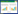扩展功能
文章信息
- 宋建华, 杨永刚, 胡文哲, 陈乐天, 许玫英
- SONG Jian-Hua, YANG Yong-Gang, HU Wen-Zhe, CHEN Le-Tian, XU Mei-Ying
- 活性污泥中电活性微生物的富集筛选研究进展
- Advances of enrichment and screening of electroactive microorganisms from activated sludge
- 微生物学通报, 2019, 46(8): 2090-2100
- Microbiology China, 2019, 46(8): 2090-2100
- DOI: 10.13344/j.microbiol.china.190398
-
文章历史
- 收稿日期: 2019-05-06
- 接受日期: 2019-06-17
- 网络首发日期: 2019-06-19
2. 广东省微生物研究所 广东省科学院 广东省菌种保藏与应用重点实验室 广东 广州 510070;
3. 华南应用微生物国家重点实验室 广东 广州 510070
2. Guangdong Provincial Key Laboratory of Microbial Culture Collection and Application, Guangdong Institute of Microbiology, Guangdong Academy of Sciences, Guangzhou, Guangdong 510070, China;
3. State Key Laboratory of Applied Microbiology Southern China, Guangzhou, Guangdong 510070, China
电活性微生物(Electroactive microorganisms,EAMs)是指能够通过细胞电子传递链与胞外电子供体或受体发生电子交换的一类微生物[1-3]。目前所发现的EAMs主要分为两大类,一类可以将自身代谢过程中所产生的电子传递给胞外的电子受体,称作产电型微生物(Exoelectrogenic microorganisms);另一类可以接收胞外的电子来完成自身的呼吸代谢,称为电营养型微生物(Electrotrophic microorganisms)[4-5]。这些微生物参与了多种重要的元素循环过程,在污染治理、生物能源和绿色化工等方面展现出广阔的应用前景[6-12]。
活性污泥法利用活性污泥中微生物的呼吸代谢作用对废水中的污染物进行有氧或厌氧降解转化,是目前应用最为广泛的废水处理方法[13]。EAMs是活性污泥微生物种群的重要组成部分,在废水中污染物降解转化、资源化(再生水回用)和能源化(厌氧发酵产甲烷、生物制氢和电能产生)过程中扮演着重要角色[14-21]。为了更好地认识和了解活性污泥中EAMs的种类及其功能活性特点,加速废水处理过程,促进其资源化和能源化进程,近年来科学家以活性污泥作为接种源,建立了多种EAMs富集选育方法,获得了多种新型的EAMs。本文重点综述了活性污泥EAMs的富集和筛选方法,总结了从活性污泥中分离获得的EAMs菌种资源信息,同时还分析了EAMs富集选育中所存在的问题,并展望了未来的研究方向,以期进一步提高EAMs菌种资源的挖掘效率,加速以EAMs为核心驱动力的废水资源化与能源化技术发展。
1 电活性微生物的富集和筛选 1.1 电活性微生物的富集由于EAMs在活性污泥中的相对丰度较低,为了提高EAMs的分离选育效率,通常需要对活性污泥中的EAMs进行富集。已建立的EAMs富集方法主要是利用EAMs所具有的胞外电子传递功能特性,包括Fe3+还原富集法、微生物燃料电池(Microbial fuel cells,MFCs)电极富集法、微生物电解池(Microbial electrolysis cells,MECs)电极富集法和恒电位仪-电化学系统富集法。这几种方法均具有各自的反应特点和优缺点,具体如表 1所示。
| 方法 Methods | 特征 Feature | 优点 Advantage | 缺点 Disadvantage | 参考文献 References |
| Fe3+ | Fe3+ was reduced to Fe2+ on anaerobic conditions | Convenient; Commercial | Poor specificity; Low efficiency | [22-23] |
| MFCs | The method based on current production from the bacterial metabolism | Obvious; High community diversity on enrichment | Time-consuming; Low efficiency on enrichment of new species | [24-25] |
| MECs | A potential is applied to the anode, hydrogen production from cathode | Strong selectivity | Noncommercial; Needs power supply | [26] |
| Potentiostat-controlled bioelectrochemical cells | The method based on three-electrode set-up | The potential of electrode can be controlled | [27] |
铁是自然界中最丰富的具有氧化还原活性的金属元素,主要以铁氧化物的形式普遍存在于多种环境中[22]。Fe3+还原富集法最早用于富集筛选铁还原菌,其基本原理是Fe3+作为一种胞外电子受体可以接收来自EAMs呼吸代谢所产生的电子被还原为Fe2+,从而通过检测Fe3+的还原情况来判断EAMs的富集效果。由于前期研究发现的EAMs大部分是典型的铁还原菌[4],因此越来越多的研究团队选用该方法来富集筛选EAMs。Zhang等以蔗糖废水中的厌氧污泥作为接种源,利用Fe2O3作为电子受体,富集获得了以Desulfovibrio为优势物种、具有电活性的铁还原菌群[28]。Shen等则以污水处理厂的厌氧污泥作为接种物,使用Fe(Ⅲ)-EDTA作为电子受体,富集获得了能够降解1, 4-二噁烷能力的具有电活性的铁还原菌群[29]。尽管该方法成本较低,但是随着研究的深入,发现并非所有的EAMs都能进行Fe3+还原[30-31],也并非所有的铁还原菌都具有电化学活性[32],该方法针对性差、效率低的缺点逐渐凸显[22-23]。
1.1.2 MFCs电极富集法MFCs电极富集法主要是以MFCs阳极室的电极作为微生物胞外呼吸的电子受体从而富集EAMs (图 1)。MFCs根据有无质子交换膜可分为两种构型:单室MFCs和双室MFCs。Xing等采用污水处理厂初沉池污泥作为接种物,利用单室MFCs作为富集装置,获得了以Firmicutes为优势物种的电活性菌群,并从中分离出一株具有脱氮功能的EAM——Comamonas denitrificans[30]。Zhou等选用生活污水处理厂的活性污泥作为接种源,利用双室MFCs富集分离到一株兼性厌氧的EAM——Citrobacter freundii LZ-1[33]。Park等在探索底物预驯化过程对MFC阳极微生物群落结构影响的相关研究中发现,在没有任何底物进行预驯化的情况下,以活性污泥为接种源的MFC阳极富集到大量的Geobacter[34]。除此之外,Zhao等在研究底物转化对MFC阳极微生物群落的影响时发现,乳酸作为底物也会使Geobacter在阳极得到富集[35]。目前,多数实验研究主要采用双室MFCs来对活性污泥中的EAMs进行富集[36-38],但是常规双室MFCs装置需占用较大空间且运行时间较长[24]。为此,Zuo等开发设计了U-型管双室MFCs装置,不仅克服了常规双室MFCs存在的不足,而且该装置可以使阳极室中原本呈悬浮态的EAMs也得到富集[31]。Yu等选取污水处理厂初沉池污泥作为接种源,采用自行设计的U-型管MFCs装置,富集获得了具有一定电活性的Ochrobactrum sp.、Achromobacter sp.和Acinetobacter sp.[39]。MFCs电极富集法效果明显直观、针对性强。然而MFCs装置和材料的成本也较高,运行时间较长,且对于新的EAM的富集效果并不理想[40]。
|
| 图 1 MFC/MEC富集原理示意图 Figure 1 Schematic diagram of the MFC/MEC for enrichment 注:单室MFC/MEC不含质子交换膜,双室MFC/MEC被质子交换膜分隔成阴阳两极室;MFC无需外界能量输入,MEC需外界提供能量. Note: Single-chamber MFC/MEC does not contain a proton exchange membrane, whereas dual-chamber MFC/MEC contains a proton exchange membrane to separate cathode compartment and anode compartment; The MEC needs power supply, while MFC does not. |
|
|
MFCs电极富集法的基本原理是在MECs中微生物氧化其中的基质产生电子和质子,在阴极室通过化学或者生物催化作用结合生成氢气,而产电型EAMs主要被富集在阳极表面和阳极液体中,而生物阴极中会存在少量的电营养型EAMs[26]。MECs与MFCs的基本结构相似,也具有阴阳两极,以及连接阴阳两极的外电路[41-42],不同在于MECs需要一个外加电压,因而MECs系统消耗电能(图 1)。MECs也有2种不同构型,分别为单室和双室(有无质子膜)。Zhao等选取污水处理厂二沉池的剩余活性污泥作为接种物,以单室MECs作为富集装置,获得了高效促进活性污泥中甲烷产生以Geobacter为主要物种的产电菌群[43]。Liu等以污水处理厂的厌氧污泥(初沉池和二沉池污泥进行混合)为接种源,乙酸钠为电子供体,利用双室MECs装置富集获得了具有较高库仑效率的EAMs混合培养物(包括Desulfovibrio、Rhodopseudomonas、Shewanella和Geobacter)[44]。Nam等的研究发现,微生物电解池可以用来处理纤维素废水,而且可以实现废水中蛋白质的去除,其阳极富集到的细菌以Deltaproteobacteria为主,古菌则主要是Methanobacterium[45]。Yin等的研究发现,当生物炭(Biochar,BC)作为催化剂投加到MEC后,阳极上产电菌Thermincola的丰度则大幅度降低[46]。该方法选择性较强,然而富集过程中需要额外提供能量,导致其运行成本较高,同时也比较耗时[40]。
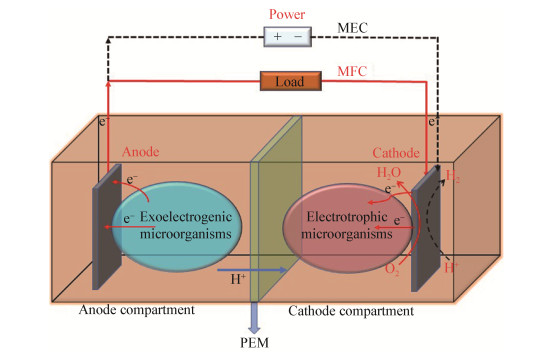
1.1.4 恒电位仪-电化学系统富集法恒电位仪-电化学系统富集法主要利用电化学系统的工作电极作为微生物胞外电子传递的电子受体而实现对EAMs的富集(图 2)。恒电位仪是用来控制工作电极(阳极或阴极)电位的一种设备。恒电位仪控制的生物电化学系统主要是由一个工作电极(电活性生物膜形成)、一个用来平衡电荷的对电极以及一个参比电极组成[27]。Yoon等利用三电极电化学电池装置,以污水处理厂的厌氧污泥为接种物,人工合成废水作为电子供体,短时间内(一周)就富集获得了具有稳定产电能力的EAMs混合培养物[47]。Liu等的研究发现,通过连续的电化学选择和生物膜驯化过程可以提高生物膜的产电能力,而且在其工作电极形成了以Geobacter为主的电活性生物膜[48]。Doyle等也利用此方法,以乳酸盐作为底物,在工作电极上富集获得了以Geobacter metallireducens为主要物种的电活性菌群,并从中分离获得了2株纯培养物[49]。相比于MFCs,该系统可以更好地对EAMs的特性进行量化,而且能够稳定控制工作电极的电势,因此理论上可以有效地富集到更多的EAMs。但是该系统的成本要远远高于MFCs,所以目前在EAMs富集上的应用有限。
|
| 图 2 恒电位仪-电化学系统富集原理示意图 Figure 2 Schematic diagram of potentiostat-electrochemical system for enrichment |
|
|
为了更好地分离获取EAMs,需要对富集到的微生物群落中的功能菌株进行特异性地筛选,以便进一步认识和了解EAMs的分类及其功能活性机理。MFCs和MECs等生物电化学系统是筛选EAMs的经典方法,已经有较多相关的文章[25, 40, 50]报道。但由于该方法通常需要电化学分析设备,成本较高,操作较为繁琐,且需要多学科知识背景,近些年研究者们建立了越来越多的快速筛选方法。我们重点介绍WO3纳米探针显色法、过氧化物酶显色法和染料还原法3种方法(表 2)。
| 方法 Methods | 特征 Feature | 优点 Advantage | 缺点 Disadvantage | 参考文献 References |
| WO3 probe | WO3 nanorods accepting electrons resulted color change | High throughput; Rapid; Sensitive | Complex synthesis of WO3 nanorods | [51] |
| TMB | A colorimetric assay based on immunomagnetic capture and bacterial intrinsic peroxidase activity | High throughput; High efficiency; Quantitative | Unsuitable for evaluating exoelectrogenic ability | [52] |
| Dyes-reducing | A photometric assay based on high-polarity dyes degradation outside the cell | High throughput; Convenient; Commercial | Unable to evaluate exoelectrogenic ability of mixed cultures | [32] |
WO3纳米探针显色WO3是一种具有六角结构的纳米材料,拥有独特的比色特性[53],因其具有良好的导电性、选择性和生物相容性,已经被广泛用作电致变色探针[54-55]。WO3纳米探针显色法的基本原理是利用WO3接收来自微生物的电子后会发生明显的颜色变化,从而实现EAMs的检测和筛选。WO3纳米探针可以快速捕获来自EAMs的电子,使其颜色由白色变成蓝色,捕获的电子越多则颜色就变得越深[56]。而且从微生物到WO3的胞外电子传递方式与微生物到电极的胞外电子传递方式十分相似。Yuan等利用合成的WO3纳米探针,以96孔板作为反应装置,对分离于活性污泥的12株菌进行电化学活性筛选[51, 57]。Yang等采用WO3纳米探针耦合MPN (The most probable number)法来实现对来自城市污水处理厂样品中EAMs的快速检测和计数[56]。Marques等则利用微波水热合成法合成WO3纳米颗粒,然后与未经处理的打印纸相融合形成一层活性传感器来检测EAMs[58]。利用WO3纳米探针可以快速、直观和有效地对EAMs进行检测和筛选,其成本较低且可实现对EAMs高通量的鉴定和分离,但是WO3纳米材料的合成相对复杂。
1.2.2 过氧化物酶显色法外膜的细胞色素c (Outer membrane c-type cytochromes,OMCs)是胞外呼吸的核心组分,它们可以将来自细胞质和内膜氧化酶的电子传递到胞外[59-60]。OMCs是含铁卟啉的酶蛋白,可通过色原/过氧化物系统起到催化作用[61]。铁卟啉的过氧化物酶活性已经被用来描述参与微生物胞外呼吸功能蛋白的特性[62]。过氧化物酶显色法的基本原理是利用四甲基联苯胺(3, 3′, 5, 5′-Tetramethylbenzidine,TMB)与过氧化物酶反应发生明显的颜色变化(变成蓝色)来检测微生物的胞外呼吸能力,完成对EAMs的检测筛选。本方法实验过程主要在96孔板上完成,操作简单而且反应比较迅速,因此可快速、高通量地对微生物的胞外呼吸能力进行测定,从而检测筛选出具有胞外呼吸能力的微生物。Wen等利用该方法实现了对电活性微生物Shewanella oneidensis的快速检测[63]。Zhou等采用此方法完成了对Escherichia coli和Geobacter sulfurreducens PCA-1电活性的快速鉴定[52]。TMB具有检测灵敏度高、稳定性好、安全无毒等优点,然而该方法无法对微生物的胞外产电能力进行评估[52]。
1.2.3 染料还原法染料按照其分子结构性质可分为高极性染料和低极性染料,根据相似相溶原理,低极性染料可以进入到细胞内,而高极性染料很难进入到细胞内。染料还原法筛选EAMs的基本原理是基于高极性染料在厌氧条件下可以作为胞外电子受体被微生物还原脱色,因此可通过颜色变化筛选具有胞外电子传递能力的微生物。我们团队利用这一方法,从纺织印染废水活性污泥中分离获得了具有典型电活性的脱色希瓦氏菌(Shewanella decolorationis S12)[64]。此外,我们还运用荧光共振能量转移技术,设计了高极性的染料荧光探针NA-OG,实现对EAMs的快速分离选育[65]。Xiao等也利用此方法实现了对Shewanella oneidensis MR-1及其突变株电活性的快速检测[32]。该方法既可以借助96孔板也可以利用流式细胞仪开展工作,通过监测染料的脱色情况评估微生物的胞外电子传递能力,从而实现对EAMs的高通量快速筛选,具有操作简便、成本低、效率高的优点[32]。然而随着研究的深入,研究发现并非所有能实现对高极性染料还原脱色的微生物都具有电活性[66-68],所以该方法也需要进一步的完善和优化。
2 从活性污泥中获得的EAMs为了更好地认识和掌握活性污泥中EAMs的功能活性特点,充分发挥EAMs在废水资源化和能源化过程中的作用,研究者已从活性污泥中富集分离了近40株EAMs。到目前为止所获取的EAMs纯培养物基本上都归属于细菌,且主要来自Proteobacteria、Firmicutes和Actinobacteria,尤其以Proteobacteria居多(图 3)。比如Rezaei等利用U型管MFCs富集分离到了可以利用纤维素作为底物进行产电的Enterobacter cloacae[70];Xing等从MFCs阳极生物膜上获得了具有脱氮功能的电活性菌Comamonas denitrificans[30];Wu等从MFCs的阴极获得了能够还原六价铬的电化学活性菌Bacillus sp.等[89]。除细菌外,研究者也从活性污泥中获得了具有电活性的真菌,如Lee等以厌氧活性污泥作为接种源,从MFCs的阳极生物膜上分离到了具有产电能力的酵母菌Candida sp. IR11[95]。总体而言,到目前为止从活性污泥中分离选育的EAMs都以产电型微生物为主,菌属种类仍相当有限,产电效率也较低。这在很大程度上是由于活性污泥EAMs往往需要比较独特的生长和营养条件,已有的富集和筛选方法仍然存在一定的局限性所造成的。
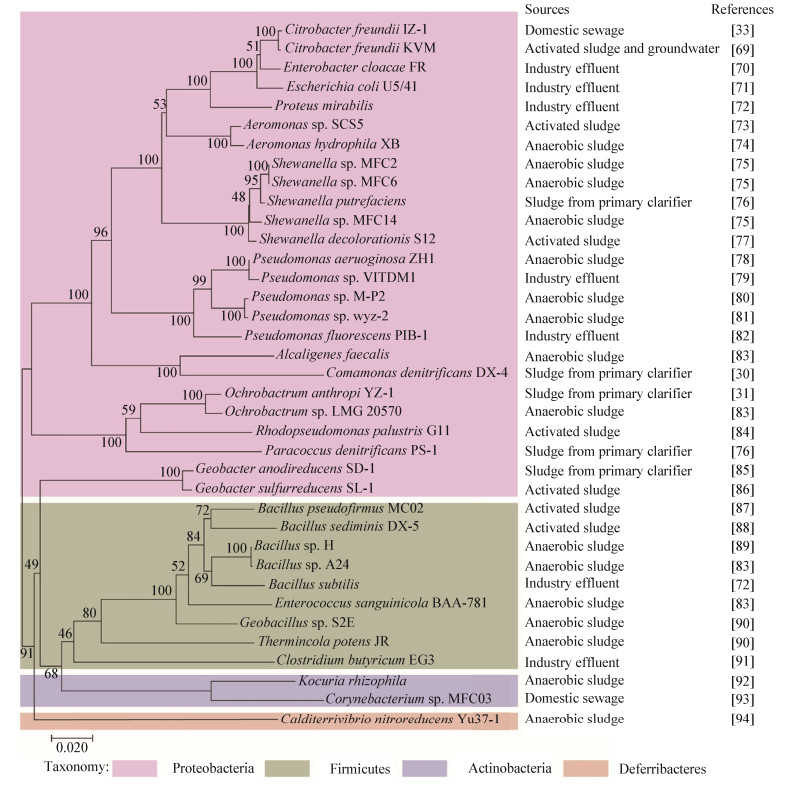
|
| 图 3 从活性污泥中已分离的电活性细菌 Figure 3 Electroactive bacteria isolated from activated sludge |
|
|
近几年快速发展的高通量测序技术也为我们了解和获取活性污泥中尚未实现人工培养的EAMs基因组成信息提供了很多便利[96-98]。Eddie等通过测定生物阴极微生物群落的宏基因组,成功获得了尚未能进行人工培养的电营养型微生物Tenderia electrophaga的完整基因组,并进一步结合宏转录组和宏蛋白组的结果,推测该菌可以利用来自阴极的电子固定CO2[99]。Mei等通过测定不同温度下MFCs阳极生物膜的细菌16S rRNA基因序列,发现在低温条件下生物膜的优势菌为Zoogloea、Simplicispira等未曾报道有电活性的菌属所组成[97]。Xiao等通过焦磷酸测序分析了7个不同阳极的微生物群落,发现了2个未曾报道具有电活性的核心微生物,并通过电活性测试验证了这2株菌均为电活性菌[96]。虽然通过高通量测序技术不能获得电活性菌的实体培养物,但是为进一步认识和了解活性污泥中的电活性微生物提供了新途径。随着流式细胞仪、微流控技术、单细胞分选等技术的发展和普及,以及培养基组分的不断优化,高通量测序技术将会为分离获取活性污泥中的电活性微生物提供有力的技术支撑。
3 问题与展望尽管研究者们对活性污泥中EAMs的富集筛选进行了大量的努力和尝试,但是目前所获得的EAMs菌种资源仍然十分有限,种类也比较单一。一方面是由于所采用的富集选育方法仍不能很好地满足长期生长于活性污泥这个复杂群体中EAMs的生长和营养需求,另一方面是由于活性污泥中存在着某些EAMs需要与周围的微生物互营共生。为了建立新型高效的EAMs富集选育技术,提高活性污泥中EAMs菌种资源的挖掘效率,重点需要从以下几个方面开展工作:
(1) 亟待发展基于废水能源化过程的EAMs富集培养技术
在废水能源化过程中,EAMs需要协同反应器中的多种功能微生物,将废水中有机污染物化学键中储存的化学能转化为电能,进一步通过胞外呼吸将电子传递到电极上,完成能量转换。脱离了特定反应条件所富集培养的EAMs往往难以与系统中的其它功能微生物建立紧密而有效的协同合作关系。
(2) 亟待建立基于高通量培养组的EAMs高效选育技术
在具有独特絮状结构的活性污泥中,EAMs往往与周围的生物和非生物组成代谢网络共同发挥作用。利用近几年快速发展的微生物培养组学(Microbial Culturomics),通过结合原始菌群梯度稀释、培养基成分优化、MALDI-TOF质谱检测、核糖体RNA基因全长测序等方法手段,可以在保证代谢网络完整性的条件下,大幅度提高核心功能微生物单细胞分离培养和物种鉴定的通量。
(3) 亟待开展基于合成生物学的复合功能EAMs理性设计研究
由于废水能源化过程涉及底物分解转化及电能传递等多个环节,需要复杂多样的代谢途径共同参与。随着高通量组学技术的广泛应用,将逐渐阐明废水能源化过程的微生物代谢网络特点,明确其核心关键功能基因或蛋白质。在此基础上,借助合成生物学技术手段,通过理性设计和构建废水能源化复合功能EAMs,有望突破传统微生物分离选育技术的局限性。
| [1] |
Ramírez-Vargas CA, Arias CA, Carvalho P, et al. Electroactive biofilm-based constructed wetland (EABB-CW): A mesocosm-scale test of an innovative setup for wastewater treatment[J]. Science of the Total Environment, 2019, 659: 796-806. DOI:10.1016/j.scitotenv.2018.12.432 |
| [2] |
Pandey P, Shinde VN, Deopurkar RL, et al. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery[J]. Applied Energy, 2016, 168: 706-723. DOI:10.1016/j.apenergy.2016.01.056 |
| [3] |
Kumar R, Singh L, Zularisam AW. Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications[J]. Renewable and Sustainable Energy Reviews, 2016, 56: 1322-1336. DOI:10.1016/j.rser.2015.12.029 |
| [4] |
Logan BE, Rossi R, Ragab A, et al. Electroactive microorganisms in bioelectrochemical systems[J]. Nature Reviews Microbiology, 2019, 17(5): 307-319. |
| [5] |
Jing XY, Chen SS, Zhou SG. Advances in electroactive microorganisms with capability for extracellular electron uptake[J]. Acta Microbiologica Sinica, 2018, 58(1): 19-27. (in Chinese) 靖宪月, 陈姗姗, 周顺桂. 吸收胞外电子的电活性微生物[J]. 微生物学报, 2018, 58(1): 19-27. |
| [6] |
Luan FB, Liu Y, Griffin AM, et al. Iron(Ⅲ)-bearing clay minerals enhance bioreduction of nitrobenzene by Shewanella putrefaciens CN32[J]. Environmental Science & Technology, 2015, 49(3): 1418-1426. |
| [7] |
Logan BE. Exoelectrogenic bacteria that power microbial fuel cells[J]. Nature Reviews Microbiology, 2009, 7(5): 375-381. DOI:10.1038/nrmicro2113 |
| [8] |
Jiang Y, May HD, Lu L, et al. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation[J]. Water Research, 2019, 149: 42-55. DOI:10.1016/j.watres.2018.10.092 |
| [9] |
Zhen GY, Lu XQ, Kumar G, et al. Microbial electrolysis cell platform for simultaneous waste biorefinery and clean electrofuels generation: Current situation, challenges and future perspectives[J]. Progress in Energy and Combustion Science, 2017, 63: 119-145. DOI:10.1016/j.pecs.2017.07.003 |
| [10] |
Sciarria TP, Batlle-Vilanova P, Colombo B, et al. Bio-electrorecycling of carbon dioxide into bioplastics[J]. Green Chemistry, 2018, 20(17): 4058-4066. DOI:10.1039/C8GC01771A |
| [11] |
Srikanth S, Kumar M, Puri SK. Bio-electrochemical system (BES) as an innovative approach for sustainable waste management in petroleum industry[J]. Bioresource Technology, 2018, 265: 506-518. DOI:10.1016/j.biortech.2018.02.059 |
| [12] |
Zhang YC, Jiang ZH, Liu Y. Application of electrochemically active bacteria as anodic biocatalyst in microbial fuel cells[J]. Chinese Journal of Analytical Chemistry, 2015, 43(1): 155-163. DOI:10.1016/S1872-2040(15)60800-3 |
| [13] |
Bitton G. Wastewater Microbiology 4th ed[M]. Washington: Wiley-Blackwell, 2010: 281-318.
|
| [14] |
Joicy A, Song YC, Lee CY. Electroactive microorganisms enriched from activated sludge remove nitrogen in bioelectrochemical reactor[J]. Journal of Environmental Management, 2019, 233: 249-257. |
| [15] |
Wang K, Sheng YX, Cao HB, et al. Impact of applied current on sulfate-rich wastewater treatment and microbial biodiversity in the cathode chamber of microbial electrolysis cell (MEC) reactor[J]. Chemical Engineering Journal, 2017, 307: 150-158. DOI:10.1016/j.cej.2016.07.106 |
| [16] |
Feng Q, Song YC, Yoo K, et al. Bioelectrochemical enhancement of direct interspecies electron transfer in upflow anaerobic reactor with effluent recirculation for acidic distillery wastewater[J]. Bioresource Technology, 2017, 241: 171-180. DOI:10.1016/j.biortech.2017.05.073 |
| [17] |
Feng Q, Song YC, Ahn Y. Electroactive microorganisms in bulk solution contribute significantly to methane production in bioelectrochemical anaerobic reactor[J]. Bioresource Technology, 2018, 259: 119-127. DOI:10.1016/j.biortech.2018.03.039 |
| [18] |
Hua MD, He H, Fu G, et al. 17β-estradiol removal by electrochemical technology in the presence of electrochemically active bacteria in aerobic aquatic environments[J]. Environmental Engineering Science, 2019, 36(3): 316-325. |
| [19] |
Liu ZH, Zhou AJ, Zhang JG, et al. Hydrogen recovery from waste activated sludge: role of free nitrous acid in a prefermentation-microbial electrolysis cells system[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(3): 3870-3878. |
| [20] |
Sato K, Kawaguchi H, Kobayashi H. Bio-electrochemical conversion of carbon dioxide to methane in geological storage reservoirs[J]. Energy Conversion and Management, 2013, 66: 343-350. DOI:10.1016/j.enconman.2012.12.008 |
| [21] |
Xia T, Zhang XL, Wang HM, et al. Power generation and microbial community analysis in microbial fuel cells: A promising system to treat organic acid fermentation wastewater[J]. Bioresource Technology, 2019, 284: 72-79. DOI:10.1016/j.biortech.2019.03.119 |
| [22] |
Fuller SJ, McMillan DGG, Renz MB, et al. Extracellular electron transport-mediated Fe(Ⅲ) reduction by a community of alkaliphilic bacteria that use flavins as electron shuttles[J]. Applied and Environmental Microbiology, 2014, 80(1): 128-137. DOI:10.1128/AEM.02282-13 |
| [23] |
Li XM, Liu TX, Liu L, et al. Dependence of the electron transfer capacity on the kinetics of quinone-mediated Fe(Ⅲ) reduction by two iron/humic reducing bacteria[J]. RSC Advances, 2014, 4(5): 2284-2290. DOI:10.1039/C3RA45458D |
| [24] |
Choi S. Microscale microbial fuel cells: Advances and challenges[J]. Biosensors and Bioelectronics, 2015, 69: 8-25. DOI:10.1016/j.bios.2015.02.021 |
| [25] |
Choi G, Hassett DJ, Choi S. A paper-based microbial fuel cell array for rapid and high-throughput screening of electricity-producing bacteria[J]. Analyst, 2015, 140(12): 4277-4283. DOI:10.1039/C5AN00492F |
| [26] |
Call DF, Logan BE. A method for high throughput bioelectrochemical research based on small scale microbial electrolysis cells[J]. Biosensors and Bioelectronics, 2011, 26(11): 4526-4531. DOI:10.1016/j.bios.2011.05.014 |
| [27] |
Tang Y, Deng DD, Zhou L, et al. Analysis of electricity generation and community of electroactive biofilms enriched from various wastewater treatment stages[J]. Journal of Electroanalytical Chemistry, 2017, 803: 72-80. DOI:10.1016/j.jelechem.2017.09.004 |
| [28] |
Zhang JX, Zhang YB, Chang JH, et al. Biological sulfate reduction in the acidogenic phase of anaerobic digestion under dissimilatory Fe (Ⅲ)--reducing conditions[J]. Water Research, 2013, 47(6): 2033-2040. DOI:10.1016/j.watres.2013.01.034 |
| [29] |
Shen WR, Chen H, Pan SS. Anaerobic biodegradation of 1, 4-dioxane by sludge enriched with iron-reducing microorganisms[J]. Bioresource Technology, 2008, 99(7): 2483-2487. DOI:10.1016/j.biortech.2007.04.054 |
| [30] |
Xing DF, Cheng SA, Logan BE, et al. Isolation of the exoelectrogenic denitrifying bacterium Comamonas denitrificans based on dilution to extinction[J]. Applied Microbiology and Biotechnology, 2010, 85(5): 1575-1587. DOI:10.1007/s00253-009-2240-0 |
| [31] |
Zuo Y, Xing DF, Regan JM, et al. Isolation of the exoelectrogenic bacterium Ochrobactrum anthropi YZ-1 by using a U-tube microbial fuel cell[J]. Applied and Environmental Microbiology, 2008, 74(10): 3130-3137. DOI:10.1128/AEM.02732-07 |
| [32] |
Xiao X, Liu QY, Li TT, et al. A high-throughput dye-reducing photometric assay for evaluating microbial exoelectrogenic ability[J]. Bioresource Technology, 2017, 241: 743-749. DOI:10.1016/j.biortech.2017.06.013 |
| [33] |
Zhou L, Deng DD, Zhang YC, et al. Isolation of a facultative anaerobic exoelectrogenic strain LZ-1 and probing electron transfer mechanism in situ by linking UV/Vis spectroscopy and electrochemistry[J]. Biosensors and Bioelectronics, 2017, 90: 264-268. DOI:10.1016/j.bios.2016.11.059 |
| [34] |
Park Y, Cho H, Yu J, et al. Response of microbial community structure to pre-acclimation strategies in microbial fuel cells for domestic wastewater treatment[J]. Bioresource Technology, 2017, 233: 176-183. DOI:10.1016/j.biortech.2017.02.101 |
| [35] |
Zhao YG, Zhang Y, She ZL, et al. Effect of substrate conversion on performance of microbial fuel cells and anodic microbial communities[J]. Environmental Engineering Science, 2017, 34(9): 666-674. DOI:10.1089/ees.2016.0604 |
| [36] |
Liao CM, Wu JL, Zhou L, et al. Repeated transfer enriches highly active electrotrophic microbial consortia on biocathodes in microbial fuel cells[J]. Biosensors and Bioelectronics, 2018, 121: 118-124. DOI:10.1016/j.bios.2018.08.066 |
| [37] |
Zafar Z, Ayaz K, Sharafat I, et al. Enrichment of electricigenic biofilm for synchronized generation of electric current and waste water treatment in microbial fuel cells[J]. International Journal of Electrochemical Science, 2018, 13(5): 4424-4437. |
| [38] |
Chen JF, Zhang LH, Hu YY, et al. Bacterial community shift and incurred performance in response to in situ microbial self-assembly graphene and polarity reversion in microbial fuel cell[J]. Bioresource Technology, 2017, 241: 220-227. DOI:10.1016/j.biortech.2017.05.123 |
| [39] |
Yu J, Cho S, Kim S, et al. Comparison of exoelectrogenic bacteria detected using two different methods: U-tube microbial fuel cell and plating method[J]. Microbes and Environments, 2012, 27(1): 49-53. DOI:10.1264/jsme2.ME11205 |
| [40] |
Doyle LE, Marsili E. Methods for enrichment of novel electrochemically-active microorganisms[J]. Bioresource Technology, 2015, 195: 273-282. DOI:10.1016/j.biortech.2015.07.025 |
| [41] |
Zhang YF, Angelidaki I. Microbial electrolysis cells turning to be versatile technology: Recent advances and future challenges[J]. Water Research, 2014, 56: 11-25. DOI:10.1016/j.watres.2014.02.031 |
| [42] |
Bo T, Zhai HY, Ji M. Applications of microbial electrolysis cell in hydrogen production[J]. Modern Chemical Industry, 2017, 37(8): 50-54. (in Chinese) 薄涛, 翟洪艳, 季民. 微生物电解池在氢气制备中的应用[J]. 现代化工, 2017, 37(8): 50-54. |
| [43] |
Zhao ZQ, Zhang YB, Ma WC, et al. Enriching functional microbes with electrode to accelerate the decomposition of complex substrates during anaerobic digestion of municipal sludge[J]. Biochemical Engineering Journal, 2016, 111: 1-9. DOI:10.1016/j.bej.2016.03.002 |
| [44] |
Liu WZ, Wang AJ, Sun D, et al. Characterization of microbial communities during anode biofilm reformation in a two-chambered microbial electrolysis cell (MEC)[J]. Journal of Biotechnology, 2012, 157(4): 628-632. DOI:10.1016/j.jbiotec.2011.09.010 |
| [45] |
Nam JY, Yates MD, Zaybak Z, et al. Examination of protein degradation in continuous flow, microbial electrolysis cells treating fermentation wastewater[J]. Bioresource Technology, 2014, 171: 182-186. DOI:10.1016/j.biortech.2014.08.065 |
| [46] |
Yin CK, Shen YW, Yuan RX, et al. Sludge-based biochar-assisted thermophilic anaerobic digestion of waste-activated sludge in microbial electrolysis cell for methane production[J]. Bioresource Technology, 2019, 284: 315-324. DOI:10.1016/j.biortech.2019.03.146 |
| [47] |
Yoon SM, Choi CH, Kim M, et al. Enrichment of electrochemically active bacteria using a three-electrode electrochemical cell[J]. Journal of Microbiology and Biotechnology, 2007, 17(1): 110-115. |
| [48] |
Liu Y, Harnisch F, Fricke K, et al. Improvement of the anodic bioelectrocatalytic activity of mixed culture biofilms by a simple consecutive electrochemical selection procedure[J]. Biosensors and Bioelectronics, 2008, 24(4): 1006-1011. DOI:10.1016/j.bios.2008.08.001 |
| [49] |
Doyle LE, Yung PY, Mitra SD, et al. Electrochemical and genomic analysis of novel electroactive isolates obtained via potentiostatic enrichment from tropical sediment[J]. Journal of Power Sources, 2017, 356: 539-548. DOI:10.1016/j.jpowsour.2017.03.147 |
| [50] |
Szöllősi A, Rezessy-Szabó JM, Hoschke Á, et al. Novel method for screening microbes for application in microbial fuel cell[J]. Bioresource Technology, 2015, 179: 123-127. DOI:10.1016/j.biortech.2014.12.004 |
| [51] |
Yuan SJ, Li WW, Cheng YY, et al. A plate-based electrochromic approach for the high-throughput detection of electrochemically active bacteria[J]. Nature Protocols, 2014, 9(1): 112-119. DOI:10.1038/nprot.2013.173 |
| [52] |
Zhou SG, Wen JL, Chen JH, et al. Rapid measurement of microbial extracellular respiration ability using a high-throughput colorimetric assay[J]. Environmental Science & Technology Letters, 2015, 2(2): 26-30. |
| [53] |
Sharma I, Ghangrekar MM, Biswal RC, et al. Bioelectrogenesis detection of inoculums using electrochromic tungsten oxide and performance evaluation in microbial fuel cells[J]. Journal of the Electrochemical Society, 2016, 163(3): F183-F189. DOI:10.1149/2.0381603jes |
| [54] |
Sugawara Y, Sakaizawa Y, Shibata A, et al. Detection of hydrogen distribution in pure iron using WO3 thin film[J]. ISIJ International, 2018, 58(10): 1860-1867. DOI:10.2355/isijinternational.ISIJINT-2018-236 |
| [55] |
He H, Yuan SJ, Tong ZH, et al. Characterization of a new electrochemically active bacterium, Lysinibacillus sphaericus D-8, isolated with a WO3 nanocluster probe[J]. Process Biochemistry, 2014, 49(2): 290-294. DOI:10.1016/j.procbio.2013.11.008 |
| [56] |
Yang ZC, Cheng YY, Zhang F, et al. Rapid detection and enumeration of exoelectrogenic bacteria in lake sediments and a wastewater treatment plant using a coupled WO3 nanoclusters and most probable number method[J]. Environmental Science & Technology Letters, 2016, 3(4): 133-137. |
| [57] |
Yuan SJ, He H, Sheng GP, et al. A photometric high-throughput method for identification of electrochemically active bacteria using a WO3 nanocluster probe[J]. Scientific Reports, 2013, 3: 1315. DOI:10.1038/srep01315 |
| [58] |
Marques AC, Santos L, Costa MN, et al. Office paper platform for bioelectrochromic detection of electrochemically active bacteria using tungsten trioxide nanoprobes[J]. Scientific Reports, 2015, 5: 9910. DOI:10.1038/srep09910 |
| [59] |
Shi L, Richardson DJ, Wang ZM, et al. The roles of outer membrane cytochromes of Shewanella and Geobacter in extracellular electron transfer[J]. Environmental Microbiology Reports, 2009, 1(4): 220-227. DOI:10.1111/j.1758-2229.2009.00035.x |
| [60] |
Wen JL, He DG, Yu Z, et al. In situ detection of microbial c-type cytochrome based on intrinsic peroxidase-like activity using screen-printed carbon electrode[J]. Biosensors and Bioelectronics, 2018, 113: 52-57. DOI:10.1016/j.bios.2018.04.049 |
| [61] |
Shelnutt JA, Song XZ, Ma JG, et al. Nonplanar porphyrins and their significance in proteins[J]. Chemical Society Reviews, 1998, 27(1): 31-42. |
| [62] |
Goodhew CF, Brown KR, Pettigrew GW. Haem staining in gels, a useful tool in the study of bacterial c-type cytochromes[J]. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1986, 852(2/3): 288-294. |
| [63] |
Wen JL, Zhou SG, Chen JH. Colorimetric detection of Shewanella oneidensis based on immunomagnetic capture and bacterial intrinsic peroxidase activity[J]. Scientific Reports, 2014, 4: 5191. |
| [64] |
Xu MY, Guo J, Cen YH, et al. Shewanella decolorationis sp. nov., a dye-decolorizing bacterium isolated from activated sludge of a waste-water treatment plant[J]. International Journal of Systematic and Evolutionary Microbiology, 2005, 55(1): 363-368. DOI:10.1099/ijs.0.63157-0 |
| [65] |
Liu F, Xu MY, Chen XJ, et al. Novel strategy for tracking the microbial degradation of azo dyes with different polarities in living cells[J]. Environmental Science & Technology, 2015, 49(19): 11356-11362. |
| [66] |
He XL, Song C, Li YY, et al. Efficient degradation of Azo dyes by a newly isolated fungus Trichoderma tomentosum under non-sterile conditions[J]. Ecotoxicology and Environmental Safety, 2018, 150: 232-239. DOI:10.1016/j.ecoenv.2017.12.043 |
| [67] |
Herkommerová K, Dostál J, Pichová I. Decolorization and detoxification of textile wastewaters by recombinant Myceliophthora thermophila and Trametes trogii laccases[J]. 3 Biotech, 2018, 8(12): 505. DOI:10.1007/s13205-018-1525-3 |
| [68] |
Kiran S, Huma T, Jalal F, et al. Lignin degrading system of Phanerochaete chrysosporium and its exploitation for degradation of synthetic dyes wastewater[J]. Polish Journal of Environmental Studies, 2019, 28(3): 1749-1757. DOI:10.15244/pjoes/89575 |
| [69] |
Venkidusamy K, Hari AR, Megharaj M. Petrophilic, Fe(Ⅲ) reducing exoelectrogen Citrobacter sp. KVM11, isolated from hydrocarbon fed microbial electrochemical remediation systems[J]. Frontiers in Microbiology, 2018, 9: 349. DOI:10.3389/fmicb.2018.00349 |
| [70] |
Rezaei F, Xing DF, Wagner R, et al. Simultaneous cellulose degradation and electricity production by Enterobacter cloacae in a microbial fuel cell[J]. Applied and Environmental Microbiology, 2009, 75(11): 3673-3678. DOI:10.1128/AEM.02600-08 |
| [71] |
Brunner S, Klessing T, Dötsch A, et al. Efficient bioelectrochemical conversion of industrial wastewater by specific strain isolation and community adaptation[J]. Frontiers in Bioengineering and Biotechnology, 2019, 7: 23. DOI:10.3389/fbioe.2019.00023 |
| [72] |
Gunasekaran G, Chongdar S, Naragoni S, et al. Microbial fuel cell constructed with micro-organisms isolated from industry effluent[J]. International Journal of Hydrogen Energy, 2011, 36(22): 14914-14922. DOI:10.1016/j.ijhydene.2011.03.031 |
| [73] |
Sharma SCD, Feng CJ, Li JW, et al. Electrochemical characterization of a novel exoelectrogenic bacterium strain SCS5, isolated from a mediator-less microbial fuel cell and phylogenetically related to Aeromonas jandaei[J]. Microbes and Environments, 2016, 31(3): 213-225. DOI:10.1264/jsme2.ME15185 |
| [74] |
Zhang JX, Zhang YB, Liu BQ, et al. A direct approach for enhancing the performance of a microbial electrolysis cell (MEC) combined anaerobic reactor by dosing ferric iron: Enrichment and isolation of Fe(Ⅲ) reducing bacteria[J]. Chemical Engineering Journal, 2014, 248: 223-229. DOI:10.1016/j.cej.2014.02.102 |
| [75] |
Kan JJ, Hsu L, Cheung ACM, et al. Current production by bacterial communities in microbial fuel cells enriched from wastewater sludge with different electron donors[J]. Environmental Science & Technology, 2011, 45(3): 1139-1146. |
| [76] |
Kiely PD, Call DF, Yates MD, et al. Anodic biofilms in microbial fuel cells harbor low numbers of higher-power-producing bacteria than abundant genera[J]. Applied Microbiology and Biotechnology, 2010, 88(1): 371-380. DOI:10.1007/s00253-010-2757-2 |
| [77] |
Hong YG, Xu MY, Guo J, et al. Respiration and growth of Shewanella decolorationis S12 with an azo compound as the sole electron acceptor[J]. Applied and Environmental Microbiology, 2007, 73(1): 64-72. DOI:10.1128/AEM.01415-06 |
| [78] |
Nor MHM, Mubarak MFM, Elmi HSA, et al. Bioelectricity generation in microbial fuel cell using natural microflora and isolated pure culture bacteria from anaerobic palm oil mill effluent sludge[J]. Bioresource Technology, 2015, 190: 458-465. DOI:10.1016/j.biortech.2015.02.103 |
| [79] |
Manogari R, Daniel DK. Isolation, characterization and assessment of Pseudomonas sp. VITDM1 for electricity generation in a microbial fuel cell[J]. Indian Journal of Microbiology, 2015, 55(1): 8-12. DOI:10.1007/s12088-014-0491-7 |
| [80] |
Sun J, Cai BH, Zhang YP, et al. Regulation of biocathode microbial fuel cell performance with respect to azo dye degradation and electricity generation via the selection of anodic inoculum[J]. International Journal of Hydrogen Energy, 2016, 41(9): 5141-5150. DOI:10.1016/j.ijhydene.2016.01.114 |
| [81] |
Wang YZ, Wang AJ, Zhou AJ, et al. Electrode as sole electrons donor for enhancing decolorization of azo dye by an isolated Pseudomonas sp. WYZ-2[J]. Bioresource Technology, 2014, 152: 530-533. DOI:10.1016/j.biortech.2013.11.001 |
| [82] |
Kaushik A, Jadhav SK. Conversion of waste to electricity in a microbial fuel cell using newly identified bacteria: Pseudomonas fluorescens[J]. International Journal of Environmental Science and Technology, 2017, 14(8): 1771-1780. DOI:10.1007/s13762-017-1260-z |
| [83] |
Rabaey K, Boon N, Siciliano SD, et al. Biofuel cells select for microbial consortia that self-mediate electron transfer[J]. Applied and Environmental Microbiology, 2004, 70(9): 5373-5382. DOI:10.1128/AEM.70.9.5373-5382.2004 |
| [84] |
Lai YC, Liang CM, Hsu SC, et al. Polyphosphate metabolism by purple non-sulfur bacteria and its possible application on photo-microbial fuel cell[J]. Journal of Bioscience and Bioengineering, 2017, 123(6): 722-730. DOI:10.1016/j.jbiosc.2017.01.012 |
| [85] |
Sun D, Wang AJ, Cheng SA, et al. Geobacter anodireducens sp. nov., an exoelectrogenic microbe in bioelectrochemical systems[J]. International Journal of Systematic and Evolutionary Microbiology, 2014, 64(10): 3485-3491. |
| [86] |
Nielsen PH, Frølund B, Spring S, et al. Microbial Fe(Ⅲ) reduction in activated sludge[J]. Systematic and Applied Microbiology, 1997, 20(4): 645-651. DOI:10.1016/S0723-2020(97)80037-9 |
| [87] |
Ma C, Zhuang L, Zhou SG, et al. Alkaline extracellular reduction: isolation and characterization of an alkaliphilic and halotolerant bacterium, Bacillus pseudofirmus MC02[J]. Journal of Applied Microbiology, 2012, 112(5): 883-891. DOI:10.1111/j.1365-2672.2012.05276.x |
| [88] |
Yu Z, Wang YQ, Qin DX, et al. Bacillus sediminis sp. nov., isolated from an electroactive biofilm[J]. Antonie van Leeuwenhoek, 2013, 104(6): 1109-1116. DOI:10.1007/s10482-013-0032-0 |
| [89] |
Wu XY, Ren XQ, Owens G, et al. A facultative electroactive chromium(Ⅵ)-reducing bacterium aerobically isolated from a biocathode microbial fuel cell[J]. Frontiers in Microbiology, 2018, 9: 2883. DOI:10.3389/fmicb.2018.02883 |
| [90] |
Wrighton KC, Agbo P, Warnecke F, et al. A novel ecological role of the Firmicutes identified in thermophilic microbial fuel cells[J]. The ISME Journal, 2008, 2(11): 1146-1156. DOI:10.1038/ismej.2008.48 |
| [91] |
Park HS, Kim BH, Kim HS, et al. A novel electrochemically active and Fe(Ⅲ)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell[J]. Anaerobe, 2001, 7(6): 297-306. DOI:10.1006/anae.2001.0399 |
| [92] |
Luo JM, Li M, Zhou MH, et al. Characterization of a novel strain phylogenetically related to Kocuria rhizophila and its chemical modification to improve performance of microbial fuel cells[J]. Biosensors and Bioelectronics, 2015, 69: 113-120. DOI:10.1016/j.bios.2015.02.025 |
| [93] |
Liu M, Yuan Y, Zhang LX, et al. Bioelectricity generation by a Gram-positive Corynebacterium sp. strain MFC03 under alkaline condition in microbial fuel cells[J]. Bioresource Technology, 2010, 101(6): 1807-1811. DOI:10.1016/j.biortech.2009.10.003 |
| [94] |
Fu Q, Kobayashi H, Kawaguchi H, et al. A thermophilic gram-negative nitrate-reducing bacterium, Calditerrivibrio nitroreducens, exhibiting electricity generation capability[J]. Environmental Science & Technology, 2013, 47(21): 12583-12590. |
| [95] |
Lee YY, Kim TG, Cho KS. Isolation and characterization of a novel electricity-producing yeast, Candida sp. IR11[J]. Bioresource Technology, 2015, 192: 556-563. DOI:10.1016/j.biortech.2015.06.038 |
| [96] |
Xiao Y, Zheng Y, Wu S, et al. Pyrosequencing reveals a core community of anodic bacterial biofilms in bioelectrochemical systems from China[J]. Frontiers in Microbiology, 2015, 6: 1410. |
| [97] |
Mei XX, Xing DF, Yang Y, et al. Adaptation of microbial community of the anode biofilm in microbial fuel cells to temperature[J]. Bioelectrochemistry, 2017, 117: 29-33. DOI:10.1016/j.bioelechem.2017.04.005 |
| [98] |
Kouzuma A, Ishii S, Watanabe K. Metagenomic insights into the ecology and physiology of microbes in bioelectrochemical systems[J]. Bioresource Technology, 2018, 255: 302-307. DOI:10.1016/j.biortech.2018.01.125 |
| [99] |
Eddie BJ, Wang Z, Malanoski AP, et al. 'Candidatus Tenderia electrophaga', an uncultivated electroautotroph from a biocathode enrichment[J]. International Journal of Systematic and Evolutionary Microbiology, 2016, 66(6): 2178-2185. DOI:10.1099/ijsem.0.001006 |
 2019, Vol. 46
2019, Vol. 46