扩展功能
文章信息
- 王浩, 吴爱姣, 刘保兴, 刘润进, 陈应龙
- WANG Hao, WU Ai-Jiao, LIU Bao-Xing, LIU Run-Jin, CHEN Ying-Long
- 菌根真菌多样性与植物多样性的相互作用研究进展
- Interactions between mycorrhizal fungal diversity and plant diversity: a review
- 微生物学通报, 2020, 47(11): 3918-3932
- Microbiology China, 2020, 47(11): 3918-3932
- DOI: 10.13344/j.microbiol.china.190956
-
文章历史
- 收稿日期: 2019-11-18
- 接受日期: 2020-01-02
- 网络首发日期: 2020-02-21
2. 西北农林科技大学水土保持研究所 陕西 杨凌 712100;
3. 中国科学院大学 北京 100049;
4. 青岛农业大学菌根生物技术研究所 山东 青岛 266109;
5. The UWA Institute of Agriculture, and School of Agriculture and Environment, The University of Western Australia, Perth WA 6009, Australia
2. Institute of Soil and Water Conservation, Northwest A & F University, Yangling, Shaanxi 712100, China;
3. University of Chinese Academy of Sciences, Beijing 100049, China;
4. Institute of Mycorrhizal Biotechnology, Qingdao Agricultural University, Qingdao, Shandong 266109, China;
5. The UWA Institute of Agriculture, and School of Agriculture and Environment, The University of Western Australia, Perth WA 6009, Australia
菌根共生体是自然或半自然生态系统中最丰富和重要的生命有机体。在陆生维管植物中,72%和2%可以分别形成丛枝菌根(arbuscular mycorrhizas,AM)和外生菌根(ectomycorrhizal mycorrhizas,ECM),1.5%形成欧石南类菌根,10%形成兰科菌根,另外,约7%的菌根分类地位尚不明确(有报道不形成或形成非典型的菌根结构),只有8%为非菌根(受根系特征、生长环境等影响)[1]。尽管少数植物根系不形成菌根,但能与其他植物内生真菌形成共生体,因此,植物根系与真菌的共生是普遍的。
菌根真菌帮助寄主植物获取更多或难利用的无机和有机养分,寄主植物回馈自身固定的碳源于真菌,从而维持真菌正常的生活史、共生体构建及其功能运作。这一共生关系不仅对植物从水生向陆生的演化发挥了重要作用,而且菌根真菌与植物不同营养级之间的互作影响生态系统中两者的共存、多样性维持与生产力,以及资源的转移循环等[2-4]。在菌根系统中,不同的菌根真菌可以调节养分的数量与质量影响寄主植物的养分吸收、生长发育,相反,不同寄主植物也能利用碳源供给的变化影响菌根真菌菌丝生长、分枝、侵染定殖及产孢[5]。因而,菌根真菌多样性与植物多样性的相互作用,驱动着生态系统过程与功能。一方面,菌根真菌多样性能决定植物多样性、生态系统生产力与变异性[6-8];另一方面,寄主植物多样性在某种程度上也调控着菌根真菌多样性及其群落组成结构与功能[9-10]。鉴于AM和ECM是菌根系统中最普遍的两种共生类型,本文归纳了丛枝菌根真菌(AMF)或外生菌根真菌(ECMF)多样性与植物多样性的相互作用特点,及可能存在的影响因素与机制,旨在为相关研究提供可借鉴的思路。
1 菌根真菌与寄主植物二者物种多样性的比较全球已描述的AMF为314种,隶属于球囊菌门1纲4目11科25属(http://www.amf-phylogeny.com,截止2019年5月30日),其中,以球囊霉属(Glomus)的菌种数最多,分布最广,是AMF优势属。根据rDNA序列多态性推测,AMF物种数比形态描述的种数可能高出10倍[11],并不断有新种或新科属的发表[12-13],但AMF的寄主植物超过20万种[14];隶属于担子菌纲与子囊菌纲的ECMF约2万种[15],其寄主包括松科、壳斗科、龙香脑科、桦木科、杨柳科和柏科等木本植物和部分草本植物6 000−7 000种[16],很显然,AMF的物种多样性显著低于其寄主的物种多样性,而ECMF的物种多样性则高于其寄主植物的物种多样性。
由此可见,AMF是寄主“广适型”,相对而言,ECMF是寄主“专化型”。这种差异现象的可能解释是:(1) ECMF具有多元发生和有性繁殖,AMF为单元发生且缺失有性繁殖;(2) ECMF较高的物种丰富度归因于其具有获取多样性的异质土壤资源的特性,而AMF主要吸收无机养分(3)低水平AMF物种多样性还因为其物种形成中的低选择压(或缺失通过寄主专化性以形成物种)。尽管AMF物种多样性低于ECMF,但与AMF共生的植物存在极高的物种丰富度,造成AMF与ECMF的寄主植物物种多样性差异的原因尚不清楚,特别是AMF也能侵染少数ECMF的寄主植物形成AM。因此,菌根真菌与寄主植物的共生演化机制值得深入探究。
2 植物多样性对菌根真菌多样性的影响 2.1 植物物种多样性对菌根真菌多样性的影响在明尼苏达州3年田间试验中,在含16种植物的样方中AMF产孢量比单种植物样方中的多30%−150%,孢子体积增大40%−70%,表明植物物种多样性影响孢子数量和体积,并且具大孢子AMF [巨孢囊霉(Gigaspora spp.)和盾巨孢囊霉(Scutellospora spp.)]的孢子密度随寄主植物物种多样性增加而显著升高,而具小孢子AMF [毛氏无梗囊霉(Acaulospora morrowiae)、根内根孢囊霉(Rhizophagus intraradices)和类球囊霉(Paraglomus spp.)]的孢子密度对植物物种多样性的反应各异[17]。Chen等对我国亚热带地区浙江常山县的3年田间试验发现,杂草物种丰富度的增加(0、4、8和12)直接影响土壤碳与氮含量以及显著提高了植物生物量积累,而且AMF孢子数目随杂草丰富度增加而显著增加[18]。Landis等对美国威斯康星州橡树热带稀树草原的考察表明,植物物种多样性和AMF物种多样性之间存在正相关,而且该相关性随土壤肥力提高而增强[19]。在不同生境中,热带雨林的AMF物种多样性最高,其次为草原和温带森林,最后为农田与污染区域,农田中低的AMF物种多样性与低的植物物种多样性相关[20]。Ehinger等的研究还表明,根内根孢囊霉的相同菌株在不同磷水平下接种不同种寄主植物时,经过几代后菌株生长适合性相关特征和遗传组成发生改变,并发展形成不同的基因型[21],表明寄主植物多样性与环境因素能共同影响AMF的遗传变异与基因型多样性。寄主植物物种(尤其在属水平上)多样性与ECMF物种多样性之间也具有显著正相关关系[22]。
2.2 植物基因型多样性对菌根真菌多样性的影响Siefert等对全球629个植物群落与36个功能特征进行Meta分析表明,植物的平均种内特征变异(intraspecific trait variation,ITV)分别占群落内和群落间总特征变异的25%和32%,并且ITV表现为个体水平(如株高)大于器官水平,叶片养分(如氮、磷含量)大于叶片形态(如叶面积、厚度)[23]。植物种内不同基因型之间具有显著的形态和生理特征变异[24],而且其表型变异可能高于种间,相比物种多样性,种内基因型多样性对决定群落组成与结构同等重要[25]。
植物基因型多样性较高的群落能维持更高的植物物种多样性[26-27],Johnson等的研究表明,尽管基因型多样性高的植物种群根系定殖的菌根真菌物种丰富度较低,但植物基因型多样性通过间接作用于植物物种多样性而影响菌根真菌物种多样性[28]。rDNA ITS (internal transcribed spacer)序列相似性分析表明,欧洲云杉(Picea abies)的不同基因型直接或间接影响ECMF多样性和其群落组成,ECMF多样性及其群落组成与寄主植物的生长速率(生物量)相关[29]。Lamit等也发现,不同的杨树(Populus)基因型影响ECMF侵染率、担子菌门(Basidiomycota)真菌与子囊菌门(Ascomycota)真菌的比率以及群落组成[30]。不难推测,植物种内基因型多样性可以直接通过个体水平上关键特征变化和间接通过引起物种多样性变化,进而影响菌根真菌多样性。
2.3 植物功能群多样性对菌根真菌多样性的影响功能群可根据植物所利用资源的类型或对特定生态因子变化的响应的不同而划分。Torrecillas等调查发现,在同一区域内生长的一年生植物红雀麦(Bromus rubens)根系的AMF多样性高于多年生草本植物Brachypodium retusum,不同功能群的植物根系定殖的菌根真菌群落组成存在差异[31]。但在同一半干旱石膏土壤中生长的两种多年生植物Herniaria fruticosa和狗舌草耳廓(Senecio auricula)的根系具有不同的AMF群落组成,并且各植物根系的AMF多样性均高于一年生植物红雀麦[32],这种差异现象可能源于AMF侵染定殖的季节变化;豆科植物和非豆科植物根系的AMF群落组成也不同[33],Zheng等针对青藏高原高寒草甸的研究还发现,在长期单一种植中,多年生植物垂穗披碱草(Elymus nutans)根系的AMF多样性显著高于一年生植物燕麦(Avena sativa)和箭舍豌豆(Vicia sativa)[34]。然而,一年生植物与多年生植物Nassella pulchra的间作(植物功能群多样性的增加)对Nassella pulchra根系AMF群落组成产生影响[35]。利用SSU rRNA的RFLP序列分析,Chifflot等发现杨树与大豆间作比传统人工杨树林具有更高的AMF物种多样性,植物功能群多样性的增加使AMF的物种丰富度提高[36]。
3 植物多样性影响菌根真菌多样性的可能因素 3.1 寄主植物的偏好性末端限制性片段长度多态性(terminal-restriction fragment length polymorphism,T-RFLP)分析显示,同一试验小区生长的3种禾草植物毛状剪股颖(Agrostis capillaris)、紫羊茅(Festuca rubra)和早熟禾(Poa pratensis)根系AMF群落组成的多样性水平显著不同,证实了存在寄主对AMF的偏好性[37]。对马达加斯加岛和乌干达半干旱地区辣木(Moringa spp.)根系AMF群落结构进行RFLP和系统发生分析表明,根内根孢囊霉和弯丝硬囊霉(Sclerocystis sinuosa)是该地区的常见种[38]。爱尔兰境内白车轴草(Trifolium repens)和黑麦草(Lolium perenne)根系定殖不同的AMF群落[39]。西班牙东南地中海气候的半干旱草原中5种一年生草本植物和1种多年生草本植物根系的AMF群落不同,聚类分析显示寄主植物对AMF具有偏好性[40]。另外,在爱沙尼亚中部针叶林和阔叶林交错带寄主植物根系具有显著不同的AMF群落[41]。虽然AMF具有寄主广适性,24%的全球取样位点中寄主植物根系定殖不同的AMF群落[42]。
寄主植物偏好性也是影响ECMF群落组成的驱动因素之一。ITS-RFLP分析表明地中海地区刺叶栎(Quercus ilex)和草莓树(Arbutus unedo)根系的ECMF物种相似度很低(共有种数低于15%)[43]。Tedersoo等对塔斯马尼亚岛湿硬叶森林ECMF的rDNA-ITS大亚基序列分析发现,寄主植物对ECMF虽没有直接专一性,但表现强偏好性[44]。Carriconde等调查统计新喀里多尼亚地区假山毛榉(Nothofagus aequilateralis)优势种雨林、栎胶木(Arillastrum gummiferum)优势种雨林及其混交林中的ECMF共有28个谱系和311个OTU,发现寄主植物偏好性和密度影响ECMF群落组成,促成雨林中ECMF的高物种多样性[45]。寄主植物偏好性使不同菌根真菌所受碳源或其他利益有所不同,进而影响真菌生存生长,并导致菌根真菌群落组成和多样性的变化。
此外,Oehl等对中欧温带气候区9个草地和7个耕地调查发现,土地利用强度和土壤类型是影响AMF群落组成与结构的主要因素,而植物物种多样性的影响次之[46]。Hazard等的考察发现,爱尔兰境内白车轴草和黑麦草根系定殖不同的AMF群落,并且局部环境因素(主要包括土壤类型与pH、降雨量)显著影响AMF群落组成[39]。在爱沙尼亚Järvselja地区原始森林中测序鉴定的6种寄主植物根系AMF群落组成类似[47],北美北极圈两种共存的植物北极柳(Salix arctica)和山地仙女木(Dryas integrifolia)根系ECMF丰富度相似[48],表明寄主植物以外的环境条件等因素也可能对菌根真菌多样性的变化产生影响,即寄主植物偏好性对菌根真菌多样性的影响也存在环境依赖性。
3.2 寄主植物多样性驱动的初级生产力或其他利益植物物种多样性或基因型多样性决定地上净初级生产力,植物生产力的变化很大程度上影响着菌根真菌生长和多样性的变化[49-50]。光合作用是植物生产力的生理基础和构成植物生产力的主要因素,Bever等发现,寄主植物将光合碳源优先分配给对自身生长更有利的AMF,其多度也相应增加[51]。山毛榉(Fagus sylvatica)的碳源(包括叶片生产和根内储存)供给决定着根系ECMF侵染率与其物种多样性,当光合碳源不足时,根储存碳可能对ECMF生存更为重要并且偏利碳需求弱的ECMF物种[52]。Koorem等的研究也认为,不同寄主植物的光合能力(光合产物量)可能是决定AMF物种多样性格局的驱动因素[53]。de Deyn等则认为,与寄主植物生产力相比,AMF多度对寄主个体特性及其丰富度更敏感,较高的寄主植物物种多样性使AMF多度增加[54]。TaqMan Real-time PCR分析表明,AMF物种多样性主要由植物物种丰富度与功能特征决定,而植物生产力和土壤特性对AMF群落仅有微弱影响[55]。因此推测,由于寄主植物偏好性使不同菌根真菌所接受的碳源或其他利益不同,进而影响菌根真菌生长和其多样性。
3.3 寄主植物的生命周期/生长季利用rRNA RFLP分析表明,寄主植物Tetragastris panamensis幼苗生长过程中,早期定殖的AMF偶见种取代优势种,AMF物种多样性发生下降,不同年龄段幼苗根系具有显著不同的AMF种群,寄主植物的不同年龄可能影响AMF群落组成[56]。寄主蓝铃花(Hyacinthoides non-scripta)在其生长季早期(秋季和冬季)与盾巨孢囊霉属真菌共生,到后期(春季)时,则与无梗囊霉属(Acaulospora)和球囊霉属真菌共生[57]。寄主植物光合碳源供给的季节变化调控AMF群落组成的时间动态,导致冬季和夏季AMF群落组成与结构显著不同[58]。
同样,不同年龄斑克松(Pinus banksiana)群丛的光合碳源分配的变化可能驱动ECMF群落组成的变化[59]。
随时间推移,定殖龙脑香(Shorea leprosula)幼苗根系的ECMF群落中部分ECMF发生消失或相对多度下降[60]。Twieg等利用PCR-RFLP分析不同年龄的花旗松(Pseudotsuga menziesii)和纸皮桦(Betula papyrifera)混交林发现,ECMF多样性在不同年龄的林分中不同:5年的最低,26年的最高,65年的处于稳定水平且群落组成与100年的类似[61]。西班牙北部刺叶栎森林不同季节ECMF相对多度变化不大,但ECMF物种丰富度在秋季最高,夏季最低[62]。不同演替阶段的次生林中ECMF群落组成显著不同,并且年老森林中环境选择和扩散限制决定ECMF群落组成,但幼龄、中龄森林或整个森林中ECMF群落只受环境选择作用的影响[63]。然而,Richard等考察了法国科西嘉岛地中海地区刺叶栎森林的ECMF群落组成,发现寄主刺叶栎年龄没有明显影响其根部ECMF多样性[43]。可见,植物在不同生命周期/生长季的光合能力及碳源供给能力不同,或与环境因素共同作用,而影响菌根真菌多样性。
4 菌根真菌多样性对植物多样性的影响 4.1 菌根真菌物种多样性对植物多样性的影响AMF物种丰富度较低时,AMF的变化导致植物物种组成及群落结构波动剧烈,当AMF物种丰富度增加时,植物磷素吸收、生长量及其多样性均显著提高[6]。Vogelsang等也发现,AMF物种丰富度的增加提高了植物物种生产力和多样性[8]。Koch等则认为,在同一营养级水平上,AMF (或寄主植物)生产力随各自丰富度的增加而增加,但AMF物种多样性和植物物种多样性对双方生产力的效应互不依赖,表明同一营养级类群内存在相似的调控多样性-生产力关系的生态过程;但AMF生产力与寄主植物生产力的无相关性,可能与试验所用系统发生近似的AMF物种有关[64]。目前鲜见ECMF物种多样性影响寄主植物多样性方面的报道。
4.2 菌根真菌基因型多样性对植物多样性的影响AMF具有极高的种内遗传变异性(孢子形成中核的随机分配或不同菌丝融合引起遗传物质交换),无性繁殖时可形成遗传组成或核型不同的孢子[65]即基因型多样性。ECMF也具有丰富的种内基因型多样性,例如,单一欧洲山杨(Populus tremula)个体根系除了存在高水平ECMF物种多样性外,对其中的土生空团菌(Cenococcum geophilum) rRNA基因鉴定发现23个ITS基因型[66]。真菌种群内基因型个体间的相互接触使它们呈现明显不同的形态和生长反应,进而影响其功能特性[67]。Johnson等认为,菌根真菌种内基因型个体间的表型与生理功能存在着高度变异,种内基因型多样性可能在某种程度上类似于物种多样性对植物多样性产生影响[68]。
4.3 菌根真菌功能群多样性对植物多样性的影响Öpik等根据耐逆性差异将AMF区分为不同的功能群,一些AMF (根内根孢囊霉、摩西斗管囊霉等)出现在干扰生境中且产生大量孢子(即耐干扰型),另外一些AMF出现在养分贫瘠和干扰少的生境中,形成菌丝网且很少或不产孢(即耐胁迫型)[20]。事实上,不同菌根真菌的生活史或生活策略不同,例如,不同菌根真菌的菌丝在距植物根系的不同距离/区域获取养分[69],资源利用的空间互补使植物生长得以增强。不仅如此,不同的菌根真菌呈现季节活性与时间演替[70-71],减弱了群落中真菌之间的竞争,致使接种不同AMF的某些植物的最大生长量出现在不同年(月)份[72],菌根真菌随时间推移或选择过程产生(空间、时间及功能)生态位分化并有可能发展形成不同的功能群。
因此,有理由推断菌根真菌功能群多样性的增加可能因包含更多不同的功能群而影响植物的生长、竞争与共存以及多样性,探索菌根真菌功能群多样性对植物多样性影响的研究有待加强。
5 菌根真菌多样性影响植物多样性的可能因素 5.1 菌根真菌的偏好性RFLP分析显示毛状剪股颖和白车轴草根系AMF绝大部分相同,但少数AMF显示对寄主植物的偏好性[73]。并且,具有寄主专一性或偏好性AMF的寄主植物种类范围窄[74]。根内根孢囊霉的不同基因型对不同的寄主植物大豆(Glycine max)、向日葵(Helianthus annuus)和韭葱(Allium porrum)同样存在显著偏好性[75]。当根据真菌孢子多度作为衡量其专一性或偏好性时,极大巨孢囊霉(Gigaspora gigantea)对裂稃草(Schizachyrium scoparium)和桔草(Andropogon gerardii)没有明显偏好性但均增强了它们的生长,小果球囊霉(Glomus microcarpum)偏好裂稃草且抑制寄主生长,幼套近明球囊霉(Claroideoglomus etunicatum)偏好桔草也使其生长受抑[76]。
Ishida等对两个针叶-阔叶混交林8个寄主物种根尖进行ITS-RFLP鉴定发现,有较高比例的ECMF物种具有寄主偏好性[77],不同基因型辐射松(Pinus radiata)根系的ECMF群落组成发生趋异演化,个别优势ECMF-寄主植物之间存在相容性差别或偏好性[78]。非洲热带地区和马达加斯加岛4个不同生态系统调查显示,ECMF对寄主植物仅有弱偏好性,推测在伐林和自然干扰的生态系统中,先锋种叶下珠科(Phyllanthaceae)植物促进之后豆科(Fabaceae)等植物演替出现并为其提供相容性ECMF接种物[79]。此外,南美洲圭亚那波塔罗河上游流域雨林中,ECMF对其优势豆科植物种没有偏好性,并且空间格局主要决定了ECMF群落组成[80]。表明菌根真菌对寄主偏好性也具有环境依赖性。
菌根真菌的偏好性可能导致寄主植物的养分吸收和生长效应的不同(提高或抑制),进而影响植物多样性的发生与形成。
5.2 菌根真菌多样性影响植物生长或竞争力不同种AMF对羽状短柄草(Brachypodium pinnatum)和夏枯草(Prunella vulgaris)的生长、氮磷养分分配及共存比(夏枯草生物量/两者总生物量)的影响不同[81]。3种土著AMF [摩西斗管囊霉(Funneliformis mosseae)、根内根孢囊霉和微丛球囊霉(Glomus microaggregatum)]混合接种5种匐枝植物,与未接种相比,植株磷吸收均提高但生长量增加或减少[82]。AMF不同基因型也引起植物生长的差异性,Koch等利用无菌系统与温室试验表明,根内根孢囊霉的不同基因型之间存在功能差异性(或变异性),使不同寄主植物的生长增强或受抑制[83]。也有研究发现,根内根孢囊霉某一基因型较其他基因型对寄主植物生物量的增加多达5倍[84]。
贫瘠沙壤中,单接种隐多样孢囊霉(Diversispora celata)较4种AMF混合接种对寄主的生长效益相同,表明AMF多样性选择效应的重要性并且受土壤养分制约[85]。Wagg等的研究则认为,菌根真菌物种多样性提高了植物生产力与植物间共存,菌根真菌物种之间的互补和选择效应(取决于土壤条件)对植物生产力的提高幅度分别达82%和85%;而且,在不同土壤肥力下,单接种AMF时植物的生长迥异,较高AMF丰富度能维持植物的稳定生长[85-86],所以,在不同的环境状况下高水平菌根真菌物种多样性可以“保障”植物生产力。另外,Shi等发现,选择效应(最有效AMF种)决定了植物种间竞争力,且使先锋种盐肤木(Rhus chinensis)竞争力胜于演替中后期的朴树(Celtis sinensis)和香樟(Cinnamomum camphora)[87]。
ECMF物种Rhizopogon occidentalis的不同基因型对寄主扭叶松(pinus contorta)的侵染能力与相容性各有不同,能促进或抑制寄主生长[88]。Baxter等研究发现,随ECMF物种丰富度增加,灰色桦(Betula populifolia)根系的总侵染率提高,但ECMF个体侵染率有所下降,植株地上生物量减少而根系生物量及茎叶氮或磷增加,但对植株总生物量的影响没有差异[89]。在低养分条件下,ECMF物种丰富度增加显著提高了垂枝桦(Betula pendula)地上与根系生物量,但对樟子松(Pinus sylvestris)生长的影响不显著;在高养分条件下,ECMF物种多样性与樟子松生长具有负相关,对垂枝桦生长的影响不显著[90]。Hazard等利用盆栽试验表明,ECMF种内基因型丰富度增加提高了樟子松根系生物量、ECMF根尖密度但降低菌丝长度,而ECMF种间物种丰富度却没有显著作用,然而,又发现双色蜡蘑(Laccaria bicolor)基因型丰富度增加对寄主樟子松和真菌生产力没有显著影响,ECMF种内个体效应对寄主植物生产力影响更大[91-92]。总之,与AMF类似,ECMF多样性对寄主植物生长的效应不一且依赖于环境条件。
菌根真菌(尤其AMF)对寄主植物的生长效应还与其系统发生远近相关。温室条件下AMF物种多样性的增加并未提高植物生产力,该试验所用的4种AMF均为球囊霉属[72],但在van der Heijden等1998年试验中使用了5种不同的AMF属且显著促进寄主植物生长[6],因而,可能在属而非种或基因型水平上,AMF多样性对寄主植物生长效应最强[93]。Maherali等研究证实,试验初期当采用巨孢囊霉科(Gigasporaceae)、无梗囊霉科(Acaulosporaceae)和球囊霉科(Glomeraceae)的系统发育关系邻近的8个物种时(相比采用其中1或2个科),接种一年后,AMF群落的实际物种丰富度最高,寄主植物生物量也较高[94],表明不同科(系统发生相对较远) AMF多样性与寄主植物的生长的正相关性。Gosling等通过温室试验表明AMF物种丰富度增加提高了洋葱(Allium cepa)地上部生物量及磷、铜含量,但AMF物种数目超过3种时,其作用变得不显著,其中球囊霉科真菌效应最大,而无梗囊霉属和裂盾囊霉属(Racocetra)真菌的效应不显著,并且还发现当寄主植物受胁迫时AMF物种多样性效益才更为显现[95]。
5.3 植物的菌根依赖性植物群落中不同植物的菌根依赖性与受益不一,一些植物更依赖菌根共生体以维持生长,菌根依赖性强的植物比菌根依赖性弱的植物获益更多[7, 96]。当豆科植物(AMF依赖性强)百脉根(Lotus corniculatus)或禾草植物(AMF依赖性弱)羊茅(Festuca ovina)单一栽培时,AMF接种均能使寄主植物生长增强;混合栽培时,AMF接种增强百脉根的生长和竞争力但抑制羊茅生长,而且,不同AMF基因型对寄主植物竞争力与相对多度的影响程度不同[97]。
Wagg等通过试验发现,AMF接种更利于菌根依赖性强的豆科植物红车轴草(Trifolium pratense)生长,降低多花黑麦草(Lolium multiflorum)对红车轴草的生长抑制,植物间的竞争力差异变小,并且AMF丰富度增加提高了它们的生物量生产及红车轴草的产量[85];在接种AMF时较低植物丰富度即可获得最大生产力,而未接种AMF时则需要较高植物丰富度以提高群落生产力[98]。这表明菌根依赖性强的植物在没有AMF存在时难以有效获取养分,竞争力不敌邻近植物;AMF的出现介导菌根依赖性强的植物对养分的高效获取,提高总的养分可利用水平且缩小植物之间的竞争差异。
事实上,在群落演替早期,AMF促进次优势种的幼苗建成与相对多度,而提高群落植物物种丰富度,而到群落演替中后期AMF可能使植物物种丰富度降低[99-100]。Hartnett等在美国堪萨斯州东北区的老龄高草草原保护区的试验发现,相比C3禾草植物和非禾草植物,C4禾草植物是专性菌根营养型或菌根依赖性更强,AM共生增强C4禾草植物的生长与竞争力,致使该草原C4禾草植物优势度升高从而降低了植物物种均匀度及多样性;然而,杀真菌剂施用由于抑制AMF而降低了优势种C4禾草植物的多度,提高营兼性菌根营养的次优势种即C3禾草和非禾草植物的多度,使整体植物多样性提高,但对地上总生物量没有影响[101]。O’Connor等在澳大利亚南部半干旱草地野外试验中也发现,与对照区相比,施用杀真菌剂使菌根依赖性强的优势种小苜蓿(Medicago minima)的生长及其竞争力降低,促进次优势种的非菌根植物Carrichtera annua和弱菌根植物Salvia verbenaca生长,从而提高了植物物种均匀度和多样性指数[102]。Urcelay等进一步利用模型模拟试验证明,当群落中次优势种菌根依赖时,AMF可能通过增强其生长与竞争力,导致植物多样性提高;当优势种高度菌根依赖时,AMF可能进一步增强其生长与竞争力,导致植物多样性下降[103]。
菌根真菌生长发育中产生广泛的菌丝网(common mycelia networks,CMN),把周围各种各样的植物联结为一体。菌根真菌可能通过CMN在种内/间植物之间转移分配资源(如氮、磷和碳)介导植物生产力/竞争力,寄主植物既可从菌根共生体直接获益,也可通过CMN维持及转运资源来改善邻近植物生长,提高其防御共同天敌或固氮能力(豆科植物)从而间接受益[104],但同一或不同营养级间经由CMN发生的养分“库-源”间转移的认识还极其有限。
6 菌根真菌多样性与植物多样性之间的互作机制通常认为,生物多样性影响生态系统过程与功能的机制主要包括互补效应和选择效应,因此其也适于解释菌根真菌多样性和植物多样性之间的相互作用关系(图 1)。
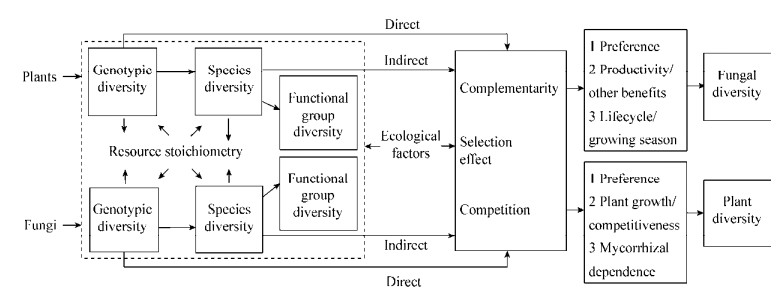
|
| 图 1 菌根真菌多样性与植物多样性的相互作用过程 Figure 1 The processes of interactions between mycorrhizal fungal diversity and plant diversity |
|
|
互补效应包括生态位互补和种间正相互作用。生态位互补是随生物多样性的增加,也提高了功能特征多样性[105],有利于实现在不同空间、时间范围内有效资源的充分利用,促进较高多样性下物种间共存的稳定和较高生产力的维持等[106]。例如,Jansa等用近明球囊霉(Claroideoglomus claroideum)和根内根孢囊霉同时接种韭葱与单接种相比植物的磷吸收更多[107],事实上,AMF物种多样性的增加能增强AMF的菌丝养分获取能力及对不同养分利用的功能互补性,从而维持了较高的植物生产力及其多样性[6]。
种间正相互作用是(尤指在变动或胁迫环境中)一些个体/物种的存在(通过创造有利小生境)能益于另一些个体/物种生存生长[108],从而使生产力及多样性提高。草地冠层建群种通过CMN向下层次优势种转移光合产物可能促进植物物种多样性增加[109]。一些寄主植物通过CMN的维持并转运资源,提高邻近幼苗植物生长发育[110],这对发生干扰后或演替事件时植物的建成非常重要。
选择效应是特定或少数的快速生长(竞争优势)的个体/物种(系统中多样性增加时其出现的概率更大)起决定作用,当快速生长个体/物种保持高产时为正选择效应,若未保持高产时即为负选择效应[111]。Vogelsang等发现,随AMF物种丰富度的增加,个体AMF或选择效应决定了植物物种多样性和生产力的提高且受土壤磷水平依赖[8],Wilkinson等发现ECMF种内基因型或种间物种丰富度增加提高了真菌自身的生产力及CO2释放,且受氮水平影响,ECMF个体的选择与互补作用驱动多样性效应[112-113]。因此,互补和选择效应往往同时发挥作用,在群落建立早期以选择效应为主,且随时间发展,互补效应逐渐增强并占主导[114]。
此外,生物个体之间由于生态位相似而对共有资源、空间等的竞争也能弱化于生态系统过程与功能。根内根孢囊霉和近明球囊霉对寄主植物新疆三肋果(Tripleurospermum inodorum)或拂子茅(Calamagrostis epigejos)侵染率/竞争力相似,共接种(相比单接种)时反而抑制寄主植物生长[115];低磷条件下,植物接种单种AMF (Glomus sp.)时的生物量积累高于同时接种6种AMF,表明AMF种内间的竞争不利于或部分抵制了最有效AMF的效应(即选择效应)[116]。菌根真菌也与寄主植物竞争土壤养分,例如,在有机氮源供给下何氏球囊霉(Glomus hoi)或摩西斗管囊霉与寄主长叶车前(Plantago lanceolata)竞争吸收分解的无机氮[117],根外菌丝体富集和固定大量氮,使寄主植物生长受抑制。一些AMF仍可以侵染非菌根植物(特别是当其处于邻近菌根植物的CMN中时)形成发育不完全的丛枝结构,其根内菌丝吸收非菌根植物的碳源而不提供养分,AMF (或菌根植物)与非菌根植物的互作通常限制后者生长[118]。同时,菌根真菌与植物之间存在的相互偏好性、依赖程度及养分的化学计量分配也影响双方的互作及多样性发生过程。
7 展望菌根共生双方的多样性对维持生态系统的正常服务功能及可持续性具有不可替代的作用。当今人类活动干扰已使众多生物的种类与数量急剧下降或濒临灭绝,也可能导致与其共生的伙伴的数量减少或灭绝,所以对菌根真菌多样性与植物多样性之间的互作关系的研究与总结尤为重要。
根据以往研究工作中存在的问题,对今后研究开展提出以下建议:
(1) 以菌根或菌根真菌孢子/子实体形态学特征为依据的传统分类方法,可能低估菌根真菌的实际物种多样性,例如,产孢菌根真菌会掩盖非产孢菌根真菌的潜在多样性。结合并建立一套基于分子生物学的菌根真菌分离鉴定技术,有利于弥补传统分类的缺陷和完善整个分类体系。
(2) 在菌根真菌多样性的控制试验中,大多数研究仅局限于少数代表性的菌根真菌物种或基因型,未来试验应考虑扩大采用更为广泛的物种,尤其同时涉及种内的不同基因型,其他类型菌根(如欧石楠类菌根真菌在特定范围寄主植物根系中广泛分布)多样性互作的研究与试验也有待探索。
(3) 在人为控制植物物种多样性的田间试验中,低水平植物物种多样性小区控制通过频繁除草来保持,可能利于那些耐干扰如球囊霉属真菌的生存繁殖[55],而且该类试验多是针对幼龄植物群落,不能反映较老的建群群落,随着时间的发展,已稳固定殖的菌根真菌抑制外来菌根真菌的侵染[119],在年老的植物群落中,菌根真菌物种多样性变得稳定[120-121],其物种多样性有可能下降。对其互作关系的探明,亟需发展采用更有效的物种多样性观测与试验手段。
(4) 菌根共生体存在由丛枝菌根向外生菌根或非菌根演化趋势,多功能性的中间型丛枝菌根-外生菌根共生体具有更高生态功能可塑性与功能冗余,非菌根植物丧失菌根共生的直系同源基因(如共生体形成不同阶段的调控因子),然而,在一定条件下非菌根植物仍可被菌根真菌侵染且产生发育不完全或退化的丛枝菌根表型[122]。因此需要尝试利用新的比较系统发生分析方法和试验设计手段以揭示共生不相容性进化的遗传限制和调控共生的冗余机制。
(5) 寄主植物-菌根真菌之间分子对话的信号物质、相互识别与共生的机制可能比所预想的更为复杂多样化。Bogar等对落叶松(Larix occidentalis)幼苗分根后接种3种乳牛肝菌Suillus Spectabilis、S. clintonianus和S. grisellus,尽管3种ECMF单接种时的侵染率和对寄主的效益类似,但S. spectabilis与S. clintonianus或S. grisellus两两接种时,其侵染定殖总受抑制,可能是S. clintonianus或S. grisellus侵染时诱导寄主植物根系特异性防御反应的产生[123],引起寄主植物-真菌选择偏好性和共生相容性维持的遗传变异可能部分来自菌根真菌对植物适应性进化(或反之),其中内在的遗传机制有待系统阐明。
(6) 种内基因型个体间的表型与生理功能也包含着巨大变异性,一定程度上与物种多样性类似的方式直接改变着生态系统过程与功能,同时也塑造着植物-菌根真菌双方表型、生理以及功能特征的多样化。应用基因组测序与种群基因组学可以在关键功能特征上揭示产生个体变异的遗传学基础,并利用群体遗传学途径,发掘这些功能特征在菌根真菌-植物共生关系演变和多样性形成过程的意义。此外,菌根共生在时空上受生态因子(非生物因素和生物因素)的反馈作用[124],从不同学科与组织水平上综合研究菌根真菌-植物-生态因子互作富有挑战却尤为必要。
(7) 有研究认为,植物群落通过植物特征多样性的变化来驱动生态系统功能运作[125]。同样,菌根真菌孢子形态、核糖体基因序列等特征可用于鉴定分类菌根真菌外,其他特征(如侵染速率、侵染率、产孢、泡囊和辅助细胞形成等)也存在功能意义(影响土壤团聚体形成与稳定性、养分循环、植物生长等)[126-128],例如,AMF表型如总菌丝长度、菌丝密度和侵染率与寄主植物紫花苜蓿(Medicago sativa)生长反应变量(茎生物量、磷含量)呈正相关[129]。如同植物特征数据库(即TRY),可以试图建立一套量化菌根真菌特征的方法及数据库,构建菌根真菌特征与其系统发生、功能的联系,例如,根据AMF单位菌丝长度磷吸收的特异性来鉴定AMF[130],以及菌根真菌特征与植物关键特征(株高、比叶面积和叶片干物质含量等)的相关性,该数据库不仅加深菌根真菌-植物之间多样性互作的认识,对于菌根应用、提高作物抗逆性及生产力具有潜在价值。
| [1] |
Brundrett MC, Tedersoo L. Evolutionary history of mycorrhizal symbioses and global host plant diversity[J]. New Phytologist, 2018, 220(4): 1108-1115. DOI:10.1111/nph.14976 |
| [2] |
Feng B, Yang ZL. Ectomycorrhizal symbioses: diversity of mycobionts and molecular mechanisms that entail the development of ectomycorrhizae[J]. Scientia Sinica Vitae, 2019, 49(4): 436-444. (in Chinese) 冯邦, 杨祝良. 外生菌根共生:共生菌根多样性及菌根形成的分子机制[J]. 中国科学:生命科学, 2019, 49(4): 436-444. |
| [3] |
Chomicki G, Weber M, Antonelli A, et al. The impact of mutualisms on species richness[J]. Trends in Ecology & Evolution, 2019, 34(8): 698-711. |
| [4] |
Liu RJ, Wang L. Biological Symbiotics[M]. Beijing: Science Press, 2018: 137-166. (in Chinese) 刘润进, 王琳. 生物共生学[M]. 北京: 科学出版社, 2018: 137-166. |
| [5] |
Wang H, Fang Y, Liu RJ, et al. Recent advances in the studies of nutrient transportation, metabolism, utilization and regulation in arbuscular mycorrhizas[J]. Plant Physiology Journal, 2018, 54(11): 1645-1658. (in Chinese) 王浩, 方燕, 刘润进, 等. 丛枝菌根中养分转运、代谢、利用与调控研究的最新进展[J]. 植物生理学报, 2018, 54(11): 1645-1658. |
| [6] |
van der Heijden MGA, Klironomos JN, Ursic M, et al. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity[J]. Nature, 1998, 396(6706): 69-72. DOI:10.1038/23932 |
| [7] |
van der Heijden MGA. Arbuscular mycorrhizal fungi as a determinant of plant diversity: in search of underlying mechanisms and general principles[A]//van der Heijden MGA, Sanders IR. Mycorrhizal Ecology[M]. Heidelberg, Germany: Springer Verlag, 2002: 243-265
|
| [8] |
Vogelsang KM, Reynolds HL, Bever JD. Mycorrhizal fungal identity and richness determine the diversity and productivity of a tallgrass prairie system[J]. New Phytologist, 2006, 172(3): 554-562. DOI:10.1111/j.1469-8137.2006.01854.x |
| [9] |
Johnson D, IJdo M, Genney DR, et al. How do plants regulate the function, community structure, and diversity of mycorrhizal fungi?[J]. Journal of Experimental Botany, 2005, 56(417): 1751-1760. DOI:10.1093/jxb/eri192 |
| [10] |
Liu RJ, Wang FY. Selection of appropriate host plants used in trap culture of arbuscular mycorrhizal fungi[J]. Mycorrhiza, 2003, 13(3): 123-127. DOI:10.1007/s00572-002-0207-4 |
| [11] |
Krüger M, Krüger C, Walker C, et al. Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level[J]. New Phytologist, 2012, 193(4): 970-984. DOI:10.1111/j.1469-8137.2011.03962.x |
| [12] |
Błaszkowski J, Kozłowska A, Niezgoda P, et al. A new genus, Oehlia with Oehlia diaphana comb. nov. and an emended description of Rhizoglomus vesiculiferum comb. nov. in the Glomeromycotina[J]. Nova Hedwigia, 2018, 107(3/4): 501-518. |
| [13] |
Schüßler A, Walker C. Archaeospora ecuadoriana sp. nov. from a mountainous biodiversity hotspot area in Ecuador, and transfer of Palaeospora spainiae to Archaeospora, as A. spainiae comb. nov.[J]. Mycorrhiza, 2019, 29(5): 435-443. DOI:10.1007/s00572-019-00913-2 |
| [14] |
Öpik M, Davison J, Moora M, et al. DNA-based detection and identification of glomeromycota: the virtual taxonomy of environmental sequences[J]. Botany, 2014, 92(2): 135-147. DOI:10.1139/cjb-2013-0110 |
| [15] |
Martin F, Kohler A, Murat C, et al. Unearthing the roots of ectomycorrhizal symbioses[J]. Nature Reviews Microbiology, 2016, 14(12): 760-773. DOI:10.1038/nrmicro.2016.149 |
| [16] |
Tedersoo L, Brundrett MC. Evolution of ectomycorrhizal symbiosis in plants[A]//Tedersoo L. Biogeography of Mycorrhizal Symbiosis[M]. Cham, Switzerland: Springer, 2017: 407-467
|
| [17] |
Burrows RL, Pfleger FL. Arbuscular mycorrhizal fungi respond to increasing plant diversity[J]. Canadian Journal of Botany, 2002, 80(2): 120-130. DOI:10.1139/b01-138 |
| [18] |
Chen X, Tang JJ, Fang ZG, et al. Effects of weed communities with various species numbers on soil features in a subtropical orchard ecosystem[J]. Agriculture, Ecosystems & Environment, 2004, 102(3): 377-388. |
| [19] |
Landis FC, Gargas A, Givnish TJ. Relationships among arbuscular mycorrhizal fungi, vascular plants and environmental conditions in oak savannas[J]. New Phytologist, 2004, 164(3): 493-504. DOI:10.1111/j.1469-8137.2004.01202.x |
| [20] |
Öpik M, Moora M, Liira J, et al. Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe[J]. Journal of Ecology, 2006, 94(4): 778-790. DOI:10.1111/j.1365-2745.2006.01136.x |
| [21] |
Ehinger M, Koch AM, Sanders IR. Changes in arbuscular mycorrhizal fungal phenotypes and genotypes in response to plant species identity and phosphorus concentration[J]. New Phytologist, 2009, 184(2): 412-423. DOI:10.1111/j.1469-8137.2009.02983.x |
| [22] |
Gao C, Shi NN, Liu YX, et al. Host plant genus-level diversity is the best predictor of ectomycorrhizal fungal diversity in a Chinese subtropical forest[J]. Molecular Ecology, 2013, 22(12): 3403-3414. DOI:10.1111/mec.12297 |
| [23] |
Siefert A, Violle C, Chalmandrier L, et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities[J]. Ecology Letters, 2015, 18(12): 1406-1419. DOI:10.1111/ele.12508 |
| [24] |
Whitlock R, Grime JP, Burke T. Genetic variation in plant morphology contributes to the species-level structure of grassland communities[J]. Ecology, 2010, 91(5): 1344-1354. DOI:10.1890/08-2098.1 |
| [25] |
Hughes AR, Inouye BD, Johnson MTJ, et al. Ecological consequences of genetic diversity[J]. Ecology Letters, 2008, 11(6): 609-623. DOI:10.1111/j.1461-0248.2008.01179.x |
| [26] |
Booth RE, Grime JP. Effects of genetic impoverishment on plant community diversity[J]. Journal of Ecology, 2003, 91(5): 721-730. DOI:10.1046/j.1365-2745.2003.00804.x |
| [27] |
Whitlock R, Phil Grime J, Booth R, et al. The role of genotypic diversity in determining grassland community structure under constant environmental conditions[J]. Journal of Ecology, 2007, 95(5): 895-907. DOI:10.1111/j.1365-2745.2007.01275.x |
| [28] |
Johnson D, Anderson IC, Williams A, et al. Plant genotypic diversity does not beget root-fungal species diversity[J]. Plant and Soil, 2010, 336(1/2): 107-111. |
| [29] |
Korkama T, Pakkanen A, Pennanen T. Ectomycorrhizal community structure varies among Norway spruce (Picea abies) clones[J]. New Phytologist, 2006, 171(4): 815-824. DOI:10.1111/j.1469-8137.2006.01786.x |
| [30] |
Lamit LJ, Holeski LM, Flores-Rentería L, et al. Tree genotype influences ectomycorrhizal fungal community structure: ecological and evolutionary implications[J]. Fungal Ecology, 2016, 24: 124-134. DOI:10.1016/j.funeco.2016.05.013 |
| [31] |
Torrecillas E, del Mar Alguacil M, Roldán A. Differences in the AMF diversity in soil and roots between two annual and perennial gramineous plants co-ocurring in a Mediterranean, semiarid degraded area[J]. Plant and Soil, 2012, 354(1/2): 97-106. |
| [32] |
Alguacil MM, Torrecillas E, Roldán A, et al. Perennial plant species from semiarid gypsum soils support higher AMF diversity in roots than the annual Bromus rubens[J]. Soil Biology and Biochemistry, 2012, 49: 132-138. DOI:10.1016/j.soilbio.2012.02.024 |
| [33] |
Scheublin TR, Ridgway KP, Young JPW, et al. Nonlegumes, legumes, and root nodules harbor different arbuscular mycorrhizal fungal communities[J]. Applied and Environmental Microbiology, 2004, 70(10): 6240-6246. DOI:10.1128/AEM.70.10.6240-6246.2004 |
| [34] |
Zheng Y, Chen L, Luo CY, et al. Plant identity exerts stronger effect than fertilization on soil arbuscular mycorrhizal fungi in a sown pasture[J]. Microbial Ecology, 2016, 72(3): 647-658. DOI:10.1007/s00248-016-0817-6 |
| [35] |
Hausmann NT, Hawkes CV. Plant neighborhood control of arbuscular mycorrhizal community composition[J]. New Phytologist, 2009, 183(4): 1188-1200. DOI:10.1111/j.1469-8137.2009.02882.x |
| [36] |
Chifflot V, Rivest D, Olivier A, et al. Molecular analysis of arbuscular mycorrhizal community structure and spores distribution in tree-based intercropping and forest systems[J]. Agriculture, Ecosystems & Environment, 2009, 131(1/2): 32-39. |
| [37] |
Vandenkoornhuyse P, Ridgway KP, Watson IJ, et al. Co-existing grass species have distinctive arbuscular mycorrhizal communities[J]. Molecular Ecology, 2003, 12(11): 3085-3095. DOI:10.1046/j.1365-294X.2003.01967.x |
| [38] |
Yamato M, Ikeda S, Iwase K. Community of arbuscular mycorrhizal fungi in drought-resistant plants, Moringa spp., in semiarid regions in Madagascar and Uganda[J]. Mycoscience, 2009, 50(2): 100-105. DOI:10.1007/S10267-008-0459-8 |
| [39] |
Hazard C, Gosling P, van der Gast CJ, et al. The role of local environment and geographical distance in determining community composition of arbuscular mycorrhizal fungi at the landscape scale[J]. The ISME Journal, 2013, 7(3): 498-508. DOI:10.1038/ismej.2012.127 |
| [40] |
Torrecillas E, Alguacil MM, Roldán A. Host preferences of arbuscular mycorrhizal fungi colonizing annual herbaceous plant species in semiarid mediterranean prairies[J]. Applied and Environmental Microbiology, 2012, 78(17): 6180-6186. DOI:10.1128/AEM.01287-12 |
| [41] |
Öpik M, Metsis M, Daniell TJ, et al. Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest[J]. New Phytologist, 2009, 184(2): 424-437. DOI:10.1111/j.1469-8137.2009.02920.x |
| [42] |
Lekberg Y, Waller LP. What drives differences in arbuscular mycorrhizal fungal communities among plant species?[J]. Fungal Ecology, 2016, 24: 135-138. DOI:10.1016/j.funeco.2016.05.012 |
| [43] |
Richard F, Millot S, Gardes M, et al. Diversity and specificity of ectomycorrhizal fungi retrieved from an old-growth Mediterranean forest dominated by Quercus ilex[J]. New Phytologist, 2005, 166(3): 1011-1023. DOI:10.1111/j.1469-8137.2005.01382.x |
| [44] |
Tedersoo L, Jairus T, Horton BM, et al. Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers[J]. New Phytologist, 2008, 180(2): 479-490. DOI:10.1111/j.1469-8137.2008.02561.x |
| [45] |
Carriconde F, Gardes M, Bellanger JM, et al. Host effects in high ectomycorrhizal diversity tropical rainforests on ultramafic soils in New Caledonia[J]. Fungal Ecology, 2019, 39: 201-212. DOI:10.1016/j.funeco.2019.02.006 |
| [46] |
Oehl F, Laczko E, Bogenrieder A, et al. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities[J]. Soil Biology and Biochemistry, 2010, 42(5): 724-738. DOI:10.1016/j.soilbio.2010.01.006 |
| [47] |
Saks Ü, Davison J, Öpik M, et al. Root-colonizing and soil-borne communities of arbuscular mycorrhizal fungi in a temperate forest understorey[J]. Botany, 2014, 92(4): 277-285. DOI:10.1139/cjb-2013-0058 |
| [48] |
Timling I, Dahlberg A, Walker DA, et al. Distribution and drivers of ectomycorrhizal fungal communities across the North American Arctic[J]. Ecosphere, 2012, 3(11): 111. |
| [49] |
Crutsinger GM, Collins MD, Fordyce JA, et al. Plant genotypic diversity predicts community structure and governs an ecosystem process[J]. Science, 2006, 313(5789): 966-968. DOI:10.1126/science.1128326 |
| [50] |
Zak DR, Holmes WE, White DC, et al. Plant diversity, soil microbial communities, and ecosystem function: are there any links?[J]. Ecology, 2003, 84(8): 2042-2050. DOI:10.1890/02-0433 |
| [51] |
Bever JD, Richardson SC, Lawrence BM, et al. Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism[J]. Ecology Letters, 2009, 12(1): 13-21. |
| [52] |
Druebert C, Lang C, Valtanen K, et al. Beech carbon productivity as driver of ectomycorrhizal abundance and diversity[J]. Plant, Cell & Environment, 2009, 32(8): 992-1003. |
| [53] |
Koorem K, Tulva I, Davison J, et al. Arbuscular mycorrhizal fungal communities in forest plant roots are simultaneously shaped by host characteristics and canopy-mediated light availability[J]. Plant and Soil, 2017, 410(1/2): 259-271. |
| [54] |
de Deyn GB, Quirk H, Bardgett RD. Plant species richness, identity and productivity differentially influence key groups of microbes in grassland soils of contrasting fertility[J]. Biology Letters, 2011, 7(1): 75-78. DOI:10.1098/rsbl.2010.0575 |
| [55] |
KÖnig S, Wubet T, Dormann CF, et al. TaqMan real-time PCR assays to assess arbuscular mycorrhizal responses to field manipulation of grassland biodiversity: effects of soil characteristics, plant species richness, and functional traits[J]. Applied and Environmental Microbiology, 2010, 76(12): 3765-3775. DOI:10.1128/AEM.02951-09 |
| [56] |
Husband R, Herre EA, Young JPW. Temporal variation in the arbuscular mycorrhizal communities colonising seedlings in a tropical forest[J]. FEMS Microbiology Ecology, 2002, 42(1): 131-136. DOI:10.1111/j.1574-6941.2002.tb01002.x |
| [57] |
Merryweather J, Fitter A. The arbuscular mycorrhizal fungi of Hyacinthoides non-scripta I. diversity of fungal taxa[J]. New Phytologist, 1998, 138(1): 117-129. DOI:10.1046/j.1469-8137.1998.00888.x |
| [58] |
Dumbrell AJ, Ashton PD, Aziz N, et al. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing[J]. New Phytologist, 2011, 190(3): 794-804. DOI:10.1111/j.1469-8137.2010.03636.x |
| [59] |
Visser S. Ectomycorrhizal fungal succession in Jack pine stands following wildfire[J]. New Phytologist, 1995, 129(3): 389-401. DOI:10.1111/j.1469-8137.1995.tb04309.x |
| [60] |
See LS, Alexander IJ. The dynamics of ectomycorrhizal infection of Shorea leprosula seedlings in Malaysian rain forests[J]. New Phytologist, 1996, 132(2): 297-305. DOI:10.1111/j.1469-8137.1996.tb01849.x |
| [61] |
Twieg BD, Durall DM, Simard SW. Ectomycorrhizal fungal succession in mixed temperate forests[J]. New Phytologist, 2007, 176(2): 437-447. DOI:10.1111/j.1469-8137.2007.02173.x |
| [62] |
de Román M, de Miguel AM. Post-fire, seasonal and annual dynamics of the ectomycorrhizal community in a Quercus ilex L. forest over a 3-year period[J]. Mycorrhiza, 2005, 15(6): 471-482. DOI:10.1007/s00572-005-0353-6 |
| [63] |
Gao C, Zhang Y, Shi NN, et al. Community assembly of ectomycorrhizal fungi along a subtropical secondary forest succession[J]. New Phytologist, 2015, 205(2): 771-785. DOI:10.1111/nph.13068 |
| [64] |
Koch AM, Antunes PM, Klironomos JN. Diversity effects on productivity are stronger within than between trophic groups in the arbuscular mycorrhizal symbiosis[J]. PLoS one, 2012, 7(5): e36950. DOI:10.1371/journal.pone.0036950 |
| [65] |
Koch AM, Kuhn G, Fontanillas P, et al. High genetic variability and low local diversity in a population of arbuscular mycorrhizal fungi[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(8): 2369-2374. DOI:10.1073/pnas.0306441101 |
| [66] |
Bahram M, Põlme S, Kõljalg U, et al. A single European aspen (Populus tremula) tree individual may potentially harbour dozens of Cenococcum geophilum ITS genotypes and hundreds of species of ectomycorrhizal fungi[J]. FEMS Microbiology Ecology, 2011, 75(2): 313-320. DOI:10.1111/j.1574-6941.2010.01000.x |
| [67] |
Malik M, Vilgalys R. Somatic incompatibility in fungi[A]//Worrall JJ. Structure and Dynamics of Fungal Populations[M]. Dordrecht, the Netherlands: Springer, 1999: 123-138
|
| [68] |
Johnson D, Martin F, Cairney JWG, et al. The importance of individuals: intraspecific diversity of mycorrhizal plants and fungi in ecosystems[J]. New Phytologist, 2012, 194(3): 614-628. DOI:10.1111/j.1469-8137.2012.04087.x |
| [69] |
Thonar C, Schnepf A, Frossard E, et al. Traits related to differences in function among three arbuscular mycorrhizal fungi[J]. Plant and Soil, 2011, 339(1/2): 231-245. |
| [70] |
Oehl F, Sieverding E, Ineichen K, et al. Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms[J]. Agriculture, Ecosystems & Environment, 2009, 134(3/4): 257-268. |
| [71] |
Chen YL, Brundrett MC, Dell B. Effects of ectomycorrhizas and vesicular-arbuscular mycorrhizas, alone or in competition, on root colonization and growth of Eucalyptus globulus and E. urophylla[J]. New Phytologist, 2000, 146(3): 545-555. DOI:10.1046/j.1469-8137.2000.00663.x |
| [72] |
van der Heijden MGA, Streitwolf-Engel R, Riedl R, et al. The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland[J]. New Phytologist, 2006, 172(4): 739-752. DOI:10.1111/j.1469-8137.2006.01862.x |
| [73] |
Vandenkoornhuyse P, Husband R, Daniell TJ, et al. Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem[J]. Molecular Ecology, 2002, 11(8): 1555-1564. DOI:10.1046/j.1365-294X.2002.01538.x |
| [74] |
Helgason T, Merryweather JW, Young JPW, et al. Specificity and resilience in the arbuscular mycorrhizal fungi of a natural woodland community[J]. Journal of Ecology, 2007, 95(4): 623-630. |
| [75] |
Croll D, Wille L, Gamper HA, et al. Genetic diversity and host plant preferences revealed by simple sequence repeat and mitochondrial markers in a population of the arbuscular mycorrhizal fungus Glomus intraradices[J]. New Phytologist, 2008, 178(3): 672-687. DOI:10.1111/j.1469-8137.2008.02381.x |
| [76] |
Castelli JP, Casper BB. Intraspecific AM fungal variation contributes to plant-fungal feedback in a serpentine grassland[J]. Ecology, 2003, 84(2): 323-336. |
| [77] |
Ishida TA, Nara K, Hogetsu T. Host effects on ectomycorrhizal fungal communities: insight from eight host species in mixed conifer-broadleaf forests[J]. New Phytologist, 2007, 174(2): 430-440. DOI:10.1111/j.1469-8137.2007.02016.x |
| [78] |
Hoeksema JD, Hernandez JV, Rogers DL, et al. Geographic divergence in a species-rich symbiosis: interactions between monterey pines and ectomycorrhizal fungi[J]. Ecology, 2012, 93(10): 2274-2285. DOI:10.1890/11-1715.1 |
| [79] |
Tedersoo L, Bahram M, Jairus T, et al. Spatial structure and the effects of host and soil environments on communities of ectomycorrhizal fungi in wooded savannas and rain forests of Continental Africa and Madagascar[J]. Molecular Ecology, 2011, 20(14): 3071-3080. DOI:10.1111/j.1365-294X.2011.05145.x |
| [80] |
Smith ME, Henkel TW, Catherine Aime M, et al. Ectomycorrhizal fungal diversity and community structure on three co-occurring leguminous canopy tree species in a Neotropical rainforest[J]. New Phytologist, 2011, 192(3): 699-712. DOI:10.1111/j.1469-8137.2011.03844.x |
| [81] |
van der Heijden MGA, Wiemken A, Sanders IR. Different arbuscular mycorrhizal fungi alter coexistence and resource distribution between co-occurring plant[J]. New Phytologist, 2003, 157(3): 569-578. DOI:10.1046/j.1469-8137.2003.00688.x |
| [82] |
Sudová R. Different growth response of five co-existing stoloniferous plant species to inoculation with native arbuscular mycorrhizal fungi[J]. Plant Ecology, 2009, 204(1): 135-143. DOI:10.1007/s11258-009-9576-5 |
| [83] |
Koch AM, Croll D, Sanders IR. Genetic variability in a population of arbuscular mycorrhizal fungi causes variation in plant growth[J]. Ecology Letters, 2006, 9(2): 103-110. |
| [84] |
Angelard C, Colard A, Niculita-Hirzel H, et al. Segregation in a mycorrhizal fungus alters rice growth and symbiosis-specific gene transcription[J]. Current Biology, 2010, 20(13): 1216-1221. DOI:10.1016/j.cub.2010.05.031 |
| [85] |
Wagg C, Jansa J, Stadler M, et al. Mycorrhizal fungal identity and diversity relaxes plant-plant competition[J]. Ecology, 2011, 92(6): 1303-1313. DOI:10.1890/10-1915.1 |
| [86] |
Wagg C, Jansa J, Schmid B, et al. Belowground biodiversity effects of plant symbionts support aboveground productivity[J]. Ecology Letters, 2011, 14(10): 1001-1009. DOI:10.1111/j.1461-0248.2011.01666.x |
| [87] |
Shi NN, Gao C, Zheng Y, et al. Arbuscular mycorrhizal fungus identity and diversity influence subtropical tree competition[J]. Fungal Ecology, 2016, 20: 115-123. DOI:10.1016/j.funeco.2015.12.007 |
| [88] |
Hoeksema JD, Piculell BJ, Thompson JN. Within-population genetic variability in mycorrhizal interactions[J]. Communicative & Integrative Biology, 2009, 2(2): 110-112. |
| [89] |
Baxter JW, Dighton J. Ectomycorrhizal diversity alters growth and nutrient acquisition of grey birch (Betula populifolia) seedlings in host-symbiont culture conditions[J]. New Phytologist, 2001, 152(1): 139-149. DOI:10.1046/j.0028-646x.2001.00245.x |
| [90] |
Jonsson LM, Nilsson MC, Wardle DA, et al. Context dependent effects of ectomycorrhizal species richness on tree seedling productivity[J]. Oikos, 2001, 93(3): 353-364. DOI:10.1034/j.1600-0706.2001.930301.x |
| [91] |
Hazard C, Kruitbos L, Davidson H, et al. Contrasting effects of intra- and interspecific identity and richness of ectomycorrhizal fungi on host plants, nutrient retention and multifunctionality[J]. New Phytologist, 2017, 213(2): 852-863. DOI:10.1111/nph.14184 |
| [92] |
Hazard C, Kruitbos L, Davidson H, et al. Strain identity of the ectomycorrhizal fungus Laccaria bicolor is more important than richness in regulating plant and fungal performance under nutrient rich conditions[J]. Frontiers in Microbiology, 2017, 8: 1874. DOI:10.3389/fmicb.2017.01874 |
| [93] |
Hart MM, Klironomos JN. Diversity of arbuscular mycorrhizal fungi and ecosystem functioning[A]//van der Heijden MGA, Sanders IR. Mycorrhizal Ecology[M]. Heidelberg, Germany: Springer Verlag, 2002: 225-242
|
| [94] |
Maherali H, Klironomos JN. Influence of phylogeny on fungal community assembly and ecosystem functioning[J]. Science, 2007, 316(5832): 1746-1748. DOI:10.1126/science.1143082 |
| [95] |
Gosling P, Jones J, Bending GD. Evidence for functional redundancy in arbuscular mycorrhizal fungi and implications for agroecosystem management[J]. Mycorrhiza, 2016, 26(1): 77-83. DOI:10.1007/s00572-015-0651-6 |
| [96] |
Wang XJ, Wang Q, Jin L, et al. Arbuscular mycorrhizal fungi in the rhizosphere soil of poisonous plants depressed the growth of pasture grasses in the Tibetan Plateau Alpine meadow[J]. Ecosystem Health and Sustainability, 2019, 5(1): 226-236. DOI:10.1080/20964129.2019.1673215 |
| [97] |
Scheublin TR, van Logtestijn RSP, van der Heijden MGA. Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species[J]. Journal of Ecology, 2007, 95(4): 631-638. |
| [98] |
Klironomos JN, McCune J, Hart M, et al. The influence of arbuscular mycorrhizae on the relationship between plant diversity and productivity[J]. Ecology Letters, 2000, 3(2): 137-141. |
| [99] |
Gange AC, Brown VK, Sinclair GS. Vesicular-arbuscular mycorrhizal fungi: a determinant of plant community structure in early succession[J]. Functional Ecology, 1993, 7(5): 616-622. DOI:10.2307/2390139 |
| [100] |
Gange AC, Brown VK. Soil food web components affect plant community structure during early succession[J]. Ecological Research, 2002, 17(2): 217-227. |
| [101] |
Hartnett DC, Wilson GWT. Mycorrhizae influence plant community structure and diversity in tallgrass prairie[J]. Ecology, 1999, 80(4): 1187-1195. DOI:10.1890/0012-9658(1999)080[1187:MIPCSA]2.0.CO;2 |
| [102] |
O'Connor PJ, Smith SE, Smith FA. Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland[J]. New Phytologist, 2002, 154(1): 209-218. DOI:10.1046/j.1469-8137.2002.00364.x |
| [103] |
Urcelay C, Díaz S. The mycorrhizal dependence of subordinates determines the effect of arbuscular mycorrhizal fungi on plant diversity[J]. Ecology Letters, 2003, 6(5): 388-391. DOI:10.1046/j.1461-0248.2003.00444.x |
| [104] |
Wagg C, Veiga R, van der Heijden MGA. Facilitation and antagonism in mycorrhizal networks[A]//Horton TR. Mycorrhizal Networks[M]. Dordrecht, Netherlands: Springer, 2015: 203-226
|
| [105] |
Loreau M, Naeem S, Inchausti P, et al. Biodiversity and ecosystem functioning: current knowledge and future challenges[J]. Science, 2001, 294(5543): 804-808. DOI:10.1126/science.1064088 |
| [106] |
Lehman CL, Tilman D. Biodiversity, stability, and productivity in competitive communities[J]. The American Naturalist, 2000, 156(5): 534-552. DOI:10.1086/303402 |
| [107] |
Jansa J, Smith FA, Smith SE. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi?[J]. New Phytologist, 2008, 177(3): 779-789. DOI:10.1111/j.1469-8137.2007.02294.x |
| [108] |
Mulder CPH, Uliassi DD, Doak DF. Physical stress and diversity-productivity relationships: the role of positive interactions[J]. Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(12): 6704-6708. DOI:10.1073/pnas.111055298 |
| [109] |
Grime JP, Mackey JML, Hillier SH, et al. Floristic diversity in a model system using experimental microcosms[J]. Nature, 1987, 328(6129): 420-422. DOI:10.1038/328420a0 |
| [110] |
van der Heijden MGA, Horton TR. Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems[J]. Journal of Ecology, 2009, 97(6): 1139-1150. DOI:10.1111/j.1365-2745.2009.01570.x |
| [111] |
Tilman D, Reich PB, Knops J, et al. Diversity and productivity in a long-term grassland experiment[J]. Science, 2001, 294(5543): 843-845. DOI:10.1126/science.1060391 |
| [112] |
Wilkinson A, Solan M, Taylor AFS, et al. Intraspecific diversity regulates fungal productivity and respiration[J]. PLoS One, 2010, 5(9): e12604. DOI:10.1371/journal.pone.0012604 |
| [113] |
Wilkinson A, Solan M, Alexander I, et al. Species richness and nitrogen supply regulate the productivity and respiration of ectomycorrhizal fungi in pure culture[J]. Fungal Ecology, 2012, 5(2): 211-222. DOI:10.1016/j.funeco.2011.08.007 |
| [114] |
Cardinale BJ, Wright JP, Cadotte MW, et al. Impacts of plant diversity on biomass production increase through time because of species complementarity[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(46): 18123-18128. DOI:10.1073/pnas.0709069104 |
| [115] |
Janoušková M, Seddas P, Mrnka L, et al. Development and activity of Glomus intraradices as affected by co-existence with Glomus claroideum in one root system[J]. Mycorrhiza, 2009, 19(6): 393-402. DOI:10.1007/s00572-009-0243-4 |
| [116] |
Mickelson SM, Kaeppler SM. Evaluation of six mycorrhizal isolates for their ability to promote growth of maize genotypes under phosphorus deficiency[J]. Maydica, 2005, 50(2): 137-146. |
| [117] |
Hodge A, Fitter AH. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(31): 13754-13759. DOI:10.1073/pnas.1005874107 |
| [118] |
Lekberg Y, Rosendahl S, Olsson PA. The fungal perspective of arbuscular mycorrhizal colonization in 'nonmycorrhizal' plants[J]. New Phytologist, 2015, 205(4): 1399-1403. DOI:10.1111/nph.13118 |
| [119] |
Werner GDA, Kiers ET. Order of arrival structures arbuscular mycorrhizal colonization of plants[J]. New Phytologist, 2015, 205(4): 1515-1524. DOI:10.1111/nph.13092 |
| [120] |
Deinlein U, Stephan AB, Horie T, et al. Plant salt-tolerance mechanisms[J]. Trends in Plant Science, 2014, 19(6): 371-379. DOI:10.1016/j.tplants.2014.02.001 |
| [121] |
Wallander H, Johansson U, Sterkenburg E, et al. Production of ectomycorrhizal mycelium peaks during canopy closure in Norway spruce forests[J]. New Phytologist, 2010, 187(4): 1124-1134. DOI:10.1111/j.1469-8137.2010.03324.x |
| [122] |
Cosme M, Fernandez I, van der Heijden MGA, et al. Non-mycorrhizal plants: the exceptions that prove the rule[J]. Trends in Plant Science, 2018, 23(7): 577-587. DOI:10.1016/j.tplants.2018.04.004 |
| [123] |
Bogar L, Peay K, Kornfeld A, et al. Plant-mediated partner discrimination in ectomycorrhizal mutualisms[J]. Mycorrhiza, 2019, 29(2): 97-111. DOI:10.1007/s00572-018-00879-7 |
| [124] |
Shi ZY, Zhang XL, Xu SX, et al. Mycorrhizal relationship in lupines: a review[J]. Legume Research-An International Journal, 2017, 40(6): 965-973. |
| [125] |
de Deyn GB, Cornelissen JHC, Bardgett RD. Plant functional traits and soil carbon sequestration in contrasting biomes[J]. Ecology Letters, 2008, 11(5): 516-531. DOI:10.1111/j.1461-0248.2008.01164.x |
| [126] |
Koide RT, Fernandez C, Malcolm G. Determining place and process: functional traits of ectomycorrhizal fungi that affect both community structure and ecosystem function[J]. New Phytologist, 2014, 201(2): 433-439. DOI:10.1111/nph.12538 |
| [127] |
van der Heijden MGA, Scheublin TR. Functional traits in mycorrhizal ecology: their use for predicting the impact of arbuscular mycorrhizal fungal communities on plant growth and ecosystem functioning[J]. New Phytologist, 2007, 174(2): 244-250. DOI:10.1111/j.1469-8137.2007.02041.x |
| [128] |
Wurzburger N, Clemmensen KE. From mycorrhizal fungal traits to ecosystem properties-and back again[J]. Journal of Ecology, 2018, 106(2): 463-467. DOI:10.1111/1365-2745.12922 |
| [129] |
Avio L, Pellegrino E, Bonari E, et al. Functional diversity of arbuscular mycorrhizal fungal isolates in relation to extraradical mycelial networks[J]. New Phytologist, 2006, 172(2): 347-357. DOI:10.1111/j.1469-8137.2006.01839.x |
| [130] |
Munkvold L, Kjøller R, Vestberg M, et al. High functional diversity within species of arbuscular mycorrhizal fungi[J]. New Phytologist, 2004, 164(2): 357-364. DOI:10.1111/j.1469-8137.2004.01169.x |
 2020, Vol. 47
2020, Vol. 47