扩展功能
文章信息
- 李娜, 张蕊, 黄遵锡, 周峻沛
- LI Na, ZHANG Rui, HUANG Zun-Xi, ZHOU Jun-Pei
- β-木糖苷酶的生物活性物质转化功能研究进展
- Research progress in bioactive substances transformation by β-xylosidases
- 微生物学通报, 2020, 47(7): 2290-2299
- Microbiology China, 2020, 47(7): 2290-2299
- DOI: 10.13344/j.microbiol.china.200201
-
文章历史
- 收稿日期: 2020-03-08
- 网络首发日期: 2020-06-04
2. 生物能源持续开发利用教育部工程研究中心 云南 昆明 650500;
3. 云南省生物质能与环境生物技术重点实验室 云南 昆明 650500
2. Engineering Research Center of Sustainable Development and Utilization of Biomass Energy, Ministry of Education, Kunming, Yunnan 650500, China;
3. Key Laboratory of Yunnan for Biomass Energy and Biotechnology of Environment, Kunming, Yunnan 650500, China
β-木糖苷酶属于糖苷水解酶(glycoside hydrolase,GH),是主要的木聚糖降解酶之一,可应用到食品、饲料、造纸和能源等领域。除了降解低聚木糖和对硝基苯基-β-D-木糖苷(4-nitrophenyl-β-D-xylopyranoside,pNPX)外,很多β-木糖苷酶还能降解对硝基苯基-α-L-阿拉伯吡喃糖苷(4-nitrophenyl-α-L-arabinopyranoside,pNPA)、对硝基苯基-α-L-阿拉伯呋喃糖苷(4-nitrophenyl-α-L-arabinofuranoside,pNPAf),以及对硝基苯基-β-D-葡萄糖苷(4-nitrophenyl- β-D-glucopyranoside,pNPG)。目前,已报道的β-木糖苷酶有100余个,其来源于细菌、真菌、放线菌、宏基因组及高等植物,分布在糖苷水解酶第1、3、5、28、30、39、43、51、52、54、116和120家族中。近些年,研究人员克隆和表达了越来越多的木糖苷酶基因,从中发现了一些具有生物活性物质转化功能的β-木糖苷酶。
β-木糖苷酶的生物活性物质转化功能包括两方面:(1)一些β-木糖苷酶具有转糖基功能,可将木糖基转移到醇类、糖类和其他受体的基团上,分别形成烷基木糖、木寡糖或异质寡糖和其他木糖糖基化的物质,这些木糖糖基化的产物具有表面活性剂、抗微生物、抗氧化和神经保护等作用,在食品、医药和化妆品等领域具有潜在的应用价值[1-3];(2)一些β-木糖苷酶具有特殊的水解功能,可以切除三七皂苷R1和R2及ASI、XDT、花青素上的木糖基团,分别形成人参皂苷Rg1和Rh1、10-去乙酰紫杉醇(10-deacetylpaclitaxel,DT)、环黄芪醇(cycloastragenol,CA)和花色素,这些去除了木糖基的产物具有重要的生物活性,比如抗癌、抗炎、抗氧化和提高记忆等[4-5]。与化学和物理合成方法相比,这种酶催化的方法在生物活性物质的转化上具有高效性、立体选择性和环保性等优势[6]。
1 β-木糖苷酶的转糖基功能如表 1所示,在GH3、GH5、GH39、GH43、GH52和GH54家族中发现了具有转糖基活性的β-木糖苷酶,且GH3和GH39家族中具有转糖基活性的β-木糖苷酶较多。这些β-木糖苷酶皆来源于微生物,且主要来源于细菌。
| 家族 Family |
来源 Source |
名称 Enzyme name |
供体 Donor |
受体 Receptor |
产物 Product |
转化率 Conversion rate (receptor: rate) |
| GH3 (Fungus) | Humicola insolens Y1[7] | Xyl3A | pNPX | pNPX | pNPX2; X2 | NR |
| Neurospora crassa ST A[8] | NR | X2/X3 | X2/X3 | X3/X4 | NR | |
| Talaromyces amestolkiae[1-2] | rBxTW1 | XOS/pNPX | XOS/pNPX/Phenols/ Alcohols/Sugars |
XOS, xylosylated phenols, new oligosaccharides, etc. | Sugars: < 70%, Alcohols: > 70%, Phenols: < 30% | |
| GH3 | Cellulomonas bogoriensis[9] | CbXyl3A | X2 | X2 | XOS | NR |
| GH5 | Phanerochaete chrysosporium[10] | rPcXyl5 | pNPX | Alcohols | Alkyl xylosides | NR |
| GH39 (Bacteria) | Bacillus halodurans C-125[11] | XylBH39 | pNPX/XOS | Alcohols | Alkyl xylosides | |
| Sphingomonas sp.[12-13] | JB13GH39 | pNPX | Alcohols/Sugars | Alkyl xylosides, new oligosaccharides | NR | |
| Thermoanaerobacterium saccharolyticum B6A-RI[14] | NR | pNPX/X2 | pNPX/X/X2 | XOS | 30% | |
| GH43 | Thermobifida fusca TM51[15-16] | TfBXyl43 | X2/pNPX | pAP-1-S-Xyl | pAP-1-S-X2 | < 3.2% |
| GH52 (Bacteria) | Aeromonas caviae ME-1[17] | XysB | XOS | XOS | XOS | NR |
| Geobacillus thermodenitrificans[18] | XsidB | pNPX | Alcohols | Alkyl xylosides | NR | |
| GH54 | Trichoderma koningii G-39[19] | Abf | pNPX | Alcohols | Alkyl xylosides | 30% |
| NR | T. saccharolyticum JW/SL-YS485[20] | XylC | pNPX | Alcohols | Alkyl xylosides | NR |
| 注:NR:未报道;X3:木三糖;X4:木四糖;pAP-1-S-Xyl:对氨基硫苯基-β-D-吡喃木糖苷;XOS:木寡糖. Note: NR: Not reported; X3: Xylotriose; X4: Xylotetraose; pAP-1-S-Xyl: p-aminothiophenyl-β-D-xylopyranoside; XOS: Xylooligosaccharide. |
||||||
糖苷水解酶催化的反应实质是2个关键氨基酸参与的酸碱催化反应。具有转糖基活性的糖苷水解酶通常采用保留型催化机制,与反转型催化机制相比,保留型催化机制的水解过程具有以下几个特点:(1)相距较近的质子供体(酸性氨基酸)和亲核试剂(碱性氨基酸)导致底物和水分子不能同时进入酶的活性中心;(2)质子供体首先攻击底物糖苷键中的氧原子,促使糖苷键断裂,而后亲核试剂攻击糖基异头碳形成一个底物-酶中间体,接着质子供体协助水分子进攻糖基碳正离子完成双取代反应,在转糖基过程中,糖苷配基与水分子竞争结合到底物-酶中间体上,并与中间体进一步作用形成糖基化物质,因此,转糖基和水解反应是两个相互可逆的过程[21-22]。反转型催化机制不能形成过渡态中间体,但是在GH43家族(反转型催化机制)中也发现了具有转糖基活性的β-木糖苷酶[15-16],其机制有待深入研究。
简单地说,β-木糖苷酶催化的转糖基过程是供体和受体结合的过程。供体有pNPX、木二糖(X2)和木寡糖(xylooligosaccharide,XOS),这些供体作为底物可被β-木糖苷酶水解生成木糖基团。受体,即取代水分子与底物-酶中间体结合的糖苷配基,可以是不同链长的醇类、糖类和酚类等,β-木糖苷酶对不同受体的转糖基有不同的特点。
1.1 醇类受体在β-木糖苷酶转糖基作用中,醇类是常用的受体。如图 1所示,供体的木糖基团和受体醇类(甲醇、乙醇和丙醇等)通过β-木糖苷酶的催化作用结合形成烷基糖苷,烷基糖苷具有表面活性剂和抗微生物的作用[3]。值得注意的是,大多数β-木糖苷酶对醇类受体的转糖基效率随着受体碳链的增加而减小,这与酶活性位点上的疏水性氨基酸有关,也可能是因为小分子的醇更容易进入到酶的催化部位[2, 11, 18]。另外,Muzard等[11, 23]推测短碳链醇类的羟基与酶活性位点之间的互斥作用较弱,而长碳链醇类由于疏水作用和空间位阻不容易接近酶活性位点。Ochs等[24]研究了来源于B. halodurans的β-木糖苷酶BhXyl39结合位点中的空间位阻和极性对醇类受体的转糖基效率的影响,发现284位的酪氨酸(Y284)可以结合供体并将其传达至受体,而F116和F167涉及到醇类受体的传递。Nieto-Domínguez等[2]发现BxTW1对正丙醇和正丁醇受体的转糖基效率分别高于异丙醇和异丁醇,因此推测醇类受体中的伯醇基更容易发生转糖基反应。
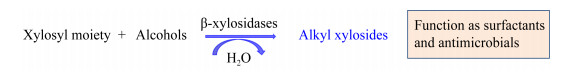
|
| 图 1 β-木糖苷酶以醇类为受体的转糖基作用 Figure 1 The transxylosylation of β-xylosidases with alcohols as receptors |
|
|
在β-木糖苷酶的转糖基过程中,常见的糖类受体有葡萄糖、半乳糖、甘露糖、木糖、木二糖和木三糖。在β-木糖苷酶的催化作用下,这些糖类受体分别与木糖基结合形成新的寡糖,如葡萄糖-木糖(图 2),这些新的寡糖可能具有益生元的作用。Nieto-Domínguez等[2]发现BxTW1对受体葡萄糖、甘露糖和半乳糖的转糖基效率依次降低,且转糖基反应均发生在这些醛糖受体的伯醇羟基上,原因可能是:D-葡萄糖C6位的伯醇羟基与其他几个羟基不在一个中心轴上,D-甘露糖的伯醇羟基与C2位的羟基在同一中心轴位置,D-半乳糖的伯醇羟基与C4位的羟基在同一中心轴位置且相距较近,即当伯醇羟基与其他羟基在同一中心轴位置或者相距较近时会降低转糖基效率。Nieto-Domínguez等[2]还发现木二糖和木三糖可以同时作为供体和受体,BxTW1对这两个受体的转糖基效率似乎和木寡糖的降解效率成互补关系;同时还发现木二糖和木三糖的浓度在0−80 mmol/L之间时,转糖基效率随着底物浓度升高而升高,浓度在20−80 mmol/L之间时,相同浓度木二糖的转糖基效率高于木三糖。
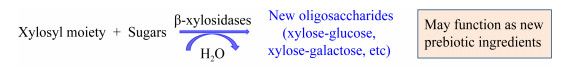
|
| 图 2 β-木糖苷酶以糖类为受体的转糖基作用 Figure 2 The transxylosylation of β-xylosidases with sugars as receptors |
|
|
羟基酪醇(hydroxytyrosol,HT)是具有抗炎和神经保护作用的酚类物质,还可以预防心血管疾病、癌症和其他慢性疾病[25-29]。天然HT的生物利用度弱、易被分解,糖基化后的HT具有更强的生物利用度、稳定性、抗氧化性、溶解度和生物安全性(图 3)[1]。其他具有抗氧化作用的酚类有对苯二酚(hydroquinone,HQ)和邻苯二酚等,其中HQ能够抑制酪氨酸酶,使皮肤产生美白的效果,但其对人体具有伤害作用[1, 30-31]。HQ被木糖糖基化后,对人体的伤害作用降低,但仍具有抑制酪氨酸酶的作用[30-31]。邻苯二酚是很多药物,如治疗支气管哮喘、高血压、帕金森和心肌梗死等疾病的药物的母核结构,木糖糖基化的邻苯二酚具有更高的生物活性[32-34]。Nieto-Domínguez等[1]研究发现β-木糖苷酶BxTW1对HT和HQ受体具有转糖基作用,但是对邻苯二酚受体却无转糖基作用;他们同时发现当反应体系中供体木二糖和受体HT的浓度分别为350 mmol/L和300 mmol/L时,转化率达到最高(26.6%),而HT的浓度低于或高于300 mmol/L时转化率均降低;在相同的反应时间内,当反应体系中木二糖和HQ的浓度分别为160 mmol/L和110 mmol/L时转化率达到最高(4.7%),低于或高于上述浓度时转化率均降低。此外,BxTW1对HT的转糖基效率明显高于HQ,通过NMR和MS对产物进行分析后,进一步发现BxTW1对受体伯醇基的糖基化具有偏好性[1]。
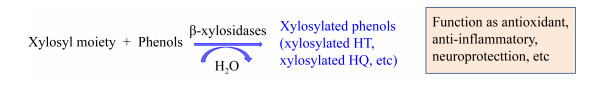
|
| 图 3 β-木糖苷酶以酚类为受体的转糖基作用 Figure 3 The transxylosylation of β-xylosidases with phenols as receptors |
|
|
Jain等[18]研究了β-木糖苷酶XsidB利用醇类和糖类作为受体的转糖基过程中供体(pNPX)浓度对转化率的影响:当pNPX浓度达到50 mmol/L时才能检测到转糖基产物,浓度为70 mmol/L时转化率最高。
综上所述,转糖基过程是供体和受体同时存在的复杂的双取代反应,其复杂性主要体现在:(1)该反应过程是可逆的;(2)反应体系中供体、受体及产物的浓度等因素影响反应进行的方向,从而影响转糖基效率;(3)不同的酶有不同的水解和转糖基活性。目前,尚无文献报道如何改造木糖苷酶以提高其转糖基效率。从保留型催化机制的角度分析,可以用其他反应介质替代水溶液来减少甚至除去反应体系中的水分子,这有利于糖基配体与中间体结合,从而提高转糖基效率;从受体选择的角度分析,β-木糖苷酶对醇类、糖类和酚类的转糖基效率依次降低,这与受体的结构和羟基位置有关,因而可对受体进行基团的修饰以提高转糖基效率;从β-木糖苷酶的催化特性和结构特性入手,深入研究酶与底物的亲和、释放等过程中影响转糖基效率的关键氨基酸、相互作用力(氢键、Cation-π等)、局部结构摆动等,从而指导β-木糖苷酶的改造,提高转糖基效率;另外,通过定向进化也可提高转糖基效率。
2 β-木糖苷酶对木糖糖基化物质的转化功能β-木糖苷酶不仅能通过转糖基作用使某些物质木糖糖基化,还能切除某些天然物质上的木糖基团,从而生成具有生物活性的物质。这些带有木糖基团的天然物质有三七皂苷R1和R2及XDT、ASI、花青素等。
2.1 三七皂苷R1和R2的转化皂苷上的糖基被糖苷水解酶切除后可生成稀有皂苷,稀有皂苷具有重要的生物活性。如图 4所示,三七皂苷R1和R2上的C6位连接一个葡萄糖基,葡萄糖基又通过β-1, 2-糖苷键连接一个木糖基,β-木糖苷酶能切割β-1, 2-糖苷键使三七皂苷R1和R2分别转化为人参皂苷Rg1和Rh1;人参皂苷Rg1和Rh1具有抗癌、抗氧化、抗炎和增强记忆的功能[13, 35]。目前报道的具有转化三七皂苷R1和R2的β-木糖苷酶均在GH39家族中,分别来源于T. thermosaccharolyticum、Sphingomonas sp.、D. thermophilum的TtGH39[35]、JB13GH39[13]、Xln-DT[36]对三七皂苷R1和R2的转化率均大于90% (表 2)。本实验室通过序列比对、分子对接及突变验证等方法揭示了GH39 β-木糖苷酶转化三七皂苷R1和R2 (切割β-1, 2-糖苷键)涉及的保守位点,并通过系统发育树分析进一步提出:其他具有β-1, 4-木糖苷酶活性的GH39 β-木糖苷酶均具有切割β-1, 2-糖苷键的功能[13]。
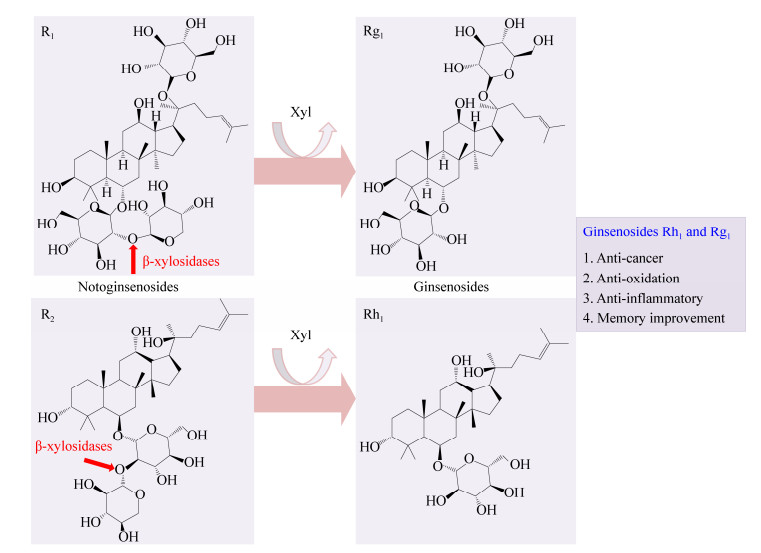
|
| 图 4 β-木糖苷酶将三七皂苷转化为人参皂苷 Figure 4 The transformation of notoginsenosides by β-xylosidases to produce ginsenosides |
|
|
| 家族 Family |
来源 Source |
名称 Enzyme name |
底物 Substrate |
产物 Product |
转化率 Conversion rate (%) |
| GH3 | Lentinula edodes[37] | LXYL-P1−1 | XDT | DT | NR |
| GH3 | Lentinula edodes[37] | LXYL-P1−2 | XDT | DT | 100 |
| GH3 | Dictyoglomus turgidum[38] | Dt-Xyl3 | XDT | DT | 98 |
| NR | Enterobacter sp. CGMCC2487[39] | NR | XDT | DT | 92 |
| NR | Cellulosimicrobium cellulans F16[40] | NR | XDT | DT | > 98 |
| GH3 | Brunfelsia calycina[41] | BcXyl | Anthocyanins | Anthocyanidins | NR |
| GH3 | Candida molischiana[42] | BGLN | Anthocyanins | Anthocyanidins | NR |
| GH3 | Bjerkandera adusta[43] | BadGluc | Anthocyanins | Anthocyanidins | NR |
| GH39 | Thermoanaerobacterium thermosaccharolyticum[35] | TtGH39 | Notoginsenosides R1 and R2 | Ginsenosides Rg1 and Rh1 | 100 |
| GH39 | Sphingomonas sp.[13] | JB13GH39 | Notoginsenosides R1 and R2 | Ginsenosides Rg1 and Rh1 | R1: 100; R2: 90.3 |
| GH39 | Dictyoglomus thermophilum[36] | Xln-DT | Notoginsenosides R1 and astragaloside IV |
Ginsenosides Rg1 and cycloastragenol |
R1: 100; IV: 88.9 |
| 注:NR:未报道. Note: NR: Not reported. |
|||||
不同皂苷上的糖基种类和位置不同,降解这些糖基化的皂苷需要不同催化功能的糖苷水解酶,根据催化功能可以对这些酶进行系统分类[44]。例如,葡糖基可在多个位置上对人参皂苷糖基化,降解这些葡糖基化的人参皂苷需要不同的葡糖基水解酶,国际酶学委员会(enzyme commission of IUB,EC)将这些葡糖基水解酶归类于EC 3.2.1.191−195中。本实验室提出:降解三七皂苷R1和R2的GH39 β-木糖苷酶应该被归类于新的EC子类中[13]。
2.2 黄芪甲苷IV的转化从黄芪中提取的黄芪甲苷IV (ASI)的含量远比环黄芪醇(cycloastragenol,CA)的含量高,但是CA的抗衰老作用更有效[36]。如图 5所示,ASI上C3位的木糖基和C6位的葡萄糖基分别被β-木糖苷酶和β-葡萄糖苷酶切除后生成CA[36]。β-木糖苷酶转化ASI的相关报道很少,目前仅Li等[36]报道来源于D. thermophilum的GH39 β-木糖苷酶Xln-DT能切除ASI上的木糖基,其转化率高达88.9% (表 2)。
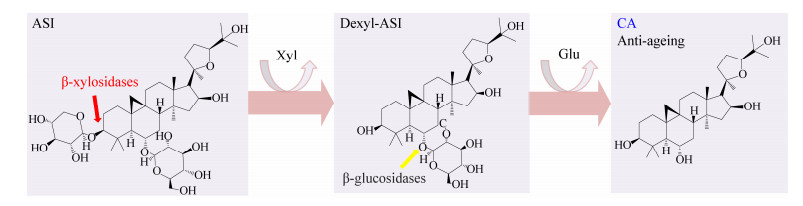
|
| 图 5 β-木糖苷酶将黄芪甲苷IV(ASI)转化为环黄芪醇(CA) Figure 5 The transformation of ASI by β-xylosidases to produce CA 注:黄色箭头代表β-葡萄糖苷酶进一步切除葡萄糖基后形成CA. Note: Yellow arrow indicates that β-glucosidases work together with β-xylosidases to form the end product CA. |
|
|
目前,药用紫杉醇主要从红豆杉中提取,但其在红豆杉中的含量约为0.02%,而其类似物7-木糖-10-去乙酰紫杉醇含量约为0.5%,常被作为废弃物处理[45]。如图 6所示,XDT上C7位的木糖基被切除后形成DT,DT进一步被酰化后形成紫杉醇[45]。目前,具有XDT转化功能的β-木糖苷酶均存在于GH3家族中,其转化率均在90%以上(表 2)。其中Cheng等[37]对来源于L. edodes的LXYL-P1-1和LXYL-P1-2的相关结构特点进行了进一步研究:LXYL-P1-1的2个单点突变体I368T (表示368位的异亮氨酸突变为苏氨酸,以下表述与此类似)和I368E对XDT的转化活性升高,双点突变体V91S/I368E和A72T/V91S的转化活性高于单点突变体I368E,表明I368是转化XDT的关键氨基酸,同时I368、V91和A72在转化过程中存在相互作用;LXYL-P1-2突变体T368E的转化活性升高,同样证明T368是LXYL-P1-2中转化XDT的关键氨基酸。Yang等[46]得到LXYL-P1-2和底物XDT复合物晶体,经序列比对和结构分析,发现A381-P382是LXYL-P1-2中区别于其他大部分同家族(GH3) β-木糖苷酶的特殊序列,该序列与DT的结合有关;含A381-P382位点的Loop结构(379–383)和另外4个Loop结构(220–232、324–328、446–450和529–530)共同形成了与DT结合的口袋;另外,LXYL-P1-2中和底物结合的部位与紫杉醇作用的微管蛋白具有相似的结构特点,因此,进一步揭示LXYL-P1-2的结构特点可以为紫杉醇新型药物载体的开发提供参考。
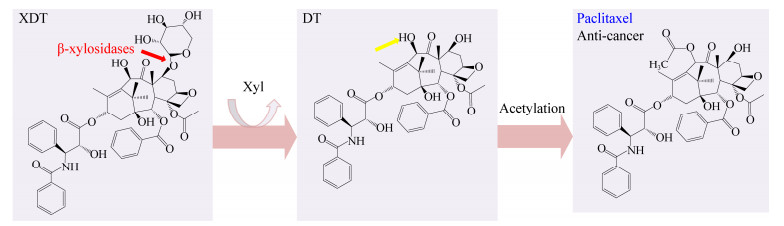
|
| 图 6 β-木糖苷酶将XDT转化为紫杉醇 Figure 6 The transformation of XDT by β-xylosidases to produce paclitaxel 注:黄色箭头代表乙酰化酶进一步酰化后形成紫杉醇. Note: Yellow arrow indicates that β-xylosidases work together with acetylases to form the end product paclitaxel. |
|
|
花青素(anthocyanins)是植物中负责颜色变化的主要物质,是黄酮类化合物,母核结构为2-苯基苯并吡喃(图 7),被称为花色元;母核结构上的R1、R2、R3、R4是不同的取代基,从而形成了250余种花青素[47]。R3、R4可以被糖基取代(包括木糖基团)[48]。花青素可作为食物色素添加剂,具有营养价值和抗氧化作用[47-48]。通过糖苷水解酶的水解作用,可去除花青素中的糖基,生成能更有效地发挥生物活性作用的花色素[41] (图 7)。有些β-木糖苷酶能去除花青素中的木糖基[41-43],目前已报道具有此功能的β-木糖苷酶均存在于GH3中,但此类β-木糖苷酶的结构特性还有待进一步研究(表 2)。
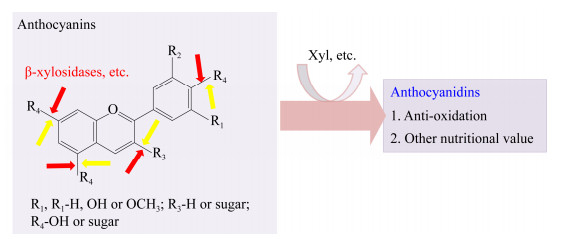
|
| 图 7 β-木糖苷酶将花青素转化为花色素 Figure 7 The transformation of anthocyanins by β-xylosidases to produce anthocyanidins 注:黄色箭头代表其他糖苷水解酶切割其他糖基. Note: Yellow arrows indicate that other glycosidases cleave other sugar groups to form anthocyanidins. |
|
|
与化学和物理方法相比,酶法往往具有特异性强、条件温和、环保、可控和可持续等优点,因此,利用木糖苷酶获得烷基糖苷、新型寡糖、木糖化酚类、稀有皂苷、紫杉醇、环黄芪醇和花色素等生物活性物质,具有重要的研究和应用价值。近年报道了一些具有生物活性物质转化功能的β-木糖苷酶,其中具有转糖基功能的β-木糖苷酶存在于GH3、GH5、GH39、GH43、GH52和GH54家族中,具有转化三七皂苷R1和R2、XDT、ASI和花青素的β-木糖苷酶存在于GH3和GH39家族中,但是,具有生物活性物质转化功能的β-木糖苷酶和能被β-木糖苷酶转化的天然物质以及转化产物的应用还有待进一步研究。另外,已有研究揭示了个别β-木糖苷酶在生物活性物质转化中涉及到的基本机制、保守氨基酸位点和一些结构特性,为后续的研究奠定了基础。但要实现这些β-木糖苷酶的工业化应用,还需要对酶的转化机制、关键氨基酸和结构特性进行更深入的研究,同时对酶的高效表达、发酵、固定化及其他下游工程进行研究,解决底物特异性、产物抑制、转糖基过程中的反应趋向性、酶的产量、酶的重复利用等问题,最终提高生物活性物质转化的效率并降低成本。
| [1] |
Nieto-Domínguez M, de Eugenio LI, Peñalver P, et al. Enzymatic synthesis of a novel neuroprotective hydroxytyrosyl glycoside[J]. Journal of Agricultural and Food Chemistry, 2017, 65(48): 10526-10533. DOI:10.1021/acs.jafc.7b04176 |
| [2] |
Nieto-Domínguez M, de Eugenio LI, Barriuso J, et al. Novel pH-stable glycoside hydrolase family 3 β-xylosidase from Talaromyces amestolkiae: an enzyme displaying regioselective transxylosylation[J]. Applied and Environmental Microbiology, 2015, 81(18): 6380-6392. DOI:10.1128/AEM.01744-15 |
| [3] |
Smułek W, Kaczorek E, Hricovíniová Z. Alkyl xylosides: physico-chemical properties and influence on environmental bacteria cells[J]. Journal of Surfactants and Detergents, 2017, 20(6): 1269-1279. DOI:10.1007/s11743-017-2012-2 |
| [4] |
Kim JH, Yi YS, Kim MY, et al. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases[J]. Journal of Ginseng Research, 2017, 41(4): 435-443. DOI:10.1016/j.jgr.2016.08.004 |
| [5] |
Liu WC, Gong T, Zhu P. Advances in exploring alternative Taxol sources[J]. RSC Advances, 2016, 6(54): 48800-48809. DOI:10.1039/C6RA06640B |
| [6] |
Brusa C, Muzard M, Rémond C, et al. β-Xylopyranosides: synthesis and applications[J]. RSC Advances, 2015, 5(110): 91026-91055. DOI:10.1039/C5RA14023D |
| [7] |
Xia W, Shi PJ, Xu XX, et al. High level expression of a novel family 3 neutral β-xylosidase from Humicola insolens Y1 with high tolerance to D-xylose[J]. PLoS One, 2015, 10(2): e0117578. DOI:10.1371/journal.pone.0117578 |
| [8] |
Kirikyali N, Connerton IF. Heterologous expression and kinetic characterisation of Neurospora crassa β-xylosidase in Pichia pastoris[J]. Enzyme and Microbial Technology, 2014, 57: 63-68. DOI:10.1016/j.enzmictec.2014.02.002 |
| [9] |
Hou J. Gene cloning, expression and characterization of three glycoside hydrolases from Cellulomonas bogoriensis[D]. Changchun: Master's Thesis of Northeast Normal University, 2018 (in Chinese) 侯晶. Cellulomonas bogoriensis的三种糖苷水解酶的基因克隆、异源重组表达及酶学性质研究[D].长春: 东北师范大学硕士学位论文, 2018 http://cdmd.cnki.com.cn/Article/CDMD-10200-1018707268.htm |
| [10] |
Huy ND, Le Nguyen C, Seo J, et al. Putative endoglucanase PcGH5 from Phanerochaete chrysosporium is a β-xylosidase that cleaves xylans in synergistic action with endo-xylanase[J]. Journal of Bioscience and Bioengineering, 2015, 119(4): 416-420. DOI:10.1016/j.jbiosc.2014.09.012 |
| [11] |
Muzard M, Aubry N, Plantier-Royon R, et al. Evaluation of the transglycosylation activities of a GH 39 β-D-xylosidase for the synthesis of xylose-based glycosides[J]. Journal of Molecular Catalysis B: Enzymatic, 2009, 58(1/4): 1-5. |
| [12] |
Li N, Han XW, Xu SJ, et al. Glycoside hydrolase family 39 β-xylosidase of Sphingomonas showing salt/ethanol/trypsin tolerance, low-pH/low-temperature activity, and transxylosylation activity[J]. Journal of Agricultural and Food Chemistry, 2018, 66(36): 9465-9472. |
| [13] |
Zhang R, Li N, Xu SJ, et al. Glycoside hydrolase family 39 β-xylosidases exhibit β-1, 2-xylosidase activity for transformation of notoginsenosides: a new EC subsubclass[J]. Journal of Agricultural and Food Chemistry, 2019, 67(11): 3220-3228. DOI:10.1021/acs.jafc.9b00027 |
| [14] |
Armand S, Vieille C, Gey C, et al. Stereochemical course and reaction products of the action of β-xylosidase from Thermoanaerobacterium saccharolyticum strain B6A-RI[J]. European Journal of Biochemistry, 1996, 236(2): 706-713. DOI:10.1111/j.1432-1033.1996.00706.x |
| [15] |
Fekete CA, Kiss L. A new approach in the active site investigation of an inverting β-D-xylosidase from Thermobifida fusca TM51[J]. The Protein Journal, 2013, 32(2): 97-105. DOI:10.1007/s10930-013-9463-8 |
| [16] |
Fekete CA, Kiss L. Purification and characterization of a recombinant β-D-xylosidase from Thermobifida fusca TM51[J]. The Protein Journal, 2012, 31(8): 641-650. DOI:10.1007/s10930-012-9440-7 |
| [17] |
Suzuki T, Kitagawa E, Sakakibara F, et al. Cloning, expression, and characterization of a family 52 β-xylosidase gene (xysB) of a multiple-xylanase-producing bacterium, Aeromonas caviae ME-1[J]. Bioscience, Biotechnology, and Biochemistry, 2001, 65(3): 487-494. DOI:10.1271/bbb.65.487 |
| [18] |
Jain I, Kumar V, Satyanarayana T. Applicability of recombinant β-xylosidase from the extremely thermophilic bacterium Geobacillus thermodenitrificans in synthesizing alkylxylosides[J]. Bioresource Technology, 2014, 170: 462-469. DOI:10.1016/j.biortech.2014.07.113 |
| [19] |
Wan CF, Chen CT, Li YK, et al. Expression, purification and characterization of a bifunctional α-L-arabinofuranosidase/ β-D-xylosidase from Trichoderma koningii G-39[J]. Journal of the Chinese Chemical Society, 2007, 54(1): 109-116. DOI:10.1002/jccs.200700018 |
| [20] |
Shao WL, Xue YM, Wu AL, et al. Characterization of a novel β-xylosidase, XylC, from Thermoanaerobacterium saccharolyticum JW/SL-YS485[J]. Applied and Environmental Microbiology, 2011, 77(3): 719-726. |
| [21] |
Li ZF, Gu ZB, Du GC, et al. Structural characteristics and catalytic mechanisms of cyclodextrin glycosyltransferase[J]. China Biotechnology, 2010, 30(6): 144-150. (in Chinese) 李兆丰, 顾正彪, 堵国成, 等. 环糊精葡萄糖基转移酶的结构特征与催化机理[J]. 中国生物工程杂志, 2010, 30(6): 144-150. |
| [22] |
Chen HC, Yang SS, Xu AJ, et al. Insight into the glycosylation and hydrolysis kinetics of alpha-glucosidase in the synthesis of glycosides[J]. Applied Microbiology and Biotechnology, 2019, 103(23/24): 9423-9432. |
| [23] |
Kurakake M, Fujii T, Yata M, et al. Characteristics of transxylosylation by β-xylosidase from Aspergillus awamori K4[J]. Biochimica et Biophysica Acta (BBA)-General Subjects, 2005, 1726(3): 272-279. DOI:10.1016/j.bbagen.2005.08.009 |
| [24] |
Ochs M, Belloy N, Dauchez M, et al. Role of hydrophobic residues in the aglycone binding subsite of a GH39 β-xylosidase in alkyl xylosides synthesis[J]. Journal of Molecular Catalysis B: Enzymatic, 2013, 96: 21-26. DOI:10.1016/j.molcatb.2013.06.001 |
| [25] |
Richard N, Arnold S, Hoeller U, et al. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages[J]. Planta Medica, 2011, 77(17): 1890-1897. DOI:10.1055/s-0031-1280022 |
| [26] |
Vlavcheski F, Young M, Tsiani E. Antidiabetic effects of hydroxytyrosol: in vitro and in vivo evidence[J]. Antioxidants, 2019, 8(6): 188. DOI:10.3390/antiox8060188 |
| [27] |
Peyrol J, Riva C, Amiot MJ. Hydroxytyrosol in the prevention of the metabolic syndrome and related disorders[J]. Nutrients, 2017, 9(3): 306. DOI:10.3390/nu9030306 |
| [28] |
de Las Hazas MCL, Piñol C, Macia A, et al. Hydroxytyrosol and the colonic metabolites derived from virgin olive oil intake induce cell cycle arrest and apoptosis in colon cancer cells[J]. Journal of Agricultural and Food Chemistry, 2017, 65(31): 6467-6476. DOI:10.1021/acs.jafc.6b04933 |
| [29] |
Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols[J]. International Journal of Molecular Sciences, 2018, 19(3): 686. DOI:10.3390/ijms19030686 |
| [30] |
Dreher F, Draelos ZD, Gold MH, et al. Efficacy of hydroquinone-free skin-lightening cream for photoaging[J]. Journal of Cosmetic Dermatology, 2013, 12(1): 12-17. DOI:10.1111/jocd.12025 |
| [31] |
Chiku K, Dohi H, Saito A, et al. Enzymatic synthesis of 4-hydroxyphenyl β-D-oligoxylosides and their notable tyrosinase inhibitory activity[J]. Bioscience, Biotechnology, and Biochemistry, 2009, 73(5): 1123-1128. DOI:10.1271/bbb.80885 |
| [32] |
Sedó J, Saiz-Poseu J, Busqué F, et al. Catechol-based biomimetic functional materials[J]. Advanced Materials, 2013, 25(5): 653-701. |
| [33] |
Lee WG, Chan AH, Spasov KA, et al. Design, conformation, and crystallography of 2-naphthyl phenyl ethers as potent anti-HIV agents[J]. ACS Medicinal Chemistry Letters, 2016, 7(12): 1156-1160. DOI:10.1021/acsmedchemlett.6b00390 |
| [34] |
Kumar M, Rawat P, Rahuja N, et al. Antihyperglycemic activity of phenylpropanoyl esters of catechol glycoside and its dimers from Dodecadenia grandiflora[J]. Phytochemistry, 2009, 70(11/12): 1448-1455. |
| [35] |
Shin KC, Seo MJ, Oh DK. Characterization of β-xylosidase from Thermoanaerobacterium thermosaccharolyticum and its application to the production of ginsenosides Rg1 and Rh1 from notoginsenosides R1 and R2[J]. Biotechnology Letters, 2014, 36(11): 2275-2281. DOI:10.1007/s10529-014-1604-4 |
| [36] |
Li Q, Wu T, Qi ZP, et al. Characterization of a novel thermostable and xylose-tolerant GH 39 β-xylosidase from Dictyoglomus thermophilum[J]. BMC Biotechnology, 2018, 18(1): 29. DOI:10.1186/s12896-018-0440-3 |
| [37] |
Cheng HL, Zhao RY, Chen TJ, et al. Cloning and characterization of the glycoside hydrolases that remove xylosyl groups from 7-β-xylosyl-10-deacetyltaxol and its analogues[J]. Molecular & Cellular Proteomics, 2013, 12(8): 2236-2248. |
| [38] |
Li Q, Jiang YJ, Tong XY, et al. Cloning and characterization of the β-xylosidase from Dictyoglomus turgidum for high efficient biotransformation of 10-deacetyl-7-xylosltaxol[J]. Bioorganic Chemistry, 2020, 94: 103357. DOI:10.1016/j.bioorg.2019.103357 |
| [39] |
Wang K, Wang TT, Li JH, et al. Microbial hydrolysis of 7-xylosyl-10-deacetyltaxol to 10-deacetyltaxol[J]. Journal of Molecular Catalysis B: Enzymatic, 2011, 68(3/4): 250-255. |
| [40] |
Dou TY, Luan HW, Liu XB, et al. Enzymatic hydrolysis of 7-xylosyltaxanes by an extracellular xylosidase from Cellulosimicrobium cellulans[J]. Biotechnology Letters, 2015, 37(9): 1905-1910. DOI:10.1007/s10529-015-1867-4 |
| [41] |
Dong BY, Luo HH, Liu B, et al. BcXyl, a β-xylosidase isolated from Brunfelsia calycina flowers with anthocyanin-β-glycosidase activity[J]. International Journal of Molecular Sciences, 2019, 20(6): 1423. DOI:10.3390/ijms20061423 |
| [42] |
Sánchez-Torres P, González-Candelas L, Ramón D. Heterologous expression of a Candida molischiana anthocyanin-β-glucosidase in a wine yeast strain[J]. Journal of Agricultural and Food Chemistry, 1998, 46(1): 354-360. DOI:10.1021/jf970570r |
| [43] |
Behrens CJ, Krahe NK, Linke D, et al. BadGluc, a β-glucosidase from Bjerkandera adusta with anthocyanase properties[J]. Bioprocess and Biosystems Engineering, 2018, 41(9): 1391-1401. DOI:10.1007/s00449-018-1966-4 |
| [44] |
Shin KC, Oh DK. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides[J]. Critical Reviews in Biotechnology, 2016, 36(6): 1036-1049. DOI:10.3109/07388551.2015.1083942 |
| [45] |
Li BJ, Wang H, Gong T, et al. Improving 10-deacetylbaccatin Ⅲ-10-β-O-acetyltransferase catalytic fitness for Taxol production[J]. Nature Communications, 2017, 8(1): 15544. DOI:10.1038/ncomms15544 |
| [46] |
Yang L, Chen TJ, Wang F, et al. Structures of β-glycosidase LXYL-P1-2 reveals the product binding state of GH3 family and a specific pocket for Taxol recognition[J]. Communications Biology, 2020, 3(1): 22. DOI:10.1038/s42003-019-0744-4 |
| [47] |
Wallace TC, Giusti MM. Anthocyanins[J]. Advances in Nutrition, 2015, 6(5): 620-622. DOI:10.3945/an.115.009233 |
| [48] |
Fang J. Bioavailability of anthocyanins[J]. Drug Metabolism Reviews, 2014, 46(4): 508-520. |
 2020, Vol. 47
2020, Vol. 47




