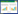扩展功能
文章信息
- 段国文, 耿新燕, 魏绪宇, 赵国琰
- DUAN Guo-Wen, GENG Xin-Yan, WEI Xu-Yu, ZHAO Guo-Yan
- 锰氧化细菌的生理生态功能与作用机制研究进展
- Advances in physiological and ecological functions of manganese oxidizing bacteria and the underlying molecular mechanisms
- 微生物学通报, 2020, 47(9): 3039-3053
- Microbiology China, 2020, 47(9): 3039-3053
- DOI: 10.13344/j.microbiol.china.200488
-
文章历史
- 收稿日期: 2020-05-19
- 接受日期: 2020-07-31
- 网络首发日期: 2020-08-13
微量元素锰(Mn)广泛分布在全球海洋和陆地土壤中,是丰度仅次于铁的第二大过渡重金属元素,也是地球上最丰富的氧化还原敏感金属之一[1-2]。Mn在3种主要价态Mn(Ⅱ)、Mn(Ⅲ)、Mn(Ⅳ)间转换,构成了自然界中的锰循环[2]。其中,高氧化状态下的锰氧化物(MnOx)具有高度的反应性,在地球化学和生态环境领域均有较为广泛的影响[3-8]。
在地球化学和海洋科学领域,基于国际科学研究计划——痕量元素及其同位素的海洋生物地球化学研究(GEOTRACES),世界各国启动了多项资助项目,聚焦各海域的锰循环过程。研究表明,锰及其30种以上的氧化物和氢氧化物影响着几乎所有其他元素的循环,包括氧、氮、碳及各种微量金属元素的循环[9]。其中,中间氧化态Mn(Ⅲ)是最近才被发现的海洋锰池的主要组成部分(占溶解锰池90%左右),广泛存在于含氧水、海底孔隙水和海洋沉积物好氧、厌氧交界处,既可作为电子受体又可作为电子供体,是一种从未被认识的可能控制海洋化学的重要因素[2]。
在环境保护和能源开发领域,作为强吸附剂和氧化剂,MnOx已被广泛应用于重金属和难降解有机物等毒性物质的修复过程中[3-5]。作为氧化剂和电催化材料,MnOx能够催化基于电子-质子等的化学反应以合成能量载体,例如铁/锰的天然半导体涂层在某种程度上类似于光合系统[5],是新能源开发的重要材料[6]。此外,锰结核是沉淀在海洋底部的一种重要的矿产资源,主要由铁、锰氧化物构成,并富集稀土元素等几十种具有重要经济价值的稀有元素[7],已成为继可燃冰之后未来深海资源开采的新选项[2, 8]。
总之,锰循环是影响全球尺度生态平衡及环境变化的重要因素之一。实现上述重要功能的途径基于锰的氧化还原过程,而关键在于锰的氧化作用。
2 锰氧化细菌的种类和分布自20世纪80年代以来,伴随着一些重要的科学发现,科学家对锰氧化过程及机制有了更为深刻的认识,而其中最关键的理论在于确立了锰氧化细菌在自然界锰氧化作用中的核心地位[10-12]。锰氧化细菌往往存在于含有大量锰以及锰循环活跃的生境中[1, 11-12],而且分布十分广泛[13]。在土壤、沙漠清漆、淡泉水、湖泊和池塘、洞穴、热液环境、受污染的沉积物以及海水、海底、河口等多种环境中都鉴定到锰氧化细菌的存在[14-15]。研究表明,海水中锰氧化细菌的总数占细菌总数的1%−10%,在海底锰结核中这一比例高达50%[16];在土壤中,锰氧化细菌的占比约为0.1%[17]。
目前已分离到的具有锰氧化活性的细菌(manganese oxidizing bacteria,MOB)主要集中在厚壁菌门(Firmicutes)、放线菌门(Actinobacteria)和α、β和γ变形菌纲(Proteobacteria)[18]。一些常见的锰氧化细菌分离株有Leptothrix sp.[19-20]、Pseudomonas spp.[21-22]、Escherichia coli[23-24]、Bacillus sp.[25]等,这些菌株被当作模式菌进行深入研究。其中,Bacillus sp. SG-1是从海洋中分离的一株锰氧化细菌,通过产生的芽胞外壁蛋白来氧化Mn(Ⅱ)[26-27];P. putida MnB1和GB-1菌株存在于淡水和土壤环境中,通过多糖-蛋白质复合物在细胞膜上氧化Mn(Ⅱ)[28-30]。除上述之外,Henkel等[31]从黑海中分离出一类化能自养型的锰氧化细菌,属于磺脲类属(Sulfurimonas),通常在硫化环境附近出现[32]。最新研究表明,异化锰还原细菌希瓦氏菌属(Shewanella oneidensis)中许多菌株在有氧条件下也能进行锰氧化并产生细颗粒状的纳米级氧化物颗粒[33]。锰氧化细菌的常见种属及具体分类详见表 1和图 1。
| 分类Class | 种属Genus | 来源Source | 参考文献References |
| 厚壁菌门 Firmicutes |
芽胞杆菌属 Bacillus |
海样表层沉积物、铁锰结核(中国湖北)、植物微生态系统等 Ocean surface sediments, Fe-Mn nodules (Hubei Province, China), plant microecosystem, etc |
[34] [35] [23] |
| 短芽胞杆菌属 Brevibacillus |
锰工厂周围的土壤(中国湖南) Soil around manganese mine (Hunan Province, China) |
[36] [37] |
|
| 放线菌门 Actinobacteria |
阿霉素属Agromyces微杆菌属Microbacterium铜绿假单胞菌属Cupriavidus | 铁锰结核周围土壤(华东地区) Soil around Fe-Mn nodules (East China) |
[38] |
| α、β、γ变形菌纲 Proteobacteria |
纤发菌属 Leptothrix |
淡水环境(锡拉丘兹熊笼溪) Freshwater (Bear Trap Creek in Syracuse) |
[20] [39] |
| 假单胞菌属Pseudomonas | 锰结核、植物微生态系统等 Mn nodules, plant microecosystem, etc |
[40] [22] |
|
| 纤维素单胞菌Cellulomonas罗氏菌属Ralstonia贪噬菌属Variovorax大肠杆菌属Escherichia | 铁锰结核周围土壤(中国华东地区) Soil around Fe-Mn nodules (East China) |
[38] [41] [24] |
|
| 土微菌属 Pedomicrobium |
污水系统 Sewage system |
[42] [43] |
|
| 赤细菌属 Erythrobacter |
海洋表层沉积物(加利福尼亚圣地亚哥湾) Ocean surface sediments (San Diego Bay, California) |
[44] | |
| Albidiferax属 | 废弃的铀矿场(德国图林根州) The former uranium-mining (Thuringia, Germany) |
[45] | |
| 柠檬酸杆菌属 Citrobacter |
生物活性炭过滤柱 Biological activated carbon filter column |
[46] | |
| 黄杆菌属 Flavobacterium |
铁锰矿床(田纳西河流域上游的山洞) Ferromanganese deposits (caves within the upper Tennessee River Basin) |
[47] | |
| 橙单胞菌属 Aurantimonas |
海峡中有氧、无氧交界处 The aerobic and anaerobic junction in the strait |
[48] | |
| 玫瑰杆菌属Roseobacter假交替单胞菌属Pseudoalteromonas海洋交替单胞菌属Alteromonas海杆菌属Marinobacter盐单胞菌属Halomonas Methylarcula Acinetobacter | 海底活火山周围的玄武岩(夏威夷岛周围) Basalt around active seabed volcanos (around Hawaii island) | [49] | |
| 泛菌属Pantoea | 湿地植物内生环境 Endogenous environment of wetland plants |
[50] | |
| 磺脲类属Sulfurimonas | 黑海中的低氧区 The suboxic zone of Black Sea |
[31] |

|
| 图 1 基于先前报道的Mn(Ⅱ)氧化细菌的16S rRNA基因序列的邻接树[14] Figure 1 The neighbour-joining (NJ) tree based on 16S rRNA gene sequences of Mn(Ⅱ) oxidizing bacteria previously reported[14] 注:自展值为1 000次重复后的百分比,仅显示高于70%的自展值;GenBank登录号在括号中给出;图距代表每个核苷酸位置0.05个取代. Note: Bootstrap values are expressed as percentages of 1 000 replications, and only bootstrap values above 70% are shown; GenBank accession numbers are given in parentheses; Bar: 0.05 substitutions per nucleotide. |
|
|
此外,在特殊的生态环境,比如植物根际内生或附生微环境也发现了锰氧化细菌的存在[50-51]。研究表明,在湿地植物柽柳、盐地碱蓬、补血草的叶片和根系组织内存在多株具有环境修复能力的内生细菌,其中有些菌株具有显著的锰氧化活性[50, 52-56]。在淡水植物丹沙的根系表面也发现了具有锰氧化活性的附生细菌[51]。
因此,锰氧化细菌具有种属多样性和分布广泛性的特点。
3 锰氧化细菌的生理生态功能 3.1 锰氧化细菌参与的反应类型在自然界中,锰矿石的主要类型为锰氧化物,但仅通过氧气条件下的曝气和沉淀难以形成锰氧化物[1]。科学家通过对原生矿化的研究,找到了微生物参与早期锰矿形成的直接证据[10],证明锰氧化细菌催化了纳米级氧化锰核的最初形成[1]。
已知存在的锰氧化物矿物有30多种[8],大多数锰矿物的基本单元都是MnO6八面体,根据锰的不同价态以及锰氧化物核心组成部分的不同,可将锰氧化物分为隧道结构和层状结构两种类型[57]。其中,隧道型锰氧化物由边缘共享的八面体链组成,主要存在于锰矿床的含氧区和海洋锰结核中,如单链结构的软锰矿和具有双链结构的钡镁锰矿等[58] (图 2)。层状结构的锰氧化物则是由边缘共享的锰八面体堆叠片组成,如水钠锰矿状氧化物等,在土壤、结核和岩石中含量丰富[15, 28]。此外,相比于隧道状锰氧化物,层状结构的锰氧化物由于矿物的对称性(六方晶系,准正交晶系)、Mn(Ⅲ)含量分布、阳离子空位数量的不同以及随机堆积排列造成的结构多样性而具有十分丰富的结构类型[30, 59-61]。

|
| 图 2 层状和隧道状锰氧化物结构的简化图[14] Figure 2 Simplified polyhedral representation of layered and tunnel Mn oxide structures[14] 注:A:六方水钠锰矿;B:三斜水钠锰矿;C:钡镁锰矿;D:软锰矿.深色阴影的八面体表示Mn3+八面体的晶格位置;三斜水钠钙锰矿(插图)描绘了平面外弯曲角(β)的简化图示. Note: A: Hexagonal birnessite; B: Triclinic birnessite; C: Todorokite; D: Pyrolusite. The dark shaded octahedra represent lattice positions of Mn3+ octahedra; Simplified illustration of the out-of-plane bending angle (β) is depicted for triclinic birnessite (inset). |
|
|
海洋细菌所诱导合成的锰氧化物多为四价态的弱晶质六方水钠锰矿相[14, 57, 62-63](图 2),但随着环境条件的变化,矿物类型会发生变化。例如,海洋芽胞杆菌Bacillus sp. SG-1可以在pH 5.0和50 mmol/L NaCl的缓冲液中将Mn(Ⅱ)氧化成六方晶系锰氧化物[58-59],但在Mn(Ⅱ)浓度较低和较高时会生成次生产物水合布塞尔矿和六方水锰矿(β-MnOOH)[63]。此外,Pecher等通过X-射线扫描透射显微镜法(scanning transmission X-ray microscopy,STXM)和X-射线近边吸收结构谱(X-ray absorption near-edge spectroscopy,XANES)观察在10 mmol/L的Mn(Ⅱ)溶液中静置培养5 d的Bacillus sp. SG-1,发现大部分芽胞周围主要为Mn(Ⅲ)氧化物,只有少量的Mn(Ⅳ)氧化物存在[64]。
土壤来源细菌的锰氧化产物形态更为多样[65],在已报道的30种锰氧化物中,土壤中可能存在的锰氧化物约16种。但是由于土壤中锰含量低、氧化锰矿物组合多样、同晶替代频繁、结晶弱等阻碍因素[66],目前能够鉴定出的只有少数几种,常见的有水羟锰矿、水钠锰矿、锂硬锰矿和钙锰矿等(图 2)。
在植物微生态系统中,研究者在黄河三角洲湿地采集的盐地碱蓬根系表面和髓部发现了黑色的锰氧化膜,并发现覆盖有黑色锰氧化膜的植株体内锰含量相对于无膜覆盖的植株较高[50-51]。从该植物中分离鉴定了2株具有锰氧化活性的内生细菌Pantoea eucrina SS01 (GenBank:MF086656.1)和Pseudomonas composti SS02 (GenBank:MF086658.1) (图 1)。利用X射线衍射(XRD)、选区电子衍射(SAED)等技术对两株菌的锰氧化产物结构进行了表征,经鉴定为MnO2和Mn2O3等[50]。将两株细菌与宿主植物共孵育,发现两株细菌能够促进植株的生长,并能显著促进盐地碱蓬表面的锰氧化膜的形成[50]。这些研究揭示了在植物微生态系统中也存在细菌介导的锰氧化作用。但由于研究较少,植物-细菌系统中锰氧化作用的分子机制和生理机制尚不清楚。
3.2 锰氧化细菌的生理功能尽管大量研究证明微生物是介导自然界锰氧化作用的主导因素,但仍不清楚为什么锰氧化细菌在长期进化中演化出了锰氧化活性,微生物从该过程中获得的生理益处是什么?总体上,目前的假说有两种,一是锰氧化过程为细菌提供营养物质;二是锰能够强化细菌抵御外界不良环境。
锰氧化过程可能为细菌提供了营养物质来源。首先,MnOx能够促进生物体对有机营养物的利用。锰氧化物可以与复杂的有机物(或腐殖质)发生反应[67],使之降解为低分子量的有机物,从而增加有机养分的可利用性。其次,锰氧化过程可能是细菌能量代谢的一部分。一方面,在厌氧条件下,微生物可以利用金属氧化物(即锰、铁或其他氧化物)作为替代性末端电子受体,从而获得通用能量[68-69];另一方面,鉴于金属氧化过程可以为特定环境(如石质铁矿)的微生物生长提供最初化学能源[70],有研究者提出Mn(Ⅱ)的氧化也可能是锰氧化细菌的化学能源[14],但尚缺乏实验证据支持[71]。
此外,锰氧化物还可以在一些条件下保护微生物免受潜在的有毒化合物、超氧化物以及氧化应激等其他不良条件的胁迫[72-73]。有研究表明,细胞内锰水平升高会使放射菌对辐射的抵抗力增强[74],并且胞内的锰蛋白和小分子具有降低超氧化物水平的能力[75-77];锰氧化物能够提高细菌对亚硝酸盐毒性的耐受力,并可能在细胞外形成一层外壳,抵抗病毒的入侵或被捕食[7]。
因此,细菌的锰氧化过程可能是微生物吸收代谢营养物质和产能代谢的部分途径,并有利于微生物适应外界胁迫。
4 细菌的锰氧化分子机制总体上讲,细菌介导锰氧化作用的模式可分为两种[12]:(1)生物诱导作用,通过细菌新陈代谢活动引起周围微环境(如pH等)的改变而有利于锰的氧化[5],或通过细菌-细菌相互作用诱导产生细胞外超氧化物,进而氧化Mn(Ⅱ)[44];(2)生物控制作用,即锰氧化过程受微生物的酶系所控制。
在生物诱导方面,通过新陈代谢,微生物可以通过升高微环境的pH、有机碳和代谢终产物(如铁载体、有机酸、多糖等)间的反应、产生H2O2和过氧化物、消耗CO2或酸类等方式来间接催化Mn(Ⅱ)氧化[18]。此外,铁载体的分泌也能在某种程度上促进生物锰氧化作用,在铁的限制下,菌株分泌铁载体吡啶酮(pyoverdine,PVD),进一步氧化Mn(Ⅱ)生成PVD-Mn(Ⅲ)络合物[78]。
在生物控制方面,多数细菌对于Mn(Ⅱ)的氧化被认为是一种酶催化的生化过程,微生物通过分泌酶,将Mn(Ⅱ)催化氧化为瞬态Mn(Ⅲ)中间体[79],进一步岐化为Mn(Ⅳ)。目前认为可能参与锰氧化催化的酶主要有三类:多铜氧化酶(multicopper oxidase,MCO)、锰过氧化物酶(manganese peroxidases,MnPs)和锰过氧化氢酶(manganese catalase,MnC)[14, 57, 76]。此外,与鞭毛合成、蛋白质转运、双组分调节系统、细胞色素C合成和碳代谢相关的基因也在一定程度上影响细菌锰氧化作用[42, 80-81],但由于相关研究较少,在本文中不作介绍。下面以研究最为详细的细菌锰氧化酶——多铜氧化酶以及锰过氧化物酶和锰过氧化氢酶为例,详细介绍细菌的锰氧化分子机制。
4.1 多铜氧化酶(multicopper oxidase,MCO)多铜氧化酶是一类活性中心含铜离子[Cu(Ⅱ)]的氧化酶,可以催化多种类型的氧化反应[82]。MCO的铜离子结合域主要由组氨酸(His)、半胱氨酸(Cys)和甲硫氨酸(Met)等组成,包含一个用于还原O2的三核Cu中心和一个与底物位点相邻的孤立Cu(Ⅱ)(1型)[83]。其中,1型Cu(Ⅱ)通过从底物中提取电子,并将其转移到三核Cu位置,将O2还原为H2O[84],从而介导各种有机底物和无机底物的催化氧化[85],同时该过程也在锰氧化细菌——芽孢杆菌属菌株PL-12和SG-1中的MCO得到了证明[86-87]。此外,细菌MCO的底物种类十分广泛,除了Mn(Ⅱ)外,还包括芳族胺和环状多元醇(苯二醇),甚至低准价过渡金属如Fe(Ⅱ)、Cu(Ⅰ)等[88]。
近年来,随着基因组数据的迅速增长,多种具有锰氧化活性的MCO在细菌中被发现,其编码基因常以基因簇形式存在,如芽孢杆菌SG-1、PL-12的mnx操纵子[42]、芽孢杆菌WH4的cotA[23]、假单胞菌属中P. putida MnB1的ccm操纵子[89]、纤发菌SS-1的mofA、mofB和mofC[90]以及土微菌ACM3067中的moxA[91]等,其中比较具有代表性的是海洋芽孢杆菌PL-12,其Mn氧化是通过孢子而不是营养细胞进行[25, 92],受到mnx操纵子的调控[93]。该操纵子编码的MCO是由一个包含锰氧化活性中心的蛋白MnxG与两种辅助蛋白MnE和MnF形成蛋白复合物[10]。但需要注意的是,并不是所有的MCO都具有直接的锰氧化活性[71, 94]。如cumA曾被鉴定为P. putida GB-1中的多铜氧化酶基因,但cumA被敲除后,并不影响该菌的锰氧化活性[94]。之后的进一步研究发现在P. putida GB-1中真正起作用的锰氧化基因是mnxG和mcoA[71],在该锰氧化过程中,MnxS1/MnxS2/MnxR双组分调控系统是必需的[70]。
在MCO的锰氧化机制研究中,MnxEFG蛋白复合物的研究最为详尽,现就该复合物的功能和催化机制进行阐述。
MnxEFG蛋白复合物分离自海洋芽孢杆菌SG-1、PL-12和MB-7,是第一个被成功纯化并以活性形式异源表达的具有锰氧化活性的多铜氧化酶,也是研究最为系统的细菌锰氧化酶[10, 87, 93]。近期,结合高精度离子淌度质谱(IM-MS)和扫描透射电子显微镜(S/TEM)技术,分子量为211 kD的MnxEFG的分子结构得到了解析,由一个MnxE3F3六聚体和一个MnxG组成的复合物,其中MnxE3F3六聚体由三重对称性的MnxE和MnxF单体组成[10, 95]。在复合物中,MnxE包含一个二硫键,可以结合一个铜离子,MnxF可以结合3个铜离子,但是亲和性低于MnxE。同时MnxEF复合体可以结合血红素,并包含由3个组氨酸配位的2型铜中心[10]。从结构中推测MnxE和MnxF可能具有氧化还原活性,参与金属或电子转移,而且在缺少MnxG的情况下,MnxEF的单独表达虽能与Cu(Ⅱ)结合形成复合物,但不能氧化Mn(Ⅱ)[96]。此外,没有辅助蛋白MnxE和MnxF的存在MnxG也不能成功表达出具有活性的多铜氧化酶[95]。
MCO通过单电子转移氧化Mn(Ⅱ),而将Mn(Ⅱ)氧化为Mn(Ⅳ)则需要转移2个电子,因此推测存在Mn(Ⅲ)中间产物,但长期以来因为技术限制,未能捕获到Mn(Ⅲ)[30, 63]。如Bargar等利用XANES等技术监测锰氧化过程中各种价态锰离子的含量,但仅检测到Mn(Ⅳ)的生成,并未观察到Mn(Ⅲ)中间产物的出现,推断在芽孢杆菌属菌株SG-1氧化过程中不会生成Mn(Ⅲ)[63]。后续在Bacillus sp. SG-1和P. putida MnB1的研究过程中也得到了同样的推论[30]。由于MCO每次只能催化底物的一个电子氧化,而Mn(Ⅱ)氧化为Mn(Ⅳ)需要2个电子转移,这使得MCO的锰氧化机理曾成为争议[71]。直到2005年,Webb等在Bacillus sp. SG-1氧化锰的过程中,通过向反应体系中加入焦磷酸钠PP,捕获到了Mn(Ⅲ)-焦磷酸盐复合中间产物[97](图 3),最终证明了中间态Mn(Ⅲ)的存在。
一般认为,MnxEFG催化Mn(Ⅱ)氧化的过程分为两步:通过单电子转移将Mn(Ⅱ)氧化为中间体过渡态Mn(Ⅲ),Mn(Ⅲ)进一步转变为Mn(Ⅳ)[98]。其中第二步反应机制尚不清楚,Mn(Ⅲ)通过歧化形成Mn(Ⅳ)还是通过酶内的第2次电子转移反应而进一步氧化仍未有定论[87]。但随着Mnx的成功纯化和结构解析,多名学者试图对其催化机制进行更深一步的阐述[95, 99-101]。其中,最近Soldatova等提出的连续多核配合物中通过氢氧化物和氧化物桥连的两步氧化锰的催化机制(图 4)最具有说服力[102],而且在光系统Ⅱ)[103-104]的O2演化的络合物和锰过氧化氢酶[44]中也发现了类似的多核Mn络合物,为该假设提供了依据。该假设[102]认为MnxG的活性发挥需要活化作用,而且Mn(Ⅲ)无法向Mnx完成直接的单电子转移,具体机理如下:
|
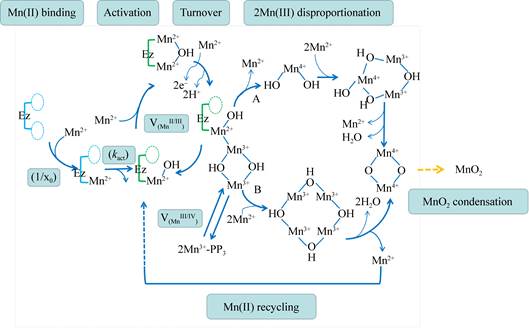
| 图 4 Mnx催化MnO2合成的假定机理[102] Figure 4 Proposed mechanism of the formation of Mnx-catalyzed MnO2[102] 注:推测的双核氧桥联的Mn(Ⅳ)产物生成途径. A:连续生成的Mn(Ⅳ)(OH)2先经歧化,再进行缩合;B:Mn(Ⅲ)(µ-OH)2Mn(Ⅲ)单元先进行自缩合,后经双歧化. Note: Proposed proceeds to Mn(Ⅳ)(μ-O)2Mn(Ⅳ) via (A) disproportionation and condensation of successively produced Mn(Ⅳ)(OH)2 units or (B) condensation of successively produced Mn(Ⅲ)(µ-OH)2Mn(Ⅲ) units followed by a double disproportionation. |
|
|
(1) MnxG的活化:首先,MnxG与Mn(Ⅱ)结合,经过水去质子化后引起酶构象的改变,从而激活酶活性,增加对Mn(Ⅱ)的亲和力。
(2) 第1个电子转移:酶活化后,第2个Mn(Ⅱ)与酶结合并与OH−桥接,形成氢氧化桥联的双核配合物Mn(Ⅱ)(μ-OH)Mn(Ⅱ),从而降低了Mn(Ⅱ)的氧化电位。电子转移到T1 Cu(Ⅱ)并生成Mn(Ⅲ),然后转移到远端部位。
(3) 第2个电子转移:另一个Mn(Ⅱ)与空位结合,重复上述过程形成第2个Mn(Ⅲ),并通过异位和去质子化产生了双核Mn(Ⅲ)复合物——Mn(Ⅲ)(µ-OH)2Mn(Ⅲ)。
(4) 双核Mn(Ⅲ)复合物通过歧化、缩合和去质子化产生双核氧桥联的Mn(Ⅳ)产物,并进一步生成MnO2。
其中,双核Mn(Ⅲ)复合物的形成使得酶得以循环利用,在整个催化过程中起着至关重要的作用[105]。
4.2 锰过氧化物酶(manganese peroxidases,MnPs)过氧化物酶(peroxidases,POD)是一类由单一肽链和铁卟啉组成的血红蛋白类氧化酶,普遍存在于动植物及微生物中,以过氧化物为电子受体而催化底物氧化,参与清除自由基、机体免疫、化合物降解等重要的生命活动[106]。锰过氧化物酶(MnPs)属于胞外分泌的过氧化物酶,在细菌、真菌以及藻类等多种微生物中都有发现[107-108],在食品工业、生物传感器设计、造纸制浆、纺织和酿酒工业、诊断试剂盒的制备和环境保护等领域有着广泛的应用前景[109]。但是细菌中锰过氧化物酶的催化机制较为欠缺,下面以研究较多的血红素锰过氧化物酶(MopA)为例进行介绍。
2009年,Anderson等在α变形菌Aurantimonas manganoxydans SI85-9A1和Erythrobacter sp. SD21中首次发现了一类动物血红素过氧化物酶同源蛋白MopA,其能够参与催化菌体的锰氧化过程[79],该酶与动物血红素过氧化物酶(animal heme peroxidase,AHP)具有很高的相似性,存在于α变形菌Aurantimonas manganoxydans SI85-9A1、Erythrobacter sp. SD21、Roseobacter sp. Azwk-3b以及P. putida GB-1中[79, 110-111]。
MopA包含两个结构域:一是具有催化活性的动物血红素过氧化物酶结构域;二是C端钙结合域[112]。研究表明,血红素过氧化物酶能够催化细菌将Mn(Ⅱ)氧化为Mn(Ⅲ)[79],但其作用机制存在争议。有结果表明,重组表达的血红素过氧化物酶能够直接催化氧化Mn(Ⅱ)[112-113];也有观点认为,过氧化物酶的主要作用是促进超氧化物的生成,而Mn(Ⅱ)的氧化过程实际是由其他酶或强氧化剂催化完成[111, 114];另有数据表明,尽管有些细菌中也存在血红素过氧化物酶的保守基因序列,但这些菌株并不能催化氧化Mn(Ⅱ)[115]。MopA的锰氧化活性能够被Cu(Ⅱ)、NAD(I)、H2O2和吡咯喹啉醌(pyrroloquinoline quinone,PQQ)等所诱导[116],其中NAD(I)是MopA发挥活性所必需的辅助因子[113]。但过氧化氢(H2O2)对MopA锰氧化活性的影响不一。一般认为,过氧化氢在血红素过氧化物酶中起关键作用:血红素过氧化物酶通过利用过氧化氢的强氧化能力初始氧化活性中心的血红素,进而完成酶的功能。如:菌株Aurantiomonas manganoxydans的锰氧化作用由过氧化物酶介导,该酶能被过氧化氢激活[79],这与典型的过氧化物酶催化机制一致。与此相反,在大肠杆菌中重组表达来自赤细菌的血红素过氧化物酶,当加入过氧化氢时,不仅不能激活其锰氧化活性,反而抑制该活性[112]。
相比于MCO,目前对MopA的认识仍十分有限,氧化机理尚不清楚。从野生菌株中分离纯化活性酶和测定酶学性质将有利于对细菌Mn(Ⅱ)氧化过程的进一步理解。
4.3 锰过氧化氢酶(manganese catalase,MnC)锰过氧化氢酶是一类不依赖血红素而发挥作用的过氧化氢酶,是含血红素催化酶在抗氧化防御中的重要替代物,也被称为假过氧化氢酶或非血红素过氧化氢酶。1961年,Delwiche等发现在Pediococci[116]和Lactobacilli[117-118]菌株中存在一类活性中心不含血红素的过氧化氢酶,能够催化细菌锰氧化[119],命名为MnC。与血红素过氧化氢酶不同,MnC的催化活性位点是双核锰配合物而不是血红素,而且对氰化物的抑制不敏感[73]。
通过分析晶体结构,表明MnC含有双核锰催化活性位点,该位点在还原的[Mn2(Ⅱ, Ⅱ)]和氧化的[Mn2(Ⅲ, Ⅲ)]的转换过程中进行双电子氧化还原循环[118]。当存在[Mn2(Ⅱ, Ⅱ)]状态时,H2O2作为氧化剂[方程式(1)],当存在[Mn2(Ⅲ, Ⅲ)]状态时,H2O2作为还原剂[方程式(2)]。此方案涉及在2个状态的循环中顺序结合2个过氧化氢分子,产生了在氧化和还原的络合物中均十分稳定的活性位点。但是与含血红素的过氧化氢酶(kcat=4×107 s−1)催化效率相比,非血红素的锰过氧化氢酶的动力学要慢得多(LPC,kcat=2×105 s−1[119];TTC,kcat=2.6×105 s−1[120])[76]。
| $ {\rm {H_2}{O_2} + \{ M{n_2}\left( {II,II} \right) + 2{H^ + }\} \to 2{H_2}O + \{ M{n_2}\left( {III,III} \right)\} } $ | (1) |
| $ {\rm {H_2}{O_2} + \{ M{n_2}\left( {III,III} \right)\} \to {O_2} + \{ M{n_2}\left( {II,II} \right) + 2{H^ + }\} } $ | (2) |
在最近的研究中,Baginski等在T. roseum中分离纯化出一种锰过氧化氢酶,并证明其同时具有过氧化物酶和邻苯二酚酶活性,但催化机理仍需进一步研究[121]。Beal等利用具有较高分辨率的电子顺磁共振(electron paramagnetic resonance,EPR)技术并结合对称性破损方法和密度泛函理论(broken symmetry-density functional theory,BS-DFT)对锰过氧化氢酶活性位点的精细电子结构进行分析[122],但是通过处于超氧化状态下的精细耦合常数计算得到的结果与仅基于实验结果的解释完全相反[123]。这表明与1位点Mn(Ⅳ)结合的水分子很可能保持完全质子化,并且在锰过氧化氢酶的超氧化状态下不会成为氢氧化物[123]。
总之,锰氧化酶在细菌的锰氧化过程中发挥着重要的作用。MnxG复合物的异源表达和结构解析以及MopA的发现和成功纯化,使得对于锰氧化酶的组成和作用机制有了更进一步的认识。但是大多数细菌中的锰氧化基因由于异源表达的障碍和蛋白纯化技术的不足致使其机制仍不明确,氧化过程中电子的转移路线以及Mn(Ⅲ)到Mn(Ⅳ)的氧化途径等问题仍有待解决。
5 展望细菌介导的锰氧化作用影响着全球尺度生态平衡和环境变化,锰氧化细菌具有种属多样性、产物多样性和分布广泛性。细菌的酶促锰氧化是一个复杂的过程,关于其生理机制和分子机制的认识尚不成熟。虽然前期研究已在细菌中找到了多种Mn(Ⅱ)氧化基因和氧化酶,并证实了它们在体内外的作用,但仍然有很多的问题亟待解决:(1)技术问题,在细菌锰氧化酶的研究过程中,蛋白的纯化、重组表达和结构解析是尚未被完全解决的技术限制问题,技术的革新和多种技术的交叉应用(如冷冻电镜技术、EPR技术与BS-DFT的结合、IM-MS与S/TEM的结合)将有利于这一问题的解决。(2)生理机制方面,仍需阐明细菌Mn(Ⅱ)氧化与能量代谢的相关性和机制;需阐明植物内生和附生微环境下锰氧化细菌的生理机制及其与植物宿主的相互作用关系。(3)来源和多样性不足,现有研究集中在少数几株模式菌株,多来自于海洋,对陆地及其他来源的菌株研究较少,应另辟蹊径,更广泛地分离鉴定不同生境来源的锰氧化细菌,深入挖掘其锰氧化机制,从而有助于在更大的研究覆盖面上揭示微生物锰氧化作用的共性和规律。
| [1] |
Hansel C, Learman D. Geomicrobiology of manganese[A]// Ehrlich's Geomicrobiology[M]. 6th ed. CRC Press, 2015: 403-452
|
| [2] |
Madison AS, Tebo BM, Mucci A, et al. Abundant porewater Mn(Ⅲ) is a major component of the sedimentary redox system[J]. Science, 2013, 341(6148): 875-878. DOI:10.1126/science.1241396 |
| [3] |
Martínez-Ruiz EB, Cooper M, Fastner J, et al. Manganese-oxidizing bacteria isolated from natural and technical systems remove cylindrospermopsin[J]. Chemosphere, 2020, 238: 124625. DOI:10.1016/j.chemosphere.2019.124625 |
| [4] |
Tran TN, Kim DG, Ko SO. Synergistic effects of biogenic manganese oxide and Mn(Ⅱ)-oxidizing bacterium Pseudomonas putida strain MnB1 on the degradation of 17 α-ethinylestradiol[J]. Journal of Hazardous Materials, 2018, 344: 350-359. DOI:10.1016/j.jhazmat.2017.10.045 |
| [5] |
Zhang Y, Tang YK, Qin ZY, et al. A novel manganese oxidizing bacterium-Aeromonas hydrophila strain DS02: Mn(Ⅱ) oxidization and biogenic Mn oxides generation[J]. Journal of Hazardous Materials, 2019, 367: 539-545. DOI:10.1016/j.jhazmat.2019.01.012 |
| [6] |
Kaim V, Kaur‐Ghumaan S. Manganese complexes: hydrogen generation and oxidation[J]. European Journal of Inorganic Chemistry, 2019, 2019(48): 5041-5051. DOI:10.1002/ejic.201900988 |
| [7] |
Zerfaβ C, Christie-Oleza JA, Soyer OS. Manganese oxide biomineralization provides protection against nitrite toxicity in a cell-density-dependent manner[J]. Applied and Environmental Microbiology, 2019, 85(2): e02129-18. |
| [8] |
Blöthe M, Wegorzewski A, Müller C, et al. Manganese-cycling microbial communities inside deep-sea manganese nodules[J]. Environmental Science & Technology, 2015, 49(13): 7692-7700. |
| [9] |
Daye M, Klepac-Ceraj V, Pajusalu M, et al. Light-driven anaerobic microbial oxidation of manganese[J]. Nature, 2019, 576(7786): 311-314. DOI:10.1038/s41586-019-1804-0 |
| [10] |
Romano CA, Zhou MW, Song Y, et al. Biogenic manganese oxide nanoparticle formation by a multimeric multicopper oxidase Mnx[J]. Nature Communications, 2017, 8(1): 746. DOI:10.1038/s41467-017-00896-8 |
| [11] |
Vasilatos C, Economou-Eliopoulos M. Fossilized bacteria in Fe-Mn-mineralization: evidence from the Legrena Valley, W. Lavrion Mine (Greece)[J]. Minerals, 2018, 8(3): 107
|
| [12] |
Tebo BM, Johnson HA, McCarthy JK, et al. Geomicrobiology of manganese(Ⅱ) oxidation[J]. Trends in Microbiology, 2005, 13(9): 421-428. DOI:10.1016/j.tim.2005.07.009 |
| [13] |
Yang JS, Cao J, Xing GL, et al. Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341[J]. Bioresource Technology, 2015, 175: 537-544. DOI:10.1016/j.biortech.2014.10.124 |
| [14] |
Hansel CM. Manganese in marine microbiology[J]. Advances in Microbial Physiology, 2017, 70: 37-83. DOI:10.1016/bs.ampbs.2017.01.005 |
| [15] |
Bargar JR, Fuller CC, Marcus MA, et al. Structural characterization of terrestrial microbial Mn oxides from Pinal Creek, AZ[J]. Geochimica et Cosmochimica Acta, 2009, 73(4): 889-910. DOI:10.1016/j.gca.2008.10.036 |
| [16] |
Liu JC, Wang OM, Li JJ, et al. Mechanisms of extracellular electron transfer in the biogeochemical manganese cycle[J]. Acta Microbiologica Sinica, 2018, 58(4): 546-559(in Chinese). (in Chinese) 刘进超, 王欧美, 李佳佳, 等. 生物地球化学锰循环中的微生物胞外电子传递机制[J]. 微生物学报, 2018, 58(4): 546-559. |
| [17] |
Yang H, Zhong J, Ji Z, et al. Advance in microbiological studies of Mn(Ⅱ) oxidizing bacteria[J]. Chinese Journal of Applied & Environmental Biology, 2008, 14(1): 143-146(in Chinese). (in Chinese) 杨宏, 钟洁, 纪娟, 等. Mn(Ⅱ)氧化细菌的微生物学研究进展[J]. 应用与环境生物学报, 2008, 14(1): 143-146. |
| [18] |
Su JM. Studies on the mechanism of bacterial Mn(Ⅱ) oxidation and characteristics of biological manganese oxides[D]. Wuhan: Doctoral Dissertation of Huazhong Agricultural University, 2015 (in Chinese) 苏键镁.细菌氧化锰的作用机理及生物锰氧化物的特性研究[D].武汉: 华中农业大学博士学位论文, 2015 |
| [19] |
El Gheriany IA, Bocioaga D, Hay AG, et al. An uncertain role for Cu(Ⅱ) in stimulating Mn(Ⅱ) oxidation by Leptothrix discophora SS-1[J]. Archives of Microbiology, 2011, 193(2): 89-93. DOI:10.1007/s00203-010-0645-x |
| [20] |
Takeda M, Kawasaki Y, Umezu T, et al. Patterns of sheath elongation, cell proliferation, and manganese(Ⅱ) oxidation in Leptothrix cholodnii[J]. Archives of Microbiology, 2012, 194(8): 667-673. DOI:10.1007/s00203-012-0801-6 |
| [21] |
Santelli CM, Chaput DL, Hansel CM. Microbial communities promoting Mn(Ⅱ) oxidation in Ashumet Pond, a historically polluted freshwater pond undergoing remediation[J]. Geomicrobiology Journal, 2014, 31(7): 605-616. DOI:10.1080/01490451.2013.875605 |
| [22] |
Geszvain K, McCarthy JK, Tebo BM. Elimination of manganese(Ⅱ, Ⅲ) oxidation in Pseudomonas putida GB-1 by a double knockout of two putative multicopper oxidase genes[J]. Applied and Environmental Microbiology, 2013, 79(1): 357-366. DOI:10.1128/AEM.01850-12 |
| [23] |
Su JM, Bao P, Bai TL, et al. CotA, a multicopper oxidase from Bacillus pumilus WH4, exhibits manganese-oxidase activity[J]. PLoS One, 2013, 8(4): e60573. |
| [24] |
Zhang Z, Zhang ZM, Chen H, et al. Surface Mn(Ⅱ) oxidation actuated by a multicopper oxidase in a soil bacterium leads to the formation of manganese oxide minerals[J]. Scientific Reports, 2015, 5(1): 10895. DOI:10.1038/srep10895 |
| [25] |
Sylvan JB, Hoffman CL, Momper LM, et al. Bacillus rigiliprofundi sp. nov., an endospore-forming, Mn-oxidizing, moderately halophilic bacterium isolated from deep subseafloor basaltic crust[J]. International Journal of Systematic and Evolutionary Microbiology, 2015, 65(Pt 6): 1992-1998
|
| [26] |
Toyoda K, Tebo BM. The effect of Ca2+ ions and ionic strength on Mn(Ⅱ) oxidation by spores of the marine Bacillus sp. SG-1[J]. Geochimica et Cosmochimica Acta, 2013, 101: 1-11
|
| [27] |
Ferreira L, Rosales E, Danko AS, et al. Bacillus thuringiensis a promising bacterium for degrading emerging pollutants[J]. Process Safety and Environmental Protection, 2016, 101: 19-26. DOI:10.1016/j.psep.2015.05.003 |
| [28] |
Villalobos M, Lanson B, Manceau A, et al. Structural model for the biogenic Mn oxide produced by Pseudomonas putida[J]. American Mineralogist, 2006, 91(4): 489-502. |
| [29] |
Zhou DH, Kim DG, Ko SO, et al. Heavy metal adsorption with biogenic manganese oxides generated by Pseudomonas putida strain MnB1[J]. Journal of Industrial and Engineering Chemistry, 2015, 24: 132-139. DOI:10.1016/j.jiec.2014.09.020 |
| [30] |
Villalobos M, Toner B, Bargar J, et al. Characterization of the manganese oxide produced by Pseudomonas putida strain MnB1[J]. Geochimica et Cosmochimica Acta, 2003, 67(14): 2649-2662. DOI:10.1016/S0016-7037(03)00217-5 |
| [31] |
Henkel JV, Dellwig O, Pollehne F, et al. A bacterial isolate from the Black Sea oxidizes sulfide with manganese(Ⅳ) oxide[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(25): 12153-12155. DOI:10.1073/pnas.1906000116 |
| [32] |
Han YC, Perner M. The globally widespread genus Sulfurimonas: versatile energy metabolisms and adaptations to redox clines[J]. Frontiers in Microbiology, 2015, 6: 989. |
| [33] |
Wright MH, Farooqui SM, White AR, et al. Production of manganese oxide nanoparticles by Shewanella species[J]. Applied and Environmental Microbiology, 2016, 82(17): 5402-5409. DOI:10.1128/AEM.00663-16 |
| [34] |
Francis CA, Tebo BM. Enzymatic manganese(Ⅱ) oxidation by metabolically dormant spores of diverse Bacillus species[J]. Applied and Environmental Microbiology, 2002, 68(2): 874-880. |
| [35] |
van Waasbergen LG, Hildebrand M, Tebo BM. Identification and characterization of a gene cluster involved in manganese oxidation by spores of the marine Bacillus sp. strain SG-1[J]. Journal of Bacteriology, 1996, 178(12): 3517-3530
|
| [36] |
Tang WW, Gong JN, Wu LJ, et al. DGGE diversity of manganese mine samples and isolation of a Lysinibacillus sp. efficient in removal of high Mn(Ⅱ) concentrations[J]. Chemosphere, 2016, 165: 277-283
|
| [37] |
Zeng XP, Zhang MT, Liu YY, et al. Manganese(Ⅱ) oxidation by the multi-copper oxidase CopA from Brevibacillus panacihumi MK-8[J]. Enzyme and Microbial Technology, 2018, 117: 79-83. DOI:10.1016/j.enzmictec.2018.04.011 |
| [38] |
Yang WH, Zhang Z, Zhang ZM, et al. Population structure of manganese-oxidizing bacteria in stratified soils and properties of manganese oxide aggregates under manganese-complex medium enrichment[J]. PLoS One, 2013, 8(9): e73778. |
| [39] |
Siering PL, Ghiorse WC. Phylogeny of the Sphaerotilus-Leptothrix group inferred from morphological comparisons, genomic fingerprinting, and 16S ribosomal DNA sequence analyses[J]. International Journal of Systematic and Evolutionary Microbiology, 1996, 46(1): 173-182. |
| [40] |
Ehrlich HL. Bacteriology of manganese nodulesⅡ. Manganese oxidation by cell-free extract from a manganese nodule bacterium[J]. Applied Microbiology, 1968, 16(2): 197-202. DOI:10.1128/AEM.16.2.197-202.1968 |
| [41] |
Zhang LM, Liu F, Tan WF, et al. Microbial DNA extraction and analyses of soil iron–manganese nodules[J]. Soil Biology and Biochemistry, 2008, 40(6): 1364-1369. DOI:10.1016/j.soilbio.2007.01.004 |
| [42] |
Larsen EI, Sly LI, McEwan AG. Manganese(Ⅱ) adsorption and oxidation by whole cells and a membrane fraction of Pedomicrobium sp. ACM 3067[J]. Archives of Microbiology, 1999, 171(4): 257-264. DOI:10.1007/s002030050708 |
| [43] |
Sly LI, Arunpairojana V, Hodgkinson MC. Pedomicrobium manganicum from drinking-water distribution systems with manganese-related "dirty water" problems[J]. Systematic and Applied Microbiology, 1988, 11(1): 75-84. DOI:10.1016/S0723-2020(88)80051-1 |
| [44] |
Francis CA, Co EM, Tebo BM. Enzymatic manganese(Ⅱ) oxidation by a marine α-proteobacterium[J]. Applied and Environmental Microbiology, 2001, 67(9): 4024-4029. DOI:10.1128/AEM.67.9.4024-4029.2001 |
| [45] |
Akob DM, Bohu T, Beyer A, et al. Identification of Mn(Ⅱ)-oxidizing bacteria from a low-pH contaminated former uranium mine[J]. Applied and Environmental Microbiology, 2014, 80(16): 5086-5097. DOI:10.1128/AEM.01296-14 |
| [46] |
Tang WW, Xia J, Zeng XP, et al. Biological characteristics and oxidation mechanism of a new manganese-oxidizing bacteria FM-2[J]. Bio-Medical Materials and Engineering, 2014, 24(1): 703-709. DOI:10.3233/BME-130858 |
| [47] |
Carmichael MJ, Carmichael SK, Santelli CM, et al. Mn(Ⅱ)-oxidizing bacteria are abundant and environmentally relevant members of ferromanganese deposits in caves of the upper Tennessee River Basin[J]. Geomicrobiology Journal, 2013, 30(9): 779-800. DOI:10.1080/01490451.2013.769651 |
| [48] |
Dick GJ, Podell S, Johnson HA, et al. Genomic insights into Mn(Ⅱ) oxidation by the marine alphaproteobacterium Aurantimonas sp. strain SI85-9A1[J]. Applied and Environmental Microbiology, 2008, 74(9): 2646-2658
|
| [49] |
Templeton AS, Staudigel H, Tebo BM. Diverse Mn(Ⅱ)-oxidizing bacteria isolated from submarine basalts at Loihi Seamount[J]. Geomicrobiology Journal, 2005, 22(3/4): 127-139. |
| [50] |
Zhao GY, Cheng JH, Sun NN, et al. Two endophytic bacterial strains modulate Mn oxidation and accumulation in the wetland plant Suaeda salsa pall[J]. Plant and Soil, 2019, 438(1/2): 223-237. |
| [51] |
Tsuji K, Asayama T, Shiraki N, et al. Mn accumulation in a submerged plant Egeria densa (Hydrocharitaceae) is mediated by epiphytic bacteria[J]. Plant, Cell & Environment, 2017, 40(7): 1163-1173. |
| [52] |
Zhao GY, Zhao LY, Xia ZJ, et al. Salinicola tamaricis spov., a heavy-metal-tolerant, endophytic bacterium isolated from the halophyte Tamarix chinensis Lour[J]. International Journal of Systematic and Evolutionary Microbiology, 2017, 67(6): 1813-1819. DOI:10.1099/ijsem.0.001868 |
| [53] |
Shang NJ, Zhu QQ, Dai MX, et al. Complete genome sequence of the heavy-metal-tolerant Endophytic type strain of Salinicola tamaricis[J]. Genome Announcements, 2018, 6(16): e00358-18. |
| [54] |
Zhao GY, Shao F, Zhang M, et al. Luteimonas rhizosphaerae sp. nov., isolated from the rhizosphere of Triticum aestivum L.[J]. International Journal of Systematic and Evolutionary Microbiology, 2018, 68(4): 1197-1203. DOI:10.1099/ijsem.0.002649 |
| [55] |
Shang NJ, Ding MJ, Dai MX, et al. Biodegradation of malachite green by an endophytic bacterium Klebsiella aerogenes S27 involving a novel oxidoreductase[J]. Applied Microbiology and Biotechnology, 2019, 103(5): 2141-2153. DOI:10.1007/s00253-018-09583-0 |
| [56] |
Zhao GY, Shang NJ. Enterobacter aerogenes for degrading malachite green in high-salinity environment: CN, 107858303B[P]. 2020-02-07 (in Chinese) 赵国琰, 尚念杰.一株在高盐环境下降解孔雀石绿的产气肠杆菌: 中国, 107858303B[P]. 2020-02-07 |
| [57] |
Tebo BM, Bargar JR, Clement BG, et al. Biogenic manganese oxides: properties and mechanisms of formation[J]. Annual Review of Earth and Planetary Sciences, 2004, 32: 287-328. DOI:10.1146/annurev.earth.32.101802.120213 |
| [58] |
Sauer K, Yachandra VK. A possible evolutionary origin for the Mn4 cluster of the photosynthetic water oxidation complex from natural MnO2 precipitates in the early ocean[J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(13): 8631-8636. DOI:10.1073/pnas.132266199 |
| [59] |
Webb SM, Tebo BM, Barger JR. Structural influences of sodium and calcium ions on the biogenic manganese oxides produced by the marine Bacillus sp. strain SG-1[J]. Geomicrobiology Journal, 2005, 22(3/4): 181-193
|
| [60] |
Webb SM, Tebo BM, Bargar JR. Structural characterization of biogenic Mn oxides produced in seawater by the marine Bacillus sp. strain SG-1[J]. American Mineralogist, 2005, 90(8/9): 1342-1357
|
| [61] |
Bodeï S, Manceau A, Geoffroy N, et al. Formation of todorokite from vernadite in Ni-rich hemipelagic sediments[J]. Geochimica et Cosmochimica Acta, 2007, 71(23): 5698-5716. DOI:10.1016/j.gca.2007.07.020 |
| [62] |
Kim HS, Pastén PA, Gaillard JF, et al. Nanocrystalline todorokite-like manganese oxide produced by bacterial catalysis[J]. Journal of the American Chemical Society, 2003, 125(47): 14284-14285. DOI:10.1021/ja0375784 |
| [63] |
Bargar JR, Tebo BM, Bergmann U, et al. Biotic and abiotic products of Mn(Ⅱ) oxidation by spores of the marine Bacillus sp. strain SG-1[J]. American Mineralogist, 2005, 90(1): 143-154
|
| [64] |
Pecher K, McCubbery D, Kneedler E, et al. Quantitative charge state analysis of manganese biominerals in aqueous suspension using scanning transmission X-ray microscopy (STXM)[J]. Geochimica et Cosmochimica Acta, 2003, 67(6): 1089-1098. DOI:10.1016/S0016-7037(02)01229-2 |
| [65] |
Zhang ZJ, Lai J, Yin H, et al. Absorption mechanisms of Cu2+ on a biogenic bixbyite-like Mn2O3 produced by Bacillus CUA isolated from soil[J]. Geochemical Transactions, 2015, 16(1): 5. DOI:10.1186/s12932-015-0020-6 |
| [66] |
Tan WF, Liu F, Li YH, et al. Mineralogy of manganese in iron-manganese nodules of several soils in China[J]. Acta Pedologica Sinica, 2000, 37(2): 192-201in Chinese). (in Chinese) 谭文峰, 刘凡, 李永华, 等. 我国几种土壤铁锰结核中的锰矿物类型[J]. 土壤学报, 2000, 37(2): 192-201. |
| [67] |
Sunda WG, Kieber DJ. Oxidation of humic substances by manganese oxides yields low-molecular-weight organic substrates[J]. Nature, 1994, 367(6458): 62-64. DOI:10.1038/367062a0 |
| [68] |
Myers CR, Nealson KH. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor[J]. Science, 1988, 240(4857): 1319-1321. DOI:10.1126/science.240.4857.1319 |
| [69] |
Venkateswaran K, Moser DP, Dollhopf ME, et al. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov.[J]. International Journal of Systematic and Evolutionary Microbiology, 1999, 49(2): 705-724
|
| [70] |
Emerson D, Fleming EJ, McBeth JM. Iron-oxidizing bacteria: an environmental and genomic perspective[J]. Annual Review of Microbiology, 2010, 64: 561-583. DOI:10.1146/annurev.micro.112408.134208 |
| [71] |
Geszvain K, Butterfield C, Davis RE, et al. The molecular biogeochemistry of manganese(Ⅱ) oxidation[J]. Biochemical Society Transactions, 2012, 40(6): 1244-1248. DOI:10.1042/BST20120229 |
| [72] |
Remucal CK, Ginder-Vogel M. A critical review of the reactivity of manganese oxides with organic contaminants[J]. Environmental Science: Processes & Impacts, 2014, 16(6): 1247-1266. |
| [73] |
Banh A, Chavez V, Doi J, et al. Manganese (Mn) oxidation increases intracellular Mn in Pseudomonas putida GB-1[J]. PLoS One, 2013, 8(10): e77835. |
| [74] |
Daly MJ, Gaidamakova EK, Matrosova VY, et al. Protein oxidation implicated as the primary determinant of bacterial radioresistance[J]. PLoS Biology, 2007, 5(4): e92. |
| [75] |
Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium[J]. Nature Reviews Microbiology, 2013, 11(7): 443-454. DOI:10.1038/nrmicro3032 |
| [76] |
Whittaker JW. Non-heme manganese catalase–the 'other' catalase[J]. Archives of Biochemistry and Biophysics, 2012, 525(2): 111-120. DOI:10.1016/j.abb.2011.12.008 |
| [77] |
Miller AF. Superoxide dismutases: ancient enzymes and new insights[J]. FEBS Letters, 2012, 586(5): 585-595. DOI:10.1016/j.febslet.2011.10.048 |
| [78] |
Hullo MF, Moszer I, Danchin A, et al. CotA of Bacillus subtilis is a copper-dependent laccase[J]. Journal of Bacteriology, 2001, 183(18): 5426-5430. DOI:10.1128/JB.183.18.5426-5430.2001 |
| [79] |
Anderson CR, Johnson HA, Caputo N, et al. Mn(Ⅱ) oxidation is catalyzed by heme peroxidases in "Aurantimonas manganoxydans" strain SI85-9A1 and Erythrobacter sp. strain SD-21[J]. Applied and Environmental Microbiology, 2009, 75(12): 4130-4138
|
| [80] |
Geszvain K, Tebo BM. Identification of a two-component regulatory pathway essential for Mn(Ⅱ) oxidation in Pseudomonas putida GB-1[J]. Applied and Environmental Microbiology, 2010, 76(4): 1224-1231. |
| [81] |
Geszvain K, Yamaguchi A, Maybee J, et al. Mn(Ⅱ) oxidation in Pseudomonas putida GB-1 is influenced by flagella synthesis and surface substrate[J]. Archives of Microbiology, 2011, 193(8): 605-614. DOI:10.1007/s00203-011-0702-0 |
| [82] |
Kosman DJ. Multicopper oxidases: a workshop on copper coordination chemistry, electron transfer, and metallophysiology[J]. JBIC Journal of Biological Inorganic Chemistry, 2010, 15(1): 15-28. |
| [83] |
Solomon EI, Sundaram UM, Machonkin TE. Multicopper oxidases and oxygenases[J]. Chemical Reviews, 1996, 96(7): 2563-2606. DOI:10.1021/cr950046o |
| [84] |
Solomon EI, Augustine AJ, Yoon J. O2 reduction to H2O by the multicopper oxidases[J]. Dalton Transactions, 2008(30): 3921-3932. DOI:10.1039/b800799c |
| [85] |
Jones SM, Solomon EI. Electron transfer and reaction mechanism of laccases[J]. Cellular and Molecular Life Sciences, 2015, 72(5): 869-883. DOI:10.1007/s00018-014-1826-6 |
| [86] |
Soldatova AV, Butterfield C, Oyerinde OF, et al. Multicopper oxidase involvement in both Mn(Ⅱ) and Mn(Ⅲ) oxidation during bacterial formation of MnO2[J]. JBIC Journal of Biological Inorganic Chemistry, 2012, 17(8): 1151-1158. DOI:10.1007/s00775-012-0928-6 |
| [87] |
Butterfield CN, Soldatova AV, Lee SW, et al. Mn(Ⅱ, Ⅲ) oxidation and MnO2 mineralization by an expressed bacterial multicopper oxidase[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(29): 11731-11735. DOI:10.1073/pnas.1303677110 |
| [88] |
Taylor RJK, Reid M, Foot J, et al. Tandem oxidation processes using manganese dioxide: discovery, applications, and current studies[J]. Accounts of Chemical Research, 2005, 38(11): 851-869. DOI:10.1021/ar050113t |
| [89] |
Caspi R, Tebo BM, Haygood MG. c-type cytochromes and manganese oxidation in Pseudomonas putida MnB1[J]. Applied and Environmental Microbiology, 1998, 64(10): 3549-3555. DOI:10.1128/AEM.64.10.3549-3555.1998 |
| [90] |
Corstjens PLAM, de Vrind JPM, Goosen T, et al. Identification and molecular analysis of the Leptothrix discophora SS-1 mofA gene, a gene putatively encoding a manganese-oxidizing protein with copper domains[J]. Geomicrobiology Journal, 1997, 14(2): 91-108. DOI:10.1080/01490459709378037 |
| [91] |
Ridge JP, Lin M, Larsen EI, et al. A multicopper oxidase is essential for manganese oxidation and laccase-like activity in Pedomicrobium sp. ACM 3067[J]. Environmental Microbiology, 2007, 9(4): 944-953
|
| [92] |
Dick GJ, Lee YE, Tebo BM. Manganese(Ⅱ)-oxidizing Bacillus spores in Guaymas Basin hydrothermal sediments and plumes[J]. Applied and Environmental Microbiology, 2006, 72(5): 3184-3190. DOI:10.1128/AEM.72.5.3184-3190.2006 |
| [93] |
Dick GJ, Torpey JW, Beveridge TJ, et al. Direct identification of a bacterial manganese(Ⅱ) oxidase, the multicopper oxidase MnxG, from spores of several different marine Bacillus species[J]. Applied and Environmental Microbiology, 2008, 74(5): 1527-1534. DOI:10.1128/AEM.01240-07 |
| [94] |
Brouwers GJ, de Vrind JPM, Corstjens PLAM, et al. CumA, a gene encoding a multicopper oxidase, is involved in Mn2+ oxidation in Pseudomonas putida GB-1[J]. Applied and Environmental Microbiology, 1999, 65(4): 1762-1768. DOI:10.1128/AEM.65.4.1762-1768.1999 |
| [95] |
Ahnert SE, Marsh JA, Hernández H, et al. Principles of assembly reveal a periodic table of protein complexes[J]. Science, 2015, 350(6266). |
| [96] |
Butterfield CN, Tao LZ, Chacón KN, et al. Multicopper manganese oxidase accessory proteins bind Cu and heme[J]. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 2015, 1854(12): 1853-1859. DOI:10.1016/j.bbapap.2015.08.012 |
| [97] |
Webb SM, Dick GJ, Bargar JR, et al. Evidence for the presence of Mn(Ⅲ) intermediates in the bacterial oxidation of Mn(Ⅱ)[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(15): 5558-5563. DOI:10.1073/pnas.0409119102 |
| [98] |
Tao LZ, Stich TA, Soldatova AV, et al. Mn(Ⅲ) species formed by the multi-copper oxidase MnxG investigated by electron paramagnetic resonance spectroscopy[J]. JBIC Journal of Biological Inorganic Chemistry, 2018, 23(7): 1093-1104. DOI:10.1007/s00775-018-1587-z |
| [99] |
Butterfield CN, Tebo BM. Substrate specificity and copper loading of the manganese-oxidizing multicopper oxidase Mnx from Bacillus sp. PL-12[J]. Metallomics, 2017, 9(2): 183-191
|
| [100] |
Tao LZ, Stich TA, Butterfield CN, et al. Mn(Ⅱ) binding and subsequent oxidation by the multicopper oxidase MnxG investigated by electron paramagnetic resonance spectroscopy[J]. Journal of the American Chemical Society, 2015, 137(33): 10563-10575. DOI:10.1021/jacs.5b04331 |
| [101] |
Tao LZ, Stich TA, Liou SH, et al. Copper binding sites in the manganese-oxidizing Mnx protein complex investigated by electron paramagnetic resonance spectroscopy[J]. Journal of the American Chemical Society, 2017, 139(26): 8868-8877. DOI:10.1021/jacs.7b02277 |
| [102] |
Soldatova AV, Tao LZ, Romano CA, et al. Mn(Ⅱ) oxidation by the multicopper oxidase complex Mnx: a binuclear activation mechanism[J]. Journal of the American Chemical Society, 2017, 139(33): 11369-11380. DOI:10.1021/jacs.7b02771 |
| [103] |
Yano J, Yachandra V. Mn4Ca cluster in photosynthesis: where and how water is oxidized to dioxygen[J]. Chemical Reviews, 2014, 114(8): 4175-4205. DOI:10.1021/cr4004874 |
| [104] |
McEvoy JP, Brudvig GW. Water-splitting chemistry of photosystem Ⅱ[J]. Chemical Reviews, 2006, 106(11): 4455-4483. DOI:10.1021/cr0204294 |
| [105] |
Soldatova AV, Romano CA, Tao LZ, et al. Mn(Ⅱ) oxidation by the multicopper oxidase complex Mnx: a coordinated two-stage Mn(Ⅱ)/(Ⅲ) and Mn(Ⅲ)/(Ⅳ) mechanism[J]. Journal of the American Chemical Society, 2017, 139(33): 11381-11391. DOI:10.1021/jacs.7b02772 |
| [106] |
Knop D, Levinson D, Makovitzki A, et al. Limits of versatility of versatile peroxidase[J]. Applied and Environmental Microbiology, 2016, 82(14): 4070-4080. DOI:10.1128/AEM.00743-16 |
| [107] |
Chowdhary P, Yadav A, Kaithwas G, et al. Distillery wastewater: a major source of environmental pollution and its biological treatment for environmental safety[A]. Singh R, Kumar S. Green Technologies and Environmental Sustainability[M]. Cham: Springer, 2017: 409-435
|
| [108] |
Zhang H, Zhang J, Zhang XY, et al. Purification and characterization of a novel manganese peroxidase from white-rot fungus Cerrena unicolor BBP6 and its application in dye decolorization and denim bleaching[J]. Process Biochemistry, 2018, 66: 222-229. DOI:10.1016/j.procbio.2017.12.011 |
| [109] |
Chowdhary P, Shukla G, Raj G, et al. Microbial manganese peroxidase: A ligninolytic enzyme and its ample opportunities in research[J]. SN Applied Sciences, 2019, 1(1): 45. DOI:10.1007/s42452-018-0046-3 |
| [110] |
Andeer PF, Learman DR, Mcilvin M, et al. Extracellular haem peroxidases mediate Mn(Ⅱ) oxidation in a marine Roseobacter bacterium via superoxide production[J]. Environmental Microbiology, 2015, 17(10): 3925-3936. DOI:10.1111/1462-2920.12893 |
| [111] |
Geszvain K, Smesrud L, Tebo BM. Identification of a third Mn(Ⅱ) oxidase enzyme in Pseudomonas putida GB-1[J]. Applied and Environmental Microbiology, 2016, 82(13): 3774-3782. DOI:10.1128/AEM.00046-16 |
| [112] |
Nakama K, Medina M, Lien A, et al. Heterologous expression and characterization of the manganese-oxidizing protein from Erythrobacter sp. strain SD21[J]. Applied and Environmental Microbiology, 2014, 80(21): 6837-6842
|
| [113] |
Medina M, Rizo A, Dinh D, et al. MopA, the Mn oxidizing protein from Erythrobactersp. SD-21, requires heme and NAD+ for Mn(Ⅱ) oxidation[J]. Frontiers in Microbiology, 2018, 9: 2671
|
| [114] |
Learman DR, Voelker BM, Vazquez-Rodriguez AI, et al. Formation of manganese oxides by bacterially generated superoxide[J]. Nature Geoscience, 2011, 4(2): 95-98. DOI:10.1038/ngeo1055 |
| [115] |
Learman DR, Hansel CM. Comparative proteomics of Mn(Ⅱ)-oxidizing and non-oxidizing Roseobacter clade bacteria reveal an operative manganese transport system but minimal Mn(Ⅱ)-induced expression of manganese oxidation and antioxidant enzymes[J]. Environmental Microbiology Reports, 2014, 6(5): 501-509. DOI:10.1111/1758-2229.12164 |
| [116] |
Delwiche EA. Catalase of Pediococcus cerevisiae[J]. Journal of Bacteriology, 1961, 81(3): 416-418. DOI:10.1128/JB.81.3.416-418.1961 |
| [117] |
Johnston MA, Delwiche EA. Isolation and characterization of the cyanide-resistant and azide-resistant catalase of Lactobacillus plantarum[J]. Journal of Bacteriology, 1965, 90(2): 352-356. DOI:10.1128/JB.90.2.352-356.1965 |
| [118] |
Barynin VV, Whittaker MM, Antonyuk SV, et al. Crystal structure of manganese catalase from Lactobacillus plantarum[J]. Structure, 2001, 9(8): 725-738. DOI:10.1016/S0969-2126(01)00628-1 |
| [119] |
Kono Y, Fridovich I. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum[J]. Journal of Biological Chemistry, 1983, 258(10): 6015-6019. |
| [120] |
Shank M, Barynin V, Dismukes GC. Protein coordination to manganese determines the high catalytic rate of dimanganese catalases. Comparison to functional catalase mimics[J]. Biochemistry, 1994, 33(51): 15433-15436
|
| [121] |
Baginski R, Sommerhalter M. A manganese catalase from Thermomicrobium roseum with peroxidase and catecholase activity[J]. Extremophiles, 2017, 21(1): 201-210. DOI:10.1007/s00792-016-0896-9 |
| [122] |
Beal NJ, Corry TA, O'Malley PJ. Comparison of experimental and broken symmetry density functional theory calculated electron paramagnetic resonance parameters for the manganese catalase active site in the superoxidized MnⅢ/MnIV state[J]. The Journal of Physical Chemistry B, 2018, 122(11): 2881-2890. DOI:10.1021/acs.jpcb.7b11649 |
| [123] |
Beal NJ, O'Malley PJ. Manganese oxidation state assignment for manganese catalase[J]. Journal of the American Chemical Society, 2016, 138(13): 4358-4361. DOI:10.1021/jacs.6b02600 |
 2020, Vol. 47
2020, Vol. 47