扩展功能
文章信息
- 吕红, 张欣, 周杨, 周集体
- LV Hong, ZHANG Xin, ZHOU Yang, ZHOU Ji-Ti
- 细菌产黄素类化合物介导的电子传递及对环境污染物的厌氧生物转化研究进展
- Bacterial flavins-mediated electron transfer and its effects on anaerobic biotransformation of environmental pollutants: a review
- 微生物学通报, 2020, 47(10): 3419-3430
- Microbiology China, 2020, 47(10): 3419-3430
- DOI: 10.13344/j.microbiol.china.200414
-
文章历史
- 收稿日期: 2020-04-26
- 接受日期: 2020-08-08
- 网络首发日期: 2020-08-18
黄素通常被认为是二甲基异恶嗪{7, 8-二甲基苯并[g]蝶啶-2, 4(3H, 10H)-二酮}骨架的衍生物,在10位上具有取代基;核黄素、黄素单核苷酸(flavin mononucleotide,FMN)和黄素腺嘌呤二核苷酸(flavin adenine dinucleotide,FAD)是黄素类化合物的主要代表[1]。照射核黄素会导致其分解,在碱性条件下产生7, 8, 10-三甲基异氧嗪,而在中性和酸性条件下产生7, 8-二甲基异氧嗪[2]。
大多数细菌能够产生核黄素,仅有极少的细菌(如棒状杆菌、乳酸杆菌和链球菌等)不能合成核黄素,需要从外部环境中获取[1]。同时,所有的细菌都能将核黄素转化为FMN和FAD[1]。黄素类化合物作为酶的辅助因子,在氧化还原反应中用于完成单、双电子和氢的转移,在分解和合成代谢中起关键作用,甚至它们参与的催化反应还可扩展到一系列不同的生化反应,包括DNA光修复、卤化和去卤化、抗生素合成、二氧活化、群体信号等[3]。随着研究的深入,人们发现部分菌株分泌到胞外的黄素可以作为一种氧化还原介体参与电子传递,介导胞外的多种过程,包括铁的获取、细胞外呼吸和物种间的相互作用[4-6]。它们具有较低的氧化还原电位,是一种理想的还原剂,适合还原环境中铁、锰氧化物及有毒污染物[7-8]。
基于这些研究,本文主要对细菌体内黄素的合成与分泌,以及其作为黄素蛋白的辅酶因子或以自由态的形式参与电子传递的过程,特别是参与环境中相关污染物降解的研究进展进行综述。
1 黄素的细菌合成与分泌核黄素由一个三磷酸鸟苷分子(guanosine- 5-triphosphate,GTP)和两个核酮糖-5-磷酸盐分子合成[1, 3],具体如图 1所示。在核黄素生物合成过程中,第一步反应是由ribA编码的GTP环水解酶Ⅱ催化的,从GTP中去除C-8,反应的产物为2, 5-二氨基-6-核糖基氨基-4(3H)-嘧啶二酮-5'-磷酸盐;然后ribD编码的嘧啶脱氨酶/还原酶催化2号位置的氨基脱氨以及核糖体侧链还原,得到5-氨基-6-核糖酸氨基-2, 4(1H, 3H)-嘧啶二酮-5'-磷酸盐;再经过进一步的去磷酸化反应得到5-氨基-6-核糖酸氨基-2, 4(1H, 3H)-嘧啶二酮。但是目前尚不清楚去磷酸化的机理,有待进一步深入研究。核酮糖-5-磷酸盐在ribB编码的3, 4-二羟基-2-丁酮-4-磷酸盐合成酶催化下生成3, 4-二羟基-2-丁酮-4-磷酸盐;然后5-氨基-6-核糖酸氨基-2, 4(1H, 3H)-嘧啶二酮和3, 4-二羟基-2-丁酮-4-磷酸盐转化为蝶啶化合物(6, 7-二甲基-8-核糖酸二氧四氢喋啶);2个蝶啶化合物分子发生歧化反应,发生4-碳单元的交换,将其中一个转化为核黄素,另一个转化为5-氨基- 6-核糖酸氨基-2, 4(1H, 3H)-嘧啶二酮。最后,核黄素合成后,再通过ribF基因编码的核黄素激酶/ FAD合酶转化为FMN,然后转化为FAD。
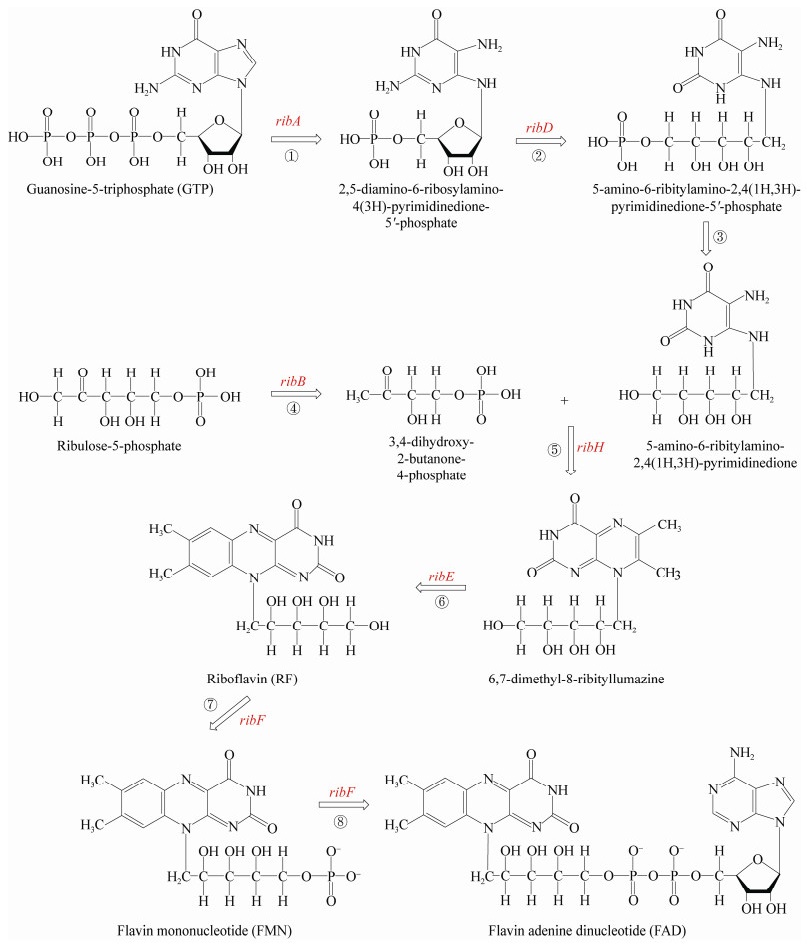
|
| 图 1 黄素类化合物的生物合成途径 Figure 1 Biosynthesis pathway of flavins |
|
|
细菌核黄素合成和调控的研究主要是在枯草芽孢杆菌和大肠杆菌中开展的[9]。如图 2所示,在这两种菌中,核黄素合成途径中编码每个酶的基因只有一个拷贝。但在一组系统发育多样化的细菌(如Shewanella oneidensis MR-1)基因组中核黄素合成基因有多个拷贝,如ribA、ribB、ribBA (双功能的ribBA基因是由2个独立的基因ribA和ribB编码)或ribE[10-11]。有研究者预测Shewanella菌中有一个核黄素合成基因表达调控因子(riboflavin,RFN)位于ribB基因的上游,主要负责调控ribB基因[11]。基于菌株MR-1可以分泌黄素到胞外发生作用,Brutinel等[9-10]推测,黄素生物合成基因的多拷贝可以实现对基因的差异调节,以响应对细胞外黄素的需求,即有效地创建了两条表达途径,ribB基因的表达可能会受到细胞内FMN浓度的控制,而ribBA基因的表达可能不受控制[10]。
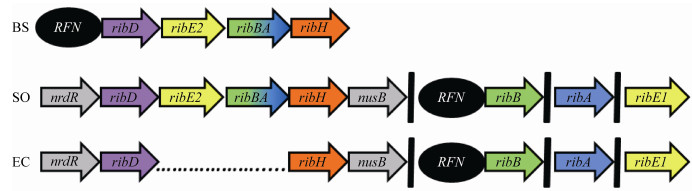
|
| 图 2 典型细菌的核黄素合成基因[10] Figure 2 Riboflavin synthesis genes of typical bacteria[10] Note: BS: Bacillus subtilis subsp. subtilis 168; SO: Shewanella oneidensis MR-1; EC: Escherichia coli MG1655. RFN: Regulation factor of riboflavin biosynthesis gene expression; nrdR: gene encoding nucleotide reductase and other gene transcription inhibitors; ribD: gene encoding pyrimidine deaminase/reductase; ribE: gene encoding riboflavin synthetase; ribBA: gene encoding 3, 4-dihydroxy-2-butanone 4-phosphate synthase, GTP cyclic hydrolase II; ribH: gene encoding 6, 7-dimethyl-8-riboacid dioxygotradine synthetase; nusB: gene encoding bacterial resistance to termination complex factors; ribB: gene encoding 3, 4-dihydroxy-2-butanone 4-phosphate synthase; ribA: Gene encoding GTP cyclic hydrolase II. |
|
|
细胞内黄素优先以FAD和FMN形式存在,其中FAD含量最高;游离细胞内核黄素含量不超过总黄素的7%[12]。核黄素在细胞质中合成为FAD,内膜转运蛋白将FAD转运至周质空间,然后在水解酶(UshA)作用下水解为FMN和磷酸腺苷,FMN穿过细胞外膜后扩散到细胞外,在胞外不稳定又可水解为核黄素[10, 12-13]。此外,在Listeria monocytogenes中FAD可以通过外膜转运蛋白(RibU和FmnA)分泌到胞外,水解后经外膜上的FMN转移酶(FmnB)运送给膜上的脂蛋白(PplA),并与其共价结合,参与胞外的电子传递[6]。据报道,细菌胞内合成的黄素不仅可以通过黄素蛋白参与电子传递,还可以分泌到胞外环境中参与电子传递[6, 13-14]。
2 胞内蛋白中的黄素参与电子传递将黄素作为辅酶因子的蛋白质通常称为黄素蛋白。FMN和FAD是比较常见的黄素蛋白的辅酶因子,其本身没有催化作用,主要是在依赖于黄素的蛋白中参与了从电子供体到电子受体的电子传递过程[14]。细菌体内含有各种黄素蛋白,其中有些黄素蛋白具有偶氮染料、铬酸盐和羰基化合物(包括醌类化合物和硝基化合物)的还原活性,这使细菌在去除环境污染物方面起到了重要的作用。
2.1 偶氮还原酶偶氮还原酶以NAD(P)H为电子供体还原裂解偶氮键。这种酶广泛存在于细菌中,并可作用于多种偶氮染料[15]。根据有无辅酶因子,细菌偶氮还原酶可分为黄素依赖性酶和无黄素酶[15]。黄素依赖性的偶氮还原酶多数以FMN为辅酶因子,少数以FAD为辅酶因子(表 1)。还有部分偶氮还原酶(如:AZRBS、AZR-BSUa和AZR-BSUi)尚未有确切证据表明其含有黄素辅酶因子(未列在表 1),但Sugiura等[35]发现它们的序列中含有可能参与FMN结合的氨基酸残基,因此推测这些蛋白可能是黄素蛋白。黄素依赖性的偶氮还原酶催化偶氮染料脱色的过程通常遵循“乒乓机理”:首先NAD(P)H还原辅酶因子FMN,其异咯嗪发色团参与氧化还原反应,从而导致催化中心形成FMNH2;随后,偶氮还原酶释放NAD(P)+,并通过FMNH2接受偶氮染料使其还原[17, 29, 36]。
| Enzyme | Clade | Source of strain | Cofactor | Electron donor | Substrate | References |
| AZR | Ⅰ | Rhodobacter sphaeroides AS1.1737 | FMN | NAD(P)H | Azo dyes, Nitroaromatic compounds, Quinones | [16-18] |
| YhdA | Ⅰ | B. subtilis 168 | FMN | NAD(P)H | Azo dyes, FMN | [19] |
| AzoABS | Ⅱ | Bacillus sp. AO1 | Flavin | NADH | Azo dyes, Sulforhodamine B, Indigocarmine, Triphenylmethane | [19] |
| AzoAEF | Ⅱ | Enterococcus faecalis ATCC 19433 | FMN | NAD(P)H | Azo dyes, Nitroaromatic compounds | [20] |
| AzoEf1 | Ⅱ | Enterococcus faecium ATCC 6569 | FMN | NAD(P)H | Azo dyes | [21] |
| AzrA | Ⅱ | Bacillus sp. B29 | FMN | NAD(P)H | Azo dyes | [22] |
| AzrB | Ⅱ | Bacillus sp. B29 | FMN | NAD(P)H | Azo dyes | [22] |
| AzrC | Ⅱ | Bacillus sp. B29 | FMN | NAD(P)H | Azo dyes | [22] |
| AzrG | Ⅱ | G. stearothermophilus ATCC 12980 | FMN | NAD(P)H | Azo dyes | [23] |
| BrAzo | Ⅱ | Brevibacillus laterosporus TISTR1911 | FMN | NAD(P)H | Azo dyes | [24] |
| YvaB | Ⅱ | B. subtilis 168 | FMN | NADH | Azo dyes, 2, 6-dichloroindophenol | [25] |
| AzoR | Ⅲ | Escherichia coli JM109 | FMN | NADH | Azo dyes, Quinones, Nitroaromatic compounds | [26] |
| AzoRo | Ⅲ | Rhodococcus opacus 1CP | FMN | NAD(P)H | Azo dyes | [27] |
| PaAzoR1 | Ⅲ | Pseudomonas aeruginosa PAO1 | FMN | NAD(P)H | Azo dyes, Quinones, Nitroaromatic compounds | [28] |
| PaAzoR2 | Ⅲ | P. aeruginosa PAO1 | FMN | NAD(P)H | Azo dyes, Nitroaromatic compounds | [28] |
| PaAzoR3 | Ⅲ | P. aeruginosa PAO1 | FMN | NAD(P)H | Azo dyes, Nitroaromatic compounds | [28] |
| PpAzoR | Ⅲ | P. putida MET94 | FMN | NAD(P)H | Azo dyes | [29] |
| Azo1 | Others | Staphylococcus aureus ATCC 25923 | FMN | NADPH | Azo dyes | [30] |
| AzoC | Others | Clostridium perfringens ATCC 3626 | Flavin | NAD(P)H | Azo dyes, Quinones | [31] |
| EF0404 | Others | Enterococcus faecalis V583 | FMN | NAD(P)H | Azo dyes, Quinones | [20] |
| PaArsH | Others | P. aeruginosa PAO1 | FMN | NAD(P)H | Azo dyes, Quinones, Nitroaromatic compounds | [32] |
| PaMdaB | Others | P. aeruginosa PAO1 | FAD | NAD(P)H | Azo dyes, Quinones, Nitroaromatic compounds | [32] |
| PaYieF | Others | P. aeruginosa PAO1 | FAD | NAD(P)H | Azo dyes, Quinones, Nitroaromatic compounds | [32] |
| SfArsH | Others | Shigella flexneri 2a | FMN | NADPH | Azo dyes, FMN | [33] |
| SmArsH | Others | Sinorhizobium meliloti Rm1021 | FMN | NADPH | Azo dyes, FMN | [34] |
偶氮还原酶在系统发育上可分为四大主要分支[15]。分支Ⅰ−Ⅲ都属于NAD(P)H依赖性FMN还原酶超家族,多数是黄素蛋白,而分支Ⅳ的成员是无黄素偶氮还原酶。分支Ⅲ除了偶氮还原酶AzoH和SoAzoR,其余都被证明是黄素蛋白。一些偶氮还原酶(Azo1、AzoC、SfArsH和SmArsH)尽管在系统发育上与分支Ⅰ−Ⅳ成员分离,但属于NAD(P)H依赖性FMN还原酶超家族。在这些黄素蛋白中,黄素通常以共价或非共价形式与蛋白结合,其存在对偶氮还原酶活性是必要的。
研究表明,部分偶氮还原酶不仅能够催化偶氮染料的还原[18-34](表 1),也可以催化还原其他底物,例如靛酚类[19]、吲哚酚[25]、金属离子[15]、硝基芳香族化合物[17, 20, 26, 28, 33]、醌类化合物[16-18, 26, 28, 31-32]和三苯基甲烷[19]等。据报道Rhodobacter sphaeroides AS1.1737中的黄素依赖性偶氮还原酶AZR能够还原Cr(Ⅵ)、醌化合物和多种硝基化合物[17-18]。Crescente等[32]发现P. aeruginosa PAO1中分离出来的黄素依赖性偶氮还原酶PaArsH、PaMdaB、PaYieF具有硝基还原酶和醌还原酶活性。Chalansonnet等[20]报道在硝基化合物还原时,Enterococcus faecalis V583中的黄素依赖性偶氮还原酶首先还原辅酶因子FMN,这一过程是独立于底物的,并遵循“乒乓机理”。然后还原态的FMN再将电子传递给底物,整个过程要比偶氮染料的还原过程更为复杂。可见,在黄素依赖性的偶氮还原酶催化这些底物还原过程中,FMN参与了电子的传递。
2.2 铬酸盐还原酶细菌铬酸盐还原酶通常以NAD(P)H为电子供体,将高毒性的六价铬还原为毒性较低的三价铬[37-38]。铬酸盐厌氧还原通常在胞外进行,因为铬酸盐还原酶大多是膜结合蛋白,如黄素还原酶和细胞色素酶等(表 2)[39-44]。少数铬酸盐还原酶是可溶性的[45],这些铬酸盐还原酶主要是黄素蛋白。
| Enzyme | Source of strain | Electron transfer mechanism | Electron donor | Cofactor | References |
| ChrR | P. putida | Single+double electrons | NAD(P)H | FMN | [39] |
| YieF | E. coli | Double electrons | NAD(P)H | FMN | [40] |
| NfsA | E. coli | Single+double electrons | NAD(P)H | FMN | [40] |
| YcnD | B. subtilis | − | NADH | FMN | [41] |
| ChrA | P. aeruginosa, Cupriavidus metallidurans | − | NADPH | FMN | [42] |
| Gh-ChrR | E. coli | − | NAD(P)H | FMN | [43] |
| NemA | E. coli | − | NADH | FMN | [44] |
| Note: −: Electron transfer mechanism was unclear. | |||||
在Cr(Ⅵ)还原过程中,由于同时产生活性氧(reactive oxygen species,ROS),细菌细胞受到氧化应激[46]。根据氧化应激产生的强度,细胞内铬酸盐还原酶可分为单电子还原酶和双电子还原酶[15]。单电子还原酶是一种依赖于黄素的酶,主要发挥催化生物合成或能量反应的作用[37]。FMN与黄素蛋白紧密结合,氧化态FMN接受一个电子形成稳定的半醌自由基(FMNH),参与Cr(Ⅵ)的还原;在单电子还原过程中,先将Cr(Ⅵ)还原成高度不稳定的Cr(Ⅴ)中间体;然后再将Cr(Ⅴ)氧化为Cr(Ⅵ),电子给予氧分子,从而产生大量ROS[37]。已鉴定属于单电子还原酶的铬酸盐还原酶主要包括来自Clostridium kluyveri的硫辛酰基脱氢酶、细胞色素c、谷胱甘肽还原酶和铁氧化还原蛋白- NADP氧化还原酶[38]。双电子还原酶是依赖NAD(P)H的酶,主要是黄素蛋白。其特征是H+ (相当于1个质子和2个电子)在反应中的转移,NAD(P)H作为电子供体将FMN还原,然后还原态的FMN将电子传递给Cr(Ⅵ);在双电子还原过程中,没有形成Cr(Ⅴ)中间体,所以反应过程中产生的活性氧要比单电子还原少得多[37]。属于双电子还原酶的铬酸盐还原酶有来自P. putida的ChrR、来自E. coli的YieF和NfsA[37-38]。研究发现,NfsA和ChrR在铬酸盐还原过程中也涉及单电子转移[39-40]。
此外,来自E. coli中的黄素蛋白NemA也具有Cr(Ⅴ)还原能力,其通过从辅助因子NAD(P)H中添加1个或2个电子来催化铬酸盐还原[44, 47]。另一个从E. coli中分离出的黄素蛋白醌还原酶ChrR也能够还原Cr(Ⅵ)[43]。从P. aeruginosa/ Cupriavidus metallidurans中分离出的ChrA属于依赖NADPH的黄素蛋白家族,Ramírez-Díaz等[48]发现其与Cr(Ⅵ)的抗性和还原有关。
3 分泌到胞外的黄素参与电子传递在厌氧条件下,微生物胞内产生的电子经胞内呼吸链传递给胞外电子受体,如不溶性电子受体含铁矿物、含锰矿物和固体电极等,这一过程称为胞外电子传递(extracellular electron transport,EET)。由于不溶性电子受体很难进入细胞内,因此在胞外的电子传递过程中游离态的黄素起到了重要的作用。下面就含铁矿物和固体电极等电子受体的胞外还原过程中黄素的作用进行阐述。
3.1 含铁矿物的还原作为地表最丰富的过渡金属元素,铁元素广泛存在于土壤、岩石以及沉积物中,主要以Fe(Ⅲ)的形式存在于固体矿物中[49]。在多种缺氧环境中,微生物对Fe(Ⅲ)的还原都是非常重要的过程,因为Fe(Ⅲ)的还原可使各类污染物中大量有机碳发生厌氧氧化;此外,通过添加氧化还原介体黄素类化合物可以促进电子传递,提高沉积物中污染物降解与Fe(Ⅲ)还原的耦合速率,使得通常在缺氧条件下也难以降解的化合物如苯、氯化物和甲基叔丁基醚等也可被分解[50]。
Shewanella oneidensis MR-1是研究Fe(Ⅲ)还原反应的模式生物。关于生物还原铁氧化物的电子传递机理,Pirbadian等[51-52]提出,Shewanella oneidensis MR-1中电子传递是通过c-型细胞色素复合物(MtrCAB-OmcA)中血红素链的多步跳跃发生的,菌株MR-1首先将代谢电子供体产生的电子传递给内膜蛋白CymA,再经周质蛋白MtrA将电子依次传递到跨膜蛋白MtrB、外膜蛋白MtrC或OmcA,最后直接将电子传递给细胞外的Fe(Ⅲ)[53]。由于菌株MR-1能够分泌黄素类物质,因此在Fe(Ⅲ)还原过程中黄素类物质起到了重要作用。相关学者分别提出了自由穿梭和氧化还原结合态辅助因子机制(图 3)。早期研究提出“自由穿梭机制”[12],但是这一机制存在无法解释氧化还原电位的问题。FMN/FMNH2的氧化还原电位为−220 mV (vs标准氢电极),而菌株MR-1细胞内MtrCAB-OmcA复合物的电位为+50 mV,介体电位比电子供体电位低270 mV。如果以这种机制促进电子传递,必须要额外吸收较多能量才能发生,这种机制显然存在矛盾。Okamoto等[54]针对这一矛盾进行了相关研究。研究发现菌株MR-1分泌的FMN可能作为辅助因子与外膜细胞色素MtrC结合形成半醌FMNH (氧化还原电位为−145 mV),通过单电子反应作为主要途径加强EET过程,并推测核黄素可能与OmcA蛋白结合,也同样以单电子反应促进EET过程。Hong等[53]在模拟分子动力学的基础上,对核黄素结合的束缚能和氧化还原特性进行分析,也推测核黄素可能与OmcA蛋白结合。
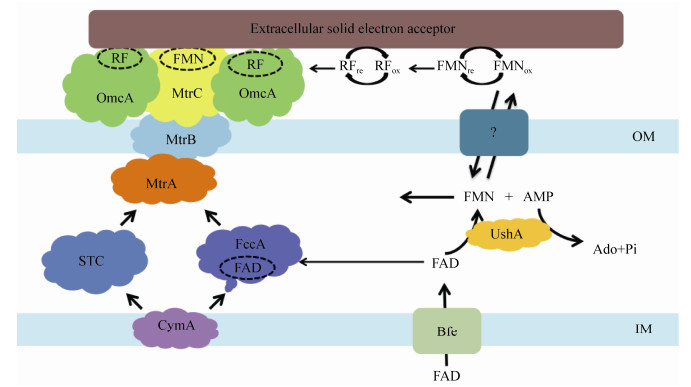
|
| 图 3 Shewanella oneidensis MR-1中黄素强化EET机理图 Figure 3 Proposed mechanism of flavins-mediated EET in Shewanella oneidensis MR-1 Note: OM: Outer membrane; IM: Inner membrane. |
|
|
von Canstein等[4]第一次证明了Shewanella菌向胞外分泌的黄素类物质是FMN和核黄素(氧化还原电位−208 mV),它们能够作为氧化还原电子穿梭体促进弱结晶铁氧化物(三价铁氧化物/Fe2+,−100到+100 mV)的还原,并且菌株MR-1分泌的微克水平的黄素类化合物就可以将电子传递效率提高3.7倍。Balasubramanian等[55]发现Methylocystis strain M、Methylosinus trichosporium OB3b和Methylococcus capsulatus (Bath)这3种甲烷菌也可以在非细胞裂解条件下分泌黄素;与Shewanella菌相类似,FAD主要在胞内,而FMN和核黄素分泌到胞外,FMN和核黄素的功能有可能作为电子穿梭体还原Fe(Ⅲ)等过渡金属,但还需要进一步证实。Fuller等[56]证明了以Tissierella、Clostridium和Alkaliphilus sp.为主体的嗜碱菌群(pH > 9.0)能够在厌氧条件下以Fe(Ⅲ)为最终电子受体,并通过初步纯化和光谱分析、高效液相色谱和电化学分析,确定了嗜碱菌群可以分泌黄素类化合物;根据Fe(Ⅲ)还原和黄素产生之间的密切联系,他们推测黄素在这个亲碱群落的胞外金属还原中起重要作用。Kotloski等[57]则通过调控bfe基因调节胞外FMN浓度,发现FMN浓度的提高对Fe(Ⅲ)底物有明显促进作用。以上研究均为黄素类物质促进含铁矿物的还原提供了有力证据。
3.2 电极的还原除了含铁矿物,研究人员对电极的生物还原也开展了广泛的研究,以期提高生物电化学系统(bioelectrochemical system,BES)的性能。BES在污染物的降解、污染场地的修复、海水淡化和清洁能源(氢气、电能)产出等方面展示着广阔的应用前景,引起人们的广泛关注。生物电化学系统可分为微生物燃料电池(microbial fuel cell,MFC)和微生物电解电池。其中,MFC已被陆续用于各种工业废水的处理(如垃圾沥出液、偶氮染料、苯酚、氯酚、2, 4, 6-三氯苯酚、砷等)。MFC处理废水的化学需氧量去除率和产电库伦效率均高达80%及以上;此外,MFC还可以同时去除废水中的氮、硫化物(硝酸盐、亚硝酸盐、铵盐、硫酸盐等)[58]。
从微生物呼吸链到阳极的电子传递是MFC技术的关键。研究表明,微生物可以通过外膜细胞色素或纳米线结构与电极直接接触传递,还可以通过电子介体间接传递。其中Shewanella sp.和Geobacter sp.是MFC产电微生物中的模式菌株。菌株MR-1胞外电子受体为电极时的EET机制同含铁矿物作为电子受体时是一致的,黄素类物质增强EET主要通过与黄素蛋白结合形式,同时存在以电子穿梭体形式的增强作用。在Geobacter sulfurreducens中,早期研究认为主要通过外膜的c型细胞色素或者纳米线的直接接触为主要机制促进EET[10]。在继菌株MR-1中发现黄素作为辅助因子与外膜c型细胞色素结合促进EET之后,在G. sulfurreducens中也发现,以电极作为电子受体时,利用自分泌的核黄素与外膜c型细胞色素结合促进了EET[58-59]。但是关于这一机制是否在MFC电极界面上存在和对产生电流的贡献方面还有待进一步的研究。
Marsili等[60]已经发现,接种菌株MR-1的MFC中,连续培养72 h的生物膜上清液中核黄素是主要产物,若从生物膜中去除核黄素,则会导致MFC的电子传递效率降低70%;Jiang等[61]也通过去除长时间培养的菌株MR-1生物膜产生的核黄素的方法,证明了MFC中的电子转移主要是通过电子介体介导的。此外,Tian等[62]通过循环伏安法和高效液相色谱法的鉴定,发现Bacillus cereus DIF1和Rhodococcus ruber DIF2可以在MFC中分泌核黄素和FMN,从而增强MFC的电子转移。从MFC中分离的另一株芽孢杆菌Bacillus megaterium LLD-1也具有分泌核黄素的能力,并将电子传递给电极[63]。这些发现为电化学活性菌分泌黄素促进MFC的胞外电子传递提供了更多证据。基于黄素的这个作用,Cao等[64]将核黄素通过电沉积的方式固定到氧化石墨烯修饰的玻璃碳阳极上,从而使阳极的电化学活性提高2倍。鉴于细菌仅能产微量的黄素类化合物,Yang等[65]通过将枯草芽孢杆菌的黄素合成基因在菌株MR-1中异源表达,将分泌的黄素浓度增加了25.7倍,提高了菌株MR-1的双向EET率,其中MFC的最大输出功率增加了约13.2倍,而内向电流增加了约15.5倍。
3.3 其他电子受体除了含铁矿物和固态电极,黄素还可以通过胞外电子传递机制催化DMSO[66]、含锰矿物[67]、溶解态重金属(包括铀U6+[68]、钒V5+[69]、锝7+[70])、黄碲矿[71]、石墨[67]、玻璃炭[72]及氧化石墨烯[73]的还原。催化机制如上所述,这里就不再赘述。进一步研究表明,当不同电子受体存在时,菌株MR-1分泌到胞外的黄素类化合物浓度不同[74]。研究还发现固定化醌如蒽醌-2-磺酸修饰的聚氨酯泡沫的存在会使希瓦氏菌产生更多的黄素类化合物[75-76]。由于固定化醌能够加速偶氮染料、硝基芳烃和氯代化合物等多种污染物的厌氧生物转化,因此在这个过程中黄素类物质的贡献及固定化醌浓度对细菌产黄素的影响还有待进一步研究。
4 展望黄素类化合物作为大多数细菌自身合成分泌的氧化还原介体,由于其有效避免了外源性介体带来的成本较高和造成二次污染的问题,从而使其介导的电子传递机理以及参与的生物修复过程得到了广泛的研究,特别是在胞外介导环境污染物的去除方面。但仍有一些尚未解决的问题,例如细菌(如菌株MR-1、Geobacter)分泌黄素的途径、分泌的机制还有待更进一步的阐明,这不仅有助于理解黄素促进EET的作用,而且有利于黄素类化合物在实际环境污染物处理中的应用。此外,在自然界中还普遍存在其他氧化还原介体如腐殖酸、吩嗪类化合物等,因此,黄素类化合物与这些氧化还原介体相互作用关系也有待进一步阐明,以便于更好地理解黄素类化合物参与的生物地球化学循环。
| [1] |
Abbas CA, Sibirny AA. Genetic control of biosynthesis and transport of riboflavin and flavin nucleotides and construction of robust biotechnological producers[J]. Microbiology and Molecular Biology Reviews, 2011, 75(2): 321-360. |
| [2] |
de Leenheer AP, Lambert WE, van Bocxlaer JF. Modern Chromatographic Analysis of Vitamins[M]. 3rd ed. New York: Marcel Dekker, 2000: 391.
|
| [3] |
Monteverde DR, Sylvan JB, Suffridge C, et al. Distribution of extracellular flavins in a coastal marine basin and their relationship to redox gradients and microbial community members[J]. Environmental Science & Technology, 2018, 52(21): 12265-12274. |
| [4] |
von Canstein H, Ogawa J, Shimizu S, et al. Secretion of flavins by Shewanella species and their role in extracellular electron transfer[J]. Applied and Environmental Microbiology, 2008, 74(3): 615-623. |
| [5] |
Light SH, Su L, Rivera-Lugo R, et al. A flavin-based extracellular electron transfer mechanism in diverse gram-positive bacteria[J]. Nature, 2018, 562(7725): 140-144. DOI:10.1038/s41586-018-0498-z |
| [6] |
Okamoto A, Saito K, Inoue, K, et al. Uptake of self-secreted flavins as bound cofactors for extracellular electron transfer in Geobacter species[J]. Energy & Environmental Science, 2014, 7(4): 1357-1361. |
| [7] |
Liu CX, Gorby YA, Zachara JM, et al. Reduction kinetics of Fe (Ⅲ), Co (Ⅲ), U (Ⅵ), Cr (Ⅵ), and Tc (Ⅶ) in cultures of dissimilatory metal-reducing bacteria[J]. Biotechnology and Bioengineering, 2002, 80(6): 637-649. DOI:10.1002/bit.10430 |
| [8] |
Ackerley DF, Gonzalez CF, Park CH, et al. Chromate-reducing properties of soluble flavoproteins from Pseudomonas putida and Escherichia coli[J]. Applied and Environmental Microbiology, 2004, 70(2): 873-882. |
| [9] |
Brutinel ED, Dean AM, Gralnick JA. Description of a riboflavin biosynthetic gene variant prevalent in the phylum Proteobacteria[J]. Journal of Bacteriology, 2013, 195(24): 5479-5486. DOI:10.1128/JB.00651-13 |
| [10] |
Brutinel ED, Gralnick JA. Shuttling happens:soluble flavin mediators of extracellular electron transfer in Shewanella[J]. Applied Microbiology and Biotechnology, 2012, 93(1): 41-48. |
| [11] |
Vitreschak AG, Rodionov DA, Mironov AA, et al. Regulation of riboflavin biosynthesis and transport genes in bacteria by transcriptional and translational attenuation[J]. Nucleic Acids Research, 2002, 30(14): 3141-3151. DOI:10.1093/nar/gkf433 |
| [12] |
Yang YG, Xu MY, Guo J, et al. Bacterial extracellular electron transfer in bioelectrochemical systems[J]. Process Biochemistry, 2012, 47(12): 1707-1714. DOI:10.1016/j.procbio.2012.07.032 |
| [13] |
Covington ED, Gelbmann CB, Kotloski NJ, et al. An essential role for UshA in processing of extracellular flavin electron shuttles by Shewanella oneidensis[J]. Molecular Microbiology, 2010, 78(2): 519-532. DOI:10.1111/j.1365-2958.2010.07353.x |
| [14] |
Pradhan SK, Singh NR, Dehury B, et al. Insights into the mode of flavin mononucleotide binding and catalytic mechanism of bacterial chromate reductases:A molecular dynamics simulation study[J]. Journal of Cellular Biochemistry, 2019, 120(10): 16990-17005. DOI:10.1002/jcb.28960 |
| [15] |
Suzuki H. Remarkable diversification of bacterial azoreductases:primary sequences, structures, substrates, physiological roles, and biotechnological applications[J]. Applied Microbiology and Biotechnology, 2019, 103(10): 3965-3978. DOI:10.1007/s00253-019-09775-2 |
| [16] |
Yan B, Zhou JT, Wang J, et al. Expression and characteristics of the gene encoding azoreductase from Rhodobacter sphaeroides AS1.1737[J]. FEMS Microbiology Letters, 2004, 236(1): 129-136. DOI:10.1111/j.1574-6968.2004.tb09638.x |
| [17] |
Liu GF, Zhou JT, Lv H, et al. Azoreductase from Rhodobacter sphaeroides AS1.1737 is a flavodoxin that also functions as nitroreductase and flavin mononucleotide reductase[J]. Applied Microbiology and Biotechnology, 2007, 76(6): 1271-1279. DOI:10.1007/s00253-007-1087-5 |
| [18] |
Liu GF, Zhou JT, Jin RF, et al. Enhancing survival of Escherichia coli by expression of azoreductase AZR possessing quinone reductase activity[J]. Applied Microbiology and Biotechnology, 2008, 80(3): 409. |
| [19] |
Deller S, Sollner S, Trenker-El-Toukhy R, et al. Characterization of a thermostable NADPH:FMN oxidoreductase from the mesophilic bacterium Bacillus subtilis[J]. Biochemistry, 2006, 45(23): 7083-7091. DOI:10.1021/bi052478r |
| [20] |
Chalansonnet V, Mercier C, Orenga S, et al. Identification of Enterococcus faecalis enzymes with azoreductases and/or nitroreductase activity[J]. BMC Microbiology, 2017, 17: 126. DOI:10.1186/s12866-017-1033-3 |
| [21] |
Macwana SR, Punj S, Cooper J, et al. Identification and isolation of an azoreductase from Enterococcus faecium[J]. Current Issues in Molecular Biology, 2010, 12(1): 43-48. |
| [22] |
Ooi T, Shibata T, Matsumoto K, et al. Comparative enzymatic analysis of azoreductases from Bacillus sp.B29[J]. Bioscience, Biotechnology, and Biochemistry, 2009, 73(5): 1209-1211. DOI:10.1271/bbb.80872 |
| [23] |
Matsumoto K, Mukai Y, Ogata D, et al. Characterization of thermostable FMN-dependent NADH azoreductase from the moderate thermophile Geobacillus stearothermophilus[J]. Applied Microbiology and Biotechnology, 2010, 86(5): 1431-1438. DOI:10.1007/s00253-009-2351-7 |
| [24] |
Lang W, Sirisansaneeyakul S, Ngiwsara L, et al. Characterization of a new oxygen-insensitive azoreductase from Brevibacillus laterosporus TISTR1911:toward dye decolorization using a packed-bed metal affinity reactor[J]. Bioresource Technology, 2013, 150: 298-306. DOI:10.1016/j.biortech.2013.09.124 |
| [25] |
Nishiya Y, Yamamoto Y. Characterization of a NADH:dichloroindophenol oxidoreductase from Bacillus subtilis[J]. Bioscience, Biotechnology, and Biochemistry, 2007, 71(2): 611-614. DOI:10.1271/bbb.60548 |
| [26] |
Mercier C, Chalansonnet V, Orenga S, et al. Characteristics of major Escherichia coli reductases involved in aerobic nitro and azo reduction[J]. Journal of Applied Microbiology, 2013, 115(4): 1012-1022. |
| [27] |
Qi JX, Schlömann M, Tischler D. Biochemical characterization of an azoreductase from Rhodococcus opacus 1CP possessing methyl red degradation ability[J]. Journal of Molecular Catalysis B:Enzymatic, 2016, 130: 9-17. DOI:10.1016/j.molcatb.2016.04.012 |
| [28] |
Ryan A, Kaplan E, Laurieri N, et al. Activation of nitrofurazone by azoreductases:multiple activities in one enzyme[J]. Scientific Reports, 2011, 1: 63. DOI:10.1038/srep00063 |
| [29] |
Mendes S, Pereira L, Batista C, et al. Molecular determinants of azo reduction activity in the strain Pseudomonas putida MET94[J]. Applied Microbiology and Biotechnology, 2011, 92(2): 393-405. DOI:10.1007/s00253-011-3366-4 |
| [30] |
Chen HZ, Hopper SL, Cerniglia CE. Biochemical and molecular characterization of an azoreductase from Staphylococcus aureus, a tetrameric NADPH-dependent flavoprotein[J]. Microbiology, 2005, 151(5): 1433-1441. DOI:10.1099/mic.0.27805-0 |
| [31] |
Morrison JM, Wright CM, John GH. Identification, isolation and characterization of a novel azoreductase from Clostridium perfringens[J]. Anaerobe, 2012, 18(2): 229-234. DOI:10.1016/j.anaerobe.2011.12.006 |
| [32] |
Crescente V, Holland SM, Kashyap S, et al. Identification of novel members of the bacterial azoreductase family in Pseudomonas aeruginosa[J]. Biochemical Journal, 2016, 473(5): 549-558. DOI:10.1042/BJ20150856 |
| [33] |
Vorontsov II, Minasov G, Brunzelle JS, et al. Crystal structure of an apo form of Shigella flexneri ArsH protein with an NADPH-dependent FMN reductase activity[J]. Protein Science, 2007, 16(11): 2483-2490. DOI:10.1110/ps.073029607 |
| [34] |
Ye J, Yang HC, Rosen BP, et al. Crystal structure of the flavoprotein ArsH from Sinorhizobium meliloti[J]. FEBS Letters, 2007, 581(21): 3996-4000. DOI:10.1016/j.febslet.2007.07.039 |
| [35] |
Sugiura W, Yoda T, Matsuba T, et al. Expression and characterization of the genes encoding azoreductases from Bacillus subtilis and Geobacillus stearothermophilus[J]. Bioscience, Biotechnology, and Biochemistry, 2006, 70(7): 1655-1665. DOI:10.1271/bbb.60014 |
| [36] |
Nakanishi M, Yatome C, Ishida N, et al. Putative ACP phosphodiesterase gene (acpD) encodes an azoreductase[J]. Journal of Biological Chemistry, 2001, 276(49): 46394-46399. DOI:10.1074/jbc.M104483200 |
| [37] |
Barak Y, Ackerley DF, Dodge CJ, et al. Analysis of novel soluble chromate and uranyl reductases and generation of an improved enzyme by directed evolution[J]. Applied and Environmental Microbiology, 2006, 72(11): 7074-7082. DOI:10.1128/AEM.01334-06 |
| [38] |
Thatoi H, Das S, Mishra J, et al. Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium:A review[J]. Journal of Environmental Management, 2014, 146: 383-399. DOI:10.1016/j.jenvman.2014.07.014 |
| [39] |
Park CH, Keyhan M, Wielinga B, et al. Purification to homogeneity and characterization of a novel Pseudomonas putida chromate reductase[J]. Applied and Environmental Microbiology, 2000, 66(5): 1788-1795. DOI:10.1128/AEM.66.5.1788-1795.2000 |
| [40] |
Ackerley DF, Gonzalez CF, Keyhan M, et al. Mechanism of chromate reduction by the Escherichia coli protein, NfsA, and the role of different chromate reductases in minimizing oxidative stress during chromate reduction[J]. Environmental Microbiology, 2004, 6(8): 851-860. DOI:10.1111/j.1462-2920.2004.00639.x |
| [41] |
Morokutti A, Lyskowski A, Sollner S, et al. Structure and function of YcnD from Bacillus subtilis, a flavin-containing oxidoreductase[J]. Biochemistry, 2005, 44(42): 13724-13733. DOI:10.1021/bi0510835 |
| [42] |
Díaz-Pérez C, Cervantes C, Campos-García J, et al. Phylogenetic analysis of the chromate ion transporter (CHR) superfamily[J]. The FEBS Journal, 2007, 274(23): 6215-6227. DOI:10.1111/j.1742-4658.2007.06141.x |
| [43] |
Eswaramoorthy S, Poulain S, Hienerwadel R, et al. Crystal structure of ChrR-a quinone reductase with the capacity to reduce chromate[J]. PLoS One, 2012, 7(4): e36017. DOI:10.1371/journal.pone.0036017 |
| [44] |
Robins KJ, Hooks DO, Rehm BHA, et al. Escherichia coli NemA is an efficient chromate reductase that can be biologically immobilized to provide a cell free system for remediation of hexavalent chromium[J]. PLoS One, 2013, 8(3): e59200. DOI:10.1371/journal.pone.0059200 |
| [45] |
Jin HJ, Zhang YF, Buchko GW, et al. Structure determination and functional analysis of a chromate reductase from Gluconacetobacter hansenii[J]. PLoS One, 2012, 7(8): e42432. DOI:10.1371/journal.pone.0042432 |
| [46] |
Cheung KH, Lai HY, Gu JD. Membrane-associated hexavalent chromium reductase of Bacillus megaterium TKW3 with induced expression[J]. Journal of Microbiology and Biotechnology, 2006, 16(6): 855-862. |
| [47] |
Roldán MD, Pérez-Reinado E, Castillo F, et al. Reduction of polynitroaromatic compounds:the bacterial nitroreductases[J]. FEMS Microbiology Reviews, 2008, 32(3): 474-500. DOI:10.1111/j.1574-6976.2008.00107.x |
| [48] |
Ramírez-Díaz MI, Díaz-Pérez C, Vargas E, et al. Mechanisms of bacterial resistance to chromium compounds[J]. Biometals, 2008, 21(3): 321-332. DOI:10.1007/s10534-007-9121-8 |
| [49] |
Liu GF, Zhu JQ, Yu HL, et al. Review on electron-shuttle-mediated microbial reduction of iron oxides minerals[J]. Earth Science, 2018, 43(S1): 157-170. (in Chinese) 柳广飞, 朱佳琪, 于华莉, 等. 电子穿梭体介导微生物还原铁氧化物的研究进展[J]. 地球科学, 2018, 43(S1): 157-170. |
| [50] |
Lovley DR, Holmes DE, Nevin KP. Dissimilatory Fe (Ⅲ) and Mn (Ⅳ) reduction[J]. Advances in Microbial Physiology, 2004, 49: 219-286. DOI:10.1016/S0065-2911(04)49005-5 |
| [51] |
Pirbadian S, El-Naggar MY. Multistep hopping and extracellular charge transfer in microbial redox chains[J]. Physical Chemistry Chemical Physics, 2012, 14(40): 13802-13808. DOI:10.1039/c2cp41185g |
| [52] |
Pirbadian S, Barchinger SE, Leung KM, et al. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(35): 12883-12888. DOI:10.1073/pnas.1410551111 |
| [53] |
Hong GY, Pachter R. Bound flavin-cytochrome model of extracellular electron transfer in Shewanella oneidensis:analysis by free energy molecular dynamics simulations[J]. The Journal of Physical Chemistry B, 2016, 120(25): 5617-5624. DOI:10.1021/acs.jpcb.6b03851 |
| [54] |
Okamoto A, Kalathil S, Deng X, et al. Cell-secreted flavins bound to membrane cytochromes dictate electron transfer reactions to surfaces with diverse charge and pH[J]. Scientific Reports, 2014, 4: 5628. |
| [55] |
Balasubramanian R, Levinson BT, Rosenzweig AC. Secretion of flavins by three species of Methanotrophic bacteria[J]. Applied and Environmental Microbiology, 2010, 76(21): 7536-7538. |
| [56] |
Fuller SJ, McMillan DGG, Renz MB, et al. Extracellular electron transport-mediated Fe (Ⅲ) reduction by a community of Alkaliphilic bacteria that use flavins as electron shuttles[J]. Applied and Environmental Microbiology, 2014, 80(1): 128-137. DOI:10.1128/AEM.02282-l3 |
| [57] |
Kotloski NJ, Gralnick JA. Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis[J]. mBio, 2013, 4(1): e00553-12. DOI:10.1128/mBio.00553-12 |
| [58] |
Wang Q, Huang LP, Yu HT, et al. Recent developments of graphene electrodes in bioelectrochemical systems[J]. Acta Physico-Chimica Sinica, 2013, 29(5): 889-896. (in Chinese) 王强, 黄丽萍, 于洪涛, 等. 用于生物电化学系统的石墨烯电极新进展[J]. 物理化学学报, 2013, 29(5): 889-896. |
| [59] |
Huang LY, Tang JH, Chen M, et al. Two modes of riboflavin-mediated extracellular electron transfer in Geobacter uraniireducens[J]. Frontiers in Microbiology, 2018, 9: 2886. DOI:10.3389/fmicb.2018.02886 |
| [60] |
Marsili E, Baron DB, Shikhare ID, et al. Shewanella secretes flavins that mediate extracellular electron transfer[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(10): 3968-3973. DOI:10.1073/pnas.0710525105 |
| [61] |
Jiang XC, Hu JS, Fitzgerald LA, et al. Probing electron transfer mechanisms in Shewanella oneidensis MR-1 using a nanoelectrode platform and single-cell imaging[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(39): 16806-16810. DOI:10.1073/pnas.1011699107 |
| [62] |
Tian T, Fan XY, Feng M, et al. Flavin-mediated extracellular electron transfer in Gram-positive bacteria Bacillus cereus DIF1 and Rhodococcus ruber DIF2[J]. RSC Advances, 2019, 9(70): 40903-40909. DOI:10.1039/C9RA08045G |
| [63] |
You LX, Liu LD, Xiao Y, et al. Flavins mediate extracellular electron transfer in Gram-positive Bacillus megaterium strain LLD-1[J]. Bioelectrochemistry, 2018, 119: 196-202. DOI:10.1016/j.bioelechem.2017.10.005 |
| [64] |
Cao YJ, Mu H, Liu W, et al. Electricigens in the anode of microbial fuel cells:pure cultures versus mixed communities[J]. Microbial Cell Factories, 2019, 18: 39. DOI:10.1186/s12934-019-1087-z |
| [65] |
Yang Y, Ding YZ, Hu YD, et al. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway[J]. ACS Synthetic Biology, 2015, 4(7): 815-823. DOI:10.1021/sb500331x |
| [66] |
Gralnick JA, Vali H, Lies DP, et al. Extracellular respiration of dimethyl sulfoxide by Shewanella oneidensis strain MR-1[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(12): 4669-4674. |
| [67] |
Bretschger O, Obraztsova A, Sturm CA, et al. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants[J]. Applied and Environmental Microbiology, 2007, 73(21): 7003-7012. DOI:10.1128/AEM.01087-07 |
| [68] |
Gao HC, Barua S, Liang YL, et al. Impacts of Shewanella oneidensis c-type cytochromes on aerobic and anaerobic respiration[J]. Microbial Biotechnology, 2010, 3(4): 455-466. DOI:10.1111/j.1751-7915.2010.00181.x |
| [69] |
Marshall MJ, Beliaev AS, Dohnalkova AC, et al. c-Type cytochrome-dependent formation of U (Ⅳ) nanoparticles by Shewanella oneidensis[J]. PLoS Biology, 2006, 4(8): e268. DOI:10.1371/journal.pbio.0040268 |
| [70] |
Myers JM, Antholine WE, Myers CR. Vanadium (Ⅴ) reduction by Shewanella oneidensis MR-1 requires menaquinone and cytochromes from the cytoplasmic and outer membranes[J]. Applied and Environmental Microbiology, 2004, 70(3): 1405-1412. |
| [71] |
Marshall MJ, Plymale AE, Kennedy DW, et al. Hydrogenase-and outer membrane c-type cytochrome-facilitated reduction of technetium (Ⅶ) by Shewanella oneidensis MR-1[J]. Environmental Microbiology, 2008, 10(1): 125-136. |
| [72] |
Coursolle D, Gralnick JA. Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR-1[J]. Molecular Microbiology, 2010, 77(4): 995-1008. |
| [73] |
Jiao YQ, Qian F, Li Y, et al. Deciphering the electron transport pathway for graphene oxide reduction by Shewanella oneidensis MR-1[J]. Journal of Bacteriology, 2011, 193(14): 3662-3665. DOI:10.1128/JB.00201-11 |
| [74] |
Rabaey K, Boon N, Siciliano SD, et al. Biofuel cells select for microbial consortia that self-mediate electron transfer[J]. Applied and Environmental Microbiology, 2014, 70(9): 5373-5382. |
| [75] |
Wang J, Lu H, Zhou Y, et al. Enhanced biotransformation of nitrobenzene by the synergies of Shewanella species and mediator-functionalized polyurethane foam[J]. Journal of Hazardous Materials, 2013, 252-253: 227-232. DOI:10.1016/j.jhazmat.2013.02.040 |
| [76] |
Zhou Y, Lu H, Wang J, et al. Catalytic performance of quinone and graphene-modified polyurethane foam on the decolorization of azo dye Acid Red 18 by Shewanella sp.RQs-106[J]. Journal of Hazardous Materials, 2018, 356: 82-90. DOI:10.1016/j.jhazmat.2018.05.043 |
 2020, Vol. 47
2020, Vol. 47




