扩展功能
文章信息
- 樊荣, 王春生, 赵蕊, 潘勇宏, 龙友华, 赵志博
- FAN Rong, WANG Chun-Sheng, ZHAO Rui, PAN Yong-Hong, LONG You-Hua, ZHAO Zhi-Bo
- 植物病原真菌1, 8-间苯二酚黑色素研究进展
- Advances in 1, 8-dihydroxynaphthalene melanin of plant fungal pathogens
- 微生物学通报, 2020, 47(11): 3671-3677
- Microbiology China, 2020, 47(11): 3671-3677
- DOI: 10.13344/j.microbiol.china.191003
-
文章历史
- 收稿日期: 2019-12-03
- 接受日期: 2020-03-06
- 网络首发日期: 2020-04-26
2. 信阳农林学院 河南 信阳 464000
2. Xinyang Agriculture and Forestry University, Xinyang, Henan 464000, China
黑色素是一种高分子量的深褐色或黑色色素,广泛存在于动物和微生物体内,是有机生命体不断适应环境胁迫与寄主共同进化的产物。黑色素有利于生物体抵抗多种不良环境胁迫,如紫外辐射、高渗胁迫、极端温度、重金属胁迫等,因此被成为“真菌护甲”[1]。许多真菌可产生黑色素,其中通过1, 8-间苯二酚(1, 8-dihydroxynaphthalene,DHN)途径合成的DHN黑色素一直是真菌黑色素研究的热点。本文重点阐述了DHN黑色素在真菌抗逆、致病和形态建成中的作用。
1 真菌黑色素合成途径根据合成途径中间产物的不同将黑色素分为3, 4-二羟基苯丙氨酸(3, 4-dihydroxyphenylalanine,L-DOPA)黑色素和DHN黑色素2种[2]。动物和少数真菌(如Cryptococcus neoformans)能形成L-DOPA黑色素,子囊菌门的许多真菌则是通过DHN途径合成黑色素[3]。但有些真菌,如申克氏胞丝菌(Sporothrix schenckii),能利用上述L-DOPA和DHN两种方式合成黑色素。
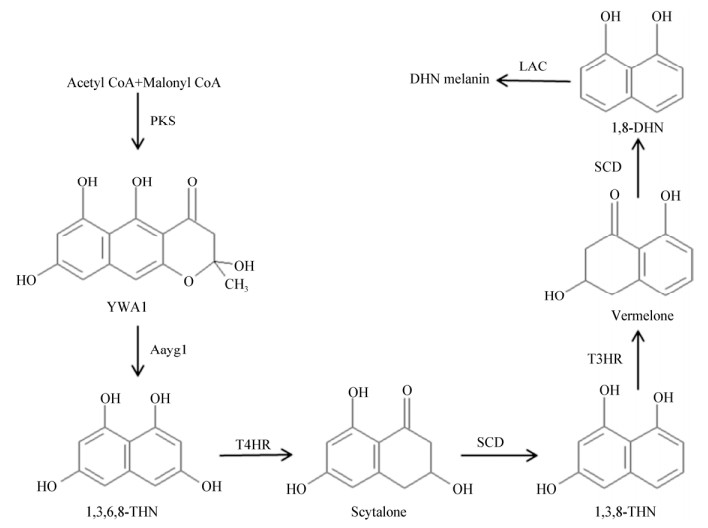
典型的DHN黑色素合成途径在大丽轮枝菌(Verticillium dahliae)黑色素研究中首次被报道,该途径包括5种关键的催化酶:聚酮合酶(polyketide synthase,PKS)、1, 3, 6, 8-四羟基萘还原酶(1, 3, 6, 8-tetra-HN reductase,T4HR)、小柱孢酮脱水酶(scytalone dehydratase,SCD)、1, 3, 8-三羟基萘还原酶(1, 3, 8-tri-HN reductase,T3HR)和漆酶(laccase,LAC)[2]。近年研究表明,烟曲霉(Aspergillus fumigatus)在合成DHN黑色素的过程中除需要上述5种催化酶外,还需要一种缩链催化酶Aayg1催化PKS产物形成中间底物1, 3, 6, 8-四羟基萘(1, 3, 6, 8-tetra-HN,1, 3, 6, 8-THN)[4],也即烟曲霉DHN黑色素合成途径包括6种主要的催化酶(图 1)[5],但模式真菌酵母中无Aayg1的同源蛋白。土传病原真菌大丽轮枝菌在合成DHN黑色素时也需要Aayg1同源基因(vayg1)的参与[5-6]。灰葡萄孢菌(Botrytis cinerea)虽有Aayg1的同源蛋白,但其同源蛋白Bayg1只参与该菌分生孢子DHN黑色素的合成,并不参与菌核DHN黑色素的合成过程[7],表明Aagy1及其同源蛋白在黑色素合成中的功能不保守。除了Aayg1,PKS在DHN黑色素合成中的作用也具有多样性。灰葡萄孢菌(B. cinerea)的DHN黑色素合成途径是非线性的,黑色素在菌核还是分生孢子中形成取决于DHN黑色素合成途径上游PKS编码基因bcpks12表达还是bcpks13表达,前者表达则在菌核中合成黑色素,后者表达则黑色素在分生孢子中合成[7]。这说明不同真菌合成DHN黑色素的方式具有多样性。最新研究发现,大丽轮枝菌跨膜蛋白VdSho1感知外界刺激后,通过激活MAPK信号模块Ste50-Ste11-Ste7将胞外信号传递到胞内,然后由Vayg1与其他调控子一起激活锌指型转录因子VdCmr1,调控相关基因表达合成黑色素[8-10]。
|
| 图 1 烟曲霉(Aspergillus fumigatus) DHN黑色素合成途径[5] Figure 1 The modified DHN melanin biosynthesis pathway in Aspergillus fumigatus[5] 注:PKS:聚酮合酶;Aayg1:缩链催化酶;T4HR:1, 3, 6, 8-四羟基萘还原酶;SCD:小柱孢酮脱水酶;T3HR:1, 3, 8-三羟基萘还原酶;LAC:漆酶.其中Aayg1首次在烟曲霉中被报道参与该菌DHN黑色素合成过程. Note: PKS: Polyketide synthase; Aayg1: A novel enzyme for hydrolytic polyketide shortening in Aspergillus fumigatus; T4HR: 1, 3, 6, 8-tetra-HN reductase; SCD: Scytalone dehydratase; T3HR: 1, 3, 8-tri-HN reductase; LAC: Laccase. The catalytic enzyme of Aayg1 was first reported to be involved in the DHN melanin biosynthesis of A. fumigatus. |
|
|
适度的电磁辐射对于生物体的存活是必要的(如光合作用),但在高能电磁波、电离辐射(如γ-射线或X-射线)或紫外线下暴露的频率和时间不当可能会对生命造成损伤,因为类似核酸或蛋白质等这些生物大分子吸收辐射能后会产生自由基和活性氧,而活性氧可导致敏感细胞内分子的结构和功能受损,从而影响正常的生命活动[11]。在重金属和不饱和烃污染的工业区以及太阳辐射水平较高的暴露环境中,黑化真菌数量较多,这可能是因为黑色素的吸收光谱宽,可作为紫外线吸收剂,增强微生物对紫外线的抗性,避免由紫外光引起的伤害[12-13]。真菌黑色素保护细胞免受辐射损伤的机制包括黑色素可对非电离辐射进行散射,且可通过改变自身化学成分和结构,发生光电效应和康普顿效应及清除自由基等一系列过程损耗辐射能,从而防御电离辐射带来的伤害[11-13]。研究表明,真菌对紫外辐照的抵抗能力与所合成黑色素的量有关。分别将外生菌根真菌暴露在0 mGy/h和404 mGy/h的γ射线下,后者产生的生物量显著少于前者[14];将真菌细胞壁表面的黑色素制成悬浮液添加使用,也可降低紫外光带来的伤害[15-16]。
2.2 DHN黑色素能增强真菌对极端温度的抗性黑色素能维持真菌在极端温度下的细胞稳定性,提高真菌抗干燥能力,这可能是由于细胞壁中黑色素的存在减小了细胞壁的孔径,增加了细胞的负电荷和疏水性,因此使黑化细胞在逆境下具有更强的生活力[12]。黑色素对真菌在高温下的存活具有重要作用。将黑化酵母菌(Aureobasdium melanogenum)细胞与6.0 mol/L HCl混匀后,在80 ℃下处理60 min仍可观察到存活的黑化细胞,但将该菌株非黑化细胞进行同样的处理,30 min后非黑化细胞全部死亡[13, 17]。通常情况下,深色分生孢子比无色菌丝体耐热能力强。果生链核盘菌(Monilinia fructicola)分生孢子缺失黑色素后对高温胁迫更为敏感,40 ℃下迅速丧失萌发能力[12]。大丽轮枝菌黑色素缺陷型突变体对高温耐受性显著降低,40 ℃下处理24 h后,黑色素缺陷型突变体的存活力仅约为野生型黑色菌株存活力的0.01倍[9]。此外,黑色素还与真菌抗寒能力有关。南极、北极和高山土壤的微生物群落中优势真菌的菌丝大多富含黑色素,以保护这些真菌不受极端温度和干旱的影响,对其在寒冷区域的长期存活起着重要作用[18-19]。适冷黑色真菌产生的黑色素类物质及参与这些菌株生化途径的催化酶,是具有生物活性新型次级代谢产物和酶的一种潜在来源[20]。
2.3 DHN黑色素能增强真菌对土壤重金属的解毒能力土壤中的重金属无法被分解,其长期富集不仅影响生态系统,而且威胁人体健康。黑色素聚合物含有芳香组分及羟基、羧基、胺基和酚等多种官能团,能与许多有机和无机分子形成分子间相互作用[21],以共价键、离子键和疏水键的形式与蛋白质、农药等结合[22],还能通过官能团之间的配位键结合土壤中的重金属,以抵抗其对细胞产生的毒性,因此被称为金属离子螯合剂[23]。蜜环菌菌索产生的黑色素能结合环境中50−100倍的Al3+、Zn2+、Fe3+和Cu2+离子,韦氏小针层孔菌(Phellinus weirii)产生的黑色假菌核能吸附土壤中的重金属离子Al3+、Cu2+、Fe3+、Mn2+、Ni2+、Pb2+、Zn2+等;真菌黑色素与土壤中重金属的结合,不仅降低甚至清除了受污染土壤中的重金属毒性,而且能促进该区域微生物多样性的重建,从而实现土壤生物修复[24-25]。基于黑色素螯合重金属离子使其失活的原理进行的土壤重金属污染修复是一种环境友好型修复技术,将受到越来越多的关注[25-26]。
3 DHN黑色素与真菌致病力的关系植物病原真菌合成黑色素的能力与其对寄主的侵染能力也具有相关性[6, 27-30]。黑色素对侵染结构为黑色的多种植物病原真菌的毒力具有正调控作用,这种调控作用主要是通过真菌产生的黑色附着胞实现的。附着胞中的黑色素可作为一种刚性结构屏障,维持附着胞细胞壁的完整性,防止附着胞内的溶质外排,对膨压形成至关重要,从而促进侵染[31]。当稻瘟菌侵染寄主时,附着胞上的一个组氨酸-天冬氨酸激酶可作为膨压感受器,感知外界膨压阈值后调控黑色素合成,调节膨压形成和入侵栓形成以完成侵染[32]。采用基因敲除的方法或外源使用黑色素合成抑制剂获得的附着胞黑色素缺陷型突变体,其致病力显著下降[27, 30]。
黑色素除了通过影响附着胞的完整性和膨压形成而对病原真菌的毒力进行调节之外,还可能影响病菌毒力因子的形成。对新月弯孢菌(Curvularia lunata)的比较基因组和比较转录组研究表明,该菌毒力增强与自身的毒素合成途径及黑色素合成途径有密切关系,且这两个途径可能有重叠[33]。此外,曲霉属真菌(Aspergillus spp.)细胞壁上的黑色素能够富集胞外Ca2+,抑制钙调蛋白信号途径,阻断自噬作用,最终达到增强致病力的作用[34-35]。
另外,黑色素不仅影响侵染结构为黑色的病原真菌的毒力,对侵染结构为无色的土传病原真菌大丽轮枝菌的致病力也有正调控作用。研究表明,黑色素合成相关基因vdpf参与大丽轮枝菌的侵染和定殖过程[36];vdHog1[37]、vdPbs2[38]和vdMcm1[39]等基因参与大丽轮枝菌黑色素合成和对黄栌的致病过程,大丽轮枝菌成功侵染棉花需要黑色素合成途径上的关键酶Vayg1[5]和VdPKS1[28]的参与。最新研究发现,大丽轮枝菌黑色素合成与对寄主棉花的侵染过程是通过跨膜蛋白VdSho1偶联的,MAPK信号模块Ste50-Ste11-Ste7参与了该偶联过程,但这种信号转导可能是寄主依赖型[10]。
然而,黑色素可能对灰霉病菌的致病过程具有负调控作用。灰霉病菌(B. cinerea)缺失黑色素不仅不会降低致病力,反而增强了突变体对寄主辣椒的毒力[40]。但黑色素并不参与所有植物病原真菌对寄主的侵染过程。核盘菌(Sclerotinia sclerotiorum)缺失黑色素合成关键基因后,突变体的致病力无显著变化[41];而且黑色素并非小麦壳针孢菌(Zymoseptoria tritici)的致病因子[42],表明在核盘菌和壳针孢菌中,黑色素合成能力与致病力无明显关系。综上所述,DHN黑色素在植物病原真菌致病过程中的作用方式多样、作用机制复杂,尚需深入研究。
4 DHN黑色素与真菌细胞发育的关系虽然黑色素并非真菌生长的必需物质,但多种真菌细胞正常发育均需黑色素。黑色素在真菌发育中的作用最初在丝状真菌中被报道,这些丝状真菌的透明菌丝、白色菌丝体、黑色附着胞、菌核、分生孢子和繁殖结构等的正常发育均需要黑色素[43]。研究表明,黑色素与分生孢子的正常形成和萌发之间具有相关性。黑色素能影响内生真菌小孢拟盘多毛孢(Pestalotiopsis microspore)分生孢子的形态发育和细胞壁完整性[44],而且可通过调控分生孢子细胞壁杆状层(rodlet layer)的修饰性棘状突起,影响分生孢子对寄主的吸附效率[45]。外源使用黑色素合成抑制剂三环唑会抑制球毛壳霉(Chaetomium globosum)分生孢子的形成和萌发[46]。黑色素还能调控链格孢菌(Alternaria alternata)分生孢子纵隔膜的数量、细胞壁完整性和菌丝形态等,这种调控可能是由激动蛋白酶SLT2介导的[47-48]。黑色素除了影响真菌菌丝和分生孢子的形态发育外,对真菌休眠结构的形成也具有正调控作用。核盘菌(S. sclerotiorum)缺失黑色素合成相关基因后,导致菌核发育受损,菌核产量显著下降[41]。大丽轮枝菌正常微菌核在发育过程中颜色由淡黄色变为黄棕色和棕色,最后变为黑色;但缺失黑色素合成关键酶Vayg1后,不仅分生孢子体积显著减小,而且微菌核形成受到抑制[6]。
Zhou等[49]从自然土壤中分离到2 031株灰葡萄孢菌(B. cinerea),其中只有5株能产生黄色菌核,其他分离株均产生黑色菌核。黄色菌核自然发生率如此之低揭示了黑色素缺陷型菌株可能对自然环境的适生性较差,从而难以长期存活。由此推测,真菌细胞发育的某些阶段中,黑色素的存在可提高真菌结构对寄主或环境的机械和化学抗性,从而提高真菌对寄主的毒力及在逆境中的存活率。因此,深入研究黑色素的合成机制对揭示真菌与寄主和环境的协同进化具有重要作用。
5 展望黑色素对真菌存活和正常行使功能具有重要作用。本文综述了真菌DHN黑色素的合成及其在真菌抗逆、致病和细胞发育中的作用。虽然围绕DHN黑色素合成的报道越来越多,但其合成的调控机制仍有待深入研究。如在模式真菌灰葡萄孢菌(B. cinerea)中,菌核和分生孢子黑色素合成的上游途径是相互独立的,但下游途径是相同的,那么不同结构利用不同途径合成黑色素的意义是什么?有哪些信号通路和关键基因调控了灰葡萄孢菌DHN黑色素的合成?为何这种调控机制在镰刀菌、轮枝菌、曲霉菌等重要植物病原真菌中没有被发现?针对以上问题,我们认为融合了比较基因组、转录组、蛋白组及代谢组学的多组学高通量测序技术和经典的生理生化分析将为解答上述问题提供新视角,这有助于阐明不同真菌合成黑色素的策略差异,进而加深我们认识黑色素对真菌进化的重要性。
许多研究表明,黑色素能增强真菌的抗逆性。但在同一种真菌中,响应不同逆境的信号途径是否有偶联?黑色素是否属于偶联信号物质的一种?如何提高黑色素对重金属吸附的效率?能否将黑色素产品与生防菌株结合使用提高生防菌株在室外的存活率?这些都属于新兴的黑色素研究和系统应用的重要方向。黑色素与侵染结构为黑色和无色的多种真菌的致病力呈正相关,那么,黑色素在这两类真菌侵染过程中的作用机制和调控机理有何不同?为何在进化过程中,有些病原真菌对寄主的毒力不依赖于黑色素的合成?相信多组学测序技术和生物信息学分析融合功能基因组学研究将加速我们对病原真菌致病机制的解析。目前,对病原菌发育机理和病害发生规律的认识不足,导致我们对许多灾害性病害的防治措施具有局限性。传统的农业防治收效慢、影响因素多,化学防治导致环境污染和有害生物抗药性产生的频率越来越快,生物防治虽然备受青睐,但难以克服在大田环境中防效不稳定的缺陷,因此急需在病害防治策略上寻求新的突破。黑色素在多种病原真菌发育、抗逆和致病过程中均发挥重要作用,可作为病害防治新的靶标。深入研究病原真菌中黑色素合成的调控机理、致病机制及其在抗逆中的应用,不仅有利于创建病害防治新措施,而且可为新型材料和环境友好型修复技术的研发提供新思路。
| [1] |
Pombeiro-Sponchiado SR, Sousa GS, Andrade JCR, et al. Production of melanin pigment by fungi and its biotechnological applications[A]//Melanin[M]. Blumenberg M. Croatia: IntechOpen, 2017
|
| [2] |
Bell AA, Wheeler MH. Biosynthesis and functions of fungal melanins[J]. Annual Review of Phytopathology, 1986, 24(1): 411-451. DOI:10.1146/annurev.py.24.090186.002211 |
| [3] |
Almeida-Paes R, Frases S, Monteiro PCF, et al. Growth conditions influence melanization of Brazilian clinical Sporothrix schenckii isolates[J]. Microbes and Infection, 2009, 11(5): 554-562. DOI:10.1016/j.micinf.2009.03.002 |
| [4] |
Fujii I, Yasuoka Y, Tsai HF, et al. Hydrolytic polyketide shortening by ayg1p, a novel enzyme involved in fungal melanin biosynthesis[J]. Journal of Biological Chemistry, 2004, 279(43): 44613-44620. DOI:10.1074/jbc.M406758200 |
| [5] |
Fan R, Xu XH, Cao YS, et al. The role of DHN melanin biosynthesis genes in microsclerotium formation in Verticillium dahliae[J]. Mycosystema, 2017, 36(12): 1608-1615. (in Chinese) 樊荣, 徐小鸿, 曹亚松, 等. 大丽轮枝菌黑色素合成相关基因与微菌核形成的关系[J]. 菌物学报, 2017, 36(12): 1608-1615. |
| [6] |
Fan R, Klosterman SJ, Wang CH, et al. Vayg1 is required for microsclerotium formation and melanin production in Verticillium dahliae[J]. Fungal Genetics and Biology, 2017, 98: 1-11. DOI:10.1016/j.fgb.2016.11.003 |
| [7] |
Schumacher J. DHN melanin biosynthesis in the plant pathogenic fungus Botrytis cinerea is based on two developmentally regulated key enzyme (PKS)-encoding genes[J]. Molecular Microbiology, 2016, 99(4): 729-748. |
| [8] |
Wang YL, Hu XP, Fang YL, et al. Transcription factor VdCmr1 is required for pigment production, protection from UV irradiation, and regulates expression of melanin biosynthetic genes in Verticillium dahliae[J]. Microbiology, 2018, 164(4): 685-696. DOI:10.1099/mic.0.000633 |
| [9] |
Fang YL, Klosterman SJ, Tian CM, et al. Insights into VdCmr1-mediated protection against high temperature stress and UV irradiation in Verticillium dahliae[J]. Environmental Microbiology, 2019, 21(8): 2977-2996. DOI:10.1111/1462-2920.14695 |
| [10] |
Li JJ, Zhou L, Yin CM, et al. The Verticillium dahliae Sho1-MAPK pathway regulates melanin biosynthesis and is required for cotton infection[J]. Environmental Microbiology, 2019, 21(12): 4852-4874. DOI:10.1111/1462-2920.14846 |
| [11] |
Cordero RJB, Casadevall A. Functions of fungal melanin beyond virulence[J]. Fungal Biology Reviews, 2017, 31(2): 99-112. |
| [12] |
Casadevall A, Cordero RJB, Bryan R, et al. Melanin, radiation, and energy transduction in fungi[J]. Microbiology Spectrum, 2017, 5(2): 1-6. |
| [13] |
Jiang H, Liu NN, Liu GL, et al. Melanin production by a yeast strain XJ5-1 of Aureobasidium melanogenum isolated from the Taklimakan desert and its role in the yeast survival in stress environments[J]. Extremophiles, 2016, 20(4): 567-577. DOI:10.1007/s00792-016-0843-9 |
| [14] |
Kothamasi D, Wannijn J, van Hees M, et al. Exposure to ionizing radiation affects the growth of ectomycorrhizal fungi and induces increased melanin production and increased capacities of reactive oxygen species scavenging enzymes[J]. Journal of Environmental Radioactivity, 2019, 197: 16-22. DOI:10.1016/j.jenvrad.2018.11.005 |
| [15] |
Zhdanova NN, Pokhodenko VD. EPR study of the dynamics of melanin accumulation in Cladosporium sp.[J]. Seriia biologicheskaia, 1970, 5: 787-789. |
| [16] |
Gonçalves RCR, Lisboa HCF, Pombeiro-Sponchiado SR. Characterization of melanin pigment produced by Aspergillus nidulans[J]. World Journal of Microbiology and Biotechnology, 2012, 28(4): 1467-1474. |
| [17] |
Rangel DEN, Finlay RD, Hallsworth JE, et al. Fungal strategies for dealing with environment- and agriculture- induced stresses[J]. Fungal Biology, 2018, 122(6): 602-612. DOI:10.1016/j.funbio.2018.02.002 |
| [18] |
Robinson CH. Cold adaptation in Arctic and Antarctic fungi[J]. New Phytologist, 2001, 151(2): 341-353. DOI:10.1046/j.1469-8137.2001.00177.x |
| [19] |
Pacelli C, Selbmann L, Zucconi L, et al. Responses of the black fungus Cryomyces antarcticus to simulated mars and space conditions on rock analogs[J]. Astrobiology, 2019, 19(2): 209-220. |
| [20] |
Rafiq M, Hassan N, Rehman M, et al. Adaptation mechanisms and applications of psychrophilic fungi[A]// Fungi in Extreme Environments: Ecological Role and Biotechnological Significance[M]. Cham: Springer, 2019: 157-174
|
| [21] |
Costa TG, Younger R, Poe C, et al. Studies on synthetic and natural melanin and its affinity for Fe(Ⅲ) ion[J]. Bioinorganic Chemistry and Applications, 2012, 2012: 712840. |
| [22] |
Kim YJ, Khetan A, Wu W, et al. Evidence of porphyrin-like structures in natural melanin pigments using electrochemical fingerprinting[J]. Advanced Materials, 2016, 28(16): 3173-3180. DOI:10.1002/adma.201504650 |
| [23] |
Maher S, Mahmoud M, Rizk M, et al. Synthetic melanin nanoparticles as peroxynitrite scavengers, photothermal anticancer and heavy metals removal platforms[J]. Environmental Science and Pollution Research, 2019. |
| [24] |
Chan WK, Wildeboer D, Garelick H, et al. Mycoremediation of heavy metal/metalloid-contaminated soil: current understanding and future prospects[A]//Fungal Applications in Sustainable Environmental Biotechnology[M]. Cham: Springer, 2016: 249-272
|
| [25] |
Łopusiewicz Ł. The isolation, purification and analysis of the melanin pigment extracted from Armillaria mellea rhizomorphs[J]. World Scientific News, 2018, 100: 135-153. |
| [26] |
Tang XK, Zhao JR, Li KQ, et al. Streptomyces cyaneochromogenes sp. nov., a blue pigment-producing actinomycete from manganese-contaminated soil[J]. International Journal of Systematic and Evolutionary Microbiology, 2019, 69(8): 2202-2207. DOI:10.1099/ijsem.0.003406 |
| [27] |
Steiner U, Oerke EC. Localized melanization of appressoria is required for pathogenicity of Venturia inaequalis[J]. Phytopathology, 2007, 97(10): 1222-1230. DOI:10.1094/PHYTO-97-10-1222 |
| [28] |
Zhang T, Zhang BS, Hua CL, et al. VdPKS1 is required for melanin formation and virulence in a cotton wilt pathogen Verticillium dahliae[J]. Science China Life Sciences, 2017, 60(8): 868-879. DOI:10.1007/s11427-017-9075-3 |
| [29] |
Griffiths SA, Cox RJ, Overdijk EJR, et al. Assignment of a dubious gene cluster to melanin biosynthesis in the tomato fungal pathogen Cladosporium fulvum[J]. PLoS One, 2018, 13(12): e0209600. DOI:10.1371/journal.pone.0209600 |
| [30] |
Li XY, Ke ZJ, Yu XJ, et al. Transcription factor CgAzf1 regulates melanin production, conidial development and infection in Colletotrichum gloeosporioides[J]. Antonie van Leeuwenhoek, 2019, 112(7): 1095-1104. DOI:10.1007/s10482-019-01243-1 |
| [31] |
He PZ, Wang YL, Wang XL, et al. The mitogen-activated protein kinase CgMK1 governs appressorium formation, melanin synthesis, and plant infection of Colletotrichum gloeosporioides[J]. Frontiers in Microbiology, 2017, 8: 2216. DOI:10.3389/fmicb.2017.02216 |
| [32] |
Ryder LS, Dagdas YF, Kershaw MJ, et al. A sensor kinase controls turgor-driven plant infection by the rice blast fungus[J]. Nature, 2019, 574(7778): 423-427. DOI:10.1038/s41586-019-1637-x |
| [33] |
Gao SG, Li YQ, Gao JX, et al. Genome sequence and virulence variation-related transcriptome profiles of Curvularia lunata, an important maize pathogenic fungus[J]. BMC Genomics, 2014, 15(1): 627. DOI:10.1186/1471-2164-15-627 |
| [34] |
Akoumianaki T, Kyrmizi I, Valsecchi I, et al. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity[J]. Cell Host & Microbe, 2016, 19(1): 79-90. |
| [35] |
Kyrmizi I, Ferreira H, Carvalho A, et al. Calcium sequestration by fungal melanin inhibits calcium-calmodulin signalling to prevent LC3-associated phagocytosis[J]. Nature Microbiology, 2018, 3(7): 791-803. DOI:10.1038/s41564-018-0167-x |
| [36] |
Luo XM, Mao HQ, Wei YM, et al. The fungal-specific transcription factor Vdpf influences conidia production, melanized microsclerotia formation and pathogenicity in Verticillium dahliae[J]. Molecular Plant Pathology, 2016, 17(9): 1364-1381. DOI:10.1111/mpp.12367 |
| [37] |
Wang YL, Tian LY, Xiong DG, et al. The mitogen-activated protein kinase gene, VdHog1, regulates osmotic stress response, microsclerotia formation and virulence in Verticillium dahliae[J]. Fungal Genetics and Biology, 2016, 88: 13-23. DOI:10.1016/j.fgb.2016.01.011 |
| [38] |
Tian LY, Wang YL, Yu J, et al. The mitogen-activated protein kinase kinase VdPbs2 of Verticillium dahliae regulates microsclerotia formation, stress response, and plant infection[J]. Frontiers in Microbiology, 2016, 7: 1532. |
| [39] |
Xiong DG, Wang YL, Tian LY, et al. MADS-Box transcription factor VdMcm1 regulates conidiation, microsclerotia formation, pathogenicity, and secondary metabolism of Verticillium dahliae[J]. Frontiers in Microbiology, 2016, 7: 1192. |
| [40] |
Zhang CH, He YF, Zhu PK, et al. Loss of bcbrn1 and bcpks13 in Botrytis cinerea not only blocks melanization but also increases vegetative growth and virulence[J]. Molecular Plant-Microbe Interactions, 2015, 28(10): 1091-1101. DOI:10.1094/MPMI-04-15-0085-R |
| [41] |
Liang Y, Xiong W, Steinkellner S, et al. Deficiency of the melanin biosynthesis genes SCD1 and THR1 affects sclerotial development and vegetative growth, but not pathogenicity, in Sclerotinia sclerotiorum[J]. Molecular Plant Pathology, 2018, 19(6): 1444-1453. DOI:10.1111/mpp.12627 |
| [42] |
Derbyshire MC, Gohari AM, Mehrabi R, et al. Author Correction: phosphopantetheinyl transferase (Ppt)-mediated biosynthesis of lysine, but not siderophores or DHN melanin, is required for virulence of Zymoseptoria tritici on wheat[J]. Scientific Reports, 2019, 9: 16536. DOI:10.1038/s41598-019-53309-9 |
| [43] |
Chen CX, Wang ZH, Feng J, et al. Sclerotia of plant pathogenic fungi[J]. Microbiology China, 2018, 45(12): 2762-2768. (in Chinese) 陈彩霞, 王泽昊, Feng J, 等. 植物病原真菌的菌核研究进展[J]. 微生物学通报, 2018, 45(12): 2762-2768. |
| [44] |
Yu X, Huo L, Liu H, et al. Melanin is required for the formation of the multi-cellular conidia in the endophytic fungus Pestalotiopsis microspora[J]. Microbiological Research, 2015, 179: 1-11. DOI:10.1016/j.micres.2015.06.004 |
| [45] |
Bayry J, Beaussart A, Dufrêne YF, et al. Surface structure characterization of Aspergillus fumigatus conidia mutated in the melanin synthesis pathway and their human cellular immune response[J]. Infection and Immunity, 2014, 82(8): 3141-3153. DOI:10.1128/IAI.01726-14 |
| [46] |
Hu Y, Hao XR, Lou J, et al. A PKS gene, pks-1, is involved in chaetoglobosin biosynthesis, pigmentation and sporulation in Chaetomium globosum[J]. Science China Life Sciences, 2012, 55(12): 1100-1108. DOI:10.1007/s11427-012-4409-5 |
| [47] |
Yago JI, Lin CH, Chung KR. The SLT2 mitogen-activated protein kinase-mediated signalling pathway governs conidiation, morphogenesis, fungal virulence and production of toxin and melanin in the tangerine pathotype of Alternaria alternata[J]. Molecular Plant Pathology, 2011, 12(7): 653-665. DOI:10.1111/j.1364-3703.2010.00701.x |
| [48] |
Kheder AA, Akagi Y, Akamatsu H, et al. Functional analysis of the melanin biosynthesis genes ALM1 and BRM2-1 in the tomato pathotype of Alternaria alternata[J]. Journal of General Plant Pathology, 2012, 78(1): 30-38. |
| [49] |
Zhou YJ, Li N, Yang JY, et al. Contrast between orange-and black-colored sclerotial isolates of Botrytis cinerea: melanogenesis and ecological fitness[J]. Plant Disease, 2018, 102(2): 428-436. DOI:10.1094/PDIS-11-16-1663-RE |
 2020, Vol. 47
2020, Vol. 47