扩展功能
文章信息
- 宁唤唤, 徐志凯, 柏银兰
- NING Huan-Huan, XU Zhi-Kai, BAI Yin-Lan
- c-di-AMP在细菌感染与免疫中的作用
- Role of c-di-AMP in bacterial infection and immunity
- 微生物学通报, 2019, 46(9): 2362-2369
- Microbiology China, 2019, 46(9): 2362-2369
- DOI: 10.13344/j.microbiol.china.180785
-
文章历史
- 收稿日期: 2018-10-11
- 接受日期: 2018-12-18
- 网络首发日期: 2019-01-28
2. 西北大学生命科学学院 陕西 西安 710069
2. College of Life Sciences, Northwest University, Xi'an, Shaanxi 710069, China
细菌通过第二信号分子感知细胞表面的信号并激活胞内靶标分子,将原始信号放大从而激活胞内一系列特异性基因的表达,最终影响细菌多种生理生化过程。环二腺苷酸(Cyclic diadenosine monophosphate,c-di-AMP)是2008年发现的一种细菌第二信号分子[1]。目前已在多种细菌中发现了c-di-AMP,它不仅调节细菌生长代谢等多种生理学过程,而且能够被宿主识别并诱导免疫应答。因此,c-di-AMP成为了细菌性感染药物和疫苗研究的新靶点。本文主要对c-di-AMP的生理功能及其在宿主抗感染免疫中的作用进行综述。
1 c-di-AMP概述2008年,Witte等在解析枯草芽胞杆菌DNA完整性扫描蛋白A (DNA integrity scanning protein A,DisA)晶体结构时发现了c-di-AMP分子,因此将DisA命名为二腺苷酸环化酶(Diadenylate cyclase,DAC),并将其具有催化活性的结构域命名为Dac结构域[1]。c-di-AMP是由两分子ATP经二腺苷酸环化酶A (DacA)催化合成的环状核苷酸分子。枯草芽胞杆菌的DisA是第一个被发现的c-di-AMP合成酶[1]。枯草芽胞杆菌中的二鸟苷酸环化酶(Diguanylate cyclase) YybT可降解细菌环状核苷酸信号分子环二鸟苷酸(Cyclic dimeric guanosine monophosphate,c-di-GMP)。YybT含有GGDEF结构域,可催化GTP合成c-di-GMP,将YybT与其他菌属中含有GGDEF结构域的蛋白命名为GGDEF结构域蛋白(GGDEF domain protein containing PDE,GdpP)[2]。随后发现,YybT含有的DHH/DHHA1结构域具有磷酸二酯酶(Phosphodiesterase,PDE)活性,可水解c-di-AMP为pApA,是c-di-AMP的水解酶。本课题组成员发现结核分枝杆菌中的Rv2837c仅含有DHH/DHHA1结构域,可将c-di-AMP降解为AMP,并将其命名为CnpB (Cyclic nucleotide phosphodiesterase,CnpB)[3]。随后课题组成员发现肺炎链球菌中存在2种含有DHH/DHHA1结构域的PDE酶:Pde1将c-di-AMP降解为pApA,Pde2可将c-di-AMP和pApA降解为AMP[4]。
目前研究表明c-di-AMP仅存在于细菌中,主要在厚壁菌门(如:芽胞杆菌、李斯特菌、葡萄球菌、链球菌)、放线菌门(如:结核分枝杆菌)和支原体中,见表 1。
| 细菌Bacteria | 合成酶Synthetases | 分解酶Degrading enzymes | 表型Phenotypes | 参考文献References |
| 枯草芽胞杆菌 Bacillus subtilis | DisA, CdaA, CdaS | GdpP, PgpH | c-di-AMP水平降低:DNA完整性降低,钾离子通道系统受损,细胞壁受损,抗生素敏感性增强,芽胞形成延迟 c-di-AMP reduced: decreased DNA integrity, impaired potassium ion channel system, weakened cell wall, increased resistance to antibiotics, delayed sporulation | [5-7] |
| 单核细胞增 生李斯特菌 Listeria monocytogenes | CdaA | PdeA, PgpH | c-di-AMP水平降低:抗生素敏感性增强,细胞壁稳定性降低c-di-AMP水平升高:酸胁迫敏感性降低,宿主细胞IFN-β应答增强,诱导细胞焦亡增加 c-di-AMP reduced: increased sensitivity to antibiotics, reduced cell wall stability c-di-AMP elevated: sensitivity towards acid stress, enhanced IFN-β response in host cells, increased cell pyroptosis | [8-9] |
| 结核分枝杆菌 Mycobacterium tuberculosis | Mtb DisA | Mtb PDE | c-di-AMP水平降低:生长略慢,毒性增强,宿主细胞IFN-β应答降低,诱导宿主细胞自噬降低 c-di-AMP水平升高:细菌长度变短,毒性降低,宿主细胞IFN-β应答增强,诱导宿主细胞自噬增加 c-di-AMP reduced: slightly slower growth rate, increased virulence, decreased IFN-β response of host cells, reduced autophagy of host cells c-di-AMP elevated: smaller cell size, decreased virulence, induced increased IFN-β response in host cells, increased induction of autophagy in host cells | [3, 10-12] |
| 耻垢分枝杆菌 Mycobacterium smegmatis | Ms DisA | Ms PDE | c-di-AMP水平降低:C12–C20脂肪酸合成下调 c-di-AMP水平升高:菌落变小,C12–C20脂肪酸合成上调 c-di-AMP reduced: reduced C12–C20 fatty acids production c-di-AMP elevated: formed small colonies, increased C12–C20 fatty acids production | [13] |
| 金黄色葡萄球菌 Staphylococcus aureus | CdaA | GdpP, Pde2 | c-di-AMP水平升高:细菌变小,肽聚糖交联增加,对靶向细胞壁、细胞膜的抗生素耐敏感性降低,钾离子通道系统受损 c-di-AMP elevated: smaller cell size, increased peptidoglycan cross-linking, reduced sensitivity against cell wall and membrane targeting antibiotics, impaired potassium ion channel system | [14-16] |
| 变形链球菌 Streptococcus mutans | CdaA | PdeA, Pde2 | c-di-AMP水平降低:对过氧化氢敏感性增加,胞外多糖产生增加 c-di-AMP水平升高:生物被膜形成增加 c-di-AMP reduced: increased sensitivity to hydrogen peroxide and enhanced polysaccharide synthesis c-di-AMP elevated: increased biofilm formation | [17-18] |
| 肺炎链球菌 Streptococcus pneumoniae | CdaA | Pde1, Pde2 | c-di-AMP水平升高:细菌长链形成受损,生长降低,钾离子通道功能受损 c-di-AMP elevated: impaired ability of long chain formation, decreased growth, and imbalance in the potassium ion channel | [4, 19] |
| 化脓链球菌 Streptococcus pyogenes | CdaA | GdpP, Pde2 | c-di-AMP水平升高:半胱氨酸蛋白酶(SpeB)活性降低,毒性降低,对抗生素敏感性降低 c-di-AMP elevated: impaired biogenesis of SpeB, decreased virulence and increased antibiotic resistance | [20] |
| 猪链球菌 Streptococcus suis (SS2) | CdaA | GdpP, Pde2 | c-di-AMP水平升高:生长变慢,生物被膜形成增加,毒性降低 c-di-AMP elevated: reduced growth, increased biofilm formation and reduced virulence | [21] |
| B群链球菌 Group B Streptococcus | NudP | CdnP | c-di-AMP水平升高:诱导IFN-β应答增强,毒力降低 c-di-AMP elevated: increased induction of IFN-β response in host cells and decreased virulence | [22] |
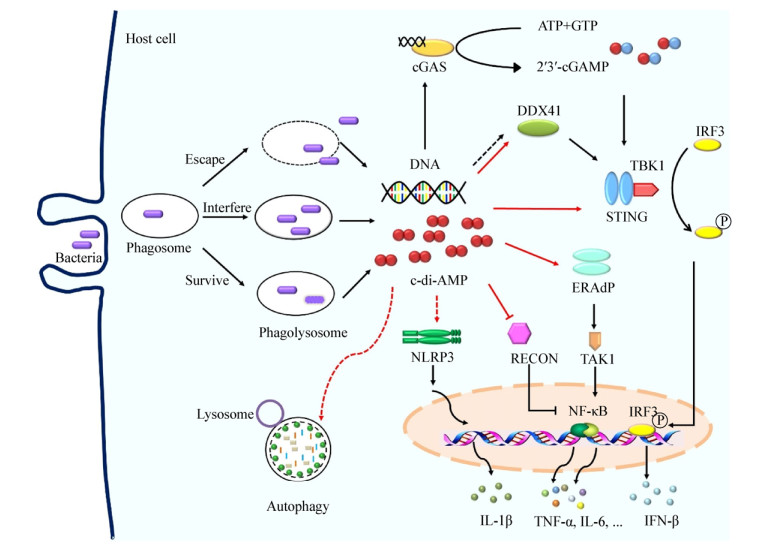
固有免疫是机体抵御微生物尤其是胞内菌感染的重要防线。哺乳动物细胞通过模式识别受体(Pattern recognition receptor,PRR)识别微生物的病原体相关的分子模式(Pathogen-associated molecular patterns,PAMP),启动固有免疫反应[23]。一些致病菌产生的c-di-AMP可被宿主细胞识别并启动下游免疫反应,被认为是固有免疫中新发现的PAMP[12]。细菌感染宿主后c-di-AMP可能同时被真核细胞中多个感应子/受体识别,从而网络式精确调控机体抗感染状态。迄今为止,在真核细胞中发现4个c-di-AMP感应子/受体(Sensor/receptor)或接头蛋白(Adaptor):干扰素基因刺激分子(Stimulator of interferon genes,STING)[24]、一种RNA解旋酶DDX41 (D-E-A-D [aspartate-glutamate-alanine-aspartate]-box polypeptide 41,DDX41)[25]、NF-κB控制还原酶(Reductase controlling NF-kB,RECON)[26]以及内质网膜接头蛋白(Endoplasmic reticulum [ER] membrane adaptor,ERAdP)[27](图 1)。细菌感染后,c-di-AMP被宿主的感应子/受体识别,从而网络式调控宿主免疫状态,进而影响细菌在宿主体内的生长及传播。因此,c-di-AMP与宿主免疫的相互作用的研究有利于细菌性疾病新疫苗的研制。
|
| 图 1 c-di-AMP调控宿主固有免疫应答 Figure 1 c-di-AMP regulates host innate immune response 注:病原菌感染后,细菌自身释放的或细菌被机体杀伤后“泄露”的c-di-AMP和DNA参与调节宿主固有免疫应答.病原菌被巨噬细胞吞噬后,细菌DNA通过cGAS或者DDX41介导STING依赖的Ⅰ型干扰素应答,c-di-AMP也可通过DDX41和STING激活宿主IFN-β应答. c-di-AMP通过激活ERAdP或者解除RECON对NF-κB的抑制效应从而激活NF-κB通路活性. c-di-AMP可诱导NLRP3依赖的炎症小体应答.此外,c-di-AMP也可激活宿主细胞自噬. Note: After the bacteria infect the host, c-di-AMP and DNA that are released by the bacteria themselves or killed by host are involved in regulating the host innate immune response. After phagocytosis by macrophage, bacterial DNA mediates STING-dependent type Ⅰ interferon responses via cGAS or DDX41, and c-di-AMP also induces IFN-β responses via DDX41 and STING. c-di-AMP activates NF-κB pathway by activating ERAdP or inhibiting RECON. c-di-AMP induces NLRP3-dependent inflammasome response. In addition, c-di-AMP activates host cell autophagy. |
|
|
胞质监督途径(Cytosolic surveillance pathway,CSP)是机体抵抗病毒、细菌感染的重要免疫防御机制。细菌感染宿主后,其DNA被宿主DNA感受器环鸟-腺苷酸合酶(Cyclic-GMP-AMP synthase,cGAS)识别,活化的cGAS合成环鸟-腺苷酸(Cyclic-GMP-AMP,cGAMP)并将感染信号传递至STING,接头分子STING募集TANK结合激酶1 (TANK-binding kinase 1,TBK1)并激活干扰素调节因子3 (Interferon regulatory factor 3,IRF3),诱导Ⅰ型干扰素产生[28]。STING是调控Ⅰ型干扰素表达的关键分子之一,在机体抵抗病毒及细菌感染中发挥重要作用。多个研究证明STING是c-di-GMP的直接受体分子[29-31]。c-di-GMP与DDX41也存在直接相互作用,而DDX41相比于STING对c-di-GMP具有更高的亲和力,同时c-di-AMP也可与DDX41结合[25]。STING不仅参与细菌DNA诱导的固有免疫,还可识别细菌特有的环状核苷酸分子c-di-AMP和c-di-GMP从而调控抗感染免疫[28]。
2010年,Woodward等[9]首次报道c-di-AMP能够直接激活宿主IFN-β应答。敲除李斯特菌c-di-AMP分解酶突变株感染巨噬细胞后IFN-β表达显著上调,而c-di-AMP合成酶的条件性去除(Conditional depletion)株感染巨噬细胞后也可诱导IFN-β应答[11]。本课题组成员前期首次报道,结核分枝杆菌Rv3586是与DisA同源的c-di-AMP合成酶[32],随后采用c-di-AMP分解酶敲除的结核分枝杆菌感染巨噬细胞,发现IFN-β应答水平升高,而该过程不依赖于NOD2[3]。Dey等发现过表达c-di-AMP合成酶或敲除c-di-AMP分解酶的结核分枝杆菌均可激活IRF,从而诱导宿主IFN-β应答水平上升,且该过程依赖于STING的激活[12],同时STING蛋白对于c-di-GMP诱导的Ⅰ型干扰素反应也是必需的[33]。用过表达Rv3586的重组卡介苗感染巨噬细胞,发现IFN-β表达显著增加[34]。上述研究表明,c-di-AMP通过STING通路介导了宿主以Ⅰ型干扰素IFN-β释放为特征的固有免疫应答。
此外,c-di-AMP能够显著诱导树突状细胞(Dendritic cell,DC) IFN-β应答,但是当c-di-AMP持续刺激DC时,可引发钙蛋白酶介导的STING蛋白的降解,使得IFN-β分泌减少[35]。提示细菌来源的c-di-AMP虽然可诱导宿主细胞免疫应答,但是这种作用可能是自限性的、不连续的,推测宿主具有相应的调控机制,以避免c-di-AMP诱导过度的免疫应答导致免疫病理损伤。
2.2 c-di-AMP促进NF-κB通路活性NF-κB是广泛存在的多功能核转录因子,其调节包括免疫识别受体、细胞因子、抗原提呈蛋白、黏附分子、趋化因子等多种靶基因表达,这些分子在抗感染及炎症反应中发挥重要作用。
NF-κB控制还原酶RECON是NF-κB通路抑制子。研究发现,c-di-AMP抑制RECON活性从而促进了炎症细胞因子IFN-β、趋化因子CCL5、CXCL11的表达,限制了李斯特菌、衣原体在宿主免疫细胞及非免疫细胞(肝脏细胞系)内的存活[26]。c-di-AMP通过抑制RECON酶活性增强促炎因子的产生,这种抗感染方式与依赖STING激活的抗感染应答是不同的。然而,李斯特菌感染肝脏细胞后,其分泌的c-di-AMP抑制了RECON活性,增强NF-κB活性及一氧化氮(NO)产生,但NO却促进了细菌在细胞间的传播(Cell-to-cell spread),这不同于以往的c-di-AMP有助于宿主清除病原菌感染机制,推测不同细菌感染宿主后c-di-AMP作用时间、方式及与宿主细胞相互作用存在差异[36]。
最新发现,内质网膜接头蛋白ERAdP缺陷小鼠感染李斯特菌后,促炎细胞因子产生减少,感染小鼠对细菌清除能力降低,小鼠生存率降低,表明ERAdP介导促炎细胞因子产生对于机体控制细菌感染至关重要[27]。c-di-AMP与ERAdP蛋白C端结合,使ERAdP形成二聚体并激活转化生长因子β激活酶1 (TGF-β activated kinase 1,TAK1),启动下游NF-κB活化,诱导促炎细胞因子TNF-α、IL-6释放增加,促进了机体抗感染免疫[27]。ERAdP-TAK1通路为清除李斯特菌感染所必需。ERAdP是c-di-AMP的直接感应子,而且c-di-AMP与ERAdP亲和力强于STING[27]。
2.3 c-di-AMP激活炎症小体NOD样受体(NOD like receptor,NLR)是一类分布于胞浆中的识别病原体的PRR分子,招募多种蛋白质组成炎症小体(Inflammasome),炎性小体激活后活化天冬氨酸特异性半胱氨酸蛋白酶1 (Caspase-1),活化的Caspase-1剪切IL-1β、IL-18使其成熟,并发挥促炎作用[37]。以IL-1β、IL-18产生为特征的炎症小体的激活是宿主防御多种病原体所必需[38]。
细菌感染宿主后,适度的炎症应答可促进机体对病原菌清除。NLR家族NLRP1、NLRP3、NLRC4以及HIN200结构域家族的黑素瘤缺乏因子2 (Absent in melanoma 2,AIM2)炎症小体可识别病原体上的PAMP,产生防御性炎症反应。其中NLRP3主要分布于巨噬细胞、中性粒细胞胞质及胞膜,与病毒和胞内菌感染关系密切,也是NLR家族研究最为深入的成员[37]。研究发现,c-di-AMP和c-di-GMP均可诱导巨噬细胞系THP-1和小鼠骨髓来源巨噬细胞(Bone marrow derived macrophage,BMDM) Caspase-1的表达增加,并释放高水平的IL-1β;NLRP3-/-小鼠的BMDM对c-di-AMP和c-di-GMP刺激的反应性缺失,表明c-di-AMP和c-di-GMP激活炎症小体应答是NLRP3依赖的,且该过程不依赖于STING分子[39]。课题组前期研究表明c-di-AMP能够诱导巨噬细胞NLRP3、IL-1β转录上调,而且过表达Rv3586的重组卡介苗可促进炎症小体关键分子NLRP3、NLRC4、IL-1β表达上调(待发表数据)。因此,c-di-AMP对炎症小体的激活作用有助于机体抗感染免疫。
2.4 c-di-AMP激活宿主细胞自噬自噬(Autophagy)是真核细胞中普遍存在的免疫防御机制。促进自噬有助于宿主对入侵病原菌(如:结核分枝杆菌、沙门氏菌、李斯特菌)的清除。微管相关蛋白质轻链3 (LC3)的表达被认为是自噬发生的金标准[40],而p62蛋白水平与自噬活性负相关[41]。
Dey等[12]首次报道,过表达c-di-AMP合成酶的重组结核分枝杆菌感染巨噬细胞后,自噬小体标志性分子LC3表达显著增加,同时LC3Ⅱ蛋白水平显著增加,表明c-di-AMP激活了自噬,且自噬的激活抑制了感染细胞内细菌的生长。本课题组成员发现c-di-AMP能够诱导巨噬细胞自噬相关基因LC3、Beclin1、Atg5及Atg7转录水平上调,但是过表达c-di-AMP合成酶的重组卡介苗并不影响LC3蛋白表达水平[37]。自噬是多基因协同完成的精密调控过程,营养匮乏、感染等因素均可诱导自噬发生[42-43],而自噬信号的缺乏和抑制是胞内菌免疫逃逸的重要原因之一,因此提高c-di-AMP水平促进细胞自噬,有助于机体抵抗病原菌感染[12]。
3 c-di-AMP诱导的黏膜免疫应答黏膜免疫系统广泛分布于呼吸道、胃肠道、泌尿生殖道黏膜下及一些外分泌腺体处的淋巴组织,是执行局部非特异性免疫功能的主要场所,对呼吸道、胃肠道及泌尿生殖道等抗感染免疫非常重要。
Ebensen等[44]将c-di-AMP作为佐剂,与β-半乳糖苷酶联合滴鼻免疫小鼠,可促进IgA和IgG,及IFN-γ、IL-2、IL-17、IL-4分泌,表明c-di-AMP作为黏膜免疫佐剂可诱导强烈的适应性免疫应答。以c-di-AMP及cGAMP与白蛋白联合免疫小鼠,也可诱导黏膜免疫应答,并且c-di-AMP相比于cGAMP能够诱导更强的体液和细胞免疫应答[45]。对其机制研究表明,c-di-AMP可促进DC成熟相关分子CD80、CD86、MHC-Ⅱ类分子的表达[45]。DC细胞膜上的CD80、CD86是T细胞表面共刺激分子CD28的配体,因此,c-di-AMP可促进T细胞的增殖和活化,进而发挥特异性免疫。课题组研究发现,c-di-AMP与结核分枝杆菌抗原联合经黏膜免疫小鼠,可诱导高水平的黏膜免疫应答,并可抵抗一定数量的结核分枝杆菌感染(待发表数据)。因此,c-di-AMP与亚单位疫苗联合用于黏膜免疫,可能获得免疫效果更好的黏膜免疫疫苗。
4 结语细菌c-di-AMP激活宿主固有免疫在抗感染中的积极作用备受关注[3, 11-12, 22, 46]。在宿主与细菌的长期作用中,宿主具备了c-di-AMP的识别、感应机制以监控感染。一方面,细菌分泌的c-di-AMP能够激活以Ⅰ型干扰素释放为特征的固有免疫应答;另一方面,细菌通过控制释放到宿主细胞中c-di-AMP的含量,逃避免疫识别而加剧感染的发生。c-di-AMP可诱导机体Ⅰ型干扰素应答、炎症因子激活及自噬发生,有助于机体抗感染免疫;但同时,c-di-AMP诱导产生的NO促进细菌的细胞间播散[36]。因此,需要进一步借助多种研究方法进行多层面深入的研究,全面审视c-di-AMP在宿主感染中发挥的作用。本课题组研究表明,c-di-AMP作为免疫佐剂单独使用时免疫效能较低,与抗原联合免疫可显著增强抗原免疫效能,同时通过提高卡介苗内源性c-di-AMP的水平,可显著提高卡介苗诱导的免疫应答水平,这将有助于新型细菌疫苗的设计[34]。此外,鉴于多种细菌采用c-di-AMP为信号分子,若用于疫苗或免疫调节剂,需要进一步研究c-di-AMP对机体正常菌群的可能的调节作用。目前对细菌c-di-AMP的研究仍处于早期阶段,c-di-AMP在宿主固有免疫和适应性免疫中的作用机制仍需进一步深入探究。
| [1] |
Witte G, Hartung S, Büttner K, et al. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates[J]. Molecular Cell, 2008, 30(2): 167-178. DOI:10.1016/j.molcel.2008.02.020 |
| [2] |
Rao F, See RY, Zhang DY, et al. YybT is a signaling protein that contains a cyclic dinucleotide phosphodiesterase domain and a GGDEF domain with ATPase activity[J]. Journal of Biological Chemistry, 2010, 285(1): 473-482. DOI:10.1074/jbc.M109.040238 |
| [3] |
Yang J, Bai YL, Zhang Y, et al. Deletion of the cyclic di-AMP phosphodiesterase gene (cnpB) in Mycobacterium tuberculosis leads to reduced virulence in a mouse model of infection[J]. Molecular Microbiology, 2014, 93(1): 65-79. DOI:10.1111/mmi.12641 |
| [4] |
Bai YL, Yang J, Zarrella TM, et al. Cyclic di-AMP impairs potassium uptake mediated by a cyclic di-AMP binding protein in Streptococcus pneumoniae[J]. Journal of Bacteriology, 2014, 196(3): 614-623. DOI:10.1128/JB.01041-13 |
| [5] |
Fahmi T, Port GC, Cho KH. c-di-AMP: An essential molecule in the signaling pathways that regulate the viability and virulence of Gram-Positive bacteria[J]. Genes, 2017, 8(8): 197. DOI:10.3390/genes8080197 |
| [6] |
Oppenheimer-Shaanan Y, Wexselblatt E, Katzhendler J, et al. c-di-AMP reports DNA integrity during sporulation in Bacillus subtilis[J]. EMBO Reports, 2011, 12(6): 594-601. DOI:10.1038/embor.2011.77 |
| [7] |
Luo Y, Helmann JD. Analysis of the role of Bacillus subtilis σM in β-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis[J]. Molecular Microbiology, 2012, 83(3): 623-639. DOI:10.1111/j.1365-2958.2011.07953.x |
| [8] |
Witte CE, Whiteley AT, Burke TP, et al. Cyclic di-AMP is critical for Listeria monocytogenes growth, cell wall homeostasis, and establishment of infection[J]. mBio, 2013, 4(3): e00282-13. |
| [9] |
Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type Ⅰ interferon response[J]. Science, 2010, 328(5986): 1703-1705. DOI:10.1126/science.1189801 |
| [10] |
Manikandan K, Sabareesh V, Singh N, et al. Two-step synthesis and hydrolysis of cyclic di-AMP in Mycobacterium tuberculosis[J]. PLoS One, 2014, 9(1): e86096. DOI:10.1371/journal.pone.0086096 |
| [11] |
Dey RJ, Dey B, Zheng Y, et al. Inhibition of innate immune cytosolic surveillance by an M. tuberculosis phosphodiesterase[J]. Nature Chemical Biology, 2017, 13(2): 210-217 http://www.ncbi.nlm.nih.gov/pubmed/28106876
|
| [12] |
Dey B, Dey RJ, Cheung LS, et al. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis[J]. Nature Medicine, 2015, 21(4): 401-406. DOI:10.1038/nm.3813 |
| [13] |
Tang Q, Luo YC, Zheng C, et al. Functional analysis of a c-di-AMP-specific phosphodiesterase MsPDE from Mycobacterium smegmatis[J]. International Journal of Biological Sciences, 2015, 11(7): 813-824. DOI:10.7150/ijbs.11797 |
| [14] |
Corrigan RM, Abbott JC, Burhenne H, et al. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress[J]. PLoS Pathogens, 2011, 7(9): e1002217. DOI:10.1371/journal.ppat.1002217 |
| [15] |
Dengler V, McCallum N, Kiefer P, et al. Mutation in the c-di-AMP cyclase dacA affects fitness and resistance of methicillin resistant Staphylococcus aureus[J]. PLoS One, 2013, 8(8): e73512. DOI:10.1371/journal.pone.0073512 |
| [16] |
Moscoso JA, Schramke H, Zhang Y, et al. Binding of cyclic di-AMP to the Staphylococcus aureus sensor kinase KdpD occurs via the universal stress protein domain and downregulates the expression of the Kdp potassium transporter[J]. Journal of Bacteriology, 2016, 198(1): 98-110. DOI:10.1128/JB.00480-15 |
| [17] |
Peng X, Zhang Y, Bai GC, et al. Cyclic di-AMP mediates biofilm formation[J]. Molecular Microbiology, 2016, 99(5): 945-959. DOI:10.1111/mmi.13277 |
| [18] |
Cheng XQ, Zheng X, Zhou XD, et al. Regulation of oxidative response and extracellular polysaccharide synthesis by a diadenylate cyclase in Streptococcus mutans[J]. Environmental Microbiology, 2016, 18(3): 904-922. DOI:10.1111/1462-2920.13123 |
| [19] |
Bai YL, Yang J, Eisele LE, et al. Two DHH subfamily 1 proteins in Streptococcus pneumoniae possess cyclic di-AMP phosphodiesterase activity and affect bacterial growth and virulence[J]. Journal of Bacteriology, 2013, 195(22): 5123-5132. DOI:10.1128/JB.00769-13 |
| [20] |
Cho KH, Kang SO. Streptococcus pyogenes c-di-AMP phosphodiesterase, GdpP, influences SpeB processing and virulence[J]. PLoS One, 2013, 8(7): e69425. DOI:10.1371/journal.pone.0069425 |
| [21] |
Du B, Ji WH, An HT, et al. Functional analysis of c-di-AMP phosphodiesterase, GdpP, in Streptococcus suis serotype 2[J]. Microbiological Research, 2014, 169(9/10): 749-758. |
| [22] |
Andrade WA, Firon A, Schmidt T, et al. Group B Streptococcus degrades cyclic-di-AMP to modulate STING-dependent type Ⅰ interferon production[J]. Cell Host & Microbe, 2016, 20(1): 49-59. |
| [23] |
Kumar S, Ingle H, Prasad DVR, et al. Recognition of bacterial infection by innate immune sensors[J]. Critical Reviews in Microbiology, 2013, 39(3): 229-246. DOI:10.3109/1040841X.2012.706249 |
| [24] |
Burdette DL, Monroe KM, Sotelo-Troha K, et al. STING is a direct innate immune sensor of cyclic di-GMP[J]. Nature, 2011, 478(7370): 515-518. DOI:10.1038/nature10429 |
| [25] |
Parvatiyar K, Zhang ZQ, Teles RM, et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type Ⅰ interferon immune response[J]. Nature Immunology, 2012, 13(12): 1155-1161. DOI:10.1038/ni.2460 |
| [26] |
McFarland AP, Luo SK, Ahmed-Qadri F, et al. Sensing of bacterial cyclic dinucleotides by the oxidoreductase RECON promotes NF-kappaB activation and shapes a proinflammatory antibacterial state[J]. Immunity, 2017, 46(3): 433-445. DOI:10.1016/j.immuni.2017.02.014 |
| [27] |
Xia PY, Wang S, Xiong Z, et al. The ER membrane adaptor ERAdP senses the bacterial second messenger c-di-AMP and initiates anti-bacterial immunity[J]. Nature Immunology, 2018, 19(2): 141-150. |
| [28] |
Marinho FV, Benmerzoug S, Oliveira SC, et al. The emerging roles of STING in bacterial infections[J]. Trends in Microbiology, 2017, 25(11): 906-918. DOI:10.1016/j.tim.2017.05.008 |
| [29] |
Yin Q, Tian Y, Kabaleeswaran V, et al. Cyclic di-GMP sensing via the innate immune signaling protein STING[J]. Molecular Cell, 2012, 46(6): 735-745. DOI:10.1016/j.molcel.2012.05.029 |
| [30] |
Ouyang SY, Song XQ, Wang YY, et al. Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding[J]. Immunity, 2012, 36(6): 1073-1086. DOI:10.1016/j.immuni.2012.03.019 |
| [31] |
Shang GJ, Zhu DY, Li N, et al. Crystal structures of STING protein reveal basis for recognition of cyclic di-GMP[J]. Nature Structural & Molecular Biology, 2012, 19(7): 725-727. |
| [32] |
Bai YL, Yang J, Zhou X, et al. Mycobacterium tuberculosis Rv3586 (DacA) is a diadenylate cyclase that converts ATP or ADP into c-di-AMP[J]. PLoS One, 2012, 7(4): e35206. DOI:10.1371/journal.pone.0035206 |
| [33] |
Sauer JD, Sotelo-Troha K, von Moltke J, et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides[J]. Infection and Immunity, 2011, 79(2): 688-694. DOI:10.1128/IAI.00999-10 |
| [34] |
Ning HH. Biological and immunological characteristics of a recombinant BCG vaccine based on c-di-AMP[D]. Xi'an: Master's Thesis of the Fourth Military Medical University, 2018 (in Chinese) 宁唤唤.环二腺苷酸(c-di-AMP)为基础的重组BCG疫苗的生物学及免疫学特性研究[D].西安: 空军军医大学硕士学位论文, 2018 |
| [35] |
Rueckert C, Rand U, Roy U, et al. Cyclic dinucleotides modulate induced type Ⅰ IFN responses in innate immune cells by degradation of STING[J]. The FASEB Journal, 2017, 31(7): 3107-3115. DOI:10.1096/fj.201601093R |
| [36] |
McFarland AP, Burke TP, Carletti AA, et al. RECON-dependent inflammation in hepatocytes enhances Listeria monocytogenes cell-to-cell spread[J]. mBio, 2018, 9(3): e00526-18. |
| [37] |
Wawrocki S, Druszczynska M. Inflammasomes in Mycobacterium tuberculosis-driven immunity[J]. Canadian Journal of Infectious Diseases and Medical Microbiology, 2017, 2017: 2309478. |
| [38] |
Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives[J]. Journal of Inflammation Research, 2015, 8: 15-27. |
| [39] |
Abdul-Sater AA, Tattoli I, Jin L, et al. Cyclic-di-GMP and cyclic-di-AMP activate the NLRP3 inflammasome[J]. EMBO Reports, 2013, 14(10): 900-906. DOI:10.1038/embor.2013.132 |
| [40] |
Yoshimori T. Autophagy: a regulated bulk degradation process inside cells[J]. Biochemical and Biophysical Research Communications, 2004, 313(2): 453-458. DOI:10.1016/j.bbrc.2003.07.023 |
| [41] |
Hu D, Wu J, Zhao RP, et al. ESAT6 inhibits autophagy flux and promotes BCG proliferation through MTOR[J]. Biochemical and Biophysical Research Communications, 2016, 477(2): 195-201. DOI:10.1016/j.bbrc.2016.06.042 |
| [42] |
Shibutani ST, Saitoh T, Nowag H, et al. Autophagy and autophagy-related proteins in the immune system[J]. Nature Immunology, 2015, 16(10): 1014-1024. DOI:10.1038/ni.3273 |
| [43] |
Ning HH, Xu ZK, Bai YL. Progress in molecular mechanisms of Mycobacterium tuberculosis regulating autophagy in host cells[J]. Chinese Journal of Cellular and Molecular Immunology, 2017, 33(6): 849-853. (in Chinese) 宁唤唤, 徐志凯, 柏银兰. 结核分枝杆菌调控宿主细胞自噬的分子机制研究进展[J]. 细胞与分子免疫学杂志, 2017, 33(6): 849-853. |
| [44] |
Ebensen T, Libanova R, Schulze K, et al. Bis-(3', 5')-cyclic dimeric adenosine monophosphate: strong Th1/Th2/Th17 promoting mucosal adjuvant[J]. Vaccine, 2011, 29(32): 5210-5220. DOI:10.1016/j.vaccine.2011.05.026 |
| [45] |
Škrnjug I, Guzmán CA, Ruecker C. Cyclic GMP-AMP displays mucosal adjuvant activity in mice[J]. PLoS One, 2014, 9(10): e110150. DOI:10.1371/journal.pone.0110150 |
| [46] |
Zhang Y, Yang J, Bai GC. Cyclic di-AMP-mediated interaction between Mycobacterium tuberculosis ΔcnpB and macrophages implicates a novel strategy for improving BCG vaccination[J]. Pathogens and Disease, 2018, 76(2): fty008. |
 2019, Vol. 46
2019, Vol. 46