并改变微生物细胞膜的结构和功能[75]。利用吐温-80和十二烷基硫酸钠处理的微生物细胞,其不饱和脂肪酸的含量明显增加,这强化了细胞膜的流动性,并促进了菲的跨膜转运速率[76]。但过高浓度的表面活性剂会破坏细胞完整性,影响菌株的正常功能和生长[74]。总之,在不影响细胞活性的前提下,适量添加表面活性剂有助于细胞对PAHs的摄取和降解。
3 影响表面活性剂强化PAHs生物降解的因素 3.1 表面活性剂的浓度一般来说,PAHs的表观溶解度随着表面活性剂浓度的增大而显著提高[7]。7.5 mg/L的吐温-80可以将菲和芘在水中的表观溶解度提高1.71倍和2.31倍;而当吐温-80浓度升高10倍后,2种PAHs的表观溶解度分别增加到2.62倍和13.25倍[77]。Congiu等将鼠李糖脂浓度从200 mg/L提高到400 mg/L时,菲和芘的表观溶解度分别从29.7%和44.9%提升到46.7%和61.7%[78]。Pan等的研究同样发现菲的溶解度随表面活性剂浓度的增加而增加[15]。
然而,当表面活性剂的浓度过高时,PAHs的表观溶解度将不再增加,甚至会降低。Peng等发现吐温-80和Triton X-100的浓度达到5 g/L之后,PAHs的去除率并未增加[22]。在Pan等的研究中,通过将Brij 30和Tergitol TMN-3 (1:1,质量比)的浓度增加为60 g/L改善了菲的降解,但随着混合表面活性剂浓度的进一步增加,菲的降解率反而降低[15]。
3.2 表面活性剂的毒性不同类型的表面活性剂对微生物的毒性大小是不同的。通常情况下,非离子表面活性剂对微生物的毒性低于离子型表面活性剂。Garon等使用SDS (阴离子)和吐温-80 (非离子)对18株真菌进行了毒性实验,结果显示前者抑制了真菌的生长,而真菌对后者表现出良好的耐受性[79]。在所有化学表面活性剂中,吐温系列的毒性最小,但是较高浓度下也会表现出毒性。研究发现,过高浓度的吐温-20和吐温-80会抑制菲和芘的微生物降解[11]。
相比于化学表面活性剂,生物表面活性剂的毒性更低,对环境更友好。但鼠李糖脂等生物表面活性剂作为一种微生物次级代谢产物,被代谢菌当作一种竞争生存空间的武器,往往会对周围其他微生物产生毒性,浓度高于其CMC的鼠李糖脂对革兰氏阳性枯草芽孢杆菌有毒性作用[12]。Fuchedzhieva等研究发现鼠李糖脂对菲降解菌蜡样芽孢杆菌的毒性,随着其浓度逐渐增加[36]。
3.3 表面活性剂作为优先底物在微生物降解PAHs的过程中,加入的表面活性剂可能会成为比PAHs更具吸引力的碳源。在某些情况下,微生物首先会利用表面活性剂,从而放弃对PAHs的降解,这使得场地修复的效率降低。Kim等利用吐温-80增加了菲的表观溶解度,却抑制了其生物降解,他们认为这是吐温-80的疏水部分作为碳源被菌株优先利用所致[13]。Ghosh等在以芘为唯一碳源的培养基中添加Triton X-100后,芘的降解受到抑制,而以Triton X-100作为唯一底物时菌株生长良好,因此认为,抑制芘生物降解的原因可能是Triton X-100成为了降解菌的优先底物;此外,研究还发现,菌株利用Triton X-100后会产生有毒代谢物,这进一步阻碍了菌株对芘的摄取[23]。对于鼠李糖脂等生物表面活性剂,由于其环境友好性,作为底物被优先降解的情况也并不少见。Wolf等发现,10 CMC的鼠李糖脂成为了降解菌株的优先碳源,抑制了PAHs的矿化[35]。
3.4 温度表面活性剂对PAHs的增溶在一定范围内与温度成正比。在表面活性剂的作用下,萘、菲和芘的溶解度随温度的升高而增加,当温度高于30 ℃时,萘的溶解度降低,这可能是由于萘的挥发速度随着温度的升高而加快,从而使萘在水溶液中的溶解度降低,溶解度的变化进一步影响了PAHs的降解[80]。Sartoros等研究了温度变化时表面活性剂对PAHs矿化的影响,结果发现,添加100 mg/L Tergitol NP-10的条件下,当温度从25 ℃降低到10 ℃后,蒽和芘的总矿化率分别从48.8%和66.1%下降到18.5%和61.5%[81]。这说明温度降低对PAHs的矿化有负面影响。
在环境温度为15-50 ℃范围内,Peng等测得PAHs的降解率随着温度的升高呈现先增后减的趋势,降解的最适温度为35 ℃;在温度高于35 ℃时,漆酶可能会发生变性而抑制降解[82]。然而,也有研究发现在10-40 ℃之间,PAHs的去除率基本不受温度的影响[22]。由此可以看出,不同情况下温度的影响尚不明确。未来,更多机理性的探索是必要的。
3.5 pH值pH值是表面活性剂增溶PAHs的关键因素。一般来说,pH值显著影响离子型表面活性剂对PAHs的增溶。在Zhou等的研究中,随着溶液pH值从4.0增加到8.0,皂角苷对菲的摩尔增溶率降低了70%[38]。不同的是,在浓度为150 mg/L和240 mg/L的鼠李糖脂溶液中,在酸性pH值(4.0-7.0)范围内,菲的表观溶解度随pH值先增后降,而pH值呈碱性(7.0-8.0)时对菲的增溶没有显著影响[83]。但Li等发现pH值由从3.0增加到13.0,PAHs的溶解度增加,在pH值为11.0时达到最大值[80]。由此可见,pH值对表面活性剂增溶PAHs的影响存在大量冲突性的结论,更多机理性的认识有待挖掘。pH值对PAHs的生物降解也有明显影响。在保持其他参数不变的情况下,随着pH从2.0升高到6.0,蒽和芘降解率呈现先增后减的趋势[82]。2种PAHs的最佳降解pH值为4.5[82]。
3.6 混合PAHs在污染土壤中,PAHs大多以混合物形式存在。已有诸多学者开展了混合PAHs的增溶研究,结果表明在表面活性剂增溶过程中,PAHs之间存在着协同或竞争作用[84-85]。萘和菲在Triton X-100胶束的增溶过程中表现出协同作用,Bernardez等推测这种可能是由于2种PAHs不同的疏水性引起的[86]。与单一PAH增溶相比,菲和芘同时加入到Triton X-100后也发生了类似的情况,两者溶解度分别增加了15.38%和18.19%[84]。与此不同的是,具有相似疏水性的芴和菲在阴离子或非离子表面活性剂溶液中的共溶解表现出减小的趋势[85]。这是因为两者竞争了同一个增溶位点。
当环境中存在2种以上PAHs时,情况会更加复杂。Hussein等开展了Triton X-100和吐温-80对3种PAHs (疏水性:蒽 > 芴≈芘)的增溶实验,研究发现,3种PAHs存在相互竞争胶束内部增溶位点的情况,疏水性更强的芴有可能从表面活性剂胶束核取代了芘,从而降低了芘的表观溶解度,宏观上表现为在有疏水性较低PAHs的存在下,疏水性较高的PAHs溶解度会增加[87]。
4 CPS中PAHs的生物降解 4.1 CPS非离子表面活性剂水溶液在高于其浊点温度或有添加物存在的条件下,溶液分相形成表面活性剂浓度很小的稀相和富集表面活性剂的凝聚相,这种两相体系称为CPS。利用CPS提取金属离子[88]、有机化合物[89]和生物活性物质[90],即浊点提取已经得到了广泛的应用。
最初,CPS主要用于疏水性有机物的生物转化。在使用分枝杆菌进行胆固醇的微生物转化时,Triton X-100和Triton X-114组成的CPS显著提高了底物的表观溶解度及传质效率[91]。随后的研究发现,利用CPS两相间底物的不均匀分配可以控制底物的输送,使微生物转化阶段的底物浓度保持在中等水平[92]。因此,CPS可以消除底物的抑制作用并提高其传质效率。
Pan等陆续开展了CPS中脂肪酶的萃取发酵和染料生物脱色的研究[75, 93]。CPS不仅提高了脂肪酶的产量,而且还起到了一定的浓缩和纯化的作用,降低了后期的分离成本[93]。在染料生物脱色方面,CPS成功实现了三苯基甲烷染料脱色并且脱毒的目的[75]。这充分说明了CPS在面对有机化合物污染时表现出优良的增强降解性能,具有可观的研究前景。
4.2 CPS中PAHs的生物降解CPS中PAHs的生物降解已被证实有效,但目前研究尚处于初级阶段,受关注较少[16]。这主要是因为表面活性剂胶束水溶液的浊点对添加物的改变非常敏感[94]。在有机物生物转化过程中,随着培养基中基质和代谢产物浓度的变化,CPS存在体系崩溃的风险[95]。在5%的Brij 30水溶液(质量体积比)形成的CPS中,菲表观溶解度的增加强化了恶臭假单胞菌DSMZ8368对菲的降解[14]。Pan等也开展了CPS中PAHs的生物降解,研究发现,不同表面活性剂形成的CPS对PAHs降解的影响也是不同的,与Triton X-114+Triton X-45、Triton X-114和Triton X-45等形成的CPS相比,Brij 30+Tergitol TMN-3体系更有利于鞘氨醇单胞菌降解菲[15]。
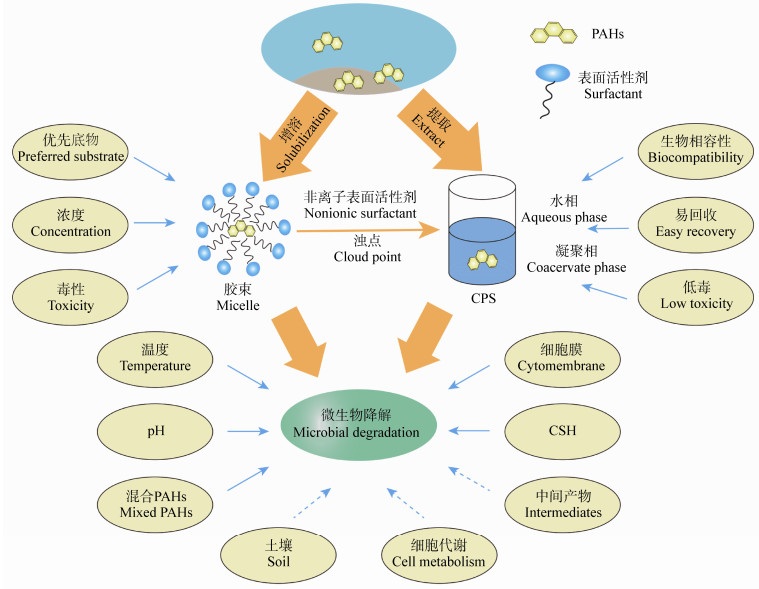
4.3 CPS和胶束体系中PAHs的生物利用度生物利用度是指有机化合物可被微生物直接降解的部分。如前文所述,表面活性剂可以在溶液中形成胶束,从而增加PAHs的表观溶解度。但是在胶束体系中,表面活性剂本身的毒性会限制微生物降解PAHs。有研究表明,在浊点体系中所使用的非离子表面活性剂,其在水环境中表现为低毒和可生物降解的特性[94]。与表面活性剂胶束水溶液相比,CPS具有良好的生物相容性和易回收性。Pan等使用由混合型非离子表面活性剂Brij 30和TMN-3分别形成胶束体系和CPS,并由萘和菲进行生物毒性实验,结果发现,在胶束体系中PAHs的生物毒性会随着表面活性剂的浓度增加而增加,但在CPS中其生物毒性一直处于较低水平,这主要是2种体系中PAHs不同的生物利用度造成的;与胶束体系相比,CPS的凝聚相消除了底物和产物对污染物的抑制作用,从而增强了生物降解性;在CPS中,由于CPS的增溶作用,PAHs被提取到凝聚相,因此,由PAHs疏水性引起的微生物降解速率限制被打破,从而促进了转移和吸收;由于大量的PAHs被提取到凝聚相,在稀相中只留下少量的PAHs,而亲水的微生物细胞存在于表面活性剂浓度很低的稀相中,因此CPS中PAHs表现出的微生物毒性更低[16]。总之,胶束体系和CPS既有联系也有区别(图 3)。后者在强化PAHs微生物降解的过程中是否会对相同影响因素产生与前者不同的响应,目前还少有研究。深入挖掘两者对PAHs微生物降解影响机制的异同,将有利于我们对各种冲突性研究成果的机理性认识。
|
| 图 3 胶束和CPS中PAHs的微生物降解 Figure 3 Microbial degradation of PAHs in micelle and CPS |
|
|
由于表面活性剂在促进微生物降解PAHs污染环境方面具有成本较低、对环境危害较小等优点,因此日益受到研究人员的重视[76, 96]。本文通过介绍表面活性剂增溶PAHs和影响微生物降解PAHs的原理,说明了表面活性剂在处理PAHs污染的优势和应用前景。随后叙述了表面活性剂影响PAHs微生物降解的不同因素[97],希望引起研究人员的重视。最后在文末介绍了一种通过表面活性剂形成CPS来促进微生物降解PAHs的新方法,并将其与胶束系统进行对比(图 3:实线部分)[16]。结果表明,CPS在促进PAHs传质方面是优于胶束系统的,目前这方面研究较少,研究前景十分可观。
尽管表面活性剂促进微生物降解PAHs的研究已较为成熟,但在以下方面仍需要深入探索(图 3虚线部分):
(1) 在PAHs的实际降解过程中,土壤颗粒之间的空隙大小和土壤本身有机相的种类也会影响表面活性剂的效果。在污染土壤的PAHs微生物降解方面,土壤本身不仅会影响表面活性剂对PAHs的增溶效果,而且还会影响土壤中微生物的流动性。所以研究土壤本身的特性显得尤为关键。
(2) 表面活性剂对微生物细胞的影响研究还不够完善。在前文已经提到表面活性剂会使微生物细胞膜表面的疏水性发生变化,但是关于微生物细胞膜通透性和细胞代谢相关变化的研究还不够深入。
(3) 表面活性剂有时会抑制PAHs的微生物降解,其原因可能是微生物代谢表面活性剂产生了有毒的中间产物[23]。虽然已经有学者发现了此类情况,但并没有对产生有毒中间产物的代谢过程进行研究,仍需深入探索。
(4) 在CPS中,PAHs微生物降解的研究目前仍处于起步阶段,有关细胞表面、环境因素和传质影响等方面的研究仍然十分匮乏,需逐步完善。
| [1] |
Wang JZ, Zhu CZ, Chen TH. PAHs in the Chinese environment: levels, inventory mass, source and toxic potency assessment[J]. Environmental Science: Processes & Impacts, 2013, 15(6): 1104-1112. |
| [2] |
Deng H, Guo GX, Qiao M. Advances in the biological indicators for toxicity assessment of polycyclic aromatic hydrocarbons contaminated soil: a review[J]. Asian Journal of Ecotoxicology, 2009, 4(1): 1-13. (in Chinese) 邓欢, 郭光霞, 乔敏. 多环芳烃污染土壤毒性评价指标的研究进展[J]. 生态毒理学报, 2009, 4(1): 1-13. |
| [3] |
Zhou ZK, Cui J, Xu P, Tang HZ. Progress in biodegradation of low molecular weight polycyclic aromatic hydrocarbons[J]. Chinese Journal of Biotechnology, 2019, 35(11): 2069-2080. (in Chinese) 周子康, 崔洁, 许平, 唐鸿志. 细菌降解低分子量多环芳烃的研究进展[J]. 生物工程学报, 2019, 35(11): 2069-2080. |
| [4] |
Megharaj M, Ramakrishnan B, Venkateswarlu K, Sethunathan N, Naidu R. Bioremediation approaches for organic pollutants: A critical perspective[J]. Environment International, 2011, 37(8): 1362-1375. DOI:10.1016/j.envint.2011.06.003 |
| [5] |
Smułek W, Sydow M, Zabielska-Matejuk J, Kaczorek E. Bacteria involved in biodegradation of creosote PAH – A case study of long-term contaminated industrial area[J]. Ecotoxicology and Environmental Safety, 2020, 187: 109843. DOI:10.1016/j.ecoenv.2019.109843 |
| [6] |
Yang M, Ke JF, Zhang QW, He XM. Effects of mixed surfactant on enhancing high concentration anthracene and pyrene removal from contaminated soil[J]. Water, Air, and Soil Pollution, 2019, 230(6): 121. DOI:10.1007/s11270-019-4172-3 |
| [7] |
An CJ, Huang GH, Wei J, Yu H. Effect of short-chain organic acids on the enhanced desorption of phenanthrene by rhamnolipid biosurfactant in soil-water environment[J]. Water Research, 2011, 45(17): 5501-5510. DOI:10.1016/j.watres.2011.08.011 |
| [8] |
Reddy PV, Karegoudar TB, Nayak AS. Enhanced utilization of fluorene by Paenibacillus sp. PRNK-6: Effect of rhamnolipid biosurfactant and synthetic surfactants[J]. Ecotoxicology and Environmental Safety, 2018, 151: 206-211. DOI:10.1016/j.ecoenv.2018.01.019 |
| [9] |
Li JL, Chen BH. Effect of nonionic surfactants on biodegradation of phenanthrene by a marine bacteria of Neptunomonas naphthovorans[J]. Journal of Hazardous Materials, 2009, 162(1): 66-73. DOI:10.1016/j.jhazmat.2008.05.019 |
| [10] |
Lamichhane S, Krishna KCB, Sarukkalige R. Surfactant-enhanced remediation of polycyclic aromatic hydrocarbons: A review[J]. Journal of Environmental Management, 2017, 199: 46-61. |
| [11] |
Aryal M, Liakopoulou-Kyriakides M. Biodegradation and kinetics of phenanthrene and pyrene in the presence of nonionic surfactants by Arthrobacter strain Sphe3[J]. Water, Air, and Soil Pollution, 2013, 224(2): 1426. DOI:10.1007/s11270-012-1426-8 |
| [12] |
Sotirova AV, Spasova DI, Galabova DN, Karpenko E, Shulga A. Rhamnolipid-biosurfactant permeabilizing effects on Gram-positive and Gram-negative bacterial strains[J]. Current Microbiology, 2008, 56(6): 639-644. DOI:10.1007/s00284-008-9139-3 |
| [13] |
Kim HS, Weber WJJ. Preferential surfactant utilization by a PAH-degrading strain: effects on micellar solubilization phenomena[J]. Environmental Science & Technology, 2003, 37(16): 3574-3580. |
| [14] |
Pantsyrnaya T, Delaunay S, Goergen JL, Guseva E, Boudrant J. Solubilization of phenanthrene above cloud point of Brij 30: A new application in biodegradation[J]. Chemosphere, 2013, 92(2): 192-195. DOI:10.1016/j.chemosphere.2013.03.025 |
| [15] |
Pan T, Deng T, Zeng XY, Dong W, Yu SJ. Extractive biodegradation and bioavailability assessment of phenanthrene in the cloud point system by Sphingomonas polyaromaticivorans[J]. Applied Microbiology and Biotechnology, 2016, 100(1): 431-437. DOI:10.1007/s00253-015-6980-8 |
| [16] |
Pan T, Liu CY, Zeng XY, Xin Q, Xu MY, Deng YW, Dong W. Biotoxicity and bioavailability of hydrophobic organic compounds solubilized in nonionic surfactant micelle phase and cloud point system[J]. Environmental Science and Pollution Research, 2017, 24(17): 14795-14801. DOI:10.1007/s11356-017-9076-4 |
| [17] |
Edwards DA, Luthy RG, Liu ZB. Solubilization of polycyclic aromatic hydrocarbons in micellar nonionic surfactant solutions[J]. Environmental Science & Technology, 1991, 25(1): 127-133. |
| [18] |
Singh A, Van Hamme JD, Ward OP. Surfactants in microbiology and biotechnology: Part 2. Application aspects[J]. Biotechnology Advances, 2007, 25(1): 99-121. DOI:10.1016/j.biotechadv.2006.10.004 |
| [19] |
Pan T, Yu SJ, Deng YW, Dong W. Advances in biodegradation research on environmental organic pollutants by surfactants[J]. Journal of Jiangxi University of Science and Technology, 2015, 36(1): 1-6, 11. (in Chinese) 潘涛, 余水静, 邓扬悟, 董伟. 应用表面活性剂强化环境有机污染物生物降解的研究进展[J]. 江西理工大学学报, 2015, 36(1): 1-6, 11. |
| [20] |
Bautista LF, Sanz R, Molina CM, González N, Sánchez D. Effect of different non-ionic surfactants on the biodegradation of PAHs by diverse aerobic bacteria[J]. International Biodeterioration & Biodegradation, 2009, 63(7): 913-922. |
| [21] |
Dave BP, Ghevariya CM, Bhatt JK, Dudhagara DR, Rajpara RK. Enhanced biodegradation of total polycyclic aromatic hydrocarbons (TPAHs) by marine halotolerant Achromobacter xylosoxidans using Triton X-100 and β-cyclodextrin – A microcosm approach[J]. Marine Pollution Bulletin, 2014, 79(1/2): 123-129. |
| [22] |
Peng S, Wu W, Chen JJ. Removal of PAHs with surfactant-enhanced soil washing: Influencing factors and removal effectiveness[J]. Chemosphere, 2011, 82(8): 1173-1177. DOI:10.1016/j.chemosphere.2010.11.076 |
| [23] |
Ghosh I, Mukherji S. Diverse effect of surfactants on pyrene biodegradation by a Pseudomonas strain utilizing pyrene by cell surface hydrophobicity induction[J]. International Biodeterioration & Biodegradation, 2016, 108: 67-75. |
| [24] |
Yuan SY, Wei SH, Chang BV. Biodegradation of polycyclic aromatic hydrocarbons by a mixed culture[J]. Chemosphere, 2000, 41(9): 1463-1468. DOI:10.1016/S0045-6535(99)00522-6 |
| [25] |
Wang CP, Liu HB, Li J, Sun HW. Degradation of PAHs in soil by Lasiodiplodia theobromae and enhanced benzo[a]pyrene degradation by the addition of Tween-80[J]. Environmental Science and Pollution Research, 2014, 21(18): 10614-10625. DOI:10.1007/s11356-014-3050-1 |
| [26] |
Machate T, Noll H, Behrens H, Kettrup A. Degradation of phenanthrene and hydraulic characteristics in a constructed wetland[J]. Water Research, 1997, 31(3): 554-560. DOI:10.1016/S0043-1354(96)00260-6 |
| [27] |
Gong X, Xu XY, Gong ZQ, Li XJ, Jia CY, Guo MX, Li HB. Remediation of PAH-contaminated soil at a gas manufacturing plant by a combined two-phase partition system washing and microbial degradation process[J]. Environmental Science and Pollution Research, 2015, 22(16): 12001-12010. DOI:10.1007/s11356-015-4466-y |
| [28] |
Rodrigues A, Nogueira R, Melo LF, Brito AG. Effect of low concentrations of synthetic surfactants on polycyclic aromatic hydrocarbons (PAH) biodegradation[J]. International Biodeterioration & Biodegradation, 2013, 83: 48-55. |
| [29] |
Zhou Y, Zhang JG, Su EZ, Wei GD, Ma YS, Wei DZ. Phenanthrene biodegradation by an indigenous Pseudomonas sp. ZJF08 with TX100 as surfactant[J]. Annals of Microbiology, 2008, 58(3): 439-442. DOI:10.1007/BF03175540 |
| [30] |
Chen G, Strevett KA, Vanegas BA. Naphthalene, phenanthrene and surfactant biodegradation[J]. Biodegradation, 2001, 12(6): 433-442. DOI:10.1023/A:1015068930512 |
| [31] |
Wang CP, Yu L, Zhang ZY, Wang BL, Sun HW. Tourmaline combined with Phanerochaete chrysosporium to remediate agricultural soil contaminated with PAHs and OCPs[J]. Journal of Hazardous Materials, 2014, 264: 439-448. DOI:10.1016/j.jhazmat.2013.10.073 |
| [32] |
Pei XH, Zhan XH, Wang SM, Lin YS, Zhou LX. Effects of a biosurfactant and a synthetic surfactant on phenanthrene degradation by a Sphingomonas strain[J]. Pedosphere, 2010, 20(6): 771-779. DOI:10.1016/S1002-0160(10)60067-7 |
| [33] |
Cui CZ, Zeng C, Wan X, Chen D, Zhang JY, Shen P. Effect of rhamnolipids on degradation of anthracene by two newly isolated strains, Sphingomonas sp. 12A and Pseudomonas sp. 12B[J]. Journal of Microbiology and Biotechnology, 2008, 18(1): 63-66. |
| [34] |
Shin KH, Ahn Y, Kim KW. Toxic effect of biosurfactant addition on the biodegradation of phenanthrene[J]. Environmental Toxicology and Chemistry, 2005, 24(11): 2768-2774. DOI:10.1897/05-071R1.1 |
| [35] |
Wolf DC, Gan J. Influence of rhamnolipid biosurfactant and Brij-35 synthetic surfactant on 14C-Pyrene mineralization in soil[J]. Environmental Pollution, 2018, 243: 1846-1853. DOI:10.1016/j.envpol.2018.10.031 |
| [36] |
Fuchedzhieva N, Karakashev D, Angelidaki I. Anaerobic biodegradation of fluoranthene under methanogenic conditions in presence of surface-active compounds[J]. Journal of Hazardous Materials, 2008, 153(1/2): 123-127. |
| [37] |
Kobayashi T, Kaminaga H, Navarro RR, Iimura Y. Application of aqueous saponin on the remediation of polycyclic aromatic hydrocarbons-contaminated soil[J]. Journal of Environmental Science and Health, Part A-Toxic/Hazardous Substances & Environmental Engineering, 2012, 47(8): 1138-1145. |
| [38] |
Zhou WJ, Yang JJ, Lou LJ, Zhu LZ. Solubilization properties of polycyclic aromatic hydrocarbons by saponin, a plant-derived biosurfactant[J]. Environmental Pollution, 2011, 159(5): 1198-1204. DOI:10.1016/j.envpol.2011.02.001 |
| [39] |
Chen J, Yang JJ, Zhou WJ. Simultaneous removal of polycyclic aromatic hydrocarbons and heavy metal from co-contaminated soils by saponin, a plant-derived biosurfactant[J]. Journal of Agro-Environment Science, 2010, 29(12): 2325-2329. (in Chinese) 陈洁, 杨娟娟, 周文军. 皂角苷增强洗脱复合污染土壤中多环芳烃和重金属的作用及机理[J]. 农业环境科学学报, 2010, 29(12): 2325-2329. |
| [40] |
Davin M, Starren A, Deleu M, Lognay G, Colinet G, Fauconnier ML. Could saponins be used to enhance bioremediation of polycyclic aromatic hydrocarbons in aged-contaminated soils?[J]. Chemosphere, 2018, 194: 414-421. DOI:10.1016/j.chemosphere.2017.11.174 |
| [41] |
Schippers C, Geßner K, Müller T, Scheper T. Microbial degradation of phenanthrene by addition of a sophorolipid mixture[J]. Journal of Biotechnology, 2000, 83(3): 189-198. DOI:10.1016/S0168-1656(00)00304-7 |
| [42] |
Wu J, Zuo SS. Characteristics of glycolipids biosurfactant and its enhancement role in the biodegradation of anthracene by white rot fungi[J]. Microbiology China, 2017, 44(6): 1286-1294. (in Chinese) 吴涓, 左珊珊. 糖脂类生物表面活性剂的特性及其对白腐真菌降解蒽的强化作用[J]. 微生物学通报, 2017, 44(6): 1286-1294. |
| [43] |
Bezza FA, Chirwa EMN. The role of lipopeptide biosurfactant on microbial remediation of aged polycyclic aromatic hydrocarbons (PAHs)-contaminated soil[J]. Chemical Engineering Journal, 2017, 309: 563-576. DOI:10.1016/j.cej.2016.10.055 |
| [44] |
Blyth W, Shahsavari E, Morrison PD, Ball AS. Biosurfactant from red ash trees enhances the bioremediation of PAH contaminated soil at a former gasworks site[J]. Journal of Environmental Management, 2015, 162: 30-36. |
| [45] |
Li ZH, Wang W, Zhu LZ. Effects of mixed surfactants on the bioaccumulation of polycyclic aromatic hydrocarbons (PAHs) in crops and the bioremediation of contaminated farmlands[J]. Science of the Total Environment, 2019, 646: 1211-1218. DOI:10.1016/j.scitotenv.2018.07.349 |
| [46] |
Zhao BW, Zhu LZ, Li W, Chen BL. Solubilization and biodegradation of phenanthrene in mixed anionic-nonionic surfactant solutions[J]. Chemosphere, 2005, 58(1): 33-40. DOI:10.1016/j.chemosphere.2004.08.067 |
| [47] |
Kubicki S, Bollinger A, Katzke N, Jaeger KE, Loeschcke A, Thies S. Marine biosurfactants: biosynthesis, structural diversity and biotechnological applications[J]. Marine Drugs, 2019, 17(7): 408. DOI:10.3390/md17070408 |
| [48] |
Goswami M, Deka S. Biosurfactant production by a rhizosphere bacteria Bacillus altitudinis MS16 and its promising emulsification and antifungal activity[J]. Colloids and Surfaces B: Biointerfaces, 2019, 178: 285-296. DOI:10.1016/j.colsurfb.2019.03.003 |
| [49] |
Liu GS, Zhong H, Yang X, Liu Y, Shao BB, Liu ZF. Advances in applications of rhamnolipids biosurfactant in environmental remediation: A review[J]. Biotechnology and Bioengineering, 2018, 115(4): 796-814. DOI:10.1002/bit.26517 |
| [50] |
Yeom IT, Ghosh MM, Cox CD. Kinetic aspects of surfactant solubilization of soil-bound polycyclic aromatic hydrocarbons[J]. Environmental Science & Technology, 1996, 30(5): 1589-1595. |
| [51] |
Deshpande S, Shiau BJ, Wade D, Sabatini DA, Harwell JH. Surfactant selection for enhancing ex situ soil washing[J]. Water Research, 1999, 33(2): 351-360. DOI:10.1016/S0043-1354(98)00234-6 |
| [52] |
Makkar RS, Rockne KJ. Comparison of synthetic surfactants and biosurfactants in enhancing biodegradation of polycyclic aromatic hydrocarbons[J]. Environmental Toxicology and Chemistry, 2003, 22(10): 2280-2292. DOI:10.1897/02-472 |
| [53] |
Yu HS, Zhu LZ. Water solubility enhancement of phenanthrene and pyrene by mixed anionic-nonionic surfactant solutions[J]. Environmental Chemistry, 2004, 23(5): 485-489. (in Chinese) 余海粟, 朱利中. 混合表面活性剂对菲和芘的增溶作用[J]. 环境化学, 2004, 23(5): 485-489. DOI:10.3321/j.issn:0254-6108.2004.05.001 |
| [54] |
Guha S, Jaffé PR. Bioavailability of hydrophobic compounds partitioned into the micellar phase of nonionic surfactants[J]. Environmental Science & Technology, 1996, 30(4): 1382-1391. |
| [55] |
Guha S, Jaffé PR, Peters CA. Bioavailability of mixtures of PAHs partitioned into the micellar phase of a nonionic surfactant[J]. Environmental Science & Technology, 1998, 32(15): 2317-2324. |
| [56] |
Guha S, Jaffé PR. Biodegradation kinetics of phenanthrene partitioned into the micellar phase of nonionic surfactants[J]. Environmental Science & Technology, 1996, 30(2): 605-611. |
| [57] |
Grimberg SJ, Nagel J, Aitken MD. Kinetics of phenanthrene dissolution into water in the presence of nonionic surfactants[J]. Environmental Science & Technology, 1995, 29(6): 1480-1487. |
| [58] |
Bezza FA, Chirwa EMN. Biosurfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbons (PAHs) in creosote contaminated soil[J]. Chemosphere, 2016, 144: 635-644. DOI:10.1016/j.chemosphere.2015.08.027 |
| [59] |
Bezza FA, Chirwa EMN. Biosurfactant-assisted bioremediation of polycyclic aromatic hydrocarbons (PAHs) in liquid culture system and substrate interactions[J]. Polycyclic Aromatic Compounds, 2017, 37(5): 375-394. DOI:10.1080/10406638.2015.1129973 |
| [60] |
Bezza FA, Chirwa EMN. Bioremediation of polycyclic aromatic hydrocarbon contaminated soil by a microbial consortium through supplementation of biosurfactant produced by Pseudomonas aeruginosa strain[J]. Polycyclic Aromatic Compounds, 2016, 36(5): 848-872. DOI:10.1080/10406638.2015.1066403 |
| [61] |
Zhang D, Zhu LZ. Effects of Tween 80 on the removal, sorption and biodegradation of pyrene by Klebsiella oxytoca PYR-1[J]. Environmental Pollution, 2012, 164: 169-174. DOI:10.1016/j.envpol.2012.01.036 |
| [62] |
Zhao ZY, Selvam A, Wong JWC. Effects of rhamnolipids on cell surface hydrophobicity of PAH degrading bacteria and the biodegradation of phenanthrene[J]. Bioresource Technology, 2011, 102(5): 3999-4007. DOI:10.1016/j.biortech.2010.11.088 |
| [63] |
Hallmann E, Mędrzycka K. Wetting properties of biosurfactant (rhamnolipid) with synthetic surfactants mixtures in the context of soil remediation[J]. Annales Universitatis Mariae Curie-Sklodowska, 2015, 70(1): 29-39. DOI:10.17951/c.2015.70.1.29 |
| [64] |
Liu Y, Ma XL, Zeng GM, Zhong H, Liu ZF, Jiang YB, Yuan XZ, He XX, Lai MY, He YB. Role of low-concentration monorhamnolipid in cell surface hydrophobicity of Pseudomonas aeruginosa: adsorption or lipopolysaccharide content variation[J]. Applied Microbiology and Biotechnology, 2014, 98(24): 10231-10241. DOI:10.1007/s00253-014-5957-3 |
| [65] |
Górna H, Ławniczak Ł, Zgoła-Grześkowiak A, Kaczorek E. Differences and dynamic changes in the cell surface properties of three Pseudomonas aeruginosa strains isolated from petroleum-polluted soil as a response to various carbon sources and the external addition of rhamnolipids[J]. Bioresource Technology, 2011, 102(3): 3028-3033. DOI:10.1016/j.biortech.2010.09.124 |
| [66] |
Zhong H, Zeng GM, Yuan XZ, Fu HY, Huang GH, Ren FY. Adsorption of dirhamnolipid on four microorganisms and the effect on cell surface hydrophobicity[J]. Applied Microbiology and Biotechnology, 2007, 77(2): 447-455. DOI:10.1007/s00253-007-1154-y |
| [67] |
Owsianiak M, Szulc A, Chrzanowski Ł, Cyplik P, Bogacki M, Olejnik-Schmidt AK, Heipieper HJ. Biodegradation and surfactant-mediated biodegradation of diesel fuel by 218 microbial consortia are not correlated to cell surface hydrophobicity[J]. Applied Microbiology and Biotechnology, 2009, 84(3): 545-553. DOI:10.1007/s00253-009-2040-6 |
| [68] |
Ahimou F, Jacques P, Deleu M. Surfactin and iturin A effects on Bacillus subtilis surface hydrophobicity[J]. Enzyme and Microbial Technology, 2000, 27(10): 749-754. DOI:10.1016/S0141-0229(00)00295-7 |
| [69] |
Al-Tahhan RA, Sandrin TR, Bodour AA, Maier RM. Rhamnolipid-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: Effect on cell surface properties and interaction with hydrophobic substrates[J]. Applied and Environmental Microbiology, 2000, 66(8): 3262-3268. DOI:10.1128/AEM.66.8.3262-3268.2000 |
| [70] |
Chen G, Zhu HL. lux-Marked Pseudomonas aeruginosa lipopolysaccharide production in the presence of rhamnolipid[J]. Colloids and Surfaces B: Biointerfaces, 2005, 41(1): 43-48. DOI:10.1016/j.colsurfb.2004.11.005 |
| [71] |
Zhao SF, Li YP, Cao ZL, Wang JL. Sorption-desorption mechanisms and environmental friendliness of different surfactants in enhancing remediation of soil contaminated with polycyclic aromatic hydrocarbons[J]. Journal of Soils and Sediments, 2020, 20(7): 2817-2828. DOI:10.1007/s11368-020-02640-0 |
| [72] |
Zhong H, Liu GS, Jiang YB, Yang JZ, Liu Y, Yang X, Liu ZF, Zeng GM. Transport of bacteria in porous media and its enhancement by surfactants for bioaugmentation: A review[J]. Biotechnology Advances, 2017, 35(4): 490-504. DOI:10.1016/j.biotechadv.2017.03.009 |
| [73] |
Chen G, Qiao MQ, Zhang HY, Zhu HL. Bacterial desorption in water-saturated porous media in the presence of rhamnolipid biosurfactant[J]. Research in Microbiology, 2004, 155(8): 655-661. DOI:10.1016/j.resmic.2004.05.001 |
| [74] |
Zhang D. Surfactant controlled bacterial interfacial behaviors of PAHs and its mechanisms[D]. Zhejiang: Doctoral Dissertation of Zhejiang University, 2013 (in Chinese) 张栋. 表面活性剂对PAHs微生物界面行为的影响及调控机制[D]. 浙江: 浙江大学博士学位论文, 2013 |
| [75] |
Pan T, Ren SZ, Xu MY, Sun GP, Guo J. Extractive biodecolorization of triphenylmethane dyes in cloud point system by Aeromonas hydrophila DN322p[J]. Applied Microbiology and Biotechnology, 2013, 97(13): 6051-6055. DOI:10.1007/s00253-012-4422-4 |
| [76] |
Li F, Zhu LZ. Surfactant-modified fatty acid composition of Citrobacter sp. SA01 and its effect on phenanthrene transmembrane transport[J]. Chemosphere, 2014, 107: 58-64. DOI:10.1016/j.chemosphere.2014.03.016 |
| [77] |
Hu JP, Wu DS, Rau JY, Liu RL, Liu JS, Lai WL. DOM fractionated from pig waste affecting the solubility of PAHs along with non-ionic surfactant[J]. Desalination and Water Treatment, 2016, 57(39): 18293-18299. DOI:10.1080/19443994.2015.1091986 |
| [78] |
Congiu E, Ortega-Calvo JJ. Role of desorption kinetics in the rhamnolipid-enhanced biodegradation of polycyclic aromatic hydrocarbons[J]. Environmental Science & Technology, 2014, 48(18): 10869-10877. |
| [79] |
Garon D, Krivobok S, Wouessidjewe D, Seigle-Murandi F. Influence of surfactants on solubilization and fungal degradation of fluorene[J]. Chemosphere, 2002, 47(3): 303-309. DOI:10.1016/S0045-6535(01)00299-5 |
| [80] |
Li SD, Pi YR, Bao MT, Zhang C, Zhao DW, Li YM, Sun PY, Lu JR. Effect of rhamnolipid biosurfactant on solubilization of polycyclic aromatic hydrocarbons[J]. Marine Pollution Bulletin, 2015, 101(1): 219-225. DOI:10.1016/j.marpolbul.2015.09.059 |
| [81] |
Sartoros C, Yerushalmi L, Béron P, Guiot SR. Effects of surfactant and temperature on biotransformation kinetics of anthracene and pyrene[J]. Chemosphere, 2005, 61(7): 1042-1050. DOI:10.1016/j.chemosphere.2005.02.061 |
| [82] |
Peng X, Yuan XZ, Liu H, Zeng GM, Chen XH. Degradation of polycyclic aromatic hydrocarbons (PAHs) by laccase in rhamnolipid reversed micellar system[J]. Applied Biochemistry and Biotechnology, 2015, 176(1): 45-55. DOI:10.1007/s12010-015-1508-3 |
| [83] |
Shin KH, Kim KW, Kim JY, Lee KE, Han SS. Rhamnolipid morphology and phenanthrene solubility at different pH values[J]. Journal of Environmental Quality, 2008, 37(2): 509-514. DOI:10.2134/jeq2007.0258 |
| [84] |
Liang XJ, Guo CL, Wei YF, Lin WJ, Yi XY, Lu GN, Dang Z. Cosolubilization synergism occurrence in codesorption of PAH mixtures during surfactant-enhanced remediation of contaminated soil[J]. Chemosphere, 2016, 144: 583-590. DOI:10.1016/j.chemosphere.2015.09.038 |
| [85] |
Rouse JD, Morita T, Furukawa K, Shiau BJ. Solubilization of mixed polycyclic aromatic hydrocarbon systems using an anionic surfactant[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 325(3): 180-185. |
| [86] |
Bernardez LA, Ghoshal S. Selective solubilization of polycyclic aromatic hydrocarbons from multicomponent nonaqueous-phase liquids into nonionic surfactant micelles[J]. Environmental Science & Technology, 2004, 38(22): 5878-5887. |
| [87] |
Hussein TA, Ismail ZZ. Desorption of selected PAHs as individuals and as a ternary PAH mixture within a water-soil-nonionic surfactant system[J]. Environmental Technology, 2013, 34(3): 351-361. DOI:10.1080/09593330.2012.696718 |
| [88] |
Wongwailikhit K, Tasakorn P, Prasassarakich P, Aratono M. Gold recovery by pH-switching process via cloud point extraction[J]. Separation Science and Technology, 2003, 38(14): 3591-3607. DOI:10.1081/SS-120023420 |
| [89] |
Sun C, Liu HZ. Application of non-ionic surfactant in the microwave-assisted extraction of alkaloids from Rhizoma coptidis[J]. Analytica Chimica Acta, 2008, 612(2): 160-164. DOI:10.1016/j.aca.2008.02.040 |
| [90] |
Wang ZL, Zhao FS, Chen DJ, Li DT. Biotransformation of phytosterol to produce androsta-diene-dione by resting cells of Mycobacterium in cloud point system[J]. Process Biochemistry, 2006, 41(3): 557-561. DOI:10.1016/j.procbio.2005.09.014 |
| [91] |
Wang ZL, Zhao FS, Hao XQ, Chen DJ, Li DT. Microbial transformation of hydrophobic compound in cloud point system[J]. Journal of Molecular Catalysis B: Enzymatic, 2004, 27(4/6): 147-153. |
| [92] |
Wang ZL, Zhao FS, Chen DJ, Li DT. Cloud point system as a tool to improve the efficiency of biotransformation[J]. Enzyme and Microbial Technology, 2005, 36(4): 589-594. DOI:10.1016/j.enzmictec.2004.12.006 |
| [93] |
Pan T, Wang ZL, Xu JH, Wu ZQ, Qi HS. Extractive fermentation in cloud point system for lipase production by Serratia marcescens ECU1010[J]. Applied Microbiology and Biotechnology, 2010, 85(6): 1789-1796. DOI:10.1007/s00253-009-2257-4 |
| [94] |
Wang ZL, Dai ZW. Extractive microbial fermentation in cloud point system[J]. Enzyme and Microbial Technology, 2010, 46(6): 407-418. DOI:10.1016/j.enzmictec.2010.02.004 |
| [95] |
Wang ZL. The potential of cloud point system as a novel two-phase partitioning system for biotransformation[J]. Applied Microbiology and Biotechnology, 2007, 75(1): 1-10. DOI:10.1007/s00253-007-0873-4 |
| [96] |
Maletić SP, Beljin JM, Rončević SD, Grgić SD, Dalmacija BD. State of the art and future challenges for polycyclic aromatic hydrocarbons is sediments: sources, fate, bioavailability and remediation techniques[J]. Journal of Hazardous Materials, 2019, 365: 467-482. DOI:10.1016/j.jhazmat.2018.11.020 |
| [97] |
Ortega-Calvo JJ, Tejeda-Agredano MC, Jimenez-Sanchez C, Congiu E, Sungthong R, Niqui-Arroyo JL, Cantos M. Is it possible to increase bioavailability but not environmental risk of PAHs in bioremediation?[J]. Journal of Hazardous Materials, 2013, 261: 733-745. DOI:10.1016/j.jhazmat.2013.03.042 |
 2021, Vol. 48
2021, Vol. 48