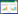扩展功能
文章信息
- 孙东年, 张昱, 秦华, 白建峰, 胡君利, 王景伟, 林先贵
- SUN Dong-Nian, ZHANG Yu, QIN Hua, BAI Jian-Feng, HU Jun-Li, WANG Jing-Wei, LIN Xian-Gui
- 电子废弃物拆解区土壤重金属污染对丛枝菌根真菌多样性的影响
- Effect of soil heavy metal contamination on arbuscular mycorrhizal fungal diversity in an e-waste dismantling area
- 微生物学通报, 2020, 47(11): 3780-3788
- Microbiology China, 2020, 47(11): 3780-3788
- DOI: 10.13344/j.microbiol.china.200629
-
文章历史
- 收稿日期: 2020-06-21
- 接受日期: 2020-08-25
- 网络首发日期: 2020-08-31
2. 中国科学院南京土壤研究所土壤与农业可持续发展国家重点实验室 江苏 南京 210008;
3. 浙江农林大学环境与资源学院 浙江省土壤污染生物修复重点实验室 浙江 杭州 311300;
4. 中国科学院大学 北京 100049
2. State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing, Jiangsu 210008, China;
3. Key Laboratory of Soil Contamination Bioremediation of Zhejiang Province, School of Environmental and Resource Sciences, Zhejiang Agriculture and Forestry University, Hangzhou, Zhejiang 311300, China;
4. University of Chinese Academy of Sciences, Beijing 100049, China
20世纪末期,中国东南部地区包括台州温岭、汕头贵屿和清远龙塘等地出现了专门从事电子废弃物拆解的行业[1]。然而,电子废弃物拆解是一把“双刃剑”,一方面能够回收大量的金属资源,减轻资源短缺造成的压力,促进地区经济增长,具有积极作用;另一方面,拆解业长期以来大多采用的是小规模和不规范的拆解方式,特别是露天拆解,致使重金属、持久性有机污染物等各种有毒有害物质大量泄露到环境中,给大气、水体和土壤造成严重污染[2],造成不容忽视的环境危害。由于大气和水体中的重金属也会以各种形式进入到土壤,而且它们在土壤中迁移能力差、滞留时间长、不能被微生物降解[3],所以电子废弃物拆解造成的土壤重金属污染问题已经引起了人们的高度重视。电子废弃物拆解、堆卸导致周边土壤普遍受到重金属污染,其中拆解、倾倒点位呈重度污染状态[4-5],这会对植物、土壤动物以及微生物等产生威胁。例如,台州某电子废弃物拆解尾渣倾倒点土壤中高浓度的重金属显著抑制向日葵生长并破坏其叶片类囊体层状结构[6],贵屿某电子废弃物拆解区域附近水稻土中线虫的数量随着重金属浓度升高而呈现波动下降[7],龙塘某电子废弃物集中处理区土壤中真菌和细菌的数量分别下降了85.6%和99.7%[8]。另外,与周边稻田相比,电子废弃物拆解现场表层土壤中含有更高浓度的镉和铜,土壤过氧化氢酶、蔗糖酶、酸性磷酸酶和脲酶等的活性下降[9]。因此,由电子废弃物拆解造成的土壤重金属污染引发的其他环境问题尤其需要关注。
丛枝菌根(arbuscular mycorrhizal,AM)真菌广泛存在于农田和自然生态系统中,能与绝大多数陆生植物的根系建立互惠共生关系[10],在重金属污染土壤中也能够侵染植物根系,改善植物的生长状况[11]。但是,重金属污染会对AM真菌群落结构和多样性产生影响,而且随着土壤重金属浓度的升高,定殖在植物根部的AM真菌多样性也会降低[12]。例如,铜污染不仅降低了海洲香薷根际土壤中AM真菌的香农指数,同时改变了其群落组成[13]。在锰污染土壤中,AM真菌的香农指数与总锰、可提取态锰的浓度均呈显著负相关关系[14]。在铅锌矿区土壤中,重金属复合污染降低了AM真菌的物种丰富度、香农指数和辛普森指数,导致刺槐根际土壤中AM真菌的群落结构发生变化[15]。研究发现,球囊霉属(Glomus)和无梗囊霉属(Acaulospora)是重金属污染土壤中最常见的AM真菌属[16-18]。尽管国内外已有大量学者关注土壤重金属与AM真菌之间的相互关系并开展了很多研究,但电子废弃物拆解造成的土壤重金属污染对AM真菌群落结构和多样性的影响尚不明确。因此,本研究以浙江省台州市路桥区某典型电子废弃物拆解场地及其周边区域为对象,采用Illumina高通量测序技术来探究电子废弃物拆解造成的土壤重金属污染对AM真菌多样性的影响,并试图甄别具有高重金属耐受性的AM真菌,以便为电子废弃物拆解区重金属污染土壤的治理提供理论依据。
1 材料与方法 1.1 样点信息2016年1月,在浙江省台州市路桥区某典型电子废弃物拆解场地及其周边区域,针对拆解/倾倒和扩散共设置12个样点(范围为28°30′39″N− 28°31′31″N,121°22′07″E−121°24′34″E),采集表层土壤(0−10 cm)。其中,拆解/倾倒区包括拆解园区(C1)、下脚料拆解点(C2)、尾渣倾倒点(Q1)和尾渣排放点(Q2)共计4个样点,扩散区包括1个池塘底泥样点(D1)和7个农田样点(N1−N7)。每个土样混匀和去除碎石等杂物后,一式两份,其中一份自然风干过筛后测定Cd、Cu、Pb、Cr、Zn、Ni等浓度[4],另一份在−40 ℃下存储以提取土壤总DNA。具体的采样点信息、土壤pH值、重金属浓度、综合污染指数及潜在生态风险指数见表 1。
| 样点 Sampling site |
pH | Cd | Cu | Pb | Cr | Zn | Ni | 综合污染指数CPI | 生态风险指数ERI |
| (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | ||||
| 拆解园区Waste recycling plant | 8.35 | 8.24 | 3 611.0 | 9 386.0 | 818.0 | 4 470 | 278.0 | 161.00 | 2 685.0 |
| 下脚料拆解点Scraps dismantling point | 6.33 | 0.44 | 107.0 | 80.2 | 103.0 | 231 | 49.4 | 2.16 | 88.0 |
| 尾渣倾倒点Tailings dumping point | 8.06 | 46.6 | 3 243.0 | 3 087.0 | 423.0 | 12 985 | 279.0 | 153.00 | 6 914.0 |
| 尾渣排放点Tailings discharge point | 6.66 | 1.07 | 167.0 | 452.0 | 99.2 | 1 055 | 48.7 | 8.34 | 227.0 |
| 池塘底泥Sediment in pond | 8.77 | 0.13 | 35.1 | 31.0 | 104.0 | 121 | 48.7 | 0.95 | 32.5 |
| 农田Farmland | 6.23 | 0.28 | 52.6 | 43.8 | 95.6 | 160 | 48.1 | 1.20 | 56.3 |
| 农田Farmland | 6.16 | 0.51 | 76.8 | 50.8 | 262.0 | 326 | 42.7 | 2.30 | 92.9 |
| 农田Farmland | 5.52 | 0.24 | 50.3 | 51.0 | 177.0 | 144 | 44.4 | 1.53 | 52.9 |
| 农田Farmland | 7.23 | 0.30 | 65.6 | 52.3 | 122.0 | 161 | 46.7 | 1.42 | 61.7 |
| 农田Farmland | 5.10 | 0.51 | 280.0 | 79.2 | 90.7 | 295 | 47.1 | 5.00 | 117.0 |
| 农田Farmland | 6.52 | 0.82 | 188.0 | 78.6 | 131.0 | 229 | 75.2 | 3.59 | 150.0 |
| 农田Farmland | 5.47 | 1.47 | 179.0 | 87.2 | 120.0 | 262 | 65.3 | 4.94 | 233.0 |
| 注:综合污染指数采用内梅罗指数法,以温黄平原土壤环境背景上限值为污染评价标准[19],Cd、Cu、Pb、Cr、Zn和Ni的权重分别为3、2、3、3、2、2[20];生态风险指数参照徐争启等[21]进行计算,为多种重金属的潜在生态风险指数之和. Note: The Nemerow index method was used to calculate the comprehensive factor pollution index (CPI). The upper limit of soil environmental background in the Wenhuang Plain was taken as the pollution evaluation standard[19]. The weights of Cd, Cu, Pb, Cr, Zn and Ni were 3, 2, 3, 3, 2 and 2, respectively[20]. The ecological risk index (ERI) was calculated according to Xu et al[21], referring to the sum of the potential ecological risk indices of various heavy metals. |
|||||||||
FastDNA® SPIN Kit for soil,MP Biomedicals公司;Premix Taq酶,TakaRa公司;QIAquick PCR纯化试剂盒,Qiagen公司。PCR仪,TakaRa公司;NanoDrop ND-1000,Thermo Scientific公司。
1.3 测定方法 1.3.1 DNA提取土壤总DNA提取采用FastDNA® SPIN Kit for soil,称取0.5 g冷冻土样,参照试剂盒内的使用说明提取总DNA。提取的总DNA用无菌水稀释10倍,储存在−30 ℃冰箱待用。
1.3.2 Illumina高通量测序以土壤总DNA的10倍稀释液为模板,以AMV4.5NF (5′-AAGCTCGTAGTTGAATTTGG-3′)和AMDGR (5′-CCCAACTATCCCTATTAATCAT-3′)为引物,对土壤样品中AM真菌的18S rRNA基因进行扩增[22]。此外,为了区分扩增产物,在每个样品的引物中添加一个5 bp的barcode序列。PCR反应体系(50 μL):DNA模板(10 ng/μL) 5 μL,Premix Taq酶25 μL,正、反向引物(10 μmol/L)各1 μL,无菌水18 μL。PCR反应条件:95 ℃ 5 min;95 ℃ 45 s,58 ℃ 45 s,72 ℃ 1 min,共35个循环;72 ℃ 7 min[23]。使用QIAquick PCR纯化试剂盒纯化PCR产物,并用NanoDrop ND-1000测定其含量。混合等量后在Illumina MiSeqTM System平台上测序。
1.4 数据处理测序结果使用微生物生态定量指标(the quantitative insights into microbial ecology,QIIME)软件包进行分析(http://qiime.org/tutorials/processing_18S_data.html)。首先,对18S rRNA基因序列进行质控(质量分 > 25、序列长度 > 200 bp),根据barcode序列将所有序列分配到相应样品后,去除barcode和引物序列。然后,以97%为阈值筛分可操作分类单元(operational taxonomic unit,OTU)[24],并从中选择丰度最高的序列作为代表序列,通过与Silva 128数据库(https://www.arbsilva.de/download/archive/qiime/)比对获得每个OTU的分类学信息后,分析AM真菌群落组成;采用香农(Shannon)、辛普森(Simpson)和Chao1指数来比较AM真菌多样性。采用Pearson相关分析对AM真菌多样性、各OTU的相对丰度与重金属污染指标进行相关性分析,解析具有重金属高耐受性的OTU,并使用t检验分析拆解/倾倒区和扩散区之间的差异显著性。所有统计分析均用SPSS 19进行,数据处理和绘图使用Excel 2013和Origin 2017进行。
2 结果与分析 2.1 AM真菌群落结构所测土壤样品AM真菌由原囊霉目(Archaeosporales)、球囊霉目(Glomerales)和多孢囊霉目(Diversisporales)组成,其中处于优势的球囊霉目约占94% (图 1A);在科水平上包括巨孢囊霉科(Gigasporaceae)、球囊霉科(Glomeraceae)和3个分类地位未定的科,其中球囊霉科占据优势地位。各样点土壤AM真菌不同科的相对丰度存在一定差异(图 1B),但按样点类型来看,拆解/倾倒区与扩散区未见明显的规律性差异。其中,除拆解点C1和C2以外,其余各土壤样品中均出现了多孢囊霉目或原囊霉目。
|
| 图 1 土壤AM真菌的群落组成(A)及其相对丰度(B) Figure 1 Soil arbuscular mycorrhizal fungal community composition (A) and the relative abundance (B) |
|
|
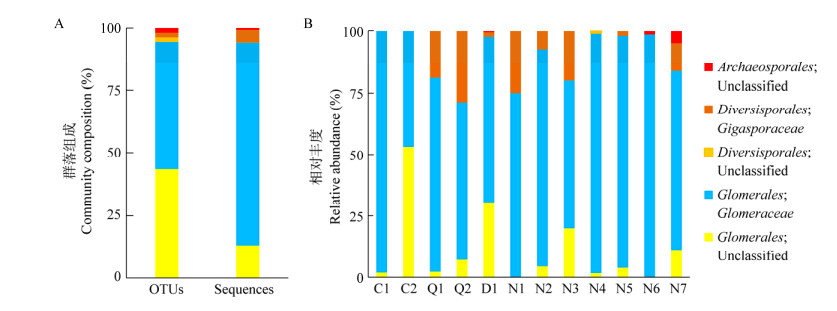
拆解/倾倒区土壤AM真菌的香农、辛普森和Chao1指数与扩散区没有显著差异(图 2);从中位值和均值来看,拆解/倾倒区高于扩散区(辛普森指数中位值除外),表明电子废弃物拆解、倾倒并没有降低土壤AM真菌多样性,甚至刺激了一些高重金属耐受型AM真菌的生长。通过Pearson相关分析发现(表 2),AM真菌多样性指数与重金属浓度、综合污染指数及潜在风险指数间并不存在显著的相关关系。
|
| 图 2 土壤AM真菌香农(A)、辛普森(B)与Chao1 (C)指数 Figure 2 Soil arbuscular mycorrhizal fungal Shannon (A), Simpson (B) and Chao1 (C) indices 注:△代表均值. Note:△ represents the mean value. |
|
|
| 重金属污染指标 Pollution parameters of heavy metals |
香农指数Shannon index | 辛普森指数Simpson index | Chao1指数Chao1 index | |||||||
| r | P | r | P | r | P | |||||
| CPI | −0.313 | 0.161 | −0.261 | 0.206 | −0.411 | 0.092 | ||||
| ERI | −0.199 | 0.268 | −0.198 | 0.268 | −0.292 | 0.179 | ||||
| Cd | −0.014 | 0.337 | −0.157 | 0.313 | −0.220 | 0.246 | ||||
| Cu | −0.320 | 0.155 | −0.262 | 0.205 | −0.426 | 0.083 | ||||
| Pb | −0.350 | 0.132 | −0.261 | 0.206 | −0.425 | 0.084 | ||||
| Cr | −0.466 | 0.063 | −0.420 | 0.087 | −0.365 | 0.122 | ||||
| Zn | −0.018 | 0.289 | −0.187 | 0.280 | −0.267 | 0.201 | ||||
| Ni | −0.316 | 0.158 | −0.263 | 0.204 | −0.427 | 0.083 | ||||
| 注:r:Pearson相关性;P:显著性(单侧). Note: r: Pearson correlation; P: Significance (one-side). |
||||||||||
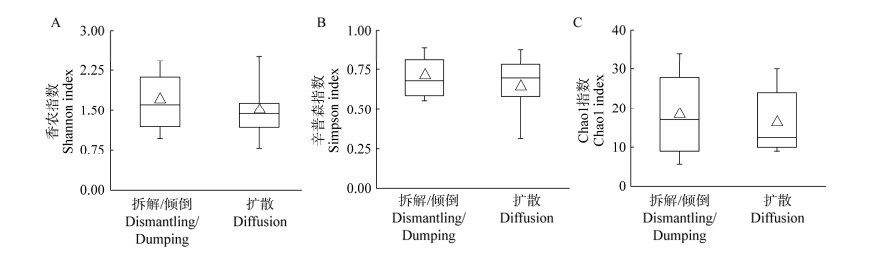
通过相关性分析发现(表 3),疑似Rhizophagus vesiculiferus的OTU (相似度≥97%)相对丰度与重金属浓度、综合污染指数及潜在生态风险指数间均呈极显著正相关关系。进一步对比中位值和均值发现(图 3),该疑似R. vesiculiferus的OTU在拆解/倾倒区土壤中的相对丰度均远高于扩散区土壤,这表明该AM真菌对土壤重金属污染具有较强的耐受性,或可作为电子拆解造成的重金属污染土壤的潜在修复材料。
| 重金属污染指标 Pollution parameters of heavy metals |
疑似泡囊根生囊霉的OTU相对丰度 Relative abundance of R. vesiculiferus-like OTUs |
|
| r | P | |
| CPI | 0.977 | 0.000 |
| ERI | 0.762 | 0.004 |
| Cd | 0.614 | 0.034 |
| Cu | 0.982 | 0.000 |
| Pb | 0.962 | 0.000 |
| Cr | 0.954 | 0.000 |
| Zn | 0.729 | 0.007 |
| Ni | 0.964 | 0.000 |
| 注:r:Pearson相关性;P:显著性(单侧). Note: r: Pearson correlation; P: Significance (one-side). |
||
|
| 图 3 土壤AM真菌群落中疑似泡囊根生囊霉的OTU相对丰度 Figure 3 Relative abundance of Rhizophagus vesiculiferus-like OTUs in soil arbuscular mycorrhizal fungal community 注:△代表均值. Note: △ represents the mean value. |
|
|
电子废弃物拆解/倾倒区和扩散区土壤AM真菌群落结构、多样性受到重金属复合污染的影响,但AM真菌多样性指数与重金属污染指标间并无显著相关性(表 2)。这与丁苏丽等[25]和赵祥伟等[26]的报道相一致,可能是因为土壤中不同种类微生物对重金属复合污染的敏感性不同,而且微生物群落之间存在着多种相互关系,偏利或竞争可能会抵消其对重金属的响应[26],因此,土壤微生物多样性指数与重金属指标间往往不呈现显著相关关系。当然,环境因子差异也会掩盖重金属污染对土壤微生物的影响。在本研究中,各采样点土壤pH存在一定差异(表 1),一方面会直接影响重金属的生物有效性和毒性作用[27-28],另一方面会因为AM真菌对pH值的适应范围不同而导致群落组成不同[29]。此外,土壤有机质、氮、磷等的差异也可能会影响AM真菌对重金属的响应[15, 30-31],多种因素互作最终导致土壤AM真菌多样性与重金属指标间无显著关系。
电子废弃物拆解/倾倒区和扩散区土壤AM真菌均由原囊霉目、球囊霉目和多孢囊霉目组成,其中球囊霉目占据优势地位(图 1A),这与广东大宝山矿区重金属污染土壤中AM真菌的群落组成情况相似[32]。尽管拆解/倾倒区比扩散区具有更加严重的土壤重金属污染(表 1),但土壤AM真菌多样性指数却未呈现显著差异(图 2),表明重金属含量升高对AM真菌群落的组成并没有造成显著的影响,这可能与部分AM真菌类群对重金属具有较强的耐受性有关[33-34]。例如,拆解/倾倒区土壤AM真菌群落中疑似R. vesiculiferus的OTU相对丰度远高于扩散区土壤,而且与重金属浓度、综合污染指数及潜在生态风险指数间均呈显著正相关关系(表 3)。在Pb (1 585 mg/kg)、Zn (525 mg/kg)和Cd (8.8 mg/kg)复合污染土壤的修复试验中发现,R. vesiculiferus的丰度随着重金属浓度的下降而降低[35]。重金属浓度升高能促进R. vesiculiferus相对丰度的增长,表明R. vesiculiferus对重金属污染具有强耐受性,或可作为重金属污染土壤的潜在修复材料。然而,目前对R. vesiculiferus的了解仍停留在污染场地调查采样及分子生物学分析阶段,其对重金属的响应机理和耐受机制尚缺乏系统研究。此外,AM真菌生态功能的发挥还会受到植物种类及土壤性质等因素的影响[29, 36]。因此,将R. vesiculiferus用于电子废弃物拆解区重金属污染土壤修复的可行性仍有待进一步探究。
4 结论(1) 电子废弃物拆解场地及周边区域土壤中均存在一定丰度的AM真菌,其中球囊霉目和球囊霉科分别为目和科水平上的优势类群。
(2) 拆解/倾倒区和扩散区土壤AM真菌群落结构存在一定差异,但土壤重金属污染对AM真菌多样性指数的影响较小,两者之间没有显著差异。
(3) 拆解/倾倒区土壤AM真菌群落中疑似R. vesiculiferus的OTU相对丰度远高于扩散区,而且与重金属浓度、综合污染指数及潜在生态风险指数间均呈显著正相关关系。
(4) 土壤AM真菌中R. vesiculiferus对重金属污染可能具有极强的耐受性,或可为重金属污染土壤的修复提供技术支撑。
| [1] |
Yuan JG, Zheng J, Chen SL, et al. Advances in the researches on investigations of pollution and environmental, ecological and health risks from E-Waste recycling activities in China[J]. Asian Journal of Ecotoxicology, 2013, 8(4): 473-486. (in Chinese) 袁剑刚, 郑晶, 陈森林, 等. 中国电子废物处理处置典型地区污染调查及环境、生态和健康风险研究进展[J]. 生态毒理学报, 2013, 8(4): 473-486. |
| [2] |
Huang HW, Zhu CL, Ren Y. Speciation and potential ecological risk of heavy metals in the soils and river sediments of the e-waste recycling region of Longtang Town[J]. Environmental Chemistry, 2015, 34(2): 254-261. (in Chinese) 黄华伟, 朱崇岭, 任源. 龙塘镇电子垃圾拆解区土壤和河流底泥重金属赋存形态及生态风险[J]. 环境化学, 2015, 34(2): 254-261. |
| [3] |
Cui DJ, Zhang YL. Current situation of soil contamination by heavy metals and research advances on the remediation techniques[J]. Chinese Journal of Soil Science, 2004, 35(3): 366-370. (in Chinese) 崔德杰, 张玉龙. 土壤重金属污染现状与修复技术研究进展[J]. 土壤通报, 2004, 35(3): 366-370. |
| [4] |
Zhang Y, Hu JL, Bai JF, et al. Contamination assessment and genesis analysis of heavy metals in farmland soil around a waste electrical and electronic equipments disassembling area[J]. Ecology and Environmental Sciences, 2017, 26(7): 1228-1234. (in Chinese) 张昱, 胡君利, 白建峰, 等. 电子废弃物拆解区周边农田土壤重金属污染评价及成因解析[J]. 生态环境学报, 2017, 26(7): 1228-1234. |
| [5] |
Wang JF, Song XF, Niu XJ, et al. Toxicology studies on soil of electronic waste recycling site to spinach[J]. Ecology and Environmental Sciences, 2014, 23(10): 1664-1670. (in Chinese) 王瑾丰, 宋小飞, 牛晓君, 等. 电子垃圾拆解区土壤上空心菜的毒理响应[J]. 生态环境学报, 2014, 23(10): 1664-1670. |
| [6] |
Zhang Y, Hu JL, Bai JF, et al. Arbuscular mycorrhizal fungi alleviate the heavy metal toxicity on sunflower (Helianthus annuus L.) plants cultivated on a heavily contaminated field soil at a WEEE-recycling site[J]. Science of the Total Environment, 2018, 628-629: 282-290. DOI:10.1016/j.scitotenv.2018.01.331 |
| [7] |
Wang YL, Wang HH, Liao JL, et al. Effects of heavy metal contamination on nematode communities in paddy soils of an e-waste recycling area[J]. Journal of Agro-Environment Science, 2015, 34(5): 874-881. (in Chinese) 王赢利, 王宏洪, 廖金铃, 等. 电子垃圾拆解地重金属污染对稻田土壤线虫群落结构的影响[J]. 农业环境科学学报, 2015, 34(5): 874-881. |
| [8] |
Yu XH. A study on the environmental risks from im-proper handling of e-waste[D]. Guangzhou: Doctoral Dissertation of Sun Yat-sen University, 2010 (in Chinese) 余晓华.电子废物不当处置环境污染风险研究[D].广州: 中山大学博士学位论文, 2010 |
| [9] |
Zhang JL, Ding JF, Lin HZ, et al. Microbial communities of farmland soils nearby e-waste dismantling site[J]. Environmental Science & Technology, 2018, 41(4): 96-102, 181. (in Chinese) 张金莲, 丁疆峰, 林浩忠, 等. 电子垃圾拆解区农田土壤微生物群落结构研究[J]. 环境科学与技术, 2018, 41(4): 96-102, 181. |
| [10] |
Willis A, Rodrigues BF, Harris PJC. The ecology of arbuscular mycorrhizal fungi[J]. Critical Reviews in Plant Sciences, 2013, 32(1): 1-20. DOI:10.1080/07352689.2012.683375 |
| [11] |
Zhang Y, Hu JL, Bai JF, et al. Intercropping with sunflower and inoculation with arbuscular mycorrhizal fungi promotes growth of garlic chive in metal-contaminated soil at a WEEE-recycling site[J]. Ecotoxicology and Environmental Safety, 2019, 167: 376-384. DOI:10.1016/j.ecoenv.2018.10.046 |
| [12] |
Zarei M, König S, Hempel S, et al. Community structure of arbuscular mycorrhizal fungi associated to Veronica rechingeri at the Anguran zinc and lead mining region[J]. Environmental Pollution, 2008, 156(3): 1277-1283. DOI:10.1016/j.envpol.2008.03.006 |
| [13] |
Yang RY, Zan ST, Tang JJ, et al. Variation in community structure of arbuscular mycorrhizal fungi associated with a Cu tolerant plant-Elsholtzia splendens[J]. Applied Soil Ecology, 2010, 44(3): 191-197. DOI:10.1016/j.apsoil.2009.12.005 |
| [14] |
Wei Y, Hou H, Li JN, et al. Molecular diversity of arbuscular mycorrhizal fungi associated with an Mn hyperaccumulator-Phytolacca americana, in Mn mining area[J]. Applied Soil Ecology, 2014, 82: 11-17. DOI:10.1016/j.apsoil.2014.05.005 |
| [15] |
Yang YY, Song YY, Scheller HV, et al. Community structure of arbuscular mycorrhizal fungi associated with Robinia pseudoacacia in uncontaminated and heavy metal contaminated soils[J]. Soil Biology and Biochemistry, 2015, 86: 146-158. DOI:10.1016/j.soilbio.2015.03.018 |
| [16] |
da Silva GA, Trufem SFB, Júnior OJS, et al. Arbuscular mycorrhizal fungi in a semiarid copper mining area in Brazil[J]. Mycorrhiza, 2005, 15(1): 47-53. DOI:10.1007/s00572-004-0293-6 |
| [17] |
Zarei M, Saleh-Rastin N, Jouzani GS, et al. Arbuscular mycorrhizal abundance in contaminated soils around a zinc and lead deposit[J]. European Journal of Soil Biology, 2008, 44(4): 381-391. DOI:10.1016/j.ejsobi.2008.06.004 |
| [18] |
González-Chávez MC, Carrillo-González R, Gutiérrez-Castorena MC. Natural attenuation in a slag heap contaminated with cadmium: the role of plants and arbuscular mycorrhizal fungi[J]. Journal of Hazardous Materials, 2009, 161(2/3): 1288-1298. |
| [19] |
Wang QH, Dong YX, Zheng W, et al. Soil geochemical baseline values and environmental background values in Zhejiang, China[J]. Geological Bulletin of China, 2007, 26(5): 590-597. (in Chinese) 汪庆华, 董岩翔, 郑文, 等. 浙江土壤地球化学基准值与环境背景值[J]. 地质通报, 2007, 26(5): 590-597. |
| [20] |
Zhou GZ, Yang FJ, Cheng JG, et al. Research on synthetic assessment method for soil environmental quality[J]. Journal of Shandong University of Science and Technology (Natural Science), 2005, 24(4): 113-115, 118. (in Chinese) 周广柱, 杨锋杰, 程建光, 等. 土壤环境质量综合评价方法探讨[J]. 山东科技大学学报:自然科学版, 2005, 24(4): 113-115, 118. |
| [21] |
Xu ZQ, Ni SJ, Tuo XG, et al. Calculation of heavy metals' toxicity coefficient in the evaluation of potential ecological risk index[J]. Environmental Science & Technology, 2008, 31(2): 112-115. (in Chinese) 徐争启, 倪师军, 庹先国, 等. 潜在生态危害指数法评价中重金属毒性系数计算[J]. 环境科学与技术, 2008, 31(2): 112-115. |
| [22] |
Lumini E, Orgiazzi A, Borriello R, et al. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach[J]. Environmental Microbiology, 2010, 12(8): 2165-2179. |
| [23] |
Cao JL, Feng YZ, Lin XG, et al. Iron oxide magnetic nanoparticles deteriorate the mutual interaction between arbuscular mycorrhizal fungi and plant[J]. Journal of Soils and Sediments, 2017, 17(3): 841-851. DOI:10.1007/s11368-016-1561-8 |
| [24] |
Edgar RC. Search and clustering orders of magnitude faster than BLAST[J]. Bioinformatics, 2010, 26(19): 2460-2461. DOI:10.1093/bioinformatics/btq461 |
| [25] |
Ding SL, Zhang QJ, Dong J, et al. Microbial community structure and its relationship to heavy metals in Shenzhen and Hong Kong mangrove sediments[J]. Chinese Journal of Ecology, 2018, 37(10): 3018-3030. (in Chinese) 丁苏丽, 张祁炅, 董俊, 等. 深港红树林沉积物微生物群落多样性及其与重金属的关系[J]. 生态学杂志, 2018, 37(10): 3018-3030. |
| [26] |
Zhao XW, Luo YM, Teng Y, et al. Genetic diversity of microbial communities in farmland soils contaminated with mixed heavy metals[J]. Acta Scientiae Circumstantiae, 2005, 25(2): 186-191. (in Chinese) 赵祥伟, 骆永明, 滕应, 等. 重金属复合污染农田土壤的微生物群落遗传多样性研究[J]. 环境科学学报, 2005, 25(2): 186-191. |
| [27] |
Zeng FR, Ali S, Zhang HT, et al. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants[J]. Environmental Pollution, 2011, 159(1): 84-91. DOI:10.1016/j.envpol.2010.09.019 |
| [28] |
Marcin C, Marcin G, Justyna MP, et al. Diversity of microorganisms from forest soils differently polluted with heavy metals[J]. Applied Soil Ecology, 2013, 64: 7-14. DOI:10.1016/j.apsoil.2012.11.004 |
| [29] |
Wang FY, Liu RJ. Effects of environmental factors on the diversity of arbuscular mycorrhizal fungi[J]. Biodiversity Science, 2001, 9(3): 301-305. (in Chinese) 王发园, 刘润进. 环境因子对AM真菌多样性的影响[J]. 生物多样性, 2001, 9(3): 301-305. |
| [30] |
Xu XH, Chen C, Zhang Z, et al. The influence of environmental factors on communities of arbuscular mycorrhizal fungi associated with Chenopodium ambrosioides revealed by MiSeq sequencing investigation[J]. Scientific Reports, 2017, 7(1): 45134. DOI:10.1038/srep45134 |
| [31] |
Zarei M, Hempel S, Wubet T, et al. Molecular diversity of arbuscular mycorrhizal fungi in relation to soil chemical properties and heavy metal contamination[J]. Environmental Pollution, 2010, 158(8): 2757-2765. DOI:10.1016/j.envpol.2010.04.017 |
| [32] |
Long LK, Yao Q, Guo J, et al. Molecular community analysis of arbuscular mycorrhizal fungi associated with five selected plant species from heavy metal polluted soils[J]. European Journal of Soil Biology, 2010, 46(5): 288-294. DOI:10.1016/j.ejsobi.2010.06.003 |
| [33] |
Liao JP, Lin XG, Cao ZH. Tolerance of mycorrhizal fungi to heavy metals and mechanisms[J]. Soils, 2003, 35(5): 370-377. (in Chinese) 廖继佩, 林先贵, 曹志洪. 内外生菌根真菌对重金属的耐受性及机理[J]. 土壤, 2003, 35(5): 370-377. |
| [34] |
Chen BD, Sun YQ, Zhang X, et al. Underlying mechanisms of the heavy metal tolerance of mycorrhizal fungi[J]. Environmental Science, 2015, 36(3): 1123-1132. (in Chinese) 陈保冬, 孙玉青, 张莘, 等. 菌根真菌重金属耐性机制研究进展[J]. 环境科学, 2015, 36(3): 1123-1132. |
| [35] |
Maček I, Šibanc N, Kavšček M, et al. Diversity of arbuscular mycorrhizal fungi in metal polluted and EDTA washed garden soils before and after soil revitalization with commercial and indigenous fungal inoculum[J]. Ecological Engineering, 2016, 95: 330-339. DOI:10.1016/j.ecoleng.2016.06.026 |
| [36] |
Xiang D, Xu TL, Li H, et al. Ecological distribution of arbuscular mycorrhizal fungi and the influencing factors[J]. Acta Ecologica Sinica, 2017, 37(11): 3597-3606. (in Chinese) 向丹, 徐天乐, 李欢, 等. 丛枝菌根真菌的生态分布及其影响因子研究进展[J]. 生态学报, 2017, 37(11): 3597-3606. |
 2020, Vol. 47
2020, Vol. 47