扩展功能
文章信息
- 阚玉敏, 蒋娜, 白凯红, 李健强, 罗来鑫
- KAN Yu-Min, JIANG Na, BAI Kai-Hong, LI Jian-Qiang, LUO Lai-Xin
- 细菌有活力但不可培养状态及其机制研究进展
- Research progress on viable but non-culturable state of bacteria
- 微生物学通报, 2020, 47(3): 880-891
- Microbiology China, 2020, 47(3): 880-891
- DOI: 10.13344/j.microbiol.china.190556
-
文章历史
- 收稿日期: 2019-07-05
- 接受日期: 2019-10-08
- 网络首发日期: 2019-11-04
有活力但不可培养(viable but non-culturable,VBNC)状态,即有活力但是不可培养状态。Xu等于1982年研究大肠杆菌(Escherichia coli)和霍乱弧菌(Vibrio cholera)时首次发现此现象[1]。在随后30多年的研究中,又陆续有多种细菌被证实遭遇环境逆境时能够进入这种状态。目前,报道能够进入VBNC状态的100多种微生物中,除布鲁氏酵母菌(Brettanomyces bruxellensis)[2]、酿酒酵母(Saccharomyces cerevisiae)[3]和季也蒙有孢汉逊酵母(Hanseniaspora guilliermondii)[4]等3种酵母外,其余均为细菌[5]。
本课题组多年来从事种传植物病原细菌相关研究,根据种子带菌检测和农业生产实际,在国内率先开展了种传病原细菌的VBNC状态研究,首次证实了番茄溃疡病菌(Clavibacter michiganensis)[6]和西瓜果斑病菌(Acidovorax citrulli)[7]的VBNC状态及其复苏条件,但在植物病原细菌VBNC状态现有相关研究中,鲜有对其形成和复苏机制的报道。本文根据课题组当前研究工作重点及已有文献报道,结合VBNC状态机制尚不明确这一空白点,从细菌VBNC状态的表型、检测方法、诱导和复苏条件、复苏菌体的致病性、VBNC状态的形成和复苏机制等方面进行了较为全面的系统性综述,期望为深入了解VBNC状态菌体的特性及其机制提供理论参考,为农业生产中预防VBNC状态病原细菌的潜在威胁提供新思路。
1 VBNC状态概述自然界中的微生物有99%以上因处于VBNC状态而无法被分离培养[8],有研究显示多种能够正常分离培养的微生物在不良环境条件胁迫下可以进入VBNC状态[9]。细菌进入VBNC状态后不能在常规培养基上生长分裂,但保持细胞的完整性和低水平的代谢活性[9]。当条件适宜时,处于VBNC状态的菌体可以恢复在培养基上生长繁殖的能力,同时其致病性也随之恢复[5, 8]。因此,VBNC菌体的存在对医学、禽畜养殖和农业生产等众多领域均具有巨大的潜在危害,被认为是自然环境中病原细菌的“储存库”,已引起了环境科学和食品工业等领域研究者的广泛关注[9-10],但在农业生产和植物病理学领域,研究者对植物病原细菌VBNC状态的研究起步较晚,基础相对薄弱[11]。
1.1 细菌VBNC状态的检测和诱导处于VBNC状态的菌体具有细胞的完整性,能够检测到较低水平的代谢活性和基因表达能力[12]。不可培养这一特性决定了VBNC状态菌体的检测只能通过分别对有活力菌体和可培养菌体计数,再用有活力菌体数减去可培养菌体数间接得到。可培养菌体计数通过常规培养平板计数得到,而有活力菌体计数基于菌体膜完整性或代谢活性有多种检测方法[11],如吖啶橙染色方法[13]、5-氰基-2, 3-二(4-甲基苯基)四唑氯化物(5-cyano-2, 3-ditolyl tetrazoliumchloride,CTC)染色方法[14]和使用细菌活力试剂盒进行染色的方法[6]。此外,基于菌体膜完整性,使用DNA结合染料EMA (ethidium monoazide)[15-16]、PMA (propidium monoazide)[17]或其改良产品PMAxx[18]等结合qPCR的方法也是VBNC状态菌体检测中的常用方法。在食品微生物检测中,荧光原位杂交(fluorescence in situ hybridization,FISH)由于耗时较少且特异性高,也常被采用[19]。
VBNC状态的诱导因素有很多,不适宜的温度及pH、光照、低氧环境、较低的氧化还原电势、二氧化硫、高压二氧化碳、高盐环境、干燥和重金属胁迫等均可使菌体进入VBNC状态[20-21]。动物病原细菌与此相关的研究内容主要集中在水、土壤等环境微生物和食品微生物中。至今已报道能够进入VBNC状态的水环境微生物有60多种,从属于20个属[22]。研究表明,大部分弧菌可在寡营养条件下经低温诱导进入VBNC状态,如霍乱弧菌(Vibrio cholera)、创伤弧菌(V. vulnificus)、副溶血性弧菌(V. parahaemolyticus)等[23-26]。大肠杆菌(Escherichia coli) O157:H7可在高压二氧化碳和紫外线杀菌过程中进入VBNC状态[21, 27];近期研究结果表明,饮用水氯化消毒也可使E. coli CMCC 44103进入VBNC状态[28];乳酸菌(Lactobacillus lindneri) BM-LL14527可在0 ℃低温和脱气的啤酒中进入VBNC状态[29]。土壤微生物如耐金属贪铜菌(Cupriavidus metallidurans)可通过寡营养结合干燥处理进入VBNC状态[30]。
植物病原细菌的VBNC状态研究起步较晚,目前报道的诱导条件大多与生产中细菌病害的防治方法相关,如根癌土壤杆菌(Agrobacterium tumefaciens)[31]、十字花科黑腐病菌(Xanthomonas campestris pv. campestris)[32]、茄科作物青枯病菌(Ralstonia solanacearum)[33]、梨火疫病菌(Erwinia amylovora)[34]、柑橘溃疡病菌(Xanthomonas axonopodis pv. citri)[35]、番茄溃疡病菌(Clavibacter michiganensis)[6]和细菌性果斑病菌(Acidovorax citrulli)[7]等均可在一定浓度铜离子诱导下进入VBNC状态。此外,木质部难养菌(Xylella fastidiosa)可在硫酸锌作用下进入VBNC状态[36],丁香假单胞菌丁香致病变种(Pseudomonas syringae pv. syringae)和烟草致病变种(P. s. pv. tabaci)则可在乙酰丁香酮作用下进入VBNC状态[37]。
1.2 VBNC状态菌体复苏及其致病性VBNC状态菌体在一定条件下可以复苏,复苏后能够侵染寄主使之发病。当前报道的VBNC状态菌体复苏方法有多种,总体可以归纳为三类:去除诱导因子、添加营养物质和接种寄主。低温条件诱导的VBNC状态菌体可通过升高温度进行复苏[38-39],添加EDTA能够复苏铜离子诱导的VBNC状态菌体[7, 40]。添加营养培养基或其他一些特定的小分子有机物如氨基酸和复苏促进因子等可使某些VBNC状态的菌体复苏[20, 41]。使广大研究者最为担心的是VBNC状态菌体可在寄主体内复苏。有研究显示,VBNC状态的溶藻弧菌(V. alginolyticus)和副溶血性弧菌(V. parahaemolyticus)能够在接种小鼠体内复苏[42],植物病原细菌如Er. amylovora和C. michiganensis也分别在接种的梨果实及幼苗和番茄植株上检测到了复苏的可培养菌体[6, 43]。
VBNC状态菌体的致病性在不同细菌中差异较大。VBNC状态的V. alginolyticus和V. parahaemolyticus接种小鼠后经一定时间能够分离得到可培养菌体,但是恢复后的菌体需要在小鼠回肠中连续两次传代才能恢复致病性[42]。单增李斯特菌(Listeria monocytogenes)处于VBNC状态时不具有致病性,但在含胚胎的蛋黄中复苏后接种小鼠即可表现致病性,表明该菌进入VBNC状态时致病性丧失是暂时的,致病性随菌体可培养性的恢复而恢复[44]。VBNC状态的植物病原细菌R. solanacearum可在番茄植株根系周围复苏并对番茄侵染致病[33]。Er. amylovora的VBNC状态菌体也可在梨果实和梨树苗上复苏并侵染致病[40, 43];VBNC状态的C. michiganensis接种番茄植株后虽然分离到了可培养菌体但植株并未表现发病症状[6];A. citrulli的VBNC状态菌体复苏后与对数期菌体致病力无显著差异,但在西瓜幼苗上直接接种VBNC状态菌体并未发病[7]。因此,深入了解VBNC状态菌体的生理特性及形成机制,对研究微生物的逆境生理、医学上疾病复发及农业生产中田间病害的侵染源具有重要意义。
2 VBNC状态形成和复苏机制关于VBNC状态菌体的形成机制尚不清楚[45]。研究者前期主要利用组学手段,通过比较VBNC状态与对数期或稳定期菌体的差异表达基因,对形成机制展开研究。Heim等通过二维场凝胶电泳比较VBNC状态与饥饿处理的Enterococcus faecalis,发现VBNC状态的菌体虽然代谢水平整体下调,但蛋白质合成相关蛋白上调,能量代谢相关蛋白表达量发生变化,这一结果表明VBNC状态是菌体应对不良环境的一种保护机制[46]。在随后V. cholera的VBNC状态研究中,发现与细胞过程相关的基因,三价铁离子ABC运输体和polB、fliG、flaC基因上调表达。而VBNC状态V. parahaemolyticus的基因组和蛋白组分析结果显示,上调的主要为非代谢相关的基因或蛋白,涉及转录、翻译、ATP合成、糖异生和抗氧化等多个途径[47-48]。Zhao等综合使用转录组学和蛋白组学对高压二氧化碳诱导的VBNC状态E. coli菌体展开研究,推断VBNC状态菌体的膜完整性、代谢活性降低、致病力下降等一系列变化,均是该状态菌体为应对恶劣的环境条件所采取的适应性策略;同时,VBNC状态的菌体能量代谢的变化主要表现在丙酮酸代谢由三羧酸循环转变为无氧呼吸途径,此呼吸代谢途径产生的ATP较少,需通过降解丝氨酸、苏氨酸、增加AMP生成和加强电子传递等途径补充ATP[49]。Pu等通过对抗生素诱导的E. coli不同程度休眠状态的菌体进行研究,发现细胞中ATP的水平影响VBNC状态的形成和复苏,VBNC状态菌体中ATP含量下降,低水平的ATP使VBNC状态菌体复苏所需时间更长[50]。Pseudomonas syringae pv. syringae经乙酰丁香酮诱导进入VBNC状态后的转录组分析结果显示,VBNC状态菌体中聚氨类物质、季胺化合物、碳水化合物等代谢与运输、能量产生和肽聚糖等相关基因均上调表达,而三型分泌系统和毒素相关基因下调表达[51]。由此表明植物病原细菌的VBNC状态也是病原菌在逆境条件下的一种适应性反应。
VBNC形成机制比较复杂,涉及多条代谢途径中的众多基因,目前研究热点主要集中在以下几个方面。
2.1 严紧反应严紧反应(stringent response)是细菌在氨基酸饥饿条件下通过减缓生长来增加氨基酸合成的一种响应机制,这种机制通过信号分子鸟苷五磷酸或鸟苷四磷酸[(p)ppGpp]来调控[5]。(p)ppGpp通过结合RNA聚合酶、影响生物合成中必需的转录过程来改变基因的表达[52]。在有关持留细菌(persister bacteria)的研究中,E. coli内(p)ppGpp通过毒素-抗毒素系统(toxin-antitoxin system,TA system)诱导持留状态[53],(p)ppGpp使多聚磷酸盐积累,从而激活Lon蛋白酶调控的蛋白水解过程,进而影响TA系统。有研究表明,严紧反应在VBNC状态形成中发挥作用,且V. cholerae中(p)ppGpp的合成基因relA、E. coli中(p)ppGpp的合成基因relA与spoT在VBNC状态菌体中均上调表达[54-55]。不能合成(p)ppGpp的突变体较野生型更快丧失可培养性,且进入VBNC状态的能力明显降低;而当(p)ppGpp过量表达时,形成VBNC状态菌体的数量明显增多[56]。有研究者认为严紧反应对VBNC状态的调控过程如图 1所示,即当细菌遭遇氨基酸饥饿时,RelA或SpoT会行使功能,增加(p)ppGpp的合成或减少(p)ppGpp的降解,(p)ppGpp抑制外切聚磷酸酶(PPX)降解多聚磷酸盐(polyphosphates),因此,导致多聚磷酸盐的积累,多聚磷酸盐能够激活Lon蛋白酶从而影响TA系统相关基因的表达,通过增加菌体细胞中游离毒素的含量来抑制翻译过程和菌体生长分裂,进而调控VBNC状态的形成[5]。

|
| 图 1 严紧反应调控持留状态或VBNC状态的分子模型[5] Figure 1 Molecular mechanism of persisters or VBNC state formation regulated by stringent response[5] 注:细菌遭遇氨基酸饥饿时,RelA或SpoT会增加(p)ppGpp的合成或减少(p)ppGpp的降解,(p)ppGpp能够抑制外切聚磷酸酶(PPX)降解多聚磷酸盐(polyphosphates),导致多聚磷酸盐的积累,多聚磷酸盐能够激活Lon/Clp蛋白酶从而影响TA系统相关基因的表达,最终导致菌体细胞中游离的毒素水平增加,游离的毒素抑制翻译过程和菌体生长分裂,进而调控VBNC状态的形成. Note: During amino acid starvation, the protein RelA or SpoT causes an increase of (p)ppGpp, resulting in the enzyme activity inhibition of exopolyphosphatase (PPX), which responsible for degrading the polyphosphates (PolyP). This results in an accumulation of PolyP, which activate of the Lon/Clp protease and affect the expression of toxin-antitoxin related genes. Eventually, the level of free toxins in bacterial cells accumulated, which inhibited the protein translation and division of the bacteria, thereby regulating the formation of VBNC state. |
|
|
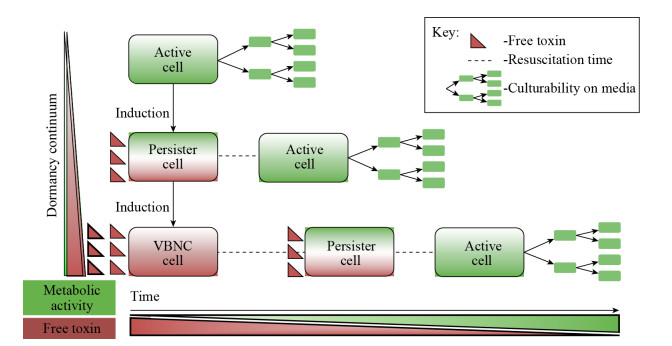
毒素-抗毒素系统(toxin-antitoxin system,TA system)在细菌中普遍存在,在细菌适应外界压力、毒性、抵御噬菌体侵染和生物膜形成等多个方面发挥作用[57]。TA系统在抗生素诱导的持留状态细菌中研究广泛,被认为是持留细菌形成的主要调控因子[58]。2015年,Ayrapetyan等[59]结合前人报道和所在实验室多年研究成果,提出了微生物休眠连续假说,该假说认为微生物的VBNC状态与细菌持留状态可以用相似的调控机制来解释,即VBNC状态也受TA系统调控,且VBNC状态是比持留状态更深程度的“休眠”。当菌体中游离毒素达到一定浓度时,正常代谢活性的菌体进入持留状态,而当压力因子持续存在时,菌体中游离毒素会进一步累积,从而使持留状态的细菌进入更深程度的休眠,即VBNC状态;当胁迫条件解除,菌体细胞中毒素含量降低,VBNC状态的细菌逐渐恢复到持留状态,如果菌体中游离的毒素进一步降低,则可使持留状态的菌体恢复为正常代谢活性,表现为恢复在常规培养基上的可培养性(图 2)。VBNC状态菌体与持留细菌有诸多共同特点,因此有学者认为VBNC状态与持留状态描述是细菌的同一种应激状态[60]。Ayrapetyan等[59]认为VBNC状态与持留状态是细菌对压力胁迫具有高度耐受性的生理状态,二者虽有诸多相似性但又有所区别。与VBNC状态相比,受抗生素胁迫的持留状态是瞬时的早期休眠状态,去除抗生素后经过较短时间即可复苏,而VBNC状态菌体的复苏条件则相对复杂且更为严苛,但TA系统对VBNC状态的调控机制与持留状态相同[5]。
|
| 图 2 微生物休眠连续假说模型[59] Figure 2 The model of microbial dormancy continuum hypothesis[59] 注:环境压力会导致抗毒素的降解,导致细胞内与其同源的毒素(红色箭头)释放.正常代谢活性的菌体中游离毒素达到一定浓度时,会进入持留状态,而当压力因子持续存在时,菌体中游离毒素会进一步累积,从而使持留状态的细菌进入更深程度的休眠,即VBNC状态;当环境胁迫条件解除时菌体细胞中毒素含量降低,持留状态的菌体和VBNC状态的细菌恢复为正常代谢活性,恢复在常规培养基上的可培养性. Note: Environmental stress induces the degradation of antitoxins, causing the liberation of their cognate toxins (red triangles). Culturable cells with normal metabolism activity will become persisters when the free toxin reached a threshold. When the stress pressure persists, more free toxins accumulated in the bacteria and resulted a deeper dormancy, which named VBNC state. When the stress conditions were removed, the toxin levels decreased, and the bacteria in persist or VBNC state recover to normal metabolic activity and culturability. |
|
|
目前,已有报道明确TA系统能够影响VBNC状态的形成[5, 59]。当过表达毒素基因时,可使细菌进入VBNC状态,而与毒素基因同源的抗毒素基因过量表达则可以扭转这种休眠状态[61-64]。已有研究表明,对细菌VBNC状态起调控作用的TA系统有hipAB[62, 64]、higBA[63]、relEB[61]、chpAKAI[61]、vapBC[65]等。
2.3 蛋白酶对抗毒素的水解Lon蛋白酶调控的蛋白质水解是联系严紧反应和TA系统调节作用的纽带,可能对VBNC状态的形成具有重要作用。Lon蛋白酶主要通过降解Ⅱ型TA系统的抗毒素、改变抗毒素与毒素的比例来影响持留状态的形成,Lon蛋白酶在VBNC状态形成中的作用还未见报道[5]。Clp蛋白酶与Lon蛋白酶具有相似的结构域,该蛋白酶能够降解E. coli和Staphylococcus aureus中多种抗毒素而导致菌体进入VBNC状态[5, 66-67]。VBNC状态Salmonella enterica菌体中的Clp蛋白酶会累积;而不能形成Clp的突变体进入VBNC状态的能力下降[5, 68]。因此,蛋白酶对抗毒素的水解作用可能在VBNC状态形成过程中发挥着重要作用。
2.4 关键调控因子目前,尚无关于VBNC状态调控关键基因的系统性报道,但有研究表明,Sigma因子(σS,RpoS)和转录调控因子OxyR对VBNC状态的形成具有一定的调控作用[41]。
RpoS是RNA聚合酶的一个亚基,在转录、翻译、蛋白质水解和蛋白活性发挥等方面均发挥作用,对细菌应对一般压力胁迫起主要调控作用[69]。RpoS参与调控VBNC状态已在多种细菌中得到证实[70]。E. coli、Salmonella enterica、Er. amylovora等细菌缺失rpoS基因后,能够更快速地进入VBNC状态,且VBNC状态维持的时间更短,即菌体更快走向死亡[56, 71-72]。VBNC状态的V. vulnificus菌体中能够检测到rpoS基因持续表达[73],且在VBNC菌体复苏后表达量显著升高,表明rpoS有利于细菌活性的维持[74]。
OxyR是LTTR (LysR type transcriptional regulators)家族的转录调控因子,多种细菌通过其对过氧化氢酶、烷基氢过氧化物还原酶及超氧化物歧化酶的激活作用来响应菌体遇到的氧化胁迫[75]。目前已证实oxyR能够调控氧化胁迫相关基因,并以此来影响细菌VBNC状态的形成及复苏[41]。V. vulnificus中oxyR的缺失突变体由于不具有过氧化氢酶活性,即使在常温下也不能在HI (heart infusion)固体培养基上生长,而处于VBNC状态;同时,低温条件下过氧化氢酶失活会致使Vibrio spp.进入VBNC状态[76]。OxyR能够调控ahpC和gst基因的表达,而AhpC和谷胱甘肽S-转移酶(glutathione s-transferase,GST)分别对V. parahaemolyticus和V. vulnificus的VBNC状态形成和维持具有重要作用[41, 77-78]。V. parahaemolyticus中ahpC2基因缺失突变体相比野生型能够快速进入VBNC状态,且能够维持VBNC状态的时间明显缩短,即菌体更快速地走向死亡[77]。关于V. vulnificus的VBNC状态研究结果表明,在寡营养环境中,过氧化氢等生长抑制物质会在菌体中积累,从而导致VBNC状态的形成[78]。上述研究表明,oxyR基因对VBNC状态形成的作用主要与活性氧及抗氧化胁迫相关[41]。
2.5 复苏促进因子与群体感应VBNC状态的形成机制尚不明确,复苏机制更是鲜有报道[45]。除在寄主体内复苏外,实验条件下VBNC状态菌体复苏具有两个十分重要的条件:一是诱导因子的去除,二是与复苏相关的特定信号分子的存在,如氨基酸、复苏促进因子(resuscitation promoting factor,Rpf)、自体诱导物等[41]。Rpf是能够促进细菌生长且对VBNC状态菌体复苏起作用的分泌蛋白,在藤黄微球菌(Micrococcus luteus)中首次报道,目前已被发现广泛存在于多种细菌中[70, 79-80]。关于Rpf促进VBNC状态菌体复苏的机制可以归纳为3种模式。第一种模式中Rpf与其他细胞信号分子作用相似,即Rpf与VBNC状态菌体表面受体结合,通过促进细胞生长而使其复苏;第二种模式和第三种模式中的Rpf均作用于VBNC状态菌体细胞壁中的肽聚糖(图 3),其中第二种模式为Rpf降解或改变VBNC状态菌体细胞壁中能够抑制菌体分裂或生长的肽聚糖成分,从而使之复苏,恢复为正常菌体,如图 3(1);第三种模式为Rpf降解肽聚糖后形成的某些产物可以充当“第二信使”,与其他因子相作用促进VBNC状态菌体的生长,从而使之复苏,恢复为正常菌体,如图 3(2)[10, 41]。

|
| 图 3 Rpf作用于肽聚糖促进VBNC状态菌体复苏的两种模式[10] Figure 3 Two viewpoints about the VBNC resuscitation mechanism of resuscitation promoting factors (Rpfs) acting on peptidoglycans[10] 注:(1):Rpf降解VBNC状态菌体细胞壁特定区域,解除这些区域中肽聚糖对菌体生长的抑制,从而促进VBNC状态菌体的复苏;(2):降解肽聚糖,其产物作为“第二信使”与其他因子作用,从而促进VBNC状态菌体的复苏. Note: (1): Rpfs are required to cleave the peptidoglycans with inhibitory properties distributed in specific area of cell wall of VBNC cells and thus promote cell division and growth again; (2): The decomposed product(s) of peptidoglycan divided by Rpfs may interact with other factors and function as "second messengers" to stimulate the resuscitation and growth of VBNC cells. |
|
|
群体感应(quorum sensing)信号分子也可以调控VBNC状态菌体的复苏。Ayrapetyan等研究发现,V. vulnificus的培养上清液可以复苏其VBNC状态菌体,而群体感应相关基因突变体的上清液则不能使之复苏[81]。此外,群体感应信号分子AI-2能够促进VBNC状态菌体的复苏,而不能合成AI-2的突变体则不能复苏,外源添加AI-2后能够使之复苏,但添加群体感应抑制物肉桂醛可以延迟野生型菌株VBNC状态的复苏[81]。该研究充分证实了群体感应信号分子对VBNC状态复苏的重要作用。
群体感应信号分子对Campylobacter jejuni和V. vulnificus的VBNC状态菌体形成和复苏均有一定影响[81-82]。目前,关于群体感应信号分子促进VBNC状态菌体复苏机制的研究较少,但有报道显示,群体感应信号分子对VBNC状态的作用可能与rpoS基因调控有关[81]。Ayrapetyan等的研究结果显示,AI-2能够通过LuxR来增强rpoS基因表达,从而诱导过氧化氢酶KatG过量表达,来抵御培养基中的过氧化氢,从而促进菌体生长和复苏。但是,作者同时指出不能排除群体感应信号分子还可能参与其他与VBNC状态复苏相关的途径来影响这一过程的可能性,因此,其具体机制还有待进一步研究[81]。
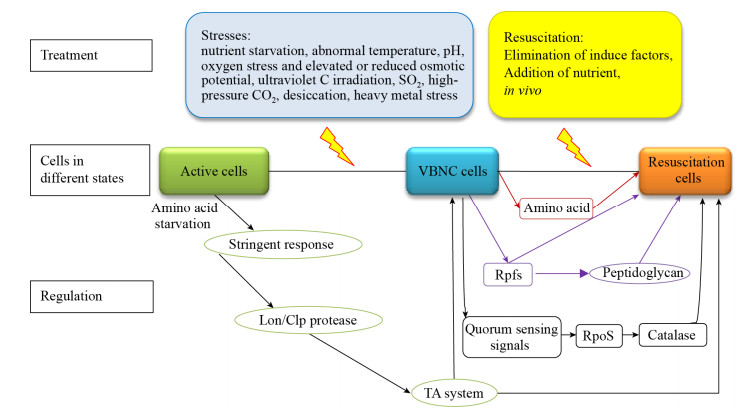
综上,研究者针对不同细菌已探索出VBNC状态的多种诱导和复苏条件。VBNC的形成和复苏机制虽目前还不十分明确,但也取得了一定进展。现有研究成果可总结为图 4所示:即代谢活跃的正常状态菌体处于不良环境时,在严紧反应、某些蛋白酶、TA系统等相关基因调控下可进入VBNC状态,丧失在常规培养基上生长的能力;而VBNC状态菌体在去除外界压力因子、培养环境中营养物质增加和接种寄主的条件下,可恢复可培养性和致病性,即VBNC状态菌体的复苏。复苏过程也受多方面的影响,如小分子物质Rpf和氨基酸、群体感应相关基因、Sigma因子RpoS和过氧化氢酶等。
|
| 图 4 VBNC状态诱导、复苏及机制研究进展示意图 Figure 4 Schematic diagram of research progress on induction, resuscitation and mechanism of VBNC state |
|
|
VBNC状态作为一种独特的生理状态,为医学领域和工农业生产带来了新的挑战和巨大潜在威胁,引起了广大研究学者的高度关注。动物及人类中很多疾病的复发,食品工业中的各种消毒方法是否安全可靠及农业生产中细菌病害的初侵染来源和再侵染等问题都与VBNC状态菌体密切相关。这一状态菌体的存在严重影响着基于细菌可培养性的传统检测方法的准确性和食品、农业生产等领域的安全性。因此,了解引起该状态形成的因素和复苏条件,进而解析其发生机制具有重要意义。经过将近40年的研究进程,目前已在VBNC状态菌体检测方面取得了重大突破,在诱导和复苏条件方面经过多方面摸索也得到了全面而详实的阐释。但就目前来看,除检测技术外,这一领域的研究成果几乎没有在实际生产中应用。我们认为,针对不同菌体的复苏处理,如果在食品微生物检测和农业生产中多种种传病原物的检测中结合使用,可大大提高检测方法的准确性,降低假阴性结果,同时使用某些不利于VBNC状态菌体复苏的因子进行处理,可以大大降低VBNC状态病原菌的潜在威胁,从而服务食品安全和农业健康生产。
当前和今后关于VBNC状态的研究热点仍将围绕调控机制展开[20]。北卡罗来纳大学的Oliver教授团队自20世纪90年代初期开展了VBNC状态的研究,他们主要以创伤弧菌等多种弧菌为研究对象,从基础研究到机制探索,取得了卓越的研究成果,为VBNC状态研究领域奠定了坚实基础[5, 12, 24, 26, 38, 59, 73, 81]。国内关于VBNC状态细菌的研究较少,本课题组以重要种传植物病原细菌为研究对象,率先开展了番茄溃疡病菌、十字花科黑腐病菌及瓜类果斑病菌的VBNC状态的诱导与复苏研究,通过转录组学、蛋白组学等手段,找到了部分与VBNC机制相关的代谢通路和关键基因[6-7, 11, 18, 83],目前正在开展基因功能探索,期待为植物病原细菌的VBNC机制研究提供重要参考。
随着二代测序技术的快速发展,结合分子遗传手段,VBNC状态的机制研究已经取得了一定进展,但具体调控通路还需要进一步探究。在VBNC状态机制研究过程中,研究者应注意VBNC状态菌体代谢水平相对较低这一特性,正确判断某一通路或基因、蛋白等表达水平变化是调控菌体进入VBNC状态还是菌体已经进入该状态后的结果。明确关键通路后展开相应基因的功能研究对解析该状态机制具有重要意义。
已报道的VBNC状态研究中,均不能排除体系中存在的死菌干扰,在未来的研究中寻找高效的诱导方法,制备死菌比率低而VBNC细菌比率高的样品,或是探索可靠的标记方法,将VBNC状态菌体与死菌区分,将VBNC状态菌体从混合的体系中分离开来,将是VBNC状态菌体机制研究的一个关键问题,对解析VBNC状态机制具有十分重要的意义。
| [1] |
Xu HS, Roberts N, Singleton FL, et al. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment[J]. Microbial Ecology, 1982, 8(4): 313-323. DOI:10.1007/BF02010671 |
| [2] |
Serpaggi V, Remize F, Recorbet G, et al. Characterization of the "viable but nonculturable" (VBNC) state in the wine spoilage yeast Brettanomyces[J]. Food Microbiology, 2012, 30(2): 438-447. DOI:10.1016/j.fm.2011.12.020 |
| [3] |
Salma M, Rousseaux S, Grand ASL, et al. Characterization of the viable but nonculturable (VBNC) state in Saccharomyces cerevisiae[J]. PLoS One, 2013, 8(10): e77600. DOI:10.1371/journal.pone.0077600 |
| [4] |
Branco P, Viana T, Albergaria H, et al. Antimicrobial peptides (AMPs) produced by Saccharomyces cerevisiae induce alterations in the intracellular pH, membrane permeability and culturability of Hanseniaspora guilliermondii cells[J]. International Journal of Food Microbiology, 2015, 205: 112-118. DOI:10.1016/j.ijfoodmicro.2015.04.015 |
| [5] |
Ayrapetyan M, Williams T, Oliver JD. Relationship between the viable but nonculturable state and antibiotic persister cells[J]. Journal of Bacteriology, 2018, 200(20): e00249-18. |
| [6] |
Jiang N, Lv QY, Xu X, et al. Induction of the viable but nonculturable state in Clavibacter michiganensis subsp. michiganensis and in planta resuscitation of the cells on tomato seedlings[J]. Plant Pathology, 2016, 65(5): 826-836. DOI:10.1111/ppa.12454 |
| [7] |
Kan YM, Jiang N, Xu X, et al. Induction and resuscitation of the viable but non-culturable (VBNC) state in Acidovorax citrulli, the causal agent of bacterial fruit blotch of cucurbitaceous crops[J]. Frontiers in Microbiology, 2019, 10: 1081. DOI:10.3389/fmicb.2019.01081 |
| [8] |
Zhang S, Ding LX, Su XM. Formation and resuscitation of the viable but non-culturable state in microorganisms[J]. Acta Microbiologica Sinica, 2018, 58(8): 1331-1339. (in Chinese) 张硕, 丁林贤, 苏晓梅. 微生物VBNC状态形成及复苏机制[J]. 微生物学报, 2018, 58(8): 1331-1339. |
| [9] |
Del Mar Lleò M, Benedetti D, Tafi MC, et al. Inhibition of the resuscitation from the viable but non-culturable state in Enterococcus faecalis[J]. Environmental Microbiology, 2007, 9(9): 2313-2320. DOI:10.1111/j.1462-2920.2007.01345.x |
| [10] |
Zhao XH, Zhong JL, Wei CJ, et al. Current perspectives on viable but non-culturable state in foodborne pathogens[J]. Frontiers in Microbiology, 2017, 8: 580. |
| [11] |
Jiang N, Li JQ, Luo LX. Progress in research on the VBNC state of plant pathogenic bacteria[J]. Acta Phytopathologica Sinica, 2013, 43(3): 249-257. (in Chinese) 蒋娜, 李健强, 罗来鑫. 植物病原细菌的VBNC状态研究进展[J]. 植物病理学报, 2013, 43(3): 249-257. DOI:10.3969/j.issn.0412-0914.2013.03.003 |
| [12] |
Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria[J]. FEMS Microbiology Reviews, 2010, 34(4): 415-425. DOI:10.1111/j.1574-6976.2009.00200.x |
| [13] |
Kogure K, Simidu U, Taga N. A tentative direct microscopic method for counting living marine bacteria[J]. Canadian Journal of Microbiology, 1979, 25(3): 415-420. DOI:10.1139/m79-063 |
| [14] |
Créach V, Baudoux AC, Bertru G, et al. Direct estimate of active bacteria: CTC use and limitations[J]. Journal of Microbiological Methods, 2003, 52(1): 19-28. DOI:10.1016/S0167-7012(02)00128-8 |
| [15] |
Luo LX, Walters C, Bolkan H, et al. Quantification of viable cells of Clavibacter michiganensis subsp. michiganensis using a DNA binding dye and a real-time PCR assay[J]. Plant Pathology, 2008, 57(2): 332-337. |
| [16] |
Casini B, Baggiani A, Totaro M, et al. Detection of viable but non-culturable legionella in hospital water network following monochloramine disinfection[J]. Journal of Hospital Infection, 2018, 98(1): 46-52. DOI:10.1016/j.jhin.2017.09.006 |
| [17] |
Rizzotti L, Levav N, Fracchetti F, et al. Effect of UV-C treatment on the microbial population of white and red wines, as revealed by conventional plating and PMA-qPCR methods[J]. Food Control, 2015, 47: 407-412. DOI:10.1016/j.foodcont.2014.07.052 |
| [18] |
Han SN, Jiang N, Lv QY, et al. Detection of Clavibacter michiganensis subsp. michiganensis in viable but nonculturable state from tomato seed using improved qPCR[J]. PLoS One, 2018, 13(5): e0196525. DOI:10.1371/journal.pone.0196525 |
| [19] |
Rohde A, Hammerl JA, Appel B, et al. FISHing for bacteria in food-A promising tool for the reliable detection of pathogenic bacteria?[J]. Food Microbiology, 2015, 46: 395-407. DOI:10.1016/j.fm.2014.09.002 |
| [20] |
Pinto D, Santos MA, Chambel L. Thirty years of viable but nonculturable state research: unsolved molecular mechanisms[J]. Critical Reviews in Microbiology, 2015, 41(1): 61-76. DOI:10.3109/1040841X.2013.794127 |
| [21] |
Zhao F, Bi XF, Hao YL, et al. Induction of viable but nonculturable Escherichia coli O157:H7 by high pressure CO2 and its characteristics[J]. PLoS One, 2013, 8(4): e62388. DOI:10.1371/journal.pone.0062388 |
| [22] |
Chen HL, Chen L, Kang YH, et al. Progress in research on viable but non-culturable state of pathogenic microorganism in water environment[J]. Chinese Journal of Veterinary Drug, 2015, 49(2): 52-57. (in Chinese) 陈亨利, 陈龙, 康元环, 等. 水环境中致病微生物活的非可培养状态研究进展[J]. 中国兽药杂志, 2015, 49(2): 52-57. |
| [23] |
Ravel J, Knight IT, Monahan CE, et al. Temperature-induced recovery of Vibrio cholerae from the viable but nonculturable state: growth or resuscitation?[J]. Microbiology, 1995, 141(2): 377-383. DOI:10.1099/13500872-141-2-377 |
| [24] |
Oliver JD. The viable but non-culturable state in the human pathogen Vibrio vulnificus[J]. FEMS Microbiology Letters, 1995, 133(3): 203-208. DOI:10.1111/j.1574-6968.1995.tb07885.x |
| [25] |
Jiang XP, Chai TJ. Survival of Vibrio parahaemolyticus at low temperatures under starvation conditions and subsequent resuscitation of viable, nonculturable cells[J]. Applied and Environmental Microbiology, 1996, 62(4): 1300-1305. DOI:10.1128/AEM.62.4.1300-1305.1996 |
| [26] |
Oliver JD. The viable but nonculturable state in bacteria[J]. The Journal of Microbiology, 2005, 43: 93-100. |
| [27] |
Zhang SH, Ye CS, Lin HR, et al. UV disinfection induces a Vbnc state in Escherichia coli and Pseudomonas aeruginosa[J]. Environmental Science & Technology, 2015, 49(3): 1721-1728. |
| [28] |
Chen S, Li X, Wang YH, et al. Induction of Escherichia coli into a VBNC state through chlorination/chloramination and differences in characteristics of the bacterium between states[J]. Water Research, 2018, 142: 279-288. DOI:10.1016/j.watres.2018.05.055 |
| [29] |
Liu JY, Li L, Li B, et al. First study on the formation and resuscitation of viable but nonculturable state and beer spoilage capability of Lactobacillus lindneri[J]. Microbial Pathogenesis, 2017, 107: 219-224. DOI:10.1016/j.micpath.2017.03.043 |
| [30] |
Giagnoni L, Arenella M, Galardi E, et al. Bacterial culturability and the viable but non-culturable (VBNC) state studied by a proteomic approach using an artificial soil[J]. Soil Biology and Biochemistry, 2018, 118: 51-58. DOI:10.1016/j.soilbio.2017.12.004 |
| [31] |
Alexander E, Pham D, Steck TR. The viable-but-nonculturable condition is induced by copper in Agrobacterium tumefaciens and Rhizobium leguminosarum[J]. Applied and Environmental Microbiology, 1999, 65(8): 3754-3756. DOI:10.1128/AEM.65.8.3754-3756.1999 |
| [32] |
Ghezzi JI, Steck TR. Induction of the viable but non-culturable condition in Xanthomonas campestris pv. campestris in liquid microcosms and sterile soil[J]. FEMS Microbiology Ecology, 1999, 30(3): 203-208. DOI:10.1111/j.1574-6941.1999.tb00648.x |
| [33] |
Grey BE, Steck TR. The viable but nonculturable state of Ralstonia solanacearum may be involved in long-term survival and plant infection[J]. Applied and Environmental Microbiology, 2001, 67(9): 3866-3872. DOI:10.1128/AEM.67.9.3866-3872.2001 |
| [34] |
Ordax M, Biosca EG, Wimalajeewa SC, et al. Survival of Erwinia amylovora in mature apple fruit calyces through the viable but nonculturable (VBNC) state[J]. Journal of Applied Microbiology, 2009, 107(1): 106-116. |
| [35] |
Del Campo R, Russi P, Mara P, et al. Xanthomonas axonopodis pv. citri enters the VBNC state after copper treatment and retains its virulence[J]. FEMS Microbiology Letters, 2009, 298(2): 143-148. DOI:10.1111/j.1574-6968.2009.01709.x |
| [36] |
Navarrete F, De La Fuente L. Response of Xylella fastidiosa to zinc: decreased culturability, increased exopolysaccharide production, and formation of resilient biofilms under flow conditions[J]. Applied and Environmental Microbiology, 2014, 80(3): 1097-1107. DOI:10.1128/AEM.02998-13 |
| [37] |
Mock NM, Baker CJ, Aver'yanov AA. Induction of a viable but not culturable (VBNC) state in some Pseudomonas syringae pathovars upon exposure to oxidation of an apoplastic phenolic, acetosyringone[J]. Physiological and Molecular Plant Pathology, 2015, 89: 16-24. DOI:10.1016/j.pmpp.2014.11.006 |
| [38] |
Oliver JD, Hite F, McDougald D, et al. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment[J]. Applied and Environmental Microbiology, 1995, 61(7): 2624-2630. DOI:10.1128/AEM.61.7.2624-2630.1995 |
| [39] |
Imazaki I, Nakaho K. Temperature-upshift-mediated revival from the sodium-pyruvate-recoverable viable but nonculturable state induced by low temperature in Ralstonia solanacearum: linear regression analysis[J]. Journal of General Plant Pathology, 2009, 75(3): 213-226. |
| [40] |
Ordax M, Marco-Noales E, López MM, et al. Survival strategy of Erwinia amylovora against copper: induction of the viable-but-nonculturable state[J]. Applied and Environmental Microbiology, 2006, 72(5): 3482-3488. DOI:10.1128/AEM.72.5.3482-3488.2006 |
| [41] |
Li L, Mendis N, Trigui H, et al. The importance of the viable but non-culturable state in human bacterial pathogens[J]. Frontiers in Microbiology, 2014, 5: 258. |
| [42] |
Baffone W, Citterio B, Vittoria E, et al. Retention of virulence in viable but non-culturable halophilic Vibrio spp.[J]. International Journal of Food Microbiology, 2003, 89(1): 31-39. DOI:10.1016/S0168-1605(03)00102-8 |
| [43] |
Santander RD, Català-Senent JF, Marco-Noales E, et al. In planta recovery of Erwinia amylovora viable but nonculturable cells[J]. Trees, 2012, 26(1): 75-82. DOI:10.1007/s00468-011-0653-8 |
| [44] |
Cappelier JM, Besnard V, Roche SM, et al. Avirulent viable but non culturable cells of Listeria monocytogenes need the presence of an embryo to be recovered in egg yolk and regain virulence after recovery[J]. Veterinary Research, 2007, 38(4): 573-583. DOI:10.1051/vetres:2007017 |
| [45] |
Nosho K, Fukushima H, Asai T, et al. cAMP-CRP acts as a key regulator for the viable but non-culturable state in Escherichia coli[J]. Microbiology, 2018, 164(3): 410-419. DOI:10.1099/mic.0.000618 |
| [46] |
Heim S, Del Mar Lleò M, Bonato B, et al. The viable but nonculturable state and starvation are different stress responses of Enterococcus faecalis, as determined by proteome analysis[J]. Journal of Bacteriology, 2002, 184(23): 6739-6745. DOI:10.1128/JB.184.23.6739-6745.2002 |
| [47] |
Lai CJ, Chen SY, Lin IH, et al. Change of protein profiles in the induction of the viable but nonculturable state of Vibrio parahaemolyticus[J]. International Journal of Food Microbiology, 2009, 135(2): 118-124. DOI:10.1016/j.ijfoodmicro.2009.08.023 |
| [48] |
Meng L, Alter T, Aho T, et al. Gene expression profiles of Vibrio parahaemolyticus in viable but non-culturable state[J]. FEMS Microbiology Ecology, 2015, 91(5): fiv035. |
| [49] |
Zhao F, Wang YT, An HR, et al. New insights into the formation of viable but nonculturable Escherichia coli O157:H7 induced by high-pressure CO2[J]. mBio, 2016, 7(4): e00961-16. |
| [50] |
Pu YY, Li YX, Jin X, et al. ATP-dependent dynamic protein aggregation regulates bacterial dormancy depth critical for antibiotic tolerance[J]. Molecular Cell, 2019, 73(1): 143-156. DOI:10.1016/j.molcel.2018.10.022 |
| [51] |
Postnikova OA, Shao J, Mock NM, et al. Gene expression profiling in viable but nonculturable (VBNC) cells of Pseudomonas syringae pv. syringae[J]. Frontiers in Microbiology, 2015, 6: 1419. |
| [52] |
Traxler MF, Summers SM, Nguyen H, et al. The global, ppGpp-mediated stringent response to amino acid starvation in Escherichia coli[J]. Molecular Microbiology, 2008, 68(5): 1128-1148. DOI:10.1111/j.1365-2958.2008.06229.x |
| [53] |
Liu KQ, Bittner AN, Wang JD. Diversity in (p)ppGpp metabolism and effectors[J]. Current Opinion in Microbiology, 2015, 24: 72-79. DOI:10.1016/j.mib.2015.01.012 |
| [54] |
Mishra A, Taneja N, Sharma M. Viability kinetics, induction, resuscitation and quantitative real-time polymerase chain reaction analyses of viable but nonculturable Vibrio cholerae O1 in freshwater microcosm[J]. Journal of Applied Microbiology, 2012, 112(5): 945-953. DOI:10.1111/j.1365-2672.2012.05255.x |
| [55] |
Magnusson LU, Farewell A, Nyström T. ppGpp: a global regulator in Escherichia coli[J]. Trends in Microbiology, 2005, 13(5): 236-242. DOI:10.1016/j.tim.2005.03.008 |
| [56] |
Boaretti M, Del Mar Lleò M, Bonato B, et al. Involvement of rpoS in the survival of Escherichia coli in the viable but non-culturable state[J]. Environmental Microbiology, 2003, 5(10): 986-996. DOI:10.1046/j.1462-2920.2003.00497.x |
| [57] |
Shidore T, Triplett LR. Toxin-antitoxin systems: implications for plant disease[J]. Annual Review of Phytopathology, 2017, 55: 161-179. DOI:10.1146/annurev-phyto-080516-035559 |
| [58] |
Maisonneuve E, Gerdes K. Molecular mechanisms underlying bacterial persisters[J]. Cell, 2014, 157(3): 539-548. DOI:10.1016/j.cell.2014.02.050 |
| [59] |
Ayrapetyan M, Williams TC, Oliver JD. Bridging the gap between viable but non-culturable and antibiotic persistent bacteria[J]. Trends in Microbiology, 2015, 23(1): 7-13. DOI:10.1016/j.tim.2014.09.004 |
| [60] |
Kim JS, Chowdhury N, Yamasaki R, et al. Viable but non-culturable and persistence describe the same bacterial stress state[J]. Environmental Microbiology, 2018, 20(6): 2038-2048. DOI:10.1111/1462-2920.14075 |
| [61] |
Pedersen K, Christensen SK, Gerdes K. Rapid induction and reversal of a bacteriostatic condition by controlled expression of toxins and antitoxins[J]. Molecular Microbiology, 2002, 45(2): 501-510. |
| [62] |
Korch SB, Hill TM. Ectopic overexpression of wild-type and mutant hipA genes in Escherichia coli: effects on macromolecular synthesis and persister formation[J]. Journal of Bacteriology, 2006, 188(11): 3826-3836. DOI:10.1128/JB.01740-05 |
| [63] |
Christensen-Dalsgaard M, Gerdes K. Two higBA loci in the Vibrio cholerae superintegron encode mRNA cleaving enzymes and can stabilize plasmids[J]. Molecular Microbiology, 2006, 62(2): 397-411. DOI:10.1111/j.1365-2958.2006.05385.x |
| [64] |
Rotem E, Loinger A, Ronin I, et al. Regulation of phenotypic variability by a threshold-based mechanism underlies bacterial persistence[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(28): 12541-12546. DOI:10.1073/pnas.1004333107 |
| [65] |
Demidenok OI, Kaprelyants AS, Goncharenko AV. Toxin-antitoxin vapBC locus participates in formation of the dormant state in Mycobacterium smegmatis[J]. FEMS Microbiology Letters, 2014, 352(1): 69-77. DOI:10.1111/1574-6968.12380 |
| [66] |
Donegan NP, Thompson ET, Fu ZB, et al. Proteolytic regulation of toxin-antitoxin systems by ClpPC in Staphylococcus aureus[J]. Journal of Bacteriology, 2010, 192(5): 1416-1422. DOI:10.1128/JB.00233-09 |
| [67] |
Diago-Navarro E, Hernández-Arriaga AM, Kubik S, et al. Cleavage of the antitoxin of the parD toxin-antitoxin system is determined by the ClpAP protease and is modulated by the relative ratio of the toxin and the antitoxin[J]. Plasmid, 2013, 70(1): 78-85. |
| [68] |
Kusumoto A, Miyashita M, Kawamoto K. Deletion in the C-terminal domain of ClpX delayed entry of Salmonella enterica into a viable but non-culturable state[J]. Research in Microbiology, 2013, 164(4): 335-341. DOI:10.1016/j.resmic.2013.01.011 |
| [69] |
Hengge R. Proteolysis of σS (RpoS) and the general stress response in Escherichia coli[J]. Research in Microbiology, 2009, 160(9): 667-676. DOI:10.1016/j.resmic.2009.08.014 |
| [70] |
Ramamurthy T, Ghosh A, Pazhani GP, et al. Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria[J]. Frontiers in Public Health, 2014, 2: 103. |
| [71] |
Kusumoto A, Asakura H, Kawamoto K. General stress sigma factor RpoS influences time required to enter the viable but non-culturable state in Salmonella enterica[J]. Microbiology and Immunology, 2012, 56(4): 228-237. DOI:10.1111/j.1348-0421.2012.00428.x |
| [72] |
Santander RD, Monte-Serrano M, Rodríguez-Herva JJ, et al. Exploring new roles for the rpoS gene in the survival and virulence of the fire blight pathogen Erwinia amylovora[J]. FEMS Microbiology Ecology, 2014, 90(3): 895-907. DOI:10.1111/1574-6941.12444 |
| [73] |
Smith B, Oliver JD. In situ and in vitro gene expression by Vibrio vulnificus during entry into, persistence within, and resuscitation from the viable but nonculturable state[J]. Applied and Environmental Microbiology, 2006, 72(2): 1445-1451. DOI:10.1128/AEM.72.2.1445-1451.2006 |
| [74] |
Berney M, Hammes F, Bosshard F, et al. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in combination with flow cytometry[J]. Applied and Environmental Microbiology, 2007, 73(10): 3283-3290. DOI:10.1128/AEM.02750-06 |
| [75] |
Wei Q, Minh PNL, Dötsch A, et al. Global regulation of gene expression by OxyR in an important human opportunistic pathogen[J]. Nucleic Acids Research, 2012, 40(10): 4320-4333. DOI:10.1093/nar/gks017 |
| [76] |
Kong IS, Bates TC, Hülsmann A, et al. Role of catalase and oxyR in the viable but nonculturable state of Vibrio vulnificus[J]. FEMS Microbiology Ecology, 2004, 50(3): 133-142. DOI:10.1016/j.femsec.2004.06.004 |
| [77] |
Wang HW, Chung CH, Ma TY, et al. Roles of alkyl hydroperoxide reductase subunit C (AhpC) in viable but nonculturable Vibrio parahaemolyticus[J]. Applied and Environmental Microbiology, 2013, 79(12): 3734-3743. DOI:10.1128/AEM.00560-13 |
| [78] |
Abe A, Ohashi E, Ren HF, et al. Isolation and characterization of a cold-induced nonculturable suppression mutant of Vibrio vulnificus[J]. Microbiological Research, 2007, 162(2): 130-138. DOI:10.1016/j.micres.2006.01.007 |
| [79] |
Mukamolova GV, Kaprelyants AS, Young DI, et al. A bacterial cytokine[J]. Proceedings of the National Academy of Sciences of the United States of America, 1998, 95(15): 8916-8921. DOI:10.1073/pnas.95.15.8916 |
| [80] |
Li ZQ, Zhang YG, Wang YY, et al. A new approach of Rpf addition to explore bacterial consortium for enhanced phenol degradation under high salinity conditions[J]. Current Microbiology, 2018, 75(8): 1046-1054. DOI:10.1007/s00284-018-1489-x |
| [81] |
Ayrapetyan M, Williams TC, Oliver JD. Interspecific quorum sensing mediates the resuscitation of viable but nonculturable Vibrios[J]. Applied and Environmental Microbiology, 2014, 80(8): 2478-2483. DOI:10.1128/AEM.00080-14 |
| [82] |
Moorhead SM, Griffiths MW. Expression and characterization of cell-signalling molecules in Campylobacter jejuni[J]. Journal of Applied Microbiology, 2011, 110(3): 786-800. |
| [83] |
Kan YM, Lyu QY, Jiang N, et al. iTRAQ-based proteomic analyses of the plant-pathogenic bacterium Acidovorax citrulli during entrance into and resuscitation from the viable but nonculturable state[J]. Journal of Proteomics, 2020, 211: 103547. DOI:10.1016/j.jprot.2019.103547 |
 2020, Vol. 47
2020, Vol. 47




