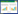扩展功能
文章信息
- 王怡静, 夏晶晶, 于景丽, 希尼尼根, 李传虹, 李新, 芦燕
- WANG Yi-Jing, XIA Jing-Jing, YU Jing-Li, Xininigen, LI Chuan-Hong, LI Xin, LU Yan
- 水分驱动半干旱区河流沉积物/土壤厌氧绳菌群落的空间异质性
- Moisture determined spatial heterogeneity of river sediment/soil Anaerolineaceae communities in semiarid region
- 微生物学通报, 2020, 47(9): 2807-2821
- Microbiology China, 2020, 47(9): 2807-2821
- DOI: 10.13344/j.microbiol.china.200247
-
文章历史
- 收稿日期: 2020-03-17
- 接受日期: 2020-07-19
- 网络首发日期: 2020-08-07
2. 内蒙古自治区环境污染控制与废物资源化重点实验室 内蒙古 呼和浩特 010021;
3. 内蒙古大学生态与环境学院 内蒙古 呼和浩特 010021;
4. 内蒙古农业大学兽医学院 内蒙古 呼和浩特 010018
2. Inner Mongolia Key Laboratory of Environmental Pollution Control & Waste Resource Reuse, Hohhot, Inner Mongolia 010021, China;
3. School of Ecology and Environment, Inner Mongolia University, Hohhot, Inner Mongolia 010021, China;
4. College of Veterinary Medicine, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia 010018, China
沉积物/土壤位于岩石圈、水圈、土壤圈、生物圈和大气圈的交界面[1],是生物地球化学循环的热区[2],具有很高的细菌生物多样性[3-5]。细菌作为生物地球化学循环的主要驱动者,能敏感地预警生态系统结构和功能的变化[3-4]。Hou等发现沉积物中厌氧绳菌的多样性高于水相[6]。大量的研究表明,厌氧绳菌分布在含盐量较高的海洋沉积物[7-8]、河流沉积物[5]、热液口[9-10]和湿地土壤[11]等环境[12-13]。例如:Abia等在南非阿皮斯河中发现厌氧绳菌是沉积物中的优势细菌[5];Cleary等发现海绵生物类型、海洋沉积物等因素解释了厌氧绳菌等细菌群落空间变异的84%[7],他们后来又证实沉积物深度和生境是潮间带河口沉积物厌氧绳菌等细菌群落空间变异的主要因素[14]。简言之,生境是驱动厌氧绳菌等细菌群落空间变异的主要因素[7, 14]。Li等证明厌氧绳菌群落是沙漠演变为绿洲过程中出现的重要性指示生物[15]。然而,关于蒙古高原半干旱区不同水分梯度下厌氧绳菌群落的空间异质性和环境驱动力等问题至今尚不明晰。
水分通常被认为是驱动半干旱区生物多样性及空间分布的限制性因子[16-17]。因此,本研究针对以下科学问题:半干旱区水生的河床环境、湿生的河漫滩环境以及旱生的草原阶地环境是否存在厌氧绳菌群落的空间分布异质性?生境变化是否为决定厌氧绳菌群落空间分布异质性的核心因素?生境本身还是其中更为核心的环境因子决定了厌氧绳菌群落的空间异质性?水分含量是否为驱动厌氧绳菌群落空间异质性的主要环境因子?利用16S rRNA基因高通量测序技术分析水生、湿生、旱生环境中厌氧绳菌群落的空间分布特征及环境驱动力,目的是揭秘水分梯度关联的河床环境、湿生河漫滩环境以及旱生草原阶地环境下厌氧绳菌群落空间异质性的核心环境驱动力,以期为半干旱区水分胁迫下河流湿地萎缩及草原荒漠化的早期预警提供科学依据。
1 材料与方法 1.1 样带选择、采样点设置及理化特性研究区域位于蒙古高原半干旱区锡林流域中游,地理坐标为43°36′−44°29′N、115°32′−117°12′E。沿着水分梯度依次采集水生的河床中心沉积物(R1)、水生的河床堆积侧沉积物(R2)、水生的牛轭湖沉积物(R3)、邻近河床的灯芯草(Juncus effusus)河滨湿地土壤(W1)、远离河床的鹅绒委陵菜(Potentilla anserina)河滨湿地土壤(W2)、邻近湿地的旱生羊草(Leymus chinensis)典型草原土壤(T1)、远离湿地的旱生大针茅(Stipa grandis)典型草原土壤(T2),沉积物/土壤的采集深度均为0−10 cm表层。采样时间为2017年7月上旬,其中水生的河床中心样带R1包括R11−R16等6个样品、水生的河床边缘样带R2包括R21−R24等4个样品、水生的牛轭湖样带R3包括R31−R35等5个样品、湿生的低河滨带W1包括W11−W14等4个样品、湿生的高河滨带W2包括W21−W23等3个样品、旱生的低阶地羊草样带T1包括T11−T16等6个样品、旱生的高阶地大针茅样带T2包括T21−T26等6个样品。34个沉积物/土壤样品布点方案及样品的检测方法参照文献[18]。受半干旱区气候影响,W和T样带地表有明显的积盐现象,沉积物/土壤的理化特性见表 1。
| 样品 Sample |
黏粒 Clay(%) |
粉粒 Silt(%) |
砂粒 Sand(%) |
水分 Moisture(%) |
pH | 盐度 DS(mg/kg) |
氨氮 AM(mg/kg) |
硝态氮 NR(mg/kg) |
总有机碳 TOC(g/kg) |
总氮 TN(g/kg) |
总磷 TP(g/kg) |
| R11 | 0.00 | 0.03 | 99.97 | 17.95 | 8.03 | 0.31 | 1.04 | 5.17 | 2.57 | 0.44 | 0.08 |
| R12 | 0.00 | 0.06 | 99.94 | 17.85 | 8.05 | 0.29 | 1.12 | 5.83 | 2.53 | 0.42 | 0.11 |
| R13 | 0.00 | 0.04 | 99.96 | 17.92 | 8.04 | 0.32 | 1.06 | 5.25 | 2.55 | 0.43 | 0.13 |
| R14 | 0.00 | 0.02 | 99.98 | 17.84 | 7.91 | 0.22 | 1.00 | 3.77 | 2.34 | 0.32 | 0.08 |
| R15 | 0.00 | 0.03 | 99.97 | 17.92 | 8.04 | 0.31 | 1.05 | 5.17 | 2.57 | 0.43 | 0.09 |
| R16 | 0.00 | 0.02 | 99.98 | 17.94 | 8.02 | 0.32 | 1.03 | 5.16 | 2.58 | 0.45 | 0.07 |
| R21 | 1.61 | 4.40 | 93.99 | 20.23 | 8.36 | 1.27 | 38.42 | 14.53 | 7.17 | 0.99 | 0.22 |
| R22 | 1.65 | 4.60 | 93.75 | 20.18 | 8.31 | 1.18 | 26.48 | 5.89 | 7.19 | 1.06 | 0.27 |
| R23 | 1.61 | 4.40 | 93.99 | 20.22 | 8.35 | 1.29 | 39.47 | 14.58 | 7.17 | 0.98 | 0.23 |
| R24 | 1.61 | 4.30 | 94.09 | 20.23 | 8.37 | 1.26 | 36.38 | 14.49 | 7.15 | 0.99 | 0.22 |
| R31 | 1.66 | 4.50 | 93.84 | 23.17 | 8.75 | 1.24 | 23.74 | 7.63 | 24.54 | 1.97 | 0.36 |
| R32 | 1.64 | 4.70 | 93.66 | 24.05 | 8.67 | 1.21 | 26.89 | 11.15 | 24.27 | 1.92 | 0.33 |
| R33 | 1.63 | 4.60 | 93.77 | 24.14 | 8.65 | 1.18 | 20.74 | 5.91 | 24.35 | 1.88 | 0.31 |
| R34 | 1.67 | 4.50 | 93.83 | 23.19 | 8.74 | 1.23 | 23.91 | 8.28 | 24.60 | 1.98 | 0.36 |
| R35 | 1.65 | 4.40 | 93.95 | 22.16 | 8.73 | 1.22 | 23.86 | 8.22 | 24.57 | 1.96 | 0.37 |
| W11 | 0.00 | 1.53 | 98.47 | 22.24 | 8.62 | 1.08 | 7.73 | 4.51 | 8.88 | 1.14 | 0.26 |
| W12 | 0.02 | 1.59 | 98.39 | 21.19 | 8.72 | 1.13 | 10.95 | 6.31 | 9.13 | 1.23 | 0.31 |
| W13 | 0.00 | 1.51 | 98.49 | 22.21 | 8.59 | 1.07 | 8.18 | 5.01 | 8.89 | 1.12 | 0.27 |
| W14 | 0.00 | 1.56 | 98.44 | 22.25 | 8.66 | 1.09 | 8.17 | 5.07 | 8.87 | 1.16 | 0.28 |
| W21 | 2.86 | 16.19 | 80.95 | 20.88 | 8.64 | 1.32 | 7.92 | 9.42 | 10.69 | 1.26 | 0.33 |
| W22 | 2.17 | 16.62 | 81.21 | 20.69 | 8.67 | 1.29 | 6.41 | 10.68 | 10.91 | 1.29 | 0.32 |
| W23 | 3.89 | 19.96 | 76.15 | 19.28 | 8.49 | 1.39 | 6.86 | 8.08 | 11.05 | 1.37 | 0.38 |
| T11 | 13.22 | 24.84 | 61.94 | 15.78 | 8.35 | 1.81 | 6.73 | 17.69 | 14.54 | 2.83 | 0.61 |
| T12 | 15.18 | 22.14 | 62.68 | 15.19 | 8.23 | 1.84 | 6.50 | 17.94 | 15.02 | 2.88 | 0.66 |
| T13 | 14.74 | 23.29 | 61.97 | 15.26 | 8.19 | 1.76 | 5.73 | 19.28 | 14.50 | 2.92 | 0.63 |
| T14 | 16.02 | 24.01 | 59.97 | 15.37 | 8.28 | 1.79 | 5.38 | 16.31 | 15.05 | 2.95 | 0.64 |
| T15 | 13.21 | 24.91 | 61.88 | 15.76 | 8.31 | 1.82 | 6.76 | 17.73 | 14.52 | 2.79 | 0.63 |
| T16 | 13.17 | 24.68 | 62.15 | 15.79 | 8.37 | 1.81 | 6.74 | 17.68 | 14.57 | 2.86 | 0.57 |
| T21 | 6.15 | 22.04 | 71.81 | 14.37 | 8.41 | 1.75 | 6.91 | 16.17 | 13.97 | 2.59 | 0.69 |
| T22 | 6.88 | 22.19 | 70.93 | 14.96 | 8.39 | 1.83 | 5.44 | 18.86 | 13.99 | 2.64 | 0.72 |
| T23 | 5.79 | 21.67 | 72.54 | 14.55 | 8.42 | 1.74 | 9.17 | 21.24 | 13.45 | 2.55 | 0.68 |
| T24 | 6.18 | 22.03 | 71.79 | 14.36 | 8.43 | 1.75 | 6.88 | 16.64 | 13.98 | 2.56 | 0.71 |
| T25 | 6.16 | 22.06 | 71.78 | 14.38 | 8.42 | 1.76 | 6.97 | 16.67 | 13.95 | 2.58 | 0.68 |
| T26 | 5.18 | 20.15 | 74.67 | 14.42 | 8.49 | 1.81 | 6.38 | 16.33 | 13.35 | 2.43 | 0.65 |
| 注:表中数值代表 3个重复的平均值;黏粒:< 2 μm;粉粒:2−20 μm;砂粒:> 20 μm. Note: The values represented the average of triplicates. Clay: < 2 μm; Silt: 2−20 μm; Sand: > 20 μm; DS: Dissolved salt; AM: Ammonia nitrogen; NR: Nitrate nitrogen; TOC: Total organic carbon; TN: Total nitrogen; TP: Total phosphorus. |
|||||||||||
Fast DNA® SPIN Kit for Soil,MP Biomedical公司;QIAquick PCR Purification Kit,Qiagen公司。QuantiFluor™-ST蓝色荧光定量系统,Promega公司。
1.3 沉积物/土壤DNA的提取、PCR、高通量测序和测序数据分析沉积物/土壤样品宏基因组DNA提取按照Fast DNA® SPIN Kit for Soil说明进行。用16S rRNA基因V3−V4区引物进行PCR扩增,引物为338F (5′-ACTCCTACGGGAGGCAGCA-3′)和806R (5′-GGACTACHVGGGTWTCTAAT-3′)。PCR反应体系和条件参见文献[18]。所有样品的PCR产物经纯化和定量后送往北京百迈客生物科技有限公司,Illumina MiSeq PE300测序平台进行高通量测序[18]。全部样品16S rRNA基因序列已上传至NCBI,登录号为SRR8835404−SRR8835447。34个样品共获得3 115 787条优质序列(459 bp),先运用UPARSE软件在97%相似性水平上聚类为43 830个可操作分类单元(operational taxonomic unit,OTU),再运用RDP classifier软件对上述OTU进行分类学(门、纲、目、科、属、种)注释获得791种细菌,最后运用SILVA数据库将34个样品获得的791种细菌在每个样品中分配的细菌种类及其种类对应的序列数进行物种组成的划分和相对丰度的计算[18]。从791种细菌中选择来自厌氧绳菌科(Anaerolineaceae) 6个属中的15种细菌进行研究。这15种细菌来自绿弯菌门(Chloroflexi)厌氧绳菌纲(Anaerolineae)厌氧绳菌目(Anaerolineales)厌氧绳菌科(Anaerolineaceae) (表 2)。目前能被明确注释的菌种有限,高通量测序数据庞大导致获得的大量种属甚至科目都无具体的名称,本研究基于“溯源命名法”将选定的物种重新命名:将没有明确分类学名称的物种(others,uncultured bacterium)向上追溯到属、科、目、纲甚至门水平直至有明确的分类学名称为止,并加注数字编号以便于后续分析(表 2)。
| 属Genus | 种Species | Average relative abundance±standard deviation (%) | ||||||
| R1 | R2 | R3 | W1 | W2 | T1 | T2 | ||
| 厌氧绳菌属 Anaerolinea |
Anaerolinea_o1 | 0.00±0.00 | 0.00±0.00 | 0.04±0.04 | 0.09±0.04 | 0.01±0.01 | 0.00±0.00 | 0.00±0.00 |
| Anaerolinea_u2 | 0.12±0.22 | 0.48±0.05 | 1.03±0.37 | 2.12±0.94 | 0.22±0.21 | 0.00±0.00 | 0.00±0.00 | |
| Anaerolinea_u3 | 0.01±0.01 | 0.08±0.01 | 0.07±0.03 | 0.08±0.04 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | |
| 纤绳菌属 Leptolinea |
Leptolinea_us4 | 0.01±0.01 | 0.46±0.07 | 0.14±0.07 | 0.13±0.05 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Leptolinea_u5 | 0.02±0.05 | 0.29±0.11 | 0.24±0.03 | 0.32±0.11 | 0.02±0.02 | 0.00±0.00 | 0.00±0.00 | |
| Leptolinea_o6 | 0.02±0.04 | 0.34±0.02 | 0.14±0.03 | 0.15±0.04 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | |
| 非培养菌属 Uncultured |
Uncultured_o7 | 0.18±0.10 | 0.81±0.07 | 0.63±0.10 | 0.57±0.21 | 0.39±0.31 | 0.14±0.03 | 0.14±0.02 |
| Uncultured_u8 | 0.36±0.26 | 2.39±0.16 | 1.10±0.17 | 1.22±0.31 | 0.46±0.10 | 0.17±0.02 | 0.15±0.04 | |
| 长绳菌属 Longilinea |
Longilinea_o9 | 0.01±0.02 | 0.20±0.02 | 0.06±0.04 | 0.11±0.05 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Longilinea_u10 | 0.00±0.01 | 0.02±0.00 | 0.05±0.05 | 0.18±0.06 | 0.01±0.01 | 0.00±0.00 | 0.00±0.00 | |
| Levilinea | Levilinea_o11 | 0.00±0.01 | 0.01±0.00 | 0.02±0.01 | 0.07±0.03 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Uncultured | Uncultured_uc12 | 0.00±0.00 | 0.00±0.00 | 0.03±0.03 | 0.09±0.05 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Other | Other_o13 | 0.02±0.05 | 0.06±0.01 | 0.15±0.03 | 0.13±0.07 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Uncultured | Uncultured_ua14 | 0.01±0.02 | 0.01±0.01 | 0.07±0.04 | 0.06±0.01 | 0.00±0.01 | 0.00±0.00 | 0.00±0.00 |
| Uncultured_us15 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.21±0.10 | 0.01±0.01 | 0.01±0.01 | |
| Note: u: Uncultured; o: Other; us: Uncultured_soil_bacterium; uc: Uncultured_Chloroflexi_bacterium; ua: Uncultured_anaerobic_bacterium. The number represented the serial number of each species. | ||||||||
用Excel 2010进行柱状图和线性回归分析等基本图形绘制。采用SPSS 22.0进行ANOVA单因素方差分析和Pearson相关性分析。用百迈客生物云计算平台(BMKCloud)内置R语言绘制Heatmap图。用CANOCO 5.0进行不同样品Anaerolineaceae群落与环境因子的冗余分析(redundancy analysis,RDA)和单个环境因子的变异权重解释度分析(variation partitioning analysis,VPA)。
2 结果与分析 2.1 高通量测序结果由图 1和表 2可知,来自厌氧绳菌科的15种菌最高相对丰度依次为Anaerolinea_u2(2.70%)、Uncultured_u8 (2.53%)、Uncultured_o7 (0.91%)、Leptolinea_us4(0.57%)、Leptolinea_u5(0.48%)、Leptolinea_o6(0.36%)、Uncultured_us15 (0.34%)、Longilinea_u10(0.26%)、Longilinea_o9(0.22%)、Other_o13 (0.21%)、Uncultured_uc12 (0.15%)、Uncultured_ua14 (0.13%)、Anaerolinea_o1(0.12%)、Anaerolinea_u3(0.12%)、Levilinea_o11(0.10%)。其中,Anaerolinea_u2和Uncultured_u8为最优势菌,相对丰度大于1.00%。Uncultured_o7和Leptolinea_us4为次优势菌,相对丰度介于0.50%−1.00%。有6种菌属于中等优势菌,相对丰度介于0.20%−0.50%。剩余5种菌相对罕见,相对丰度低于0.20%。

|
| 图 1 半干旱区锡林河系统沉积物/土壤水分梯度关联的水生、湿生、旱生环境厌氧绳菌群落及其不同种群的空间分布特征 Figure 1 Spatial distribution characteristics of sediment/soil Anaerolineaceae communities and their different populations under the water gradient associated with aquatic (R), hygrophie (W) to xerophytic (T) environments from Xilin River ecosystem in semiarid region 注:所有物种均来自厌氧绳菌科,物种缩写名称同表 2. Note: Abbreviations in all species from Anaerolineaceae family seen in Table 2. |
|
|
图 1显示,15种厌氧绳菌的累积相对丰度依次为0.35%−2.55% (R1)、4.93%−5.47% (R2)、3.01%−5.53% (R3)、1.90%−6.54% (W1)、0.79%− 2.37% (W2)、0.25%−0.38% (T1)、0.19%−0.38% (T2),沿着R1、R2、R3、W1、W2、T1、T2水分梯度,15种细菌总体呈现先增加后降低的分布趋势,在旱生环境的T1和T2样带分布最低,在湿生环境的W1样带分布最高。综上,Anaerolinea_o1、Anaerolinea_u2、Longilinea_u10、Levilinea_o11、Uncultured_uc12、Other_o13、Uncultured_us15最适合于湿生的W1环境,Leptolinea_us4、Leptolinea_u5、Leptolinea_o6、Uncultured_o7、Uncultured_u8、Longilinea_o9最适合于水生的R2环境,Anaerolinea_u3和Uncultured_ua14最适合于水生的R3环境。
图 1显示,厌氧绳菌科中的15种细菌组成及丰度各异。具体而言,Anaerolinea_o1、Anaerolinea_u2、Anaerolinea_u3、Leptolinea_us4、Leptolinea_u5、Leptolinea_o6主要分布在水生环境的R2、R3、W1样带,最高相对丰度依次出现在W13 (0.12%)、W11 (2.70%)、R33 (0.12%)、R24 (0.57%)、R22 (0.48%)、R24 (0.36%)。Uncultured_o7、Uncultured_u8、Longilinea_o9最高丰度分别为0.91%、2.53%、0.22%。Longilinea_u10、Levilinea_o11、Uncultured_uc12最高相对丰度均出现在W13,依次为0.26%、0.10%、0.15%。Other_o13、Uncultured_ua14、Uncultured_us15最高相对丰度依次出现在W14 (0.21%)、R33 (0.13%)、W21 (0.34%)。Leptolinea_us4、Leptolinea_o6、Uncultured_o7、Uncultured_u8、Longilinea_o9均在R24出现最高峰,Anaerolinea_o1、Longilinea_u10、Levilinea_o11、Uncultured_uc12均在W13出现最高峰,Anaerolinea_u3和Uncultured_ua14在R33出现最高峰,Anaerolinea_u2在W11出现最高峰。
2.3 半干旱区锡林河系统沉积物/土壤样品间厌氧绳菌群落不同种群的相似性Heatmap (图 2)显示,水生环境的R2、R3样带和湿生环境的W1、W2样带种群组成较为丰富且相似度较高,聚为一大类,水生环境的R1样带和旱生环境的T1、T2样带种群组成较为单一且相似度较高,聚为一大类。这说明本研究选择的厌氧绳菌群落能有效区分生境间的水分差异,是反映生境间水分差异的重要指示生物。
由Pearson相关性分析(表 3)可知,所有种群与含水量、氨氮(Uncultured_us15除外)、砂粒含量(Uncultured_us15除外)、pH等呈正相关关系,而且大多数达到显著(P < 0.05)或极显著(P < 0.01)水平,说明这15种菌可能是适应高水砂环境的游离型细菌。有9种菌与总有机碳也呈现正相关关系,说明这9种菌可能为异养菌,其中7种菌与TOC之间未达到显著(P > 0.05)相关水平,说明这些菌是自养偏异养的混合营养型细菌。所有的菌与其他的环境因子:黏粒、粉粒、可溶性盐、全氮、全磷、硝氮都呈现负相关关系(Uncultured_us15和粉粒、DS呈正相关关系除外),可见这些菌不适应黏粒和粉粒以及盐和营养相对丰富的环境。综上,厌氧绳菌中全部种群(Uncultured_us15除外)都是适应相对高水砂、高氨、相对低盐、高pH值、寡营养、自养或异养混合的游离型耐盐氨氧化细菌。
| 种群 Populations |
黏粒 Clay |
粉粒 Silt |
砂粒 Sand |
含水量 Moisture |
pH | 氨氮 AM |
总有机碳 TOC |
总氮 TN |
总磷 TP |
溶解性盐 DS |
硝氮 NR |
| Anaerolinea_o1 | −0.35 | −0.39* | 0.39* | 0.59** | 0.46** | 0.05 | 0.13 | −0.17 | −0.23 | −0.13 | −0.47** |
| Anaerolinea_u2 | −0.44** | −0.50** | 0.49** | 0.70** | 0.50** | 0.21 | 0.13 | −0.25 | −0.32 | −0.18 | −0.52** |
| Anaerolinea_u3 | −0.47** | −0.57** | 0.56** | 0.78** | 0.45** | 0.63** | 0.21 | −0.28 | −0.39* | −0.16 | −0.42* |
| Leptolinea_us4 | −0.35* | −0.43* | 0.41* | 0.50** | 0.16 | 0.85** | −0.06 | −0.31 | −0.36* | −0.07 | −0.12 |
| Leptolinea_u5 | −0.50** | −0.59** | 0.57** | 0.76** | 0.46** | 0.60** | 0.13 | −0.32 | −0.40* | −0.17 | −0.48** |
| Leptolinea_o6 | −0.43* | −0.53** | 0.51** | 0.61** | 0.25 | 0.86** | −0.01 | −0.36* | −0.42* | −0.12 | −0.23 |
| Uncultured_o7 | −0.47** | −0.51** | 0.51** | 0.76** | 0.44** | 0.73** | 0.15 | −0.33 | −0.41* | −0.13 | −0.38* |
| Uncultured_u8 | −0.47** | −0.55** | 0.54** | 0.65** | 0.26 | 0.84** | −0.05 | −0.41* | −0.46** | −0.16 | −0.27 |
| Longilinea_o9 | −0.41* | −0.49** | 0.48** | 0.55** | 0.20 | 0.74** | −0.13 | −0.37* | −0.40* | −0.12 | −0.23 |
| Longilinea_u10 | −0.38* | −0.43* | 0.42* | 0.59** | 0.45** | 0.07 | 0.04 | −0.22 | −0.26 | −0.15 | −0.47** |
| Levilinea_o11 | −0.36* | −0.42* | 0.41* | 0.52** | 0.36* | 0.03 | −0.02 | −0.23 | −0.26 | −0.17 | −0.45** |
| Uncultured_uc12 | −0.32 | −0.37* | 0.36* | 0.52** | 0.39* | 0.04 | 0.06 | −0.17 | −0.21 | −0.12 | −0.42* |
| Other_o13 | −0.47** | −0.56** | 0.55** | 0.80** | 0.50** | 0.46** | 0.362* | −0.21 | −0.35* | −0.21 | −0.49** |
| Uncultured_ua14 | −0.42* | −0.50** | 0.49** | 0.76** | 0.52** | 0.30 | 0.38* | −0.16 | −0.30 | −0.19 | −0.508** |
| Uncultured_us15 | −0.02 | 0.23 | −0.15 | 0.10 | 0.21 | −0.14 | −0.04 | −0.08 | −0.01 | 0.08 | −0.06 |
| 注:*:在0.05水平(双侧)上显著相关;**:在0.01水平(双侧)上显著相关. Note: *: Correlation was significant at the 0.05 level (2-tailed); **: Correlation was significant at the 0.01 level (2-tailed). |
|||||||||||
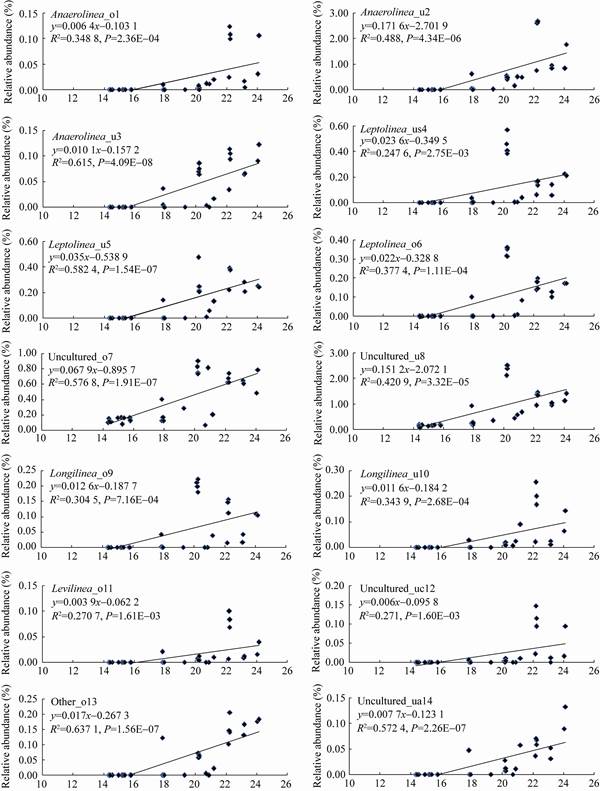
RDA (图 3A)结果显示,第1排序轴与第2排序轴的解释度分别为81.12%和4.50%。厌氧绳菌中有14个种群分布在水砂、氨、pH值较高的水生沉积物R3和邻近水源的低河滨湿地W1,而Uncultured_us15主要分布在远离水源的高河滨湿地W2。总体上,厌氧绳菌中15种菌都适应于水分含量较高的水生和湿生环境。简言之,RDA分析结果与Pearson相关性分析结果具有一致性。通过变异权重分析(图 3B),11个已知环境因子变异的总解释度高达91.0%,其中水分、AM和DS的解释度分别为64.8%、8.9%、7.5%,三者的蒙特尔检验P < 0.01。仅仅水分因子的解释度接近65.0%,说明水分是解释厌氧绳菌群落空间异质性的核心环境驱动力。通过线性回归分析(图 4)进一步证实,水分是驱动厌氧绳菌群落空间异质性的核心环境因子。
|
| 图 3 半干旱区锡林河系统沉积物/土壤厌氧绳菌群落不同种群与环境因子的冗余分析(A)及环境因子的变异权重分析(B) Figure 3 Redundancy analysis (RDA) of the different populations from sediment/soil Anaerolineaceae communities and environmental factors (A) as well as variation partitioning analysis of environmental drivers (B) from Xilin River ecosystem in semiarid region Note: Sp1: Anaerolinea_o1; Sp2: Anaerolinea_u2; Sp3: Anaerolinea_u3; Sp4: Leptolinea_us4; Sp5: Leptolinea_u5; Sp6: Leptolinea_o6; Sp7: Uncultured_o7; Sp8: Uncultured_u8; Sp9: Longilinea_o9; Sp10: Longilinea_u10; Sp11: Levilinea _o11; Sp12: Uncultured_uc12; Sp13: Other_o13; Sp14: Uncultured_ua14; Sp15: Uncultured_us15. Abbreviations in all species from Anaerolineaceae family seen in Table 2. |
|
|
|
| 图 4 半干旱区锡林河系统基于线性回归分析厌氧菌科不同种群与沉积物/土壤水分的关系 Figure 4 The relationship between different populations from Anaerolineaceae family and sediment/soil moisture based on linear regression analysis from Xilin River ecosystem in semiarid region |
|
|
Cleary等证明生境是影响厌氧绳菌等细菌群落空间变异的重要因素[7, 14],这为本研究水分梯度下厌氧绳菌种群的空间异质性和生境密切相关的结果提供了科学证据。Li等发现沙漠演变绿洲过程中厌氧绳菌转变为优势菌的研究结果[15]与本研究发现的厌氧绳菌全部种群主要分布在水生R和湿生W环境而非旱生T环境的结果具有一致性。丁苏丽等证明厌氧绳菌等是红树林表层沉积物中的优势菌[19]。Huang等证明湖泊湿地沉积物中含有丰富的厌氧绳菌[20]。Williams等揭示塞文河口沉积物具有丰富的多样性,以厌氧菌为主[21]。Sutcliffe等在富铀沉积物中发现了厌氧绳菌纲新成员[22]。薛银刚等发现厌氧绳菌纲是区分太湖不同湖区沉积物细菌群落时空异质性的关键类群[23]。靳亮等发现不可培养的厌氧绳菌目等是区分尕海湖水陆过渡带湿地细菌群落空间异质性的重要类群[24]。李本行等发现厌氧绳菌纲是人工湿地生态沟渠中的优势类群(10%),而且对湿地垂直流水分的波动响应敏感[25]。Liu等发现厌氧绳菌还是城市污泥系统的优势细菌[26]。上述文献报道[1-15, 19-28]均为本文关于厌氧绳菌不同种群主要分布在水生R和湿生W环境而非旱生T环境的研究结果提供了重要的科学证据,说明厌氧绳菌不同种群主要分布于水生和湿生环境中的结果是正确的。综上,厌氧绳菌群落是水生系统沉积物和湿地土壤中的优势细菌[1-15, 19-28],不适合生存于旱生草原土壤中[15]。
3.2 半干旱区锡林河表层沉积物/土壤厌氧绳菌群落空间异质性的环境驱动力本研究基于厌氧绳菌群落与环境因子的相关性(表 3)、RDA (图 3A)、VPA (图 3B)和线性回归(图 4)分析均证明水分是影响厌氧绳菌群落空间异质性的关键因子,这和Li等沙漠演变绿洲过程中水分增加决定厌氧绳菌转变为优势菌的研究结果[15]具有一致性。尽管没有水分驱动厌氧绳菌群落空间分布变化的研究报道,但几乎全部的文献[1-15, 19-28]都证明厌氧绳菌群落关联各级分类学水平的绿弯菌门(Chloroflexi)厌氧绳菌纲(Anaerolineae)厌氧绳菌目(Anaerolineales)厌氧绳菌科(Anaerolineaceae)厌氧绳菌属(Anaerolinea)分布在水生系统沉积物和湿地土壤中,这为本文厌氧绳菌群落种水平下的全部种群主要分布在水生系统沉积物和湿地土壤的研究结果提供了间接的科学证据,说明水分是驱动水生、湿生和旱生环境厌氧绳菌群落空间异质性的决定性因素。换句话说,厌氧绳菌群落是干涸化湿地复湿过程中出现的标志性生物(图 5)。

|
| 图 5 基于网络拓扑分析半干旱区锡林河系统厌氧绳菌不同种群与关键环境因子的关联性 Figure 5 Association of different Anaerolineaceae populations with crucial environmental factors using network topology analysis from Xilin River system in semiarid region 注:橙色、紫色、蓝色分别代表水分、氨氮、盐度3个环境因子;环境因子关联的图注越大和箭头越粗表示环境因子的影响力越大;箭头朝向菌和背离菌分别表示菌和环境因子呈现正相关、负相关关系;箭头越长表示相关系数越大(表 3).菌的全称见图 3. Note: The orange, purple and blue circles respectively represented moisture, ammonia nitrogen (AM) and dissolved salt (DS); The larger the circles or the coarser the arrows corresponding to environmental factors represented the greater their influences; The arrow oriented and deviated Anaerolineaceae populations represented the positive and negative correlation between Anaerolineaceae populations and environmental factors, respectively; The longer the arrow, the greater the correlation coefficient (Table 3). The abbreviations of Anaerolineaceae populations were shown in Figure 3. |
|
|
本研究发现厌氧绳菌种群主要分布在氨氮浓度较高的水生沉积物R2和R3中,说明氨氮对厌氧绳菌不同种群空间分布变化有重要影响,这与Zhu等发现NH4+浓度是驱动厌氧绳菌等细菌群落空间分布变化的主要环境因子[29]的结果相一致。同时,Bhattacharjee等发现厌氧绳菌是厌氧氨氧化生物反应器中重要的微生物类群[30],Chen等证实厌氧绳菌在高浓度氨水的硝化序批式反应器中丰度高达7.02%[31],Wang等证实厌氧绳菌是序批式生物膜反应器厌氧生物膜的优势微生物群落,具有氨氧化功能[32]。Cai等证实溶氧和氨氮是影响厌氧绳菌等细菌群落的主要因素[33]。综上,厌氧绳菌群落可能是氨氧化微生物,厌氧绳菌群落的空间变异可能与底物浓度密切相关(图 5)。
本研究发现盐度是影响厌氧绳菌群落空间分布变化的重要环境因子,可能和蒙古高原半干旱区沉积物及湿地土壤盐度相对较高有关系,有研究[11, 34-35]证实盐度会影响到厌氧绳菌等微生物群落结构丰度及空间分布变化,这为本研究中盐度对厌氧绳菌不同种群空间分布变化有重要影响的结果提供了科学依据。可见这些菌不适应于黏粒和粉粒以及盐度和营养相对丰富的环境。本研究野外调查表明,半干旱区内陆河系统受蒸发量大于降水量的影响,沉积物/土壤全剖面盐基饱和,表层积盐导致湿生W和旱生T土壤出现盐结皮。在盐度整体偏高的大背景下,绝大多数厌氧绳菌种群(Uncultured_us15除外)适应于盐度相对较低的环境,可能为耐盐菌(图 5)[11, 34-35]。
Wang等发现厌氧绳菌与有机碳的降解有关[12],Zhu等证实异养型的厌氧绳菌参与有机化合物的降解[29],这和本研究厌氧绳菌中部分种群属于异养菌的结果相一致。Fullerton等发现绿弯菌门多数厌氧绳菌科可能是异养菌,也可能是自养菌[36]。这进一步为本研究发现的厌氧绳菌群落中9个种群和6个种群分别与有机碳呈现正负相关的结果提供了科学依据。简言之,厌氧绳菌可能是自养菌,也可能是异养菌。早在1959年,Pavlovic发现哺乳动物肠道中存在游离型的厌氧绳菌,其隶属于不可培养的绿弯菌门[37]。但是,哺乳动物肠道中不可培养的厌氧绳菌种群和本研究中不可培养(Uncultured_o7、Uncultured_u8、Uncultured_uc12、Uncultured_ua14、Uncultured_us15)厌氧绳菌的结果具有一致性。推测原因是:(1)不管是哺乳动物肠道[37]还是本研究水生R样带都为不可培养的厌氧绳菌种群提供了相对厌氧的环境[37-39];(2)本研究所选蒙古高原半干旱区锡林河湿地和典型草原为我国的主要放牧区,表层沉积物/土壤中富集的牲畜粪便可能和肠道环境[37]具有游离型厌氧绳菌种群所需有机营养及环境条件。本研究发现灯芯草(Juncus effusus)湿地土壤(W1)中厌氧绳菌的组成和丰度明显高于鹅绒委陵菜(Potentilla anserina)湿地土壤(W2),说明地上植被组成变化可能会影响地下微生物菌群组成和空间分布变化[40-41]。
此外,R1样带6个样品均为砂质沉积物,但R14的pH、盐度、硝态氮等明显低于其他5样品(表 1)。野外调查发现位于锡林河大桥下方的R14是铁锰结核富集区,这可能是R1样带中R14厌氧绳菌的组成和丰度明显于其他5样品的原因[42]。R3样带中R33样品明显高于其他样品,可能与牛轭湖发育时间长短及发育程度有关。W1样带中W12明显低于其他样品以及W2中W21明显高于其他样品,这可能与河漫滩样带本身由水分巨大波动引起的氧化还原条件频繁交替有关[43-44]。
4 结论蒙古高原半干旱区厌氧绳菌群落沿着水分梯度呈现明显的空间异质性,全部种群优先分布在高水砂、高氨、高pH值、相对低盐的水生系统沉积物和湿地土壤中,不适合生存在全氮、硝氮、全磷、粉粒和黏粒相对较高的旱生草原土壤中。厌氧绳菌群落是干旱胁迫下退化湿地复湿过程中出现的标志性生物,厌氧绳菌群落的空间异质性主要由水分正向驱动。厌氧绳菌群落可能为异养或自养的游离型氨氧化细菌。后续我们将检测沉积物/土壤氧化还原电位(Eh)来验证氧化还原电位与水分及氨氮的关联性,探究沉积物/土壤氧化还原电位对水分和氨氮的响应,明确是水分本身还是氧化还原电位作为核心驱动力影响厌氧绳菌群落的空间异质性。
| [1] |
Nutman AP, Bennett VC, Friend CRL, et al. Rapid emergence of life shown by discovery of 3, 700-million-year-old microbial structures[J]. Nature, 2016, 537(7621): 535-538. DOI:10.1038/nature19355 |
| [2] |
Zhu GB, Wang SY, Wang WD, et al. Hotspots of anaerobic ammonium oxidation at land–freshwater interfaces[J]. Nature Geoscience, 2013, 6(2): 103-107. DOI:10.1038/ngeo1683 |
| [3] |
Wang Y, Sheng HF, He Y, et al. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of Illumina tags[J]. Applied and Environmental Microbiology, 2012, 78(23): 8264-8271. DOI:10.1128/AEM.01821-12 |
| [4] |
Bar-On YM, Phillips R, Milo R. The biomass distribution on Earth[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(25): 6506-6511. DOI:10.1073/pnas.1711842115 |
| [5] |
Abia ALK, Alisoltani A, Keshri J, et al. Metagenomic analysis of the bacterial communities and their functional profiles in water and sediments of the Apies River, South Africa, as a function of land use[J]. Science of the Total Environment, 2018, 616-617: 326-334. DOI:10.1016/j.scitotenv.2017.10.322 |
| [6] |
Hou QH, Fang Z, Zhu QM, et al. Microbial diversity in Huguangyan Maar Lake of China revealed by high-throughput sequencing[J]. Journal of Oceanology and Limnology, 2019, 37(4): 1245-1257. DOI:10.1007/s00343-019-8016-1 |
| [7] |
Cleary DFR, Polónia ARM, Becking LE, et al. Compositional analysis of bacterial communities in seawater, sediment, and sponges in the Misool coral reef system, Indonesia[J]. Marine Biodiversity, 2018, 48(4): 1889-1901. DOI:10.1007/s12526-017-0697-0 |
| [8] |
Schippers A, Kock D, H ft C, et al. Quantification of microbial communities in subsurface marine sediments of the Black Sea and off Namibia[J]. Frontiers in Microbiology, 2012, 3: 16. |
| [9] |
Gomez-Saez GV, Ristova PP, Sievert SM, et al. Relative importance of chemoautotrophy for primary production in a light exposed marine shallow hydrothermal system[J]. Frontiers in Microbiology, 2017, 8: 702. DOI:10.3389/fmicb.2017.00702 |
| [10] |
Nunoura T, Hirai M, Miyazaki M, et al. Isolation and characterization of a thermophilic, obligately anaerobic and heterotrophic marine Chloroflexi bacterium from a Chloroflexi-dominated microbial community associated with a Japanese shallow hydrothermal system, and proposal for Thermomarinilinea lacunofontalis gen. nov., sp. nov[J]. Microbes and Environments, 2013, 28(2): 228-235. DOI:10.1264/jsme2.ME12193 |
| [11] |
Yang JS, Zhan C, Li YZ, et al. Effect of salinity on soil respiration in relation to dissolved organic carbon and microbial characteristics of a wetland in the Liaohe River estuary, Northeast China[J]. Science of the Total Environment, 2018, 642: 946-953. DOI:10.1016/j.scitotenv.2018.06.121 |
| [12] |
Wang C, Liu SY, Zhang Y, et al. Bacterial communities and their predicted functions explain the sediment nitrogen changes along with submerged macrophyte restoration[J]. Microbial Ecology, 2018, 76(3): 625-636. DOI:10.1007/s00248-018-1166-4 |
| [13] |
Breuker A, K weker G, Blazejak A, et al. The deep biosphere in terrestrial sediments in the Chesapeake Bay Area, Virginia, USA[J]. Frontiers in Microbiology, 2011, 2: 156. |
| [14] |
Cleary DFR, Coelho FJRC, Oliveira V, et al. Sediment depth and habitat as predictors of the diversity and composition of sediment bacterial communities in an inter-tidal estuarine environment[J]. Marine Ecology, 2017, 38(2): e12411. DOI:10.1111/maec.12411 |
| [15] |
Li CH, Tang LS, Jia ZJ, et al. Profile changes in the soil microbial community when desert becomes oasis[J]. PLoS One, 2015, 10(10): e0139626. DOI:10.1371/journal.pone.0139626 |
| [16] |
Rocarpin P, Gachet S, Metzner K, et al. Moisture and soil parameters drive plant community assembly in Mediterranean temporary pools[J]. Hydrobiologia, 2016, 781(1): 55-66. DOI:10.1007/s10750-015-2604-7 |
| [17] |
Borchhardt N, Baum C, Mikhailyuk T, et al. Biological soil crusts of arctic svalbard—water availability as potential controlling factor for microalgal biodiversity[J]. Frontiers in Microbiology, 2017, 8: 1485. DOI:10.3389/fmicb.2017.01485 |
| [18] |
Yu JL, Xia JJ, Li CH, et al. Niche differentiation of Nitrospira and associated environmental driving forces in Xilin river basin[J]. Microbiology China, 2020, 47(5): 1418-1429. (in Chinese) 于景丽, 夏晶晶, 李传虹, 等. 锡林河流域Nitrospira的生态位分化及环境驱动力[J]. 微生物学通报, 2020, 47(5): 1418-1429. |
| [19] |
Ding SL, Zhang QJ, Dong J, et al. Microbial community structure and its relationship to heavy metals in Shenzhen and Hong Kong mangrove sediments[J]. Chinese Journal of Ecology, 2018, 37(10): 3018-3030. (in Chinese) 丁苏丽, 张祁炅, 董俊, 等. 深港红树林沉积物微生物群落多样性及其与重金属的关系[J]. 生态学杂志, 2018, 37(10): 3018-3030. |
| [20] |
Huang W, Chen X, Wang K, et al. Comparison among the microbial communities in the lake, lake wetland, and estuary sediments of a plain river network[J]. Microbiology Open, 2019, 8(2): e00644. DOI:10.1002/mbo3.644 |
| [21] |
Williams AS. The dynamic interaction between microbial biodiversity, biogeochemical activity and sedimentary geomorphology in the Severn Estuary[D]. Cardiff: Doctoral Dissertation of Cardiff University, 2015
|
| [22] |
Sutcliffe B, Chariton AA, Harford AJ, et al. Insights from the genomes of microbes thriving in uranium-enriched sediments[J]. Microbial Ecology, 2018, 75(4): 970-984. DOI:10.1007/s00248-017-1102-z |
| [23] |
Xue YG, Liu F, Jiang XD, et al. The diversity of bacterial communities in the sediment of different lake zones of Lake Taihu in winter[J]. China Environmental Science, 2018, 38(2): 719-728. (in Chinese) 薛银刚, 刘菲, 江晓栋, 等. 太湖不同湖区冬季沉积物细菌群落多样性[J]. 中国环境科学, 2018, 38(2): 719-728. |
| [24] |
Jin L, Gao XM, Du JH, et al. Peat bacterial diversity and community structure in Gahai Lake wetland in Gan'nan[J]. Microbiology China, 2016, 43(11): 2396-2404. (in Chinese) 靳亮, 高学梅, 杜建华, 等. 尕海湖湿地泥炭细菌多样性分析[J]. 微生物学通报, 2016, 43(11): 2396-2404. |
| [25] |
Li BH, Li ZH, Qu D, et al. Ecological ditches and consteructed wetland system processing thiamethoxam on farmland drainage[J]. Chinese Journal of Environmental Engineering, 2017, 11(12): 6532-6539. (in Chinese) 李本行, 李增辉, 曲丹, 等. "生态沟渠+人工湿地"系统处理农田退水中噻虫嗪[J]. 环境工程学报, 2017, 11(12): 6532-6539. |
| [26] |
Liu YC, Dong Q, Shi HC. Distribution and population structure characteristics of microorganisms in urban sewage system[J]. Applied Microbiology and Biotechnology, 2015, 99(18): 7723-7734. DOI:10.1007/s00253-015-6661-7 |
| [27] |
Kim Y, Liesack W. Differential assemblage of functional units in paddy soil microbiomes[J]. PLoS One, 2015, 10(4): e0122221. DOI:10.1371/journal.pone.0122221 |
| [28] |
Yan YW, Jiang QY, Wang JG, et al. Microbial communities and diversities in mudflat sediments analyzed using a modified metatranscriptomic method[J]. Frontiers in Microbiology, 2018, 9: 93. DOI:10.3389/fmicb.2018.00093 |
| [29] |
Zhu P, Wang YP, Shi TT, et al. Intertidal zonation affects diversity and functional potentials of bacteria in surface sediments: a case study of the Golden Bay mangrove, China[J]. Applied Soil Ecology, 2018, 130: 159-168. DOI:10.1016/j.apsoil.2018.06.003 |
| [30] |
Bhattacharjee AS, Wu S, Lawson CE, et al. Whole-community metagenomics in two different anammox configurations: process performance and community structure[J]. Environmental Science & Technology, 2017, 51(8): 4317-4327. |
| [31] |
Chen WJ, Dai XH, Cao DW, et al. Performance and microbial ecology of a nitritation sequencing batch reactor treating high-strength ammonia wastewater[J]. Scientific Reports, 2016, 6(1): 35693. DOI:10.1038/srep35693 |
| [32] |
Wang JJ, Rong HW, Zhang CS. Evaluation of the impact of dissolved oxygen concentration on biofilm microbial community in sequencing batch biofilm reactor[J]. Journal of Bioscience and Bioengineering, 2018, 125(5): 532-542. DOI:10.1016/j.jbiosc.2017.11.007 |
| [33] |
Cai W, Li Y, Niu LH, et al. New insights into the spatial variability of biofilm communities and potentially negative bacterial groups in hydraulic concrete structures[J]. Water Research, 2017, 123: 495-504. DOI:10.1016/j.watres.2017.06.055 |
| [34] |
Schneider D, Arp G, Reimer A, et al. Phylogenetic analysis of a microbialite-forming microbial mat from a hypersaline lake of the Kiritimati Atoll, Central Pacific[J]. PLoS One, 2013, 8(6): e66662. DOI:10.1371/journal.pone.0066662 |
| [35] |
Hu AY, Yu CP, Hou LY. Spatial and temporal dynamics of microbial communities in a human-perturbed estuary of China[A]//AGU Fall Meeting Abstracts[C]. San Francisco: AGU, 2015
|
| [36] |
Fullerton H, Moyer CL. Comparative single-cell genomics of Chloroflexi from the Okinawa Trough deep-subsurface biosphere[J]. Applied and Environmental Microbiology, 2016, 82(10): 3000-3008. DOI:10.1128/AEM.00624-16 |
| [37] |
Pavlovic S. Diabetes mellitus in pregnancy. (Report of a case with repeated intrauterine fetal death)[J]. Srpski Arhiv Za Celokupno Lekarstvo, 1959, 87: 687-689. |
| [38] |
Ward LM, Hemp J, Pace LA, et al. Draft genome sequence of Leptolinea tardivitalis YMTK-2, a mesophilic anaerobe from the Chloroflexi class Anaerolineae[J]. Genome Announcement, 2015, 3(6): e01356-15. |
| [39] |
Xia Y, Wang YB, Wang Y, et al. Cellular adhesiveness and cellulolytic capacity in Anaerolineae revealed by omics-based genome interpretation[J]. Biotechnology for Biofuels, 2016, 9(1): 111. |
| [40] |
Cleary DFR, Polónia ARM, Sousa AI, et al. Temporal dynamics of sediment bacterial communities in monospecific stands of Juncus maritimus and Spartina maritima[J]. Plant Biology, 2016, 18(5): 824-834. DOI:10.1111/plb.12459 |
| [41] |
Rodríguez-Caballero G, Caravaca F, Fernández-González AJ, et al. Arbuscular mycorrhizal fungi inoculation mediated changes in rhizosphere bacterial community structure while promoting revegetation in a semiarid ecosystem[J]. Science of the Total Environment, 2017, 584-585: 838-848. DOI:10.1016/j.scitotenv.2017.01.128 |
| [42] |
Ma SC, Tong M, Yuan SH, et al. Responses of the microbial community structure in Fe(II)-bearing sediments to oxygenation: the role of reactive oxygen species[J]. ACS Earth and Space Chemistry, 2019, 3(5): 738-747. DOI:10.1021/acsearthspacechem.8b00189 |
| [43] |
Meng DL, Li J, Liu TB, et al. Effects of redox potential on soil cadmium solubility: insight into microbial community[J]. Journal of Environmental Sciences, 2019, 75: 224-232. DOI:10.1016/j.jes.2018.03.032 |
| [44] |
Liang B, Wang LY, Zhou ZC, et al. High frequency of Thermodesulfovibrio spp. and Anaerolineaceae in association with Methanoculleus spp. in a long-term incubation of n-alkanes-degrading methanogenic enrichment culture[J]. Frontiers in Microbiology, 2016, 7: 1431. |
 2020, Vol. 47
2020, Vol. 47