扩展功能
文章信息
- 何苏琴, 文朝慧, 白滨, 荆卓琼, 李青青
- HE Su-Qin, WEN Zhao-Hui, BAI Bin, JING Zhuo-Qiong, LI Qing-Qing
- 甘肃省甜瓜黄萎病的病原鉴定
- Verticillium wilt of melon caused by Verticillium dahliae in Gansu province, China
- 微生物学通报, 2020, 47(3): 718-726
- Microbiology China, 2020, 47(3): 718-726
- DOI: 10.13344/j.microbiol.china.190536
-
文章历史
- 收稿日期: 2019-06-28
- 接受日期: 2019-09-03
- 网络首发日期: 2019-10-11
2. 农业农村部天水作物有害生物科学观测实验站 甘肃 天水 741200;
3. 甘肃出入境检验检疫局检验检疫综合技术中心 甘肃 兰州 730010;
4. 甘肃省农业科学院农业质量标准与检测技术研究所 甘肃 兰州 730070;
5. 农业农村部农产品质量安全风险评估实验室(兰州) 甘肃 兰州 730070
2. Scientific Observing and Experimental Station of Crop Pests in Tianshui, Ministry of Agriculture and Rural Affairs, P.R. China, Tianshui, Gansu 741200, China;
3. Comprehensive Technical Center of Gansu Entry-Exit Inspection and Quarantine Bureau, Lanzhou, Gansu 730010, China;
4. Institute of Agricultural Quality Standards and Testing Technology, Gansu Academy of Agricultural Sciences, Lanzhou, Gansu 730070, China;
5. Laboratory of Quality & Safety Risk Assessment for Agro-products (Lanzhou), Ministry of Agriculture and Rural Affairs, P.R. China, Lanzhou, Gansu 730070, China
大丽轮枝菌(Verticillium dahliae)是一种寄主范围广泛的植物病原真菌,可以侵染数百种有经济价值的植物,包括观赏花卉(松果菊,大丽菊)、蔬菜(马铃薯,莴苣)、纤维作物(棉花)、精油和调料作物(薄荷,月桂)、浆果(树莓,草莓)、果树(阿月浑子树,橄榄树)和观赏树木(枫树,黄栌)等[1-2]。大丽轮枝菌(V. dahliae)和黑白轮枝菌(V. alboatrum)的危害每年可导致全球数十亿美元的农作物损失[3-4]。
虽然大丽轮枝菌引起的植物黄萎病在全球分布广泛,但甜瓜黄萎病仅在地中海地区、欧洲和北美洲有发生的报道[4-6]。
2013年11月在甘肃省兰州市皋兰县的日光温室秋冬茬(8月下旬定植)甜瓜种植棚,发现黄萎症状的甜瓜植株,病株率约为1%。病株叶片黄化、叶缘V字形坏死、叶片萎垂,茎部维管束呈淡黄褐色,根部无明显根腐症状。本研究旨在明确该病害的病原,为病害科学防治提供依据。
1 材料与方法 1.1 主要试剂和仪器Universal Genomic DNA Extraction Kit、PCR扩增引物、Taq DNA聚合酶、琼脂糖凝胶回收试剂盒,宝生物工程(大连)有限公司。HITACHI CT15RE高速离心机,日立公司;梯度PCR扩增仪,ABI公司。
1.2 病害标样采集2013年11月7日,从甘肃省兰州市皋兰县的日光温室采集表现典型黄萎症状的甜瓜病株4株,带回实验室,用于病原菌分离。
1.3 病原菌分离以病株为单位进行病原菌的分离。采用组织分离法,每病株切取茎部变色组织2段(长度约5 mm),经75%乙醇表面消毒5 s,灭菌水冲洗4次,置PDA平板上20 ℃黑暗培养,及时挑取菌落尖端菌丝进行纯化。纯化后的菌株经单孢分离,将单孢菌株接种于PDA斜面,10 ℃保存。
1.4 致病性测定分别于2018年9月22日、11月02日和2019年4月21日,采用胚根接种法进行致病性测定[7]。甜瓜(品种:86-1)种子经55 ℃温汤浸种后,置30 ℃温箱催芽,挑选胚根长10 mm左右的发芽种子播种于盛有灭菌营养土的小花盆中(花盆直径11.5 cm,高7 cm);将菌株GLTG-2、GLTG-5接种于PDA平板中央,20 ℃培养15 d,取直径5 mm的菌丝块贴接于发芽种子的胚根上,每胚根接1枚菌丝块,接菌后覆土(覆土厚度约2 cm),以接种直径5 mm的无菌PDA培养基块为对照。每处理4−5盆,每盆2−5粒种子,常规管理。随时观察各处理发病情况,40−45 d后挖出所有试验处理植株,洗根调查根部症状;每处理取10株,切取根部变色组织(长度约5 mm),进行病原菌的重分离(方法同1.3)。
1.5 病原菌适宜生长温度测定及形态特征观察设置15、20、25和30 ℃ 4个温度。试验菌株在PDA平板上20 ℃黑暗培养14 d后,自菌落边缘取直径5 mm菌饼,接种于PDA平板中央,置不同温度下恒温黑暗培养,重复3皿。培养7 d和14 d时用十字交叉法测量菌落直径。观察不同温度条件下菌落形态。
试验菌株在PDA平板上20 ℃培养7−14 d,观察、测量分生孢子梗、瓶梗、分生孢子和微菌核的形态和大小,每个目标的测量≥30个数值。依据病菌培养特征和显微形态特征进行种类鉴定。
1.6 病原菌分子生物学鉴定 1.6.1 DNA提取及测序取在PDB中20 ℃培养7 d的菌株GLTG-2和GLTG-5的菌丝,利用Universal Genomic DNA Extraction Kit按使用说明提取总DNA。选用真菌通用引物ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′)和ITS4 (5′-TCCTCCGCTTATTGATATGC-3′)扩增其ITS基因[8]。PCR反应体系(25 μL):10×PCR Reaction Buffer 2.5 μL,dNTPs (2.5 mmol/L) 1 μL,引物ITS1/ITS4 (10 μmol/L)各0.5 μL,Taq DNA Polymerase (5 U/μL) 0.3 μL,DNA模板2 μL,ddH2O 18.2 μL。PCR反应条件:94 ℃ 3 min;94 ℃ 30 s,55 ℃ 30 s,72 ℃ 45 min,35个循环;72 ℃ 10 min,4 ℃保存。用1.0%琼脂糖凝胶电泳检测PCR产物,紫外灯下切取凝胶中的目的条带,利用琼脂糖凝胶回收试剂盒按使用说明回收纯化,测序工作委托金唯智生物科技有限公司完成。测序结果在NCBI数据库进行BLASTn分析,并提交GenBank获得登录号。
1.6.2 构建系统发育树基于试验菌株的rDNA-ITS区序列构建系统发育树,从GenBank核酸序列数据库中下载相关菌株的rDNA-ITS序列(表 1),以Gibellulopsis nigrescens为外群,用ClustalX 1.83软件对目的序列进行比对(alignment),比对后的序列用MEGA 6.0软件的NJ法构建系统发育树[9],进行1 000次Bootstrap检验计算系统树中节点的置信度。
| 序列号 Accession No. |
种名 Species |
菌株编号 Strain No. |
寄主 Host |
地理来源 Locality |
| MF149108.1 | Verticillium dahliae | At-13 | Acer truncatum | China |
| MG585719.1 | Verticillium dahliae | Vd16_9 | Ailanthus altissima | Hungary |
| MH392569.1 | Verticillium dahliae | Vd414 | Gossypium sp. | China: Tai’an |
| MH607413.1 | Verticillium dahliae | Vd_BZ2 | Ailanthus altissima | Italy |
| MH856561.1 | Verticillium dahliae | CBS 392.49 | - | - |
| MH862677.1 | Verticillium dahliae | CBS 810.97 | - | - |
| MK028533.1 | Verticillium dahliae | T_OVD-2 | Olea europaea | Pakistan |
| MK093977.1 | Verticillium dahliae | MRHf7 | Solanum tuberosum | Tunisia |
| GQ258661.1 | Verticillium tricorpus | Vt536 | Acanthus mollis subsp. mollis | Italy |
| GQ336792.1 | Verticillium tricorpus | Ls443 | Lactuca sp. | USA |
| KX756153.1 | Verticillium tricorpus | Raph-4 | Raphanus sativus | China |
| AF108473.1 | Gibellulopsis nigrescens | UAMH 6687 | - | - |
| 注:-:不详. Note: -: Unknown. |
||||
病株叶片黄化、叶缘V字形坏死、叶片萎垂,茎部维管束呈淡黄褐色,根部无明显根腐症状(图 1)。

|
| 图 1 甜瓜黄萎病田间症状 Figure 1 Symptoms of verticillium wilt of melon in field 注:A,B:发病萎蔫植株;C,D:病茎横断面;E:病茎纵切面. Note: A, B: Diseased and wilted plants; C, D: Horizontal sections of diseased stems; E: Vertical section of a diseased stem. |
|
|
共分离得到8株产生轮枝状分生孢子梗、无色单细胞分生孢子及黑色微菌核的轮枝菌属菌株。轮枝菌属真菌的病株分出率达100%。
选择显微形态特征相似,但菌落形态和生长速率不同的GLTG-2 (该类型菌株占总分出菌株的25%)和GLTG-5 (该类型菌株占总分出菌株的75%)为代表性菌株进行后续的研究工作。
2.3 致病性在不同的温度和光周期条件下,接种试验呈现出不同的结果:2018年9月22日的试验(低温+短昼长夜),接种后20 d (出苗后12 d),接种处理开始出现萎蔫病株,接种后40 d,接种GLTG-2和GLTG-5的甜瓜苗的萎蔫(枯死)率分别达70%和40%,病株矮化明显,对照未发病;2018年的11月2日的试验(高温+短昼长夜),接种后45 d,接种处理未出现萎蔫及矮化病株,对照也未发病;2019年的4月21日的试验(低温+长昼短夜),接种后40 d,接种处理未出现萎蔫及矮化病株,对照也未发病。洗根检查发现,接种处理的甜瓜根呈现不同程度的黄褐色。接种处理植株根部原接种菌的分出率达80%−100% (表 2、图 2)。
| 菌株编号 Strain No. |
接种日期 Inoculation date |
调查日期 Survey date |
光周期(昼/夜) Photoperoid (day/night) |
温度 Temperature (℃) |
接种株数 Inoculated plant number |
枯萎株率 Wilted plant rate (%) |
根部显症率 Symptomatic root rate (%) |
| GLTG-2 | 2018-09-22 | 2018-11-01 | 11.5 h/12.5 h | 18−24 | 10 | 70.0 | 100.0 |
| GLTG-5 | 2018-09-22 | 2018-11-01 | 11.5 h/12.5 h | 18−24 | 10 | 40.0 | 100.0 |
| CK | 2018-09-22 | 2018-11-01 | 11.5 h/12.5 h | 18−24 | 10 | 0.0 | 0.0 |
| GLTG-2 | 2018-11-02 | 2018-12-17 | 11 h/13 h | 22−28 | 20 | 0.0 | 100.0 |
| GLTG-5 | 2018-11-02 | 2018-12-17 | 11 h/13 h | 22−28 | 20 | 0.0 | 100.0 |
| CK | 2018-11-02 | 2018-12-17 | 11 h/13 h | 22−28 | 20 | 0.0 | 0.0 |
| GLTG-2 | 2019-04-21 | 2019-05-31 | 13 h/11 h | 18−24 | 20 | 0.0 | 100.0 |
| GLTG-5 | 2019-04-21 | 2019-05-31 | 13 h/11 h | 18−24 | 20 | 0.0 | 100.0 |
| CK | 2019-04-21 | 2019-05-31 | 13 h/11 h | 18−24 | 20 | 0.0 | 0.0 |

|
| 图 2 人工接种后40 d甜瓜发病症状 Figure 2 Symptoms of melon for 40 d after artificial inoculation 注:A:接种GLTG-2;B、D:接种GLTG-5;C、E:对照.试验条件:温度:18−24 ℃;光周期:昼/夜=11.5 h/12.5 h. Note: A: Inoculated GLTG-2; B, D: Inoculated GLTG-5; C, E: Control. Test conditions: temperature: 18−24 ℃; Photoperoid: day/night= 11.5 h/12.5 h. |
|
|
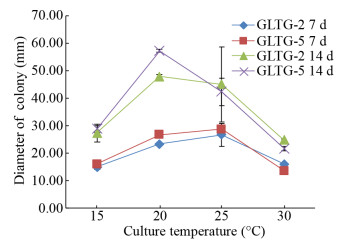
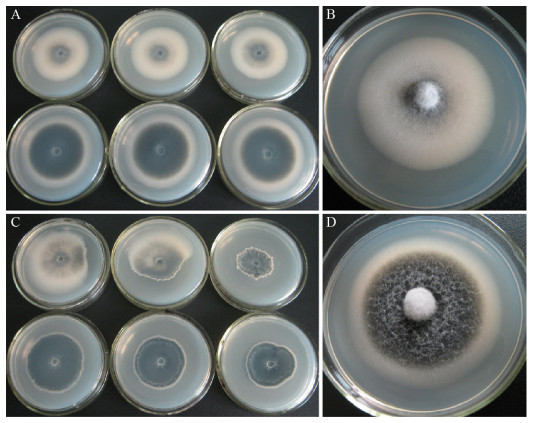
菌株GLTG-2和GLTG-5在PDA平板上培养7 d,25 ℃下平均菌落直径最大;培养14 d,20 ℃下的平均菌落直径最大(图 3)。菌落初白色,随着微菌核的形成,渐呈不同程度的黑色,气生菌丝白色稀疏,菌落边缘整齐(20 ℃培养);25 ℃培养14 d,菌株GLTG-2和GLTG-5的菌落均出现不同程度的变异(图 4)。
|
| 图 3 温度对菌株GLTG-2和GLTG-5菌丝生长的影响 Figure 3 Effect of temperature on mycelial growth of isolate GLTG-2 and GLTG-5 注:PDA培养基,黑暗培养. Note: Culture on PDA in the dark. |
|
|
|
| 图 4 菌株GLTG-2和GLTG-5在PDA培养基上培养14 d的菌落形态 Figure 4 Colony morphology of isolate GLTG-2 and GLTG-5 cultured 14 d on PDA medium 注:A:20 ℃培养,菌落背面,上排为GLTG-2,下排为GLTG-5;B:GLTG-2菌落正面,20 ℃培养;C:25 ℃培养,菌落背面,上排为GLTG-2,下排为GLTG-5;D:GLTG-5菌落正面,20 ℃培养.培养皿内径72 mm. Note: A: Back of the colony, upper row for GLTG-2, lower row for GLTG-5, at 20 ℃; B: Colony front of GLTG-2, at 20 ℃; C: Back of the colony, upper row for GLTG-2, lower row for GLTG-5, at 25 ℃; D: Colony front of GLTG-5, at 20 ℃. Petri dish inner diameter 72 mm. |
|
|
分生孢子单细胞,无色,卵圆形、长椭圆形或杆状,分生孢子形状和大小的变幅较大;分生孢子梗无色,直立或匍匐生长,单生、对生或1至多层轮生,每层轮枝上可生3−6个小梗,少数生侧枝;小梗为细长的瓶梗;微菌核黑色,葚孢型,多个微菌核可相互连接,难分彼此(图 5,表 3)。

|
| 图 5 菌株GLTG-2和GLTG-5的显微形态特征 Figure 5 Microscopic features of V. dahliae isolate GLTG-2 and GLTG-5 注:A、B、E、F:微菌核;C、G:分生孢子梗;D、H:分生孢子. A−D:菌株GLTG-2;E−H:菌株GLTG-5. PDA培养基上20 ℃培养14 d. Note: A, B, E, F: Microsclerotium; C, G: Conidiophores; D, H: Conidium. A−D: Isolate GLTG-2; E−H: Isolate GLTG-5. Cultured on PDA medium 14 d at 20 ℃. |
|
|
| Microscopic features | GLTG-2 | GLTG-5 |
| 分生孢子梗(长度) Conidiophore (Length) (μm) |
15.31−571.20 | 40.8−652.8 |
| 瓶梗(长×宽) Phialide (Length×Width) (μm) |
(12.24−41.84)×(1.02−2.04), av. (29.07±8.43)×(1.56±0.26) | (8.45−61.97)×(0.70−2.11), av. (25.40±10.33)×(1.31±0.31) |
| 分生孢子(长×宽) Conidium (Length×Width) (μm) |
(3.06−6.12)×(1.02−2.55), av. (4.05±0.73)×(1.65±0.41) | (2.82−6.34)×(0.70−2.11), av. (4.16±0.91)×(1.29±0.30) |
| 微菌核(长×宽) Microsclerotium (Length×Width) (μm) |
(30.61−115.31)×(17.35−41.84), av. (57.73±19.49)×(25.46±4.93) | (33.62−147.96)×(15.31−36.73), av. (68.14±26.05)×(28.04±4.77) |
通过与大丽轮枝菌及相近种进行形态特征比对[10-11],将菌株GLTG-2和GLTG-5均鉴定为大丽轮枝菌V. dahliae Kleb.。
2.5 病原菌的分子生物学鉴定BLASTn分析结果显示,GLTG-2 (GenBank
登录号MK252095)的rDNA-ITS序列与V. dahliae菌株MRHf7 (GenBank登录号MK093977.1)的序列相似性达99.78%,GLTG-5 (GenBank登录号MK252096)的rDNA-ITS序列与V. dahliae菌株MRHf7和Vd414 (GenBank登录号MH392569.1)的序列相似性达100.00%。相似性比对结果与形态学鉴定结果一致。
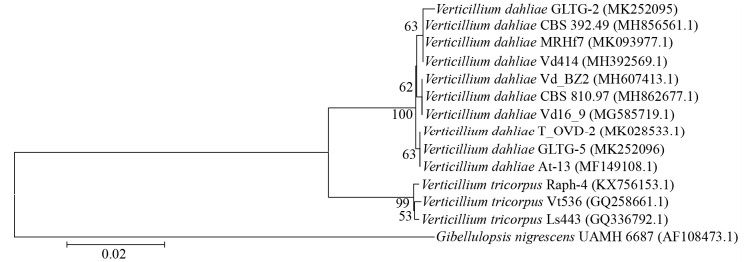
从系统发育树位置来看,两个供试菌株明显地分聚于不同的大丽轮枝菌群组中,GLTG-2与菌株CBS 392.49 (地理来源和寄主不详)、MRHf7 (地理来源:突尼斯;寄主:马铃薯Solanum tuberosum)和Vd414 (地理来源:中国泰安;寄主:棉属Gossypium sp.)聚为一组,GLTG-5与菌株T_OVD-2 (地理来源:巴基斯坦;寄主:油橄榄Olea europaea)和At-13 (地理来源:中国;寄主:五角枫Acer truncatum)聚为一组(图 6)。
|
| 图 6 试验菌株(GLTG-2和GLTG-5)基于rDNA-ITS序列构建的系统发育树 Figure 6 Phylogenetic tree based on rDNA-ITS sequences of tested isolates (GLTG-2 and GLTG-5) of Verticillium 注:分支上的数据表示Bootstrap检验的支持百分率,自展支持值(bootstrap) > 50%的显示在各个进化分支节点上;括号内为GenBank序列号;图中标尺代表位点的碱基替代率. Note: The confidence values over 50% from 1 000 replicate bootstrap samplings are shown at each node. GenBank accession numbers are shown in the parentheses. Scale bar indicates the base substitution rate. |
|
|
经病原菌分离、科赫氏法则鉴定、病原菌形态学和分子生物学分析,明确了兰州市皋兰县日光温室秋冬茬甜瓜黄萎病的病原为大丽轮枝菌(V. dahliae)。这是V. dahliae侵染甜瓜引起黄萎病在我国及亚洲地区的首次报道。
V. dahliae是一个种内遗传多样性丰富的种类,不同分离物在寄主范围、形态特征、致病性和致病类型等方面都存在明显差异[12-16]。病菌的培养特征受培养条件和培养时间的影响而变化[17],在添加NaCl的PDA平板上25 ℃培养7 d,NaCl浓度8−10 g/L时V. dahliae微菌核的产生量较NaCl浓度0−6 g/L时明显增加[18]。
V. dahliae的致病性和寄主植物的抗病性还受到环境温度和光周期的影响,短日光照周期可增加马铃薯感病和耐病品种的病害严重度[19];光周期对番茄黄萎病也影响显著,病害症状在光周期为4 h时发展更迅速、更明显,与光周期为8、12或16 h相比,感病品种的发病率也更高[20];向日葵黄萎病的症状在长日(16 h光照、8 h黑暗)下比在短日(10 h光照、14 h黑暗)下出现得更快、更严重[21];橄榄树黄萎病落叶型和非落叶型病菌的发育最佳土壤温度分别为20−24 ℃和16−20 ℃,土壤温度高于28 ℃,黄萎病的扩展速度明显降低[22];我国华中地区的落叶型病菌能较好地适应高温,在25−30 ℃下对棉花有较强的侵染力[23]。
在突尼斯,黄萎病已成为沿海地区温室甜瓜生产的一大威胁,但甜瓜黄萎病仅发生在温室晚秋栽培(8月种植,次年1月至6月成熟收获)中;夏季栽培(6月至8月成熟)的甜瓜,即使是种植在已知被V. dahliae感染的土壤中,也未见到发生黄萎病[24]。
皋兰县甜瓜黄萎病的发生特点与突尼斯[24]相似,也只在秋冬茬栽培的日光温室甜瓜中发现。在本研究中,人工接种试验结果显示出温度和光周期对甜瓜黄萎病的显著影响,虽然在3组条件下,甜瓜的根都可被试验菌株GLTG-2和GLTG-5侵染,但只在低温(18−24 ℃)和短昼长夜(昼/夜= 11.5 h/12.5 h)条件下,甜瓜植株的地上部才表现出黄萎症状,这可能也是该病害发生区域受限的原因所在。目前该病害在甘肃省内的发生和危害情况尚待开展更广泛的调查;进一步开展甜瓜黄萎病发生所需温度和光周期的阈值范围、病菌寄主范围等方面的研究将有助于了解甜瓜黄萎病的发生规律,为病害科学防控奠定基础。
| [1] |
Weiland J. An aversion to Verticillium[J]. Digger, 2013(2): 33-37. |
| [2] |
Jiménez-Díaz RM, Cirulli M, Bubici G, et al. Verticillium wilt, a major threat to olive production: current status and future prospects for its management[J]. Plant Disease, 2012, 96(3): 304-329. |
| [3] |
Klosterman SJ, Atallah ZK, Vallad GE, et al. Diversity, pathogenicity, and management of Verticillium species[J]. Annual Review of Phytopathology, 2009, 47: 39-62. |
| [4] |
Pegg GF, Brady BL. Verticillium Wilts[M]. New York: CABI Publishing, 2002: 1-432.
|
| [5] |
McKeen CD, Thorpe HJ. Pathogenic species of Verticillium in horticultural crops and weeds in southwestern Ontario[J]. Canadian Journal of Plant Science, 1973, 53(3): 615-622. |
| [6] |
Jabnoun-Khiareddine H, Daami-Remadi M, Ayed F, et al. First report of verticillium wilt of melon caused by Verticillium dahliae in Tunisia[J]. Plant Pathology, 2007, 56(4): 726. |
| [7] |
Miao ZJ, He SQ, Zhang XM, et al. Pathogens of root internal discoloration (black heart disease) in radish in Xining City of Qinghai Province, China[J]. Mycosystema, 2018, 37(4): 444-455. (in Chinese) 苗增建, 何苏琴, 张晓梅, 等. 西宁市萝卜黑心病的病原[J]. 菌物学报, 2018, 37(4): 444-455. |
| [8] |
White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics[A]//Innis MA, Gelfand DH, Sninsky JJ, et al. PCR Protocols: A Guide to Methods and Applications[M]. San Diego: Academic Press, 1990: 315-322
|
| [9] |
Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0[J]. Molecular Biology and Evolution, 2013, 30(12): 2725-2729. |
| [10] |
Smith HC. The morphology of Verticillium albo-atrum, V. dahliae, and V. tricorpus[J]. New Zealand Journal of Agricultural Research, 1965, 8(3): 450-478. |
| [11] |
Goud JKC, Termorshuizen AJ, Gams W. Morphology of Verticillium dahliae and V. tricorpus on semi-selective media used for the detection of V. dahliae in soil[J]. Mycological Research, 2003, 107(7): 822-830. |
| [12] |
Skotland CB. Pathogenic and nonpathogenic Verticillium species from south central Washington[J]. Phytopathology, 1971, 61(4): 435-436. |
| [13] |
Jabnoun-Khiareddine H, Daami-Remadi M, Barbara DJ, et al. Morphological variability within and among Verticillium species collected in Tunisia[J]. Tunisian Journal of Plant Protection, 2010, 5(1): 19-38. |
| [14] |
Bhat RG, Subbarao KV. Host range specificity in Verticillium dahliae[J]. Phytopathology, 1999, 89(12): 1218-1225. |
| [15] |
Berbegal M, Ortega A, Jiménez-Gasco MM, et al. Genetic diversity and host range of Verticillium dahliae isolates from artichoke and other vegetable crops in Spain[J]. Plant Disease, 2010, 94(4): 396-404. |
| [16] |
Ispahani SK, Goud JC, Termorshuizen AJ, et al. Host specificity, but not high-temperature tolerance, is associated with recent outbreaks of Verticillium dahliae in chrysanthemum in the Netherlands[J]. European Journal of Plant Pathology, 2008, 122(3): 437-442. |
| [17] |
Tian L, Wang KR, Lu JY. Variation and mechanism of morphology pathogenicity of Verticillium dahliae and V. alboatrum[J]. Acta Agriculturae Boreali-occidentalis Sinica, 1999, 8(4): 106-109. (in Chinese) 田黎, 王克荣, 陆家云. 轮枝菌(Verticillium dahliae和V. alboatrum)的形态、致病力变异及变异机制[J]. 西北农业学报, 1999, 8(4): 106-109. |
| [18] |
Daami-Remadi M, Oun HB, Souissi A, et al. Effects of saline irrigation water on verticillium wilt severity and tomato growth[J]. Plant Stress, 2009, 3(1): 40-48. |
| [19] |
Tsror L, Livescu L, Nachmias A. Effect of light duration and growth season on verticillium wilt in potato[J]. Phytoparasitica, 1990, 18(4): 331-339. |
| [20] |
Jones JP, Crill P, Volin RB. Effect of light duration on verticillium wilt of tomato[J]. Phytopathology, 1975, 65(5): 647-648. |
| [21] |
Sackston WE, Sheppard JW. Effect of day length on reaction of sunflowers to verticillium wilt[J]. Canadian Journal of Botany, 1973, 51(1): 23-26. |
| [22] |
Calderón R, Lucena C, Trapero-Casas JL, et al. Soil temperature determines the reaction of olive cultivars to Verticillium dahliae pathotypes[J]. PLoS One, 2014, 9(10): e110664. |
| [23] |
Xu F, Yang L, Zhang J, et al. Effect of temperature on conidial germination, mycelial growth and aggressiveness of the defoliating and nondefoliating pathotypes of Verticillium dahliae from cotton in China[J]. Phytoparasitica, 2012, 40(4): 319-327. |
| [24] |
Jabnoun-Khiareddine H, Daami-Remadi M, Ayed F, et al. Incidence of Verticillium wilt of melon in Tunisia[J]. The African Journal of Plant Science and Biotechnology, 2007, 1(1): 10-15. |
 2020, Vol. 47
2020, Vol. 47




