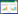扩展功能
文章信息
- 黄文茂, 韩丽珍, 王欢
- HUANG Wen-Mao, HAN Li-Zhen, WANG Huan
- 两株芽孢杆菌对花生幼苗生长及其根际土壤微生物群落结构的影响
- Effects of two Bacillus spp. strains on the growth of peanut seedling and microbial community structure in rhizosphere soil
- 微生物学通报, 2020, 47(11): 3551-3563
- Microbiology China, 2020, 47(11): 3551-3563
- DOI: 10.13344/j.microbiol.china.190977
-
文章历史
- 收稿日期: 2019-11-24
- 接受日期: 2020-02-12
- 网络首发日期: 2020-05-15
花生(Arachis hypogaea Linn.)属于地上开花地下结果的一年生豆科植物,因丰富的含油量(约52%)和蛋白质含量(约26%)已成为我国除油菜外第二大油料作物和重要的出口创汇农产品[1]。近年来,人们为了提高农作物产出,长期大量施用化肥、农药,致使作物根际土壤酶活和微生物群落多样性降低,土壤肥力受损,土壤板结严重[2-3]。因此,在农业生产活动中,兼顾土壤肥力和作物产量对我国农业的科学高效发展具有重要意义。
土壤微生物在土壤微生态系统中扮演着重要的角色,是土壤生物区系的关键性功能要素[4],在参与土壤形成与发育、物质转化和能量传递等过程中发挥重要作用,是评价土壤肥力和健康状况的一个重要指标[5-6]。土壤微生物群落多样性是维持土壤稳定性、提高土壤生产力和可持续性的基础[7-9],也是当前微生物生态学及环境科学研究的重点内容之一。研究表明,在土壤局部区域引入外源细菌后,或通过定殖于植物根际形成一定的种群密度[10-12],或只能在一定时期内存活于植物根际,但却可以通过影响土壤优势种群从而调控整个根际微生物区系的群落结构[13]。这些外源微生物的进入在一定程度可通过调控土壤中原有的微生物群落多样性而达到提高土壤肥力、改善土壤质量的作用,但引入不当也有可能会造成土壤微生态失衡[14]。因此,综合评价引入外源细菌对植物生长、土壤质量及根际土壤微生物群落多样性的影响非常重要。
芽孢杆菌(Bacillus spp.)是一类重要的微生物菌种资源,因其能产生芽孢及促生机制的多样性,在生物肥料和生物农药等领域受到广泛关注。目前对芽孢杆菌的报道主要集中在促生菌株的筛选及促生效果、生防机制等方面[15-17],而关于芽孢杆菌菌剂灌根对花生根际土壤微生物群落多样性影响的研究鲜有报道。本课题组在前期研究中,将从茶树根际分离到的2个细菌菌株HP9和HP10分别鉴定为贝莱斯芽孢杆菌(Bacillus velezensis)及坚强芽孢杆菌(Bacillus firmus),初步研究结果显示两株菌具有溶磷能力,对花生有促生作用[18]。本研究拟利用两株芽孢杆菌及混合菌株对花生进行灌根,通过高通量测序技术分析其对花生根际土壤微生物多样性和群落结构的影响,结合花生幼苗的生长特性以及根际土壤的氮磷钾有效养分变化综合评价该两株菌的促生效应,为深入探究芽孢杆菌对花生的促生机制提供理论依据。
1 材料与方法 1.1 供试菌株及试验样品供试菌株为B. velezensis HP9和B. firmus HP10,由本课题组从贵州省遵义市绥阳县宽阔水自然保护区茶园的茶树根际土壤中分离筛选得到;供试花生品种为“黔花生1号”。
1.2 主要试剂和仪器NucleoSpin® Soil Kit,Macherey-Nagel公司;Qubit® dsDNA BR Assay Kit,ThermoFisher公司;Agencourt AMPure XP Beads,Beckman Coulter公司。UV-8000紫外可见分光光度计,上海元析仪器有限公司;Qubit荧光仪、VS-24SMTI高速冷冻离心机,Vision Science公司;MiSeq测序仪,Illumina公司。
1.3 供试土壤及试验用盆试验土壤类型为黄壤,土壤采集地点位于贵州省贵阳市花溪区养牛村(E106°39′11″,N26°26′57″),经去除砾石及植物枯枝碎屑后备用。试验用盆高11.5 cm、盆口直径12 cm、盆底直径9 cm。
1.4 菌剂的制备分别取少量B. velezensis HP9和B. firmus HP10的甘油保种液至LB培养基中,180 r/min、30 ℃活化过夜,翌日转接至新鲜LB培养基中继续扩培24 h,4 ℃、5 000 r/min离心10 min后,弃去上清以无菌水重悬菌体、调节OD600值为1.0,即为HP9和HP10菌剂,混合菌剂是将以上菌悬液按1:1体积比混合制成。
1.5 试验设计及样品采集花生种子经消毒后通过培养皿滤纸法进行萌芽,待胚芽突破种皮1 cm后选取长势一致的种苗移栽至装有300 g土壤的育苗盆中,每盆种植1株。盆栽试验设4种处理:空白对照(CK)、B. velezensis HP9灌根处理组、B. firmus HP10灌根处理组、混合菌剂灌根处理组(HP9+HP10),每种处理设6个重复,共计24盆。菌液采用灌根法,每隔2日浇一次,每次5 mL,CK组采用5 mL无菌水。所有处理置于日光温室下进行培养,整个生育期未添加任何营养物质。
30 d后进行花生植株生长指标测定及其根际土壤取样,植株采集采用连土掘起法,根际土壤采集参照Courchesne等[19]方法。相同处理的6个重复分为3组(2盆/组),分别取每组的2盆根际土壤等量混合为一份土样,每个处理采得3份土样,共计12份根际土壤样本;每份土样取5 g置于−80 ℃用于根际土壤总DNA提取,剩余土样用于测定土壤氮磷钾有效养分含量。
1.6 植株生长指标及根际土壤氮、磷、钾有效养分含量测定花生植株样本地上部和地下部经清水洗净后,用滤纸吸干表面水分,分别测定植株的茎长、根长和鲜重。
根际土壤氮、磷、钾有效养分含量的测定方法[20]:碱解氮(alkali-hydrolyzed nitrogen,AN)采用碱解扩散法(GB7849-87),有效磷(available P,AP)采用碳酸氢钠法(NY/T 1121.7-2014),速效钾(available K,AK)采用乙酸铵浸提-火焰分光光度法(NY/T 889-2004)。
1.7 花生根际土壤总DNA的提取和PCR扩增根际土壤DNA经DNA Isolate Kit提取并纯化后,对细菌16S rRNA基因的V3−V4区段和真菌18S rRNA基因的ITS1区段进行PCR扩增[21-22]及测序分析,PCR扩增以带Barcode序列的特异引物进行,细菌16S rRNA基因V3−V4区段扩增引物为515F (5′-GTGCCAGCMGCCGCGGTAA-3′)和806R (5′-GGACTACHVGGGTWTCTAAT-3′);真菌18S rRNA基因ITS1区段扩增引物为ITS5-1737F (5′-GGAAGTAAAAGTCGTAACAAG G-3′)和ITS2-2043R (5'-GCTGCGTTCTTCATCG ATGC-3′)。
1.8 高通量测序数据处理采用Illumina MiSeq平台对PCR扩增获得的DNA片段进行双端(paired-end)测序(深圳华大基因科技服务有限公司)。测序下机得到的原始数据经数据过滤,截除质量值低于20的Reads末端序列,去除接头污染Reads,去除含N的Reads,去除低复杂度Reads。然后使用FLASH (fast length adjustment of short reads,V1.2.11)[23],利用重叠关系将双末端测序得到的成对Reads组装成一条序列,得到高变区Tags (最小匹配长度为15 bp,重叠区域允许错配率为0.1,去除没有Overlap关系的Reads),利用USEARCH (V7.0.1090)将Tags聚类为OTU,得到OTU代表序列后,通过RDP classifer (V2.2)软件将OTU代表序列与数据库Greengene_2013_5_99比对,并进行物种注释,置信度阈值设置为0.6。
1.9 统计与分析选择97%相似度OTU进行多样性指数计算,以Mothur (V1.30.1)进行α多样性分析,利用R软件(V3.1.1)绘制物种稀释性曲线图;通过R语言中ade4包进行主成分分析(principal component analysis,PCA);结合R语言相关软件绘制物种群落结构组分图和热图(heatmap);不同处理间花生的幼苗生长指标、根际土壤氮磷钾有效养分含量、微生物多样性指数及物种组成结构差异性通过SPSS 20.0进行One way-ANOVA方差分析及Duncan检验。
2 结果与分析 2.1 芽孢杆菌菌剂灌根对花生幼苗生长及其根际土壤氮、磷、钾有效养分的影响生长指标的测定结果显示(表 1),菌剂灌根对花生幼苗均有不同程度的促进作用(图 1)。HP9、HP10及HP9+HP10三个处理组均显著地促进了幼苗地上部的生长,相较于CK,HP9灌根的花生茎长增长31.03%,其次是混合菌剂灌根组,而HP10对根的促生作用最优,较对照增长23.70%。3个不同处理均对幼苗鲜重影响显著,尤以混合菌剂灌根处理组表现突出,较对照组增加120.97%,而HP9、HP10灌根的鲜重分别增加91.28%和59.30% (P < 0.05)。
| 处理 Treatment | 生长指标 brGrowth index | 有效养分 Available nutrients | |||||
| 茎长 Stem length (cm) |
根长 Root length (cm) |
鲜重 Plant weight (g) |
碱解氮 AN (mg/kg) |
有效磷 AP (mg/kg) |
速效钾 AK (mg/kg) |
||
| CK | 20.95±0.78c | 20.25±1.83b | 1.72±0.13c | 290.00±6.56b | 3.33±0.19a | 156.67±2.40a | |
| HP9 | 27.45±0.87a | 22.56±1.41ab | 3.29±0.18ab | 534.33±64.34a | 4.05±0.46a | 171.67±6.39a | |
| HP10 | 23.90±0.79b | 25.05±1.38a | 2.74±0.31b | 470.33±20.28a | 3.57±0.38a | 161.67±10.48a | |
| HP9+HP10 | 25.77±1.03ab | 24.58±0.98ab | 3.81±0.32a | 497.00±28.29a | 3.55±0.10a | 158.00±0.55a | |
| 注:HP9:B. velezensis HP9;HP10:B. firmus HP10;CK:对照.同列不同小写字母表示差异性显著(P < 0.05). be Note: HP9: B. velezensis HP9; HP10: B. firmus HP10; CK: Control. Different lowercase letters of the same column represent different treatment’s significant difference at 0.05 level. | |||||||

|
| 图 1 不同处理对花生幼苗生长的影响 Figure 1 Effects of different treatments on growth of peanut seedlings Note: A: HP9; B: HP10; C: HP9+HP10. HP9: B. velezensis HP9; HP10: B. firmus HP10; CK: Control. |
|
|
经芽孢杆菌菌剂灌根后,花生根际土壤的碱解氮、有效磷及速效钾含量有不同程度的提高(表 1)。其中,HP9、HP10及混合菌剂灌根组的碱解氮含量均显著增高(P < 0.05),相比对照分别提高84.35%、62.18%和71.38%;而有效磷含量分别提高21.62%、7.21%和6.61%,速效钾含量提高9.57%、3.91%和0.85%,有效磷和速效钾含量的改变未达显著水平。
2.2 花生根际土壤微生物多样性及群落结构 2.2.1 根际土壤测序结果分析利用高通量测序技术对12份土壤样本提取的DNA扩增测序并优化后,共获得高质量的细菌有效序列722 163条,平均每个样本60 180条;真菌有效序列663 580条,平均每个样本55 298条。12份土样在97%相似水平下聚类分析共得到2 842个细菌OTU,1 469个真菌OTU,所有土样的序列稀释曲线逐渐趋于平缓(图 2),说明该测序深度合理,能够比较完整地反映出土壤中细菌群落和真菌群落的结构和种类。

|
| 图 2 在97%相似水平下所有土样所观察到物种数的稀释曲线 Figure 2 Rarefaction curves of the observed species at 97% similarity level for all soil samples 注:A:细菌;B:真菌. A1−3:B. velezensis HP9;B1−3:B. firmus HP10;C1−3:B. velezensis HP9+B. firmus HP10;CK:对照;相同字母后不同数字编号表示3个重复. Note: A: Bacteria; B: Fungi. A1−3: B. velezensis HP9; B1−3: B. firmus HP10; C1−3: B. velezensis HP9+B. firmus HP10; CK: Control; The different numbers after the same letter represent 3 repetitions. |
|
|
对不同实验组OTU进行分析,与对照的2 033个OTU数相比,混合菌剂灌根处理组的细菌物种数增加了164个,而HP9和HP10处理组则有所降低,OTU分别为2 023和1 943;真菌物种数排序为CK (637) > HP9 (632) > HP9+HP10 (626) > HP10 (610)。主成分分析结果表明,经芽孢杆菌菌剂灌根的3个处理组间,花生根际土壤细菌及真菌的群落相似性高于对照组(图 3)。在97%分类水平下,对花生根际土壤的微生物群落多样性与相对丰度进行α多样性分析(表 2)。Chao1指数分析结果显示,HP9单独灌根和混合灌根均略微提高了花生根际土壤细菌和真菌的丰富度;Simpson指数结果显示灌根处理组提高了根际土壤细菌的物种多样性,降低了真菌的物种多样性,混合菌剂灌根处理组表现更明显,但处理组的多样性指数与对照组相比均未达到差异显著水平(P > 0.05)。
|
| 图 3 不同处理花生根际土壤微生物群落的主成分分析 Figure 3 Principal component analysis (PCA) of microbial communities in peanut rhizosphere soil with different treatments 注:A:B. velezensis HP9;B:B. firmus HP10;C:B. velezensis HP9+B. firmus HP10;CK:对照. Note: A: B. velezensis HP9; B: B. firmus HP10; C: B. velezensis HP9+B. firmus HP10; CK: Control. |
|
|
| 处理 Treatment | 细菌 Bacteria | 真菌 Fungi | |||||
| OTUs | Chao1 index | Simpson index | OTUs | Chao1 index | Simpson index | ||
| CK | 2 033.67±32.66a | 2 286.18±54.59a | 0.018±0.001a | 636.67±19.34a | 676.42±18.34a | 0.060±0.005a | |
| HP9 | 2 022.67±17.05a | 2 298.55±32.67a | 0.015±0.002a | 632.33±12.69a | 725.80±20.68a | 0.099±0.024a | |
| HP10 | 1 943.00±81.54a | 2 261.21±36.42a | 0.016±0.004a | 609.67±23.40a | 670.91±21.74a | 0.100±0.018a | |
| HP9+HP10 | 2 297.33±338.42a | 2 305.40±12.18a | 0.014±0.001a | 625.67±26.86a | 702.30±20.62a | 0.100±0.011a | |
| 注:HP9:B. velezensis HP9;HP10:B. firmus HP10;CK:对照.同列不同小写字母表示差异性显著(P < 0.05).
Note: HP9: B. velezensis HP9; HP10: B. firmus HP10; CK: Control. Different lowercase letters of the same column represent different treatment’s significant difference at 0.05 level. | |||||||
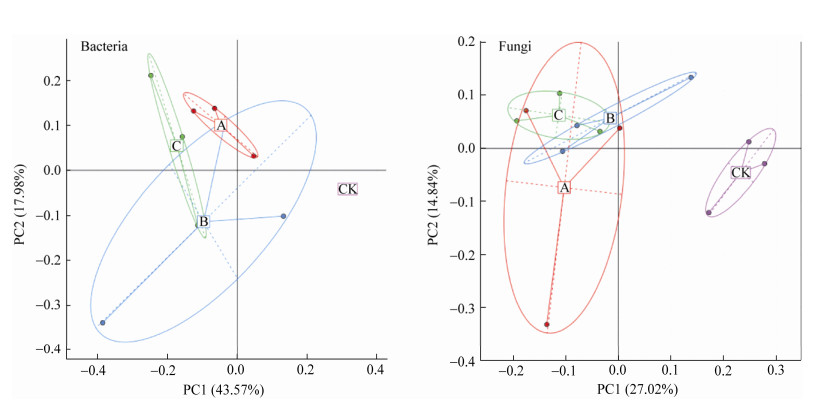
将12份花生根际土壤样品经聚类分析所获得的2 842个细菌OTU归属到27个门(图 4A),结果显示酸杆菌门(Acidobacteria)、放线菌门(Actinobacteria)、拟杆菌门(Bacteroidetes)、绿弯菌门(Chloroflexi)、浮霉菌门(Planctomycetes)、变形菌门(Proteobacteria)、疣微菌门(Verrucomicrobia)、厚壁菌门(Firmicutes)、TM7、芽单胞菌门(Gemmatimonadetes)和蓝藻门(Cyanobacteria)等11个门为花生根际土壤的优势细菌类群,约占所有细菌的97%以上。12份花生根际土壤样品经聚类分析所获得的1 469个真菌OTU归属到13个门(图 4B),包括子囊菌门(Ascomycota)、担子菌门(Basidiomycota)、Mortierellomycota、Calcarisporiellomycota、尾虫门(Cercozoa)、壶菌门(Chytridiomycota)、虫霉门(Entomophthoromycota)、根肿黑粉菌门(Entorrhizomycota)、梳霉门(Kickxellomycota)、毛霉门(Mucoromycota)、油壶菌门(Olpidiomycota)、隐真菌门(Rozellomycota)和GS19;其中优势真菌类群为子囊菌门、担子菌门和Mortierellomycota,约占所有真菌的93.05%−95.58%。
|
| 图 4 不同处理对花生根际土壤微生物群落结构的影响 Figure 4 Effects of different treatments on microbial community structure in peanut rhizosphere soil 注:A:细菌;B:真菌. A1−3:B. velezensis HP9;B1−3:B. firmus HP10;C1−3:B. velezensis HP9+B. firmus HP10;CK:对照;相同字母后不同数字编号表示3个重复. Note: A: Bacteria; B: Fungi. A1−3: B. velezensis HP9; B1−3: B. firmus HP10; C1−3: B. velezensis HP9+B. firmus HP10; CK: Control; The different numbers after the same letter represent 3 repetitions. |
|
|
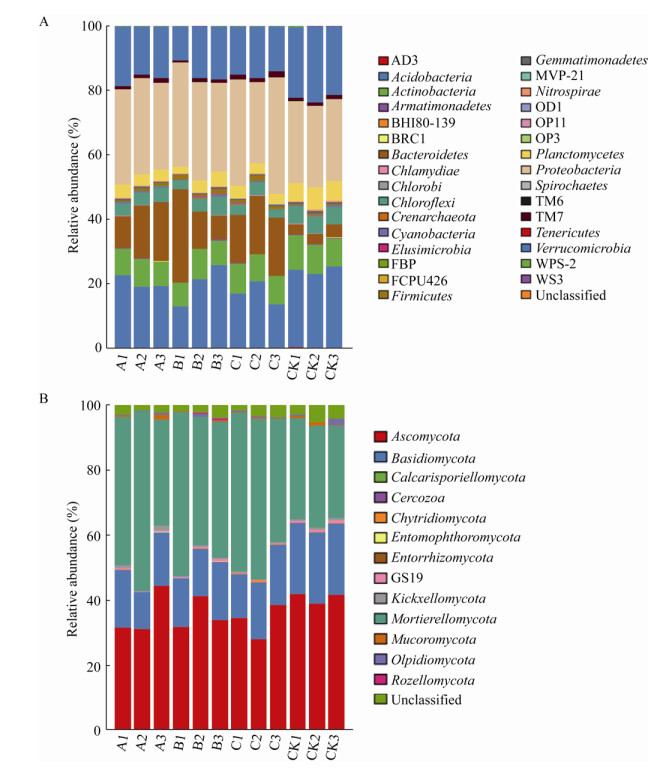
除未分类和相对丰度低于0.5%的门之外,在灌根处理组的花生根际土壤细菌中,拟杆菌门、变形菌门、厚壁菌门、蓝藻门等类群的相对丰度有不同程度升高,其中拟杆菌门丰度显著提高(P < 0.05);而酸杆菌门、疣微菌门、放线菌门、绿弯菌门、浮霉菌门等相对丰度有所降低,以疣微菌门和浮霉菌门下降最显著(P < 0.05)。此外,混合菌剂灌根处理组中芽单胞菌门和绿弯菌门的相对丰度均显著低于对照组(P < 0.05) (图 5A)。对于真菌而言,芽孢杆菌菌剂灌根显著提高了Mortierellomycota的相对丰度(P < 0.05),降低了子囊菌门、担子菌门、油壶菌门和GS19的相对丰度,其中担子菌门和GS19的降幅达到显著水平(P < 0.05) (图 5B)。
|
| 图 5 不同处理对门水平下花生根际土壤微生物(相对丰度 > 0.5%)的影响 Figure 5 Effect of different treatments on microorganisms (relative abundance > 0.5%) in peanut rhizosphere soil at the phylum level 注:A:细菌;B:真菌. HP9:B. velezensis HP9;HP10:B. firmus HP10;CK:对照; 不同小写字母表示不同处理间的差异显著(P < 0.05). Note: A: Bacteria; B: Fungi. HP9: B. velezensis HP9; HP10: B. firmus HP10; CK: Control; The different lowercases represent different treatment's significant difference at 0.05 level. |
|
|
属水平上的物种聚类热图分析显示,3个菌剂处理组根际土壤微生物的序列进化关系较对照更近,而在3个处理组间则表现为HP10及混合菌剂灌根处理组更近的序列进化关系。具体来说,细菌如农杆菌属(Agrobacterium)、节杆菌属(Arthrobacter)、芽孢杆菌属(Bacillus)、伯克霍尔德氏菌属(Burkholderia)、黄杆菌属(Flavobacterium)、Pedobacter、极地单胞菌属(Polaromonas)、假单胞菌属(Pseudomonas)、鞘脂单胞菌属(Sphingomonas)和寡养单胞菌属(Stenotrophomonas)等属的相对丰度在芽孢杆菌菌剂灌根的花生根际土壤中明显升高,而慢生根瘤菌属(Bradyrhizobium)、Candidatus_ Koribacter、Candidatus_Solibacter、Candidatus_ Xiphinematobacter、DA101和红游动菌属(Rhodoplanes)等属则相反;对于真菌而言,节丛孢属(Arthrobotrys)、镰孢属(Fusarium)、Fusicolla、土赤壳属(Ilyonectria)和被孢霉属(Mortierella)等属相比对照有所提高,鞘孢属(Chalara)、外瓶霉属(Exophiala)、Geminibasidium、Leohumicola、Lophiostoma、枝孢瓶霉属(Cladophialophora)、拟盘多毛孢属(Pestalotiopsis)和蜡壳菌属(Sebacina)等属的相对丰度在灌根处理组均不同程度降低(图 6)。此外,与对照相比,仅在芽孢杆菌菌剂灌根的花生根际土壤中检测到6个细菌属(表 3),分别为无色杆菌属(Achromobacter)、短波单胞菌属(Brevundimonas)、金黄色杆菌属(Chryseobacterium)、苍白杆菌属(Ochrobactrum)、鞘氨醇杆菌属(Sphingobacterium)和鞘氨醇盒菌属(Sphingopyxis)。
| 处理Treatment | 无色杆菌属 Achromobacter |
短波单胞菌属 Brevundimonas |
金黄色杆菌属 Chryseobacterium |
苍白杆菌属 Ochrobactrum |
鞘氨醇杆菌属 Sphingobacterium |
鞘氨醇盒菌属 Sphingopyxis |
| HP9 | 0.157±0.058 | 0.611±0.147 | 0.476±0.272 | 0.109±0.048 | 0.362±0.061 | 0.251±0.111 |
| HP10 | 0.432±0.180 | 0.662±0.331 | 2.614±2.309 | 0.563±0.353 | 3.581±3.298 | 0.192±0.130 |
| HP9+HP10 | 0.603±0.075 | 1.997±1.077 | 1.431±0.844 | 0.399±0.114 | 2.314±1.724 | 0.512±0.220 |
| Note: HP9: B. velezensis HP9; HP10: B. firmus HP10. | ||||||

|
| 图 6 不同处理花生根际土壤微生物属水平的热图和聚类分析图 Figure 6 Heatmap and cluster relationship of microorganism in peanut rhizosphere soil with different treatments at the genus level Note: A: Bacteria; B: Fungi. HP9: B. velezensis HP9; HP10: B. firmus HP10; CK: Control. |
|
|
本研究利用了2个不同种的芽孢杆菌菌株进行花生的灌根实验。贝莱斯芽孢杆菌是2005年发现的一个新种,能通过合成脂肽类物质而产生抗菌活性[24],利用该菌的促生研究报道很少。Meng等发现B. velezensis BAC03在抑制疮痂病链霉菌(Streptomyces scabies)的同时,也可通过产生吲哚乙酸、NH3等物质促进胡萝卜、辣椒、甜菜、南瓜、番茄等蔬菜的生长[25]。本研究表明,贝莱斯芽孢杆菌HP9菌株还可以促进花生幼苗茎部的明显伸长及鲜重的显著增加,对花生表现出明显的促生效应。坚强芽孢杆菌作为促生菌在其他植物上已有报道,研究发现B. firmus TG-1通过提高番茄植株的根系还原强度而增强了根系活力,进而促进番茄根部的生长[26];Khan等利用B. firmus NARS1对鹰嘴豆(Cicer arietinum)种子涂种处理后,植株的根部和茎部分别增长了36.70%和10.95%[27];本研究也发现坚强芽孢杆菌HP10处理花生对根部的促生效果更显著,其机制是否相同仍有待于进一步研究。另外,本研究还发现贝莱斯芽孢杆菌HP9和坚强芽孢杆菌HP10混合菌剂灌根处理组花生的茎部、根部整体增长更均衡统一,植株鲜重的增加也最明显,呈现出一定的协同促生效应。
据报道芽孢杆菌可通过定殖而影响土壤优势菌群,或可通过分泌抑菌物质以改变土壤微生物的群落结构[10-12, 28]。本研究中,从α多样性指数来看,处理组与对照组根际土壤细菌和真菌的多样性并无显著差异,但是主成分分析显示,与对照相比,3个芽孢杆菌灌根处理组间的细菌及真菌的群落结构更为相似。处理组根际土壤的拟杆菌门、变形菌门、TM7、厚壁菌门及蓝藻门的相对丰度均有提高,这与前人[29]研究结论基本一致。属水平上,处理组的根际土壤中如农杆菌属、节杆菌属、芽孢杆菌属、伯克霍尔德氏菌属、假单胞菌属及鞘脂单胞菌属等功能菌群的相对丰度明显提高;还检测到有6个独有的细菌属,即无色杆菌属、短波单胞菌属、金黄色杆菌属、苍白杆菌属、鞘氨醇杆菌属和鞘氨醇盒菌属。无色杆菌属[30]、短波单胞菌属[31]、苍白杆菌属[32]和鞘氨醇杆菌属[33]等被报道具有一定的促生作用。可见,芽孢杆菌菌株的灌根处理在一定程度上提高了土壤中具促生功能微生物的相对丰度。慢生根瘤菌属在处理组根际土壤中的丰度有所降低,这是否由于芽孢杆菌灌根的花生已显著提高了根际土壤中的碱解氮含量所致仍有待于进一步研究。对真菌群落结构的影响分析显示,处理组Mortierellomycota的相对丰度显著提高,属水平上的分析表明,这主要归因于被孢霉属相对丰度的增加,作为一群有益的微生物类群,被孢霉属具有溶磷、提供氮营养以及增强作物抗病性等多种促生作用[34-35]。此外,处理组子囊菌门、担子菌门、油壶菌门和GS19的相对丰度降低。据报道绝大多数植物病害都是由担子菌门病原菌引起,如最常见的白粉病、锈病和立枯病等[36],担子菌门丰度的降低在一定程度上也使花生感染该门病原菌的可能性下降。
4 结论本研究利用高通量测序技术、结合土壤氮磷钾有效养分,综合评价芽孢杆菌菌剂灌根对花生促生作用的影响。研究结果显示,贝莱斯芽孢杆菌HP9、坚强芽孢杆菌HP10及其混合菌剂灌根后影响了花生根际土壤的微生物群落结构,拟杆菌门、变形菌门及Mortierellomycota等丰度提高,明显增加了农杆菌属、节杆菌属、芽孢杆菌属、伯克霍尔德氏菌属、假单胞菌属及鞘脂单胞菌属等功能菌群的丰度,降低了酸杆菌门、疣微菌门、放线菌门、子囊菌门和担子菌门等的丰度,并检测到无色杆菌属、短波单胞菌属、金黄色杆菌属、苍白杆菌属、鞘氨醇杆菌属和鞘氨醇盒菌属等6个特有细菌属。显然,芽孢杆菌灌根处理后,具促生功能的微生物菌群明显增多,导致花生根际土壤有益微生物的活化,从而提高了根际土壤养分的有效性并促进了花生植株的生长。
| [1] |
Shen HY, Xiong HC, Guo XT, et al. A new method of Agrobacterium-mediated genetic transformation in peanut plants[J]. Plant Nutrition and Fertilizer Science, 2012, 18(2): 518-522. (in Chinese) 申红芸, 熊宏春, 郭笑彤, 等. 一种发根农杆菌介导的花生遗传转化新方法[J]. 植物营养与肥料学报, 2012, 18(2): 518-522. |
| [2] |
Wang CB, Zheng YP, Liang XY, et al. Effects of fertilization on soil fertility indices and yield of dry-land peanut[J]. Acta Ecologica Sinica, 2013, 33(4): 1300-1307. (in Chinese) 王才斌, 郑亚萍, 梁晓艳, 等. 施肥对旱地花生主要土壤肥力指标及产量的影响[J]. 生态学报, 2013, 33(4): 1300-1307. |
| [3] |
Zhao Z, Chen W, Wang H, et al. Effects of bio-manure combined with chemical fertilizer reduced in application rate on soil fertility and yield and quality of tomato[J]. Acta Pedologica Sinica, 2018, 55(5): 1243-1253. (in Chinese) 赵政, 陈巍, 王欢, 等. 木霉微生物肥与减量化肥配施对番茄产量、品质及土壤肥力的影响[J]. 土壤学报, 2018, 55(5): 1243-1253. |
| [4] |
Zhao J. Biological monitoring and assessment of soil health[J]. Soils, 2006, 38(2): 136-142. (in Chinese) 赵吉. 土壤健康的生物学监测与评价[J]. 土壤, 2006, 38(2): 136-142. |
| [5] |
Nannipieri P, Ascher J, Ceccherini MT, et al. Microbial diversity and soil functions[J]. European Journal of Soil Science, 2003, 54(4): 655-670. DOI:10.1046/j.1351-0754.2003.0556.x |
| [6] |
Romaniuk R, Giuffré L, Costantini A, et al. A comparison of indexing methods to evaluate quality of soils: the role of soil microbiological properties[J]. Soil Research, 2011, 49(8): 733-741. DOI:10.1071/SR11147 |
| [7] |
Giller D, Shaulov A, Prozorov R, et al. Disorder-induced transition to entangled vortex solid in Nd-Ce-Cu-O crystal[J]. Physical Review Letters, 1997, 79(13): 2542-2545. DOI:10.1103/PhysRevLett.79.2542 |
| [8] |
Zhang ZY, Chen H, Yang YH, et al. Effects of continuous cropping on bacterial community diversity in rhizosphere soil of Rehmannia glutinosa[J]. Chinese Journal of Applied Ecology, 2010, 21(11): 2843-2848. (in Chinese) 张重义, 陈慧, 杨艳会, 等. 连作对地黄根际土壤细菌群落多样性的影响[J]. 应用生态学报, 2010, 21(11): 2843-2848. |
| [9] |
de Feudis M, Cardelli V, Massaccesi L, et al. Altitude affects the quality of the water-extractable organic matter (WEOM) from rhizosphere and bulk soil in European beech forests[J]. Geoderma, 2017, 302: 6-13. DOI:10.1016/j.geoderma.2017.04.015 |
| [10] |
Holl FB, Chanway CP. Rhizosphere colonization and seedling growth promotion of lodgepole pine by Bacillus polymyxa[J]. Canadian Journal of Microbiology, 1992, 38(4): 303-308. DOI:10.1139/m92-050 |
| [11] |
Hatzinger PB, Alexander M. Relationship between the number of bacteria added to soil or seeds and their abundance and distribution in the rhizosphere of alfalfa[J]. Plant and Soil, 1994, 158(2): 211-222. DOI:10.1007/BF00009496 |
| [12] |
Kang YJ, Shen M, Wang HL, et al. Effects of two plant growth-promoting rhizobacteria (PGPR) on yardlong bean early seedlings growth and indigenous soil bacterial community[J]. Journal of Agro-Environment Science, 2012, 31(8): 1537-1543. (in Chinese) 康贻军, 沈敏, 王欢莉, 等. 两株PGPR对豇豆苗期生长及土著细菌群落的影响[J]. 农业环境科学学报, 2012, 31(8): 1537-1543. |
| [13] |
Buddrus-Schiemann K, Schmid M, Schreiner K, et al. Root colonization by Pseudomonas sp. DSMZ 13134 and impact on the indigenous rhizosphere bacterial community of barley[J]. Microbial Ecology, 2010, 60(2): 381-393. DOI:10.1007/s00248-010-9720-8 |
| [14] |
Han T, Zhang LM, Gao JM, et al. Correlation between root irrigation of Bacillus subtilis Tpb55 and variation of bacterial diversity in tobacco rhizosphere[J]. Acta Microbiologica Sinica, 2016, 56(5): 835-845. (in Chinese) 韩腾, 张立猛, 高加明, 等. 枯草芽孢杆菌(Bacillus subtilis) Tpb55灌根与烟草根围细菌多样性变化的相关性[J]. 微生物学报, 2016, 56(5): 835-845. |
| [15] |
Tao J, Li H, Li C. Growth-promotion and disease control effect of combination BCL-8 on tomato and growth-promotion mechanism[J]. Plant Protection, 2010, 36(1): 87-91. (in Chinese) 陶晶, 李晖, 李春. 芽胞杆菌组合BCL-8对番茄的促生防病效果及其促生机制初探[J]. 植物保护, 2010, 36(1): 87-91. |
| [16] |
Zhang DY, Liu Y, Wu Y, et al. Isolation and identification of IAA-producing strains from peanut rhizosphere and its promoting effects on peanut growth[J]. Chinese Journal of Oil Crop Sciences, 2016, 38(1): 104-110. (in Chinese) 张东艳, 刘晔, 吴越, 等. 花生根际产IAA菌的筛选鉴定及其效应研究[J]. 中国油料作物学报, 2016, 38(1): 104-110. |
| [17] |
Li YM, Wang QY, Tu WG, et al. Growth promoting activity of siderophore secreting bacteria for peanut plant under nickel stress[J]. Microbiology China, 2017, 44(8): 1882-1890. (in Chinese) 李艳梅, 王琼瑶, 涂卫国, 等. 镍胁迫下产铁载体细菌对花生的促生性[J]. 微生物学通报, 2017, 44(8): 1882-1890. |
| [18] |
Han LZ, Zhou J, Wang H. Identification and phosphorus-dissolving characteristics of two Bacillus strains promoted on peanut growth[J]. Genomics and Applied Biology, 2019, 38(9): 4066-4076. (in Chinese) 韩丽珍, 周静, 王欢. 两株对花生促生的芽孢杆菌的鉴定及溶磷特性研究[J]. 基因组学与应用生物学, 2019, 38(9): 4066-4076. |
| [19] |
Courchesne F, Gobran GR. Mineralogical variations of bulk and rhizosphere soils from a Norway spruce stand[J]. Soil Science Society of America Journal, 1997, 61(4): 1245-1249. DOI:10.2136/sssaj1997.03615995006100040034x |
| [20] |
Yin BZ, Zhen WC, Guo LG. The soil ecological-environment characteristics in wheat growing season under different tillage treatments in Haihe lowland plain[J]. Journal of Soil and Water Conservation, 2015, 29(1): 186-194. (in Chinese) 尹宝重, 甄文超, 郭丽果. 海河低平原不同耕作方式下麦田土壤生态环境特征[J]. 水土保持学报, 2015, 29(1): 186-194. |
| [21] |
Yu HT, Zhou ZX, Lu WQ. Microbial diversity of soybean meal hydrolysate treated by keratinase[J]. Microbiology China, 2019, 46(4): 729-740. (in Chinese) 于海涛, 周智旋, 陆文清. 角蛋白酶水解豆粕的微生物多样性分析[J]. 微生物学通报, 2019, 46(4): 729-740. |
| [22] |
Schmidt V, Plenz B, Pfaff M, et al. Disseminated systemic mycosis in Veiled chameleons (Chamaeleo calyptratus) caused by Chamaeleomyces granulomatis[J]. Veterinary Microbiology, 2012, 161(1/2): 145-152. |
| [23] |
Fadrosh DW, Ma B, Gajer P, et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform[J]. Microbiome, 2014, 2(1): 6. DOI:10.1186/2049-2618-2-6 |
| [24] |
Ruiz-García C, Béjar V, Martínez-Checa F, et al. Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Vélez in Málaga, southern Spain[J]. International Journal of Systematic and Evolutionary Microbiology, 2005, 55(1): 191-195. DOI:10.1099/ijs.0.63310-0 |
| [25] |
Meng QX, Jiang H, Hao JJ. Effects of Bacillus velezensis strain BAC03 in promoting plant growth[J]. Biological Control, 2016, 98: 18-26. DOI:10.1016/j.biocontrol.2016.03.010 |
| [26] |
Liu T. A study on the colonization and promoting plant growth of tomato endophytic Bacillus in Xi'ning area[D]. Xi'ning: Master's Thesis of Qinghai Normal University, 2010 (in Chinese) 刘涛.西宁地区番茄内生芽孢杆菌定殖规律及促生效应的研究[D].西宁: 青海师范大学硕士学位论文, 2010 |
| [27] |
Khan M, Patel CB. Plant growth promoting effect of Bacillus firmus strain NARS1 isolated from Central Himalayan region of India on Cicer arientnum at low temperature[A]//Proceedings of the 8th African Crop Science Society Conference[C]. El-Minia, Egypt: African Crop Science Society, 2007
|
| [28] |
Cheng HB, Chen W, Gu ZR, et al. Influence of nutritional additives on propagation and production of antifungal substance of Bacillus subtilis G3str in soil[J]. Chinese Journal of Biological Control, 2006, 22(2): 142-145. (in Chinese) 程洪斌, 陈伟, 顾真荣, 等. 营养添加剂对枯草芽孢杆菌G3str在土壤中繁殖及其抑菌物质分泌能力的影响[J]. 中国生物防治, 2006, 22(2): 142-145. |
| [29] |
He GQ, Chen SF. Influence of nitrogen-fixing, phosphate-solubilizing bacterial agents on bacterial community in wheat rhizosphere soil[J]. Journal of China Agricultural University, 2015, 20(5): 81-88. (in Chinese) 贺国强, 陈三凤. 固氮、解磷复合菌剂对小麦根际土壤细菌群落的影响[J]. 中国农业大学学报, 2015, 20(5): 81-88. |
| [30] |
Sheng H, Wang DD, Li HC, et al. Community diversity of bacteria producing ACC deaminase in rhizosphere of wheat[J]. Journal of Northwest A & F University (Natural Science Edition), 2017, 45(6): 89-95. (in Chinese) 盛浩, 汪丹丹, 李海超, 等. 小麦根际产ACC脱氨酶细菌种群的多样性研究[J]. 西北农林科技大学学报:自然科学版, 2017, 45(6): 89-95. |
| [31] |
Tong WJ, Zhang L, Xue QY, et al. Isolation of endophytic bacteria in Dendrobium loddigesii collected from different locations and comparison on their plant-growth-promoting potential[J]. Journal of Plant Resources and Environment, 2014, 23(1): 16-23. (in Chinese) 童文君, 张礼, 薛庆云, 等. 不同产地美花石斛内生细菌分离及促生潜力比较[J]. 植物资源与环境学报, 2014, 23(1): 16-23. |
| [32] |
Cui XS, Wang W, Zhang R, et al. Screening of plant growth-promoting rhizobacteria based on rhizosphere nutrition competiveness and investigation of their promoting effects[J]. Journal of Nanjing Agricultural University, 2015, 38(6): 958-966. (in Chinese) 崔晓双, 王伟, 张如, 等. 基于根际营养竞争的植物根际促生菌的筛选及促生效应研究[J]. 南京农业大学学报, 2015, 38(6): 958-966. |
| [33] |
Ji WX, Li HL, Leng X, et al. Isolation and promoting growth properties of endophytic bacteria producing ACC deaminase of Panax ginseng[J]. Journal of Jilin Agricultural University, 2019, 41(2): 168-174. (in Chinese) 姬文秀, 李虎林, 冷雪, 等. 产ACC脱氨酶人参内生细菌的分离和促生特性分析[J]. 吉林农业大学学报, 2019, 41(2): 168-174. |
| [34] |
Zhang HS, Wu XH, Li G, et al. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities[J]. Biology and Fertility of Soils, 2011, 47(5): 543. DOI:10.1007/s00374-011-0563-3 |
| [35] |
Liao XG, Fang WG, Lin LC, et al. Metarhizium robertsii produces an extracellular invertase (MrINV) that plays a pivotal role in rhizospheric interactions and root colonization[J]. PLoS One, 2013, 8(10): e78118. DOI:10.1371/journal.pone.0078118 |
| [36] |
Sheikhelzati Issa. Urumqi, korla and Ili ornamental plant fungi disease investigation[D]. Urumqi: Maste's Thesis of Xinjiang Agricultural University, 2016 (in Chinese) 谢海尔扎提·艾萨.乌鲁木齐、库尔勒和伊犁园林观赏植物真菌病害调查[D].乌鲁木齐: 新疆农业大学硕士学位论文, 2016 |
 2020, Vol. 47
2020, Vol. 47