扩展功能
文章信息
- 王彬浩, 关晓彤, 颜庆云, 贺志理
- WANG Bin-Hao, GUAN Xiao-Tong, YAN Qing-Yun, HE Zhi-Li
- 含氟有机废水处理过程活性污泥微生物群落研究进展
- Research progresses of activated sludge microbial communities in fluorine-containing organic wastewater treatment processes
- 微生物学通报, 2019, 46(8): 2020-2037
- Microbiology China, 2019, 46(8): 2020-2037
- DOI: 10.13344/j.microbiol.china.190330
-
文章历史
- 收稿日期: 2019-04-15
- 接受日期: 2019-06-10
- 网络首发日期: 2019-06-19
2. 南方海洋科学与工程广东省实验室(珠海) 广东 珠海 519000;
3. 湖南农业大学农学院 湖南 长沙 410128
2. Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Zhuhai, Guangdong 519000, China;
3. College of Agronomy, Hunan Agricultural University, Changsha, Hunan 410128, China
含氟有机物由于表面张力强、耐温性能好,并具有耐燃、绝缘、润滑等特性[1]而被广泛使用,现有上万种工业合成和加工的含氟化学品流通在农药、医药等市场[2],并与其他氟化物,包括全氟和多氟烷基物质(Per-and polyfluoroalkyl substances,PFASs)和全氟磺酸盐(Perfluorinated sulfonates,PFSAs)构成了持久性有机污染物。这些化合物释放到环境后能残留在废水、地表水、泥沙及土壤中,对生态和人类健康构成威胁[3-7]。如PFASs和PFSAs自20世纪50年代大规模生产和使用以来[8-10],在环境介质和生物体中已检测到[11-16],并被证实能引起动物和人类致癌、不孕或其他毒性[17-19]。因此,探索上述有机氟化物的微生物降解特性和机制,提高含氟有机废水的生物处理效果,减少其在环境中的残留至关重要。
利用微生物降解有机和无机污染物具有处理效率高和成本低等特点,是处理难降解有机污染废水的优选方法。活性污泥技术作为最常用的废水生物处理工艺之一,其核心是一个拥有高度复杂微生物群落的污泥生态系统,包括真核生物、细菌、古菌和病毒及相应的环境条件。在该工程系统中,活性污泥通常具有很高的生物多样性[700多个属和数千个操作分类单元(Operational taxonomical units,OTUs)][20]和生物量(一般为2-10 g/L)[21],其中常见功能菌群有EPS (Extracellular polymeric substances)产生菌[22-23]、除氮功能菌(Ammonia-oxidizing archaea,AOA;Ammonia-oxidizing bacteria,AOB;Nitrite- oxidizing bacteria,NOB)[24]、聚磷菌(PolyP-accumulating organisms,PAO)[25]和复杂有机污染物降解菌(如脱卤菌)[26]。高度多样性的微生物群落在活性污泥絮凝体中有效地聚集在一起,通过吸附、吸收和代谢来实现污染物的去除和降解,以保证废水处理效果[20, 27-28]。
研究人员通过分子技术分析含氟有机废水处理过程中活性污泥微生物群落,发现污泥中变形杆菌门、拟杆菌门、绿弯菌门、放线菌门、浮霉菌门、厚壁菌门等为优势类群,且它们所占比例随条件不同而变化[29-33],这也直接影响有机氟化物的去除效果(表 1)。
 |
同时,微生物群落还调控着活性污泥的形成、发育、功能及其稳定性。例如丝状细菌(如Chloroflexi、Sphaerotilus、Thiothrix)过度生长会引起污泥膨胀;某些功能菌(如Zoogloea)消失或某些酶(如N-acyl homoserine lactone,AHL)过分表达会使污泥絮凝体解体,影响污泥沉降性能,导致出水中泥水分离不充分,降低处理效果[52-53]。因此,研究和监测微生物群落结构,特别是核心微生物组(Core microbiome)的活性以及菌群的互作机制,对提升活性污泥的降解性能以及整个含氟有机废水处理系统的运行效率和稳定性具有指导意义[54-59]。
明确活性污泥微生物群落功能、代谢机制和降解途径对工艺设计、参数优化、生物强化、促进含氟有机废水去除效率至关重要。要矿化有机氟化物,必须破坏其稳定性较高的碳氟键(CH3-F,116 kcal/mol)[60]。研究发现活性污泥微生物可通过表达脱卤酶,利用酶促反应直接脱氟,或形成不稳定的代谢中间体,间接降解多种异源化合物。然而,一些含氟产品尤其是某些抗生素药物和全氟物质,由于其本身或其代谢产物的复杂结构和毒性,很难通过生物处理实现矿化。如氟调醇(Fluorotelomer alcohols,FTOHs)在微生物作用下能转化成多氟羧酸和全氟羧酸[61],而这些全氟烷酸(Perfluoroalkyl acids,PFAAs)却易于与有机组分络合,在微生物酶的催化下钝化形成残基,无法进一步被生物转化[62]。针对此类不能被环境中微生物完全矿化的有机氟化物,遗传操作方法为其进一步被降解提供了潜在技术。联合微生物组学、微生物组工程和基因工程技术,设计出高表达有机氟化物降解基因或高效功能微生物组是未来生物处理难降解异源污染物的重要手段[63-64]。
海洋、湖泊、河流底泥和土壤等自然环境中微生物群落特征及功能等的深入研究[65-70],为理解生物工程系统中相关微生物学机制提供了理论基础。最近,研究人员从微生物生态学的角度报道了活性污泥微生物的多样性、功能冗余、群落组装、群落互作、代谢等机制,揭示了不同活性污泥微生物群落结构与环境参数和污染物处理效果的关系[55, 71-76]。这些研究为寻找有效措施或策略来解决污水处理问题,如低效的脱氮或除磷[55, 77]、污泥膨胀[78]、较差的生物降解(含氟废水)[34]提供了指导。随着对含氟废水处理过程中活性污泥微生物群落多样性、结构功能及降解机理的进一步认知,通过解析活性污泥中功能微生物群落的生态策略,一些能提高微生物在复杂工业废水中适应力的工艺和装置将被开发和应用。
本综述聚焦于含氟有机废水处理过程中活性污泥微生物群落与含氟废水类型、处理工艺和性能之间的关系,讨论了功能微生物降解/转化有机氟化物的路径和作用机制,并展望了结合分离培养关键功能微生物和微生物组学技术解析活性污泥微生物群落构建、互作、代谢等核心科学问题,从而进一步阐明微生物降解含氟有机物的机理,并优化含氟有机废水的处理工艺。
1 制药废水处理过程活性污泥微生物群落结构和功能 1.1 微生物多样性由于处理高氨氮、高毒性制药废水的活性污泥中微生物群落非常复杂,虽然广受关注,但是其中的微生物群落结构和功能尚无明晰结论。随着分子技术的推广,当前在解析制药废水处理过程活性污泥微生物群落方面取得了一些重要进展。Zhao等[34]比较了制药废水、市政废水和其他工业废水处理厂活性污泥中的细菌组成和多样性,其平均Shannon指数分别为5.9、8.1和6.3。制药废水处理活性污泥中低α-多样性表明微生物由于受到原料、催化剂和残留抗生素等[79]物质的抑制作用,其生存/适应能力显著降低。在对8类制药废水处理污泥的微生物进行全面分析后,Thauera、Kosmotoga、Methyloversatilis、Hyphomicrobium、Petrobacter、Chelativorans、Desulfovibrio、Xanthomonadaceae和Burkholderiales确定为优势菌属。这些菌属通常具有非特异性功能,包括参与电子传递过程,如脱氮脱硫[80-82]、降解有机碳[83]和芳香类[84-85]。此外,由于制药废水对微生物群落存在较高的选择压力,这导致了不同类型制药废水之间的特定/偶发种群(Occasional groups)存在巨大差异,同时也反映出微生物多样性变化和组装过程受到确定性因素(制药废水的类型)的驱动。为了抵抗药物的抑菌作用,处理含氟类抗生素的活性污泥具有更多特异的OTU[86],如已被报道的Enterococcus、Bacteroides、Prevotella、Parabacteroides和Sphingopyxis是多种抗生素的耐药菌[87-88]。研究发现稀有类群(Rare groups)构成了制药废水污泥中微生物群落的主体,并且这些特异性耐药物种在抗生素胁迫下能从稀有物种转化为优势物种[89-90]。
1.2 微生物群落影响因素 1.2.1 接种源处理不同类型的制药废水需要特定的功能微生物类群,不同的接种源不仅影响着处理系统中的微生物群落组成,而且直接关系到废水的处理性能。将活性污泥、受污染的沟渠沉积物和渗滤系统的土壤经过30 d的废水驯化后,Kim等[38]发现尽管3种泥源之间的群落组成相差很大,但接种了活性污泥和沟渠沉积物的反应器具有较好的制药废水处理效果,这归因于接种源中土著优势菌属Sphingomonas、Beijerinckia、Methylophilus和Unknown Cytophagaceae与氟尿嘧啶(5-Fluorouracil)等药物的转化密切相关;与此同时,不同的群落组成表现出相似的降解性能说明了不同微生物接种系统存在功能冗余;由此推断,环境中可能还分布着其他有益于处理制药废水的接种源,如来自膜生物反应器的Bradyrhizobiaceae等菌群。
1.2.2 工艺类型传统制药废水处理工艺有厌氧/缺氧/好氧(Anaerobic/anoxic/oxic,A2/O)、序批式反应器(Sequencing batch reactor,SBR/Granular-SBR)、膜生物反应器(Membrane bio-reactor,MBR)或几种方法联合使用(表 1),不同处理工艺下活性污泥微生物群落差异显著。Ouyang等[30]研究了接种相同初始污泥的不同处理工艺在处理制药废水过程中的微生物群落组成,发现(UASB+A/O)系统中微生物的α-多样性最高,且Proteobacteria、Bacteroidetes、Chloroflexi和Acidobacteria是其优势菌门;在UASB装置中,富集了相对较高的Desulfovibrio、Desulfobacter、Desulfococcus和Desulfuromonas等功能菌,对去除废水中硫酸盐起重要作用;在(A/O+Biofilter)系统中,Saccharibacteria是其优势菌门,其中Candidatus Saccharibacteria bacterium菌群占据主导地位,这与该系统具有较好的NH4+-N去除性能密切相关。
1.2.3 负荷冲击处理系统中底物负荷对微生物群落具有重要影响,探究微生物对底物负荷的响应,能够解析活性污泥中微生物群落组成、多样性和功能与稳定性的关系,进而表征污泥在水质波动下的抵抗或者恢复性能。在处理含环丙沙星(Ciprofloxacin)废水的微生物燃料电池中(Microbial fuel cell,MFC)[39],运行1.5年后虽然活性污泥微生物多样性有所降低,但是Proteobacteria、Bacteroidetes、Actinobacteria丰度逐渐增加并成为优势菌,该门类下的一些功能菌属如Alcaligenes(可分泌HpaM等酶,催化目标污染物脱羧羟基化)[91-92]、Chryseobacterium (可降解恩诺沙星等有机污染物)、Myroides (降解芳香烃污染物3, 4-二氯苯胺)、Stenotrophomonas(降解环丙沙星和其他新兴污染物)、Eubacterium、Pseudomonas和Dysgonomonas显著富集[31, 93]。此外,Davids等[39]模拟了活性污泥微生物群落对高浓度布洛芬(Ibuprofen)的响应,结果表明布洛芬的选择压力导致了群落结构的显著转变,并刺激产生了对环丙沙星较高抗性且具有生物降解能力的细菌类群(Proteobacteria和Enterobacteriales)。在经过1 000 mg/L布洛芬高负荷驯化后,污泥降解环丙沙星的性能明显提高,其原因可能是γ-Proteobacteria种群的剧增及其通过调控高效药物修饰酶的编码基因而加强了微生物对环丙沙星耐药性。
2 氟化工废水处理过程活性污泥微生物群落结构和功能 2.1 常规生物处理工艺中微生物群落氟化工企业在生产含氟聚合物、含氟精细化学品和含氟药品中间体等产品过程中产生的反应废液和洗涤废水,通常具有高有机物浓度、有毒、成分复杂、强酸强碱等特点,是公认的生化性能较差的废水。虽然微生物在这类废水中的生存能力相对较弱,但对其群落多样性和结构功能研究颇受关注。Xu等[32]分析了处理氟化工园区内废水的活性污泥微生物群落,发现优势的Betaproteobacteria、Dechlorosoma和Thauera主要在厌氧和缺氧生物池富集,它们不仅能对苯等复杂有机物进行厌氧降解,还能将高氯酸根转化为氯离子,降低高氯酸盐的浓度。与此同时,含氟废水在流经处理系统后,其氟离子浓度明显提高,这表明上述优势菌可能参与了有机氟的生物降解。此外,氟化工废水污泥中一些低丰度的功能菌群也是维持系统具有良好性能的重要因素,如反硝化菌(Pseudomonas、Roseomonas)和脱硫菌(Thiobacillus)[32]。为进一步探明活性污泥中降解有机氟化物的功能微生物及其群落结构动态变化,王彬浩[33]利用提取自氟化工废水中的含氟有机物,模拟含氟废水用于驯化接种污泥。当反应器在400 mg/L TOC (Total organic carbon)负荷下,氟化物去除效率稳定在80%以上时,污泥中微生物群落发生了显著变化,Rhizobiales、Rhodocyclales和Xanthomonadale为主要优势菌群,且检测到了能够降解苯、二氯苯等含卤多环芳烃的功能微生物(含编码单加氧酶的amo基因和编码脱氢酶的mdh2基因);随后,利用驯化成熟的污泥做进一步实验研究,明确了活性污泥微生物群落受到含氟有机废水中碳氮比、盐度和重金属的显著影响;随着盐度以及重金属种类和浓度的增加微生物多样性明显降低,从而能够降解二苯噻吩(Dibenzothiophene)以及其他杂环芳香烃类(萘和蒽)的Xanthobacter表现出良好的适应能力,并成为核心菌属。这说明上述菌群在高盐度和重金属等恶劣环境条件中能占据一些特定的生态位,维持其生理和生态功能。这些在处理含氟废水污泥中优势微生物菌群的发现,以及对有机氟化物降解性能的研究有益于提高含氟有机废水的生物处理效率,同时也对利用生物强化技术处理该类废水具有指导意义。
2.2 生物强化工艺中微生物群落“生物强化”通过添加从自然界中筛选的高效菌株或通过基因工程构建的具有特定功能的高效降解菌,从而提高含氟有机废水处理效率,并促进活性污泥微生物群落的适应性转变。Ramos等[43]在颗粒污泥-序批式反应器中添加邻氟苯酚(2-Fluorophenol)降解菌后,废水中的邻氟苯酚和NH4+-N去除效率明显提高;而且当反应器遭遇邻氟苯酚负荷冲击时,添加的生物强化菌群促进了污泥中土著菌Acidobacteria的生长,能使颗粒污泥中微生物种群丰度快速恢复并维持相对稳定,并促进细菌群落转变到以Alphaproteobacteria、Betaproteobacteria和Flavobacteriia为优势菌的新群体。尽管强化菌在邻氟苯酚污染物去除方面起关键作用,但是生物强化菌在污泥絮凝体中不能保持优势地位,关于强化菌与土著菌群间的互作机制有待进一步解析。此外,在强化系统中一些与邻氟苯酚降解菌具有相似功能的重要优势种群被识别[43],如未分类根瘤菌科(Unclassified Rhizobiales)、未分类缓生根瘤菌科(Unclassified Bradyrhizobiaceae)和未分类酸杆菌Gp4 (Unclassified Acidobacteria Gp4)。Amorim等[45]在接种活性污泥的旋转生物接触器中添加对氟桂酸降解菌(Rhodococcus sp.),显著提高了反应器对COD (Chemical oxygen demand)和对氟桂酸的去除率和抗负荷冲击能力;同样地,在实验后期强化菌(Rhodococcus sp.)的基因片段在新形成的稳定细菌群体结构中消失了。综上所述,虽然生物强化菌可能并不是新菌群体系中的优势菌,但可以促进污泥中微生物群落对环境压力的适应性;此外,为使强化菌能够提高含氟废水处理效率和有效调控微生物群落,应同时考虑强化菌对有机氟化物的降解能力和微生态系统动态平衡,从而形成降解性能强和抗负荷冲击好的稳定群落。
3 消防泡沫(Aqueous film forming foam,AFFF)废水处理过程活性污泥微生物群落结构和功能AFFF废水通常具有大量含氟异构体(前驱体),由于检测含氟异构体的种类存在差异,当前活性污泥对PFASs (AFFFs的主要成分)的去除效率存在一些争议。部分污水处理厂出水PFASs浓度高于进水浓度,使得污水处理厂排放污水成为城市河流PFASs污染的主要来源[94]。而Chen等[95]研究发现活性污泥可以有效地去除废水中的全氟己烷磺酸和全氟己酸且去除效果受到处理工艺类型的影响,如PFASs的去除效果:周期循环活性污泥工艺(Cyclic activated sludge system,CASS,32.2%) > 氧化沟(Oxidation ditch,OD,17.5%) > 厌氧/缺氧/好氧(A2/O,-1.49%)。Lewis等[51]考察了AFFFs的主要成分6:2 PAPs (Polyfluoroalkyl phosphates)在活性污泥中的生物转化效能以及微生物群落动态变化,发现在培养系统中接种活性污泥比接种纯菌(FTOH-degrading Pseudomonas)具有更高效的生物转化能力。在实验进行30 d后,检测到了转化产物5:2 FTOH、PFHpA [CF3(CF2)5COOH]和PFPeA [CF3(CF2)3COOH]。尽管在生物降解结束时活性污泥中微生物多样性和丰度均有所下降,但新出现的Nitrospira sp.和Prosthecobacter dejongeii (降解雌激素等有机物)功能菌[51]显著增强了反应器处理性能。与此同时,烷烃单氧酶参与了FTOHs的降解过程[96-97],因此活性污泥中烷烃降解菌群数量的增加促进了PAPs生物转化效率。此外,Zhang等[98]研究发现Dokdonella spp.、Thauera spp.、Albidovulum spp.和Caldanaerovirga spp.是6:2 Fluorotelomer alcohol (FTOH)降解菌和耐药菌株;FTOH负荷增加,反应器群落多样性降低,微生物结构发生明显变化,成为群落结构动态变化的主要驱动力。另外,6:2 FTOH降解菌、耐药菌群(Dokdonella、Thauera、Albidovulum和Caldanaerovirga)与多氟烷基酸敏感菌(Incertaesedis、Opitutus和Bacillariophyta)同时存在于降解系统中,这表明6:2 FTOH降解系统中的微生物群落遵循生态学中“石头-剪刀-布(Rock-scissors-paper)”的共存机制[98]。
4 微生物降解或转化典型有机氟化物的途径和机制分离和鉴定高效的氟化物降解菌(Organic fluoride-degrading bacteria),研究其降解途径和脱氟机制、降解酶和功能基因,可为应用基因工程技术拓宽有机氟降解种类、提高微生物降解氟化物能力提供依据。当前,已有文献提供了多种细菌和真菌转化或降解芳香型和脂肪族有机氟化合物的证据。制药废水及氟化工废水污泥、污染土壤、湿地植物根际土和河流沉积物中分离和鉴定了多种高效有机氟降解菌(表 2),包括慢生根瘤菌(Bradyrhizobium)、红球菌属(Rhodococcus)、栖热菌属(Thermus)、假单胞菌属(Pseudomonas)、分枝杆菌属(Mycobacterium)、戈登氏菌属(Gordonia)等。其中从制药废水活性污泥中分离的高效降解细菌栖热菌属(Thermus sp.),在pH为6.5、温度在70℃时,不但可以去除超过70%的环丙沙星,还能以诺氟沙星、氧氟沙星和恩罗氟沙星等其他喹诺酮类药品作为唯一碳源,去除率分别达到63%、70%、74%[100]。Pseudomonas knackmussii B13能以对氟苯甲酸(4-Fluorobenzoate)为唯一碳源并对其进行降解[112];Ralstonia sp.能够忍耐高浓度对氟苯胺(1 250 mg/L),高盐(15 g/L)环境,在pH为6.5、温度为30℃时,高效降解对氟苯胺[110];Ralstonia sp.还能以对氟苯胺(Fluoroanilines)作为底物,以共代谢的方式将其去除[111]。Pseudomonas aeruginosa HJ4在pH为3.0-10.0、温度为35℃时可保持良好的Perfluorooctanesulfonate (PFOS)降解能力,转化率超过67%[116]。这说明活性污泥中含有多种高效的降解菌,且具有较广的有机氟降解能力,对提高含氟有机废水的处理效果起重要作用。
 |
利用基因克隆、酶活性测定和HPLC-MS/ GC-MS-MS分析代谢物方法,揭示多种降解菌的有机氟降解途径和关键基因。Microbacterium sp.和Labrys portucalensis F11等菌种在好氧环境下降解喹诺酮类抗生素时,一般包括羟基化/去氟化、去甲基化、脱羧和哌嗪环裂解等过程[122]。研究者从受氯苯污染土壤中分离出能够矿化对氟肉桂酸(4-Fluorocinnamic acid)的Arthrobacter sp. strain G1和Ralstonia sp. strain H1,并明确了该降解过程是通过β-氧化机制来实现的[109]。6:2氟调醇(6:2 FTOH,F(CF2)6CH2CH2OH)作为AFFF中基础性原料,它的生物降解性能和途径受到高度关注。Kim等[96]发现Pseudomonas oleovorans和P. butanovora能通过两种途径转化6:2 FTOH (图 1):途径Ⅰ产生x:2酮、x:2氟调醇(FTOH)和全氟己酸(PFHxA)或全氟辛酸(PFOA);途径Ⅱ产生x:3 acid (CxF2x+1CH2CH2CO2H)、x:3 Uacid (F(CF2)xCHCHCOOH)和全氟丁酸(PFBA)或PFHxA (x=n-1,n=6 or 8)。由于受到菌株类型、酶诱导剂和还原能力的影响,途径Ⅰ更容易进行。随后的研究发现P. fluorescens DSM 8341通过表达脱卤酶(Fluoroacetate-dehalogenase),能在途径Ⅱ中脱去6:2 FTOH中一个C原子,生成5:2 Uacid和4:3 acid[97]。由于PFASs包含烷烃链,通常认为烷烃单加氧酶(Alkane monooxygenase和Butane monooxygenase)在生物转化有机氟化物的过程中起重要作用。因此,烷烃单加氧酶成为判断细菌是否具有转化能力的一种检验依据。此外,Gordonia sp. NB4-1Y表达的两种酶(Nitrilotriacetate和Monooxygenases)还能将氟调聚物磺酸盐脱硫还原[119]。

|
| 图 1 烷烃降解菌和氟乙酸盐降解菌降解6:2 FTOH的途径[97] Figure 1 6:2 FTOH degradation pathways by alkane-degrading and fluoroacetate-degrading bacteria[97] 注:蓝色箭头表示A、B、C和D 4种菌均起作用的降解途径(A:Pseudomonas oleovorans;B:Pseudomonas butanovora;C:Mycobacterium vaccae JOB5;D:Pseudomonas fluorescens DSM 8341).不同菌种所起作用的途径用字母(A、B、C和D)标注;从5:3 Uacid开始的下游转化途径由Pseudomonas fluorescens DSM 8341调控;虚线框中的化合物是推测的代谢物. Note: The pathways shared by all four bacteria (A, B, C and D) are presented in blue arrows (A. Pseudomonas oleovorans; B. Pseudomonas butanovora; C. Mycobacterium vaccae JOB5; D. Pseudomonas fluorescens DSM 8341). Pathways in different bacteria are marked by their labels (A, B, C and D). The downstream pathways from 5:3 Uacid are largely performed by P. fluorescens DSM 8341. Compounds in boxes with dotted line are only predicted. |
|
|
真菌在降解有机氟污染物方面也有重要贡献。其中喹酮类药的真菌降解途径一般包括:酯化、引入羟基、脱羧、引入甲酰或乙酰基、脱氟、部分降解或由氨基取代哌嗪、在哌嗪环处形成共轭物或取代三号碳原子上羧基[107]。体外酶降解和体内细胞色素P450抑制实验表明真菌漆酶和细胞色素P450能催化含氟抗生素药物的去除,而且可能的降解途径为:脱氟或脱水→脱羧→哌嗪取代基的氧化[103];图 2所示为环丙沙星和诺氟沙星在Phanerochaete chrysosporium纯培养中的转化过程。此外,Phanerochaete sanguineus还能通过单羟基化和在N位脱甲基或脱乙基,使环丙沙星脱氟转化[103]。SF5取代的苯胺(SF5-substituted aminophenols)可以被具有N-乙酰基转移酶的链霉菌(Streptomyces griseus)转化为N-乙酰基衍生物[123]。此外,来自AFFF污染的军事基地土壤中的6种真菌(隶属Fusarium sp.,Penicilliumsp.和Aspergillus sp.)在纯培养下能够转化6:2 FTOH[120]。
微生物群落通过把单一底物作为生长碳源或者通过共代谢实现有机氟化物降解。从活性污泥或纯菌属中提取天然酶,并进行酶促实验(Enzymatic reactions),有助于准确识别特定底物的催化酶以及代谢途径。氧化还原酶和水解酶是难降解污染物污泥中较为常见的酶。Krah等在酶促降解20种微污染药品时,把最主要的生物转化途径归因于酰胺水解[124]。最近的研究发现Thaueraaromatica以4-氟甲苯或者4-氟苯甲酸为底物时,存在一条依赖于ATP的C-F键断裂途径并最终实现完全矿化,其脱氟机制如图 3所示[125]。在第Ⅰ类苯甲基辅酶还原酶(BzCoA reductase,BCR)的催化下,4-氟苯甲酰基辅酶A (4-F-BzCoA)脱氟转化成苯甲酰基辅酶A (BzCoA),此过程与伯奇还原反应类似(Birch reduction-like),并确定BCR为4-氟芳烃类物质降解的特征酶和关键酶[125]。与此同时,在研究2-氟苯甲酸盐降解机制时,Tiedt等[115]还发现了Thaueraaromatica脱氟的另一条途径:2-F苯甲酸被苯甲酸辅酶A连接酶激活后,生成的2-F苯甲基辅酶在BCR作用下开环,而后续的脱氟过程由烯酰基辅酶A水合酶/水解酶催化进行。新发现的烯酰基辅酶A水合酶通过形成α位-氟醇的脱氟路径,可能是一种具有代表性的酶促脱氟机制。尽管近年来微生物降解含氟有机物的研究取得了重要进展,然而降解机制和降解途径的多样性有待于进一步探讨,应特别关注微生物组或合成微生物群落对有机氟化物的高效降解途径及其机制。
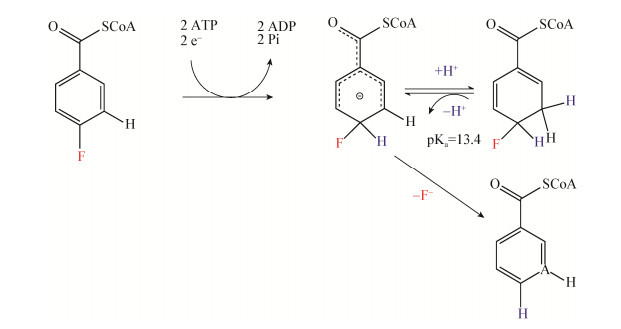
|
| 图 3 通过依赖于两个独立ATP的Ⅰ类苯甲基辅酶还原酶(BzCoA reductase,BCR)的作用,实现4-F-BzCoA潜在转化[125] Figure 3 Possible mechanism for the conversion of 4-F-BzCoA by ATP-dependent class Ⅰ BCR (BzCoA reductase)[125] 注:一种类似伯奇反应的机制,包括两个依赖于ATP的电子转移步骤和一个产生阴离子状态的质子化步骤,推测4-F-dienoyl-CoA中间体(C-3位)的pKa (蛋白激酶A)显著降低,因此在E1cB型(共轭碱单分子消除反应)过程中,本质上不可逆的氟释放是由再芳构化驱动的. Note: It is suggested a Birch-like mechanism involving two single ATP-dependent electron transfer steps and one protonation step, yielding an anionic state. During 4-F-BzCoA conversion, the pKa protein, an assumed 4-F-dienoyl-CoA intermediate (C-3 position) is significantly decreased, resulting in the essentially irreversible fluoride release in an E1cB-type elimination driven by re-aromatization. |
|
|
活性污泥微生物群落多样性、结构和功能决定了废水处理效率和工艺运行的稳定性。同时,废水特征、氟化物浓度和环境条件及工艺运行是微生物群落组装和动态变化的重要驱动力,从而影响其功能。但是,这些环境因子驱动微生物群落结构变化的生态机制尚不清楚;同时,许多研究涉及了纯培养和混合培养对有机氟化物的降解,而相关的降解途径和微生物代谢酶还需进一步探索[126-127]。微生物组学技术的快速发展将促进对复杂污泥微体系的了解,从而更好地认识含氟有机废水活性污泥处理中的微生物群体结构和功能。当前,含氟有机废水处理过程中活性污泥微生物组的相关研究将聚焦在下列几方面(图 4):

|
| 图 4 利用活性污泥微生物组技术提高含氟有机废水处理效果的概念图 Figure 4 A concept scheme for activated sludge microbiome technologies to improve fluoride-containing wastewater process performance |
|
|
(1) 解析活性污泥微生物群落多样性及其与生态系统功能和稳定性的关系
活性污泥中不同微生物在降解/转化污染和维持群落稳定方面发挥不同作用。处理不同含氟有机废水的活性污泥其微生物多样性和组成也不同,因而处理系统表现出不同的性能。运用宏基因组、宏转录组、宏蛋白组和宏代谢组多种组学技术,以活性污泥微生物群落为整体,揭示群落多样性与处理效率和稳定性的关系,为构建高效降解有机氟化物的微生物群落提供理论指导。
(2) 系统研究活性污泥中各类微生物的互作网络及其机制
在污染物降解方面,废水中污染物的降解通常由多种微生物互作完成。一方面,微生物组学(如宏基因组,宏代谢组)分析能获得含氟化合物降解微生物及功能基因、代谢产物和代谢途径的信息,为探索活性污泥微生物群落互作网络及其机制提供了数据;另一方面,运用计算机工具、数学模型、生物信息学技术和数据库平台[128],可系统地模拟活性污泥微生物群落的动态变化,分析和预测关键微生物在时间和空间上对环境的响应,阐明活性污泥微生物群落的互作网络及其机制,明确其关键微生物和功能基因,为靶向富集分离提供依据。
(3) 富集分离降解/转化有机氟化物的关键功能微生物并揭示其机制
通过了解降解/转化氟化的关键功能微生物,继而利用相关基因组信息来设计[129]高效的富集培养方案,进而运用转录组学、蛋白组学、代谢组学等技术,探索其降解/转化有机氟化物的机理[130-131]。同时,通过对微生物遗传回路、代谢途径和酶进行工程设计,利用合成生物学技术进一步增强其功能[132]。
(4) 设计和构建高效脱氟的微生物群落,提高含氟有机废水处理效率
结合微生物生态学及合成微生物生态学理论和方法,选择具有降解含氟物质的功能菌株和相关菌群,以合适比例构建高效脱氟的微生物群落,从而改善活性污泥性能,提高微生物群落的功能,增强含氟有机物的去除效率。
| [1] |
Lim XZ. Tainted water: the scientists tracing thousands of fluorinated chemicals in our environment[J]. Nature, 2019, 566(7742): 26-29. DOI:10.1038/d41586-019-00441-1 |
| [2] |
Renner R. Growing concern over perfluorinated chemicals[J]. Environmental Science & Technology, 2001, 35(7): 154A-160A. |
| [3] |
He K, Blaney L. Systematic optimization of an SPE with HPLC-FLD method for fluoroquinolone detection in wastewater[J]. Journal of Hazardous Materials, 2015, 282: 96-105. DOI:10.1016/j.jhazmat.2014.08.027 |
| [4] |
Carvalho MF, Maia AS, Tiritan ME, et al. Bacterial degradation of moxifloxacin in the presence of acetate as a bulk substrate[J]. Journal of Environmental Management, 2016, 168: 219-228. |
| [5] |
Lei XN, Lu JJ, Liu ZL, et al. Concentration and distribution of antibiotics in water-sediment system of Bosten Lake, Xinjiang[J]. Environmental Science and Pollution Research, 2015, 22(3): 1670-1678. DOI:10.1007/s11356-014-2994-5 |
| [6] |
Gao LH, Shi YL, Li WH, et al. Occurrence and distribution of antibiotics in urban soil in Beijing and Shanghai, China[J]. Environmental Science and Pollution Research, 2015, 22(15): 11360-11371. DOI:10.1007/s11356-015-4230-3 |
| [7] |
Zhang HB, Luo YM, Wu LH, et al. Residues and potential ecological risks of veterinary antibiotics in manures and composts associated with protected vegetable farming[J]. Environmental Science and Pollution Research, 2015, 22(8): 5908-5918. DOI:10.1007/s11356-014-3731-9 |
| [8] |
Lin AYC, Panchangam SC, Ciou PS. High levels of perfluorochemicals in Taiwan's wastewater treatment plants and downstream rivers pose great risk to local aquatic ecosystems[J]. Chemosphere, 2010, 80(10): 1167-1174. DOI:10.1016/j.chemosphere.2010.06.018 |
| [9] |
Prevedouros K, Cousins IT, Buck RC, et al. Sources, fate and transport of perfluorocarboxylates[J]. Environmental Science & Technology, 2006, 40(1): 32-44. |
| [10] |
Paul AG, Jones KC, Sweetman AJ. A first global production, emission, and environmental inventory for perfluorooctane sulfonate[J]. Environmental Science & Technology, 2009, 43(2): 386-392. |
| [11] |
Boiteux V, Bach C, Sagres V, et al. Analysis of 29 per- and polyfluorinated compounds in water, sediment, soil and sludge by liquid chromatography-tandem mass spectrometry[J]. International Journal of Environmental Analytical Chemistry, 2016, 96(8): 705-728. DOI:10.1080/03067319.2016.1196683 |
| [12] |
Filipovic M, Woldegiorgis A, Norström K, et al. Historical usage of aqueous film forming foam: a case study of the widespread distribution of perfluoroalkyl acids from a military airport to groundwater, lakes, soils and fish[J]. Chemosphere, 2015, 129: 39-45. DOI:10.1016/j.chemosphere.2014.09.005 |
| [13] |
Lanza HA, Cochran RS, Mudge JF, et al. Temporal monitoring of perfluorooctane sulfonate accumulation in aquatic biota downstream of historical aqueous film forming foam use areas[J]. Environmental Toxicology and Chemistry, 2017, 36(8): 2022-2029. DOI:10.1002/etc.3726 |
| [14] |
Munoz G, Desrosiers M, Duy SV, et al. Environmental occurrence of perfluoroalkyl acids and novel fluorotelomer surfactants in the freshwater fish catostomus commersonii and sediments following firefighting foam deployment at the Lac-Megantic railway accident[J]. Environmental Science & Technology, 2017, 51(3): 1231-1240. |
| [15] |
Heydebreck F, Tang JH, Xie ZY, et al. Emissions of per- and polyfluoroalkyl substances in a textile manufacturing plant in China and their relevance for workers' exposure[J]. Environmental Science & Technology, 2016, 50(19): 10386-10396. |
| [16] |
Zhang T, Li B. Occurrence, transformation, and fate of antibiotics in municipal wastewater treatment plants[J]. Critical Reviews in Environmental Science and Technology, 2011, 41(11): 951-998. DOI:10.1080/10643380903392692 |
| [17] |
Olsen GW, Lange CC, Ellefson ME, et al. Temporal trends of perfluoroalkyl concentrations in american red cross adult blood donors, 2000-2010[J]. Environmental Science & Technology, 2012, 46(11): 6330-6338. |
| [18] |
Grandjean P, Budtz-Jørgensen E. Immunotoxicity of perfluorinated alkylates: calculation of benchmark doses based on serum concentrations in children[J]. Environmental Health, 2013, 12: 35. DOI:10.1186/1476-069X-12-35 |
| [19] |
Frömel T, Knepper TP. Biodegradation of fluorinated alkyl substances[A]//De Voogt P. Reviews of Environmental Contamination and Toxicology Volume 208[M]. New York, NY: Springer, 2010: 161-177
|
| [20] |
Zhang T, Shao MF, Ye L. 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants[J]. The ISME Journal, 2012, 6(6): 1137-1147. DOI:10.1038/ismej.2011.188 |
| [21] |
Ni BJ, Xie WM, Liu SG, et al. Granulation of activated sludge in a pilot-scale sequencing batch reactor for the treatment of low-strength municipal wastewater[J]. Water Research, 2009, 43(3): 751-761. DOI:10.1016/j.watres.2008.11.009 |
| [22] |
Szabó E, Liébana R, Hermansson M, et al. Microbial population dynamics and ecosystem functions of anoxic/aerobic granular sludge in sequencing batch reactors operated at different organic loading rates[J]. Frontiers in Microbiology, 2017, 8: 770. DOI:10.3389/fmicb.2017.00770 |
| [23] |
Amorim CL, Alves M, Castro PML, et al. Bacterial community dynamics within an aerobic granular sludge reactor treating wastewater loaded with pharmaceuticals[J]. Ecotoxicology and Environmental Safety, 2018, 147: 905-912. DOI:10.1016/j.ecoenv.2017.09.060 |
| [24] |
Gonzalez-Silva BM, Jonassen KR, Bakke I, et al. Nitrification at different salinities: biofilm community composition and physiological plasticity[J]. Water Research, 2016, 95: 48-58. DOI:10.1016/j.watres.2016.02.050 |
| [25] |
Nancharaiah YV, Mohan SV, Lens PNL. Recent advances in nutrient removal and recovery in biological and bioelectrochemical systems[J]. Bioresource Technology, 2016, 215: 173-185. DOI:10.1016/j.biortech.2016.03.129 |
| [26] |
Gómez-Acata S, Vital-Jácome M, Pérez-Sandoval MV, et al. Microbial community structure in aerobic and fluffy granules formed in a sequencing batch reactor supplied with 4-chlorophenol at different settling times[J]. Journal of Hazardous Materials, 2018, 342: 606-616. DOI:10.1016/j.jhazmat.2017.08.073 |
| [27] |
Nielsen PH, Saunders AM, Hansen AA, et al. Microbial communities involved in enhanced biological phosphorus removal from wastewater-a model system in environmental biotechnology[J]. Current Opinion in Biotechnology, 2012, 23(3): 452-459. DOI:10.1016/j.copbio.2011.11.027 |
| [28] |
Ju F, Guo F, Ye L, et al. Metagenomic analysis on seasonal microbial variations of activated sludge from a full-scale wastewater treatment plant over 4 years[J]. Environmental Microbiology Reports, 2014, 6(1): 80-89. DOI:10.1111/1758-2229.12110 |
| [29] |
Marathe NP, Shetty SA, Shouche YS, et al. Limited bacterial diversity within a treatment plant receiving antibiotic-containing waste from bulk drug production[J]. PLoS One, 2016, 11(11): e0165914. DOI:10.1371/journal.pone.0165914 |
| [30] |
Ouyang EM, Liu Y, Ouyang JT, et al. Effects of different wastewater characteristics and treatment techniques on the bacterial community structure in three pharmaceutical wastewater treatment systems[J]. Environmental Technology, 2019, 40(3): 329-341. DOI:10.1080/09593330.2017.1393010 |
| [31] |
Yan WF, Wang SH, Ding R, et al. Long-term operation of electroactive biofilms for enhanced ciprofloxacin removal capacity and anti-shock capabilities[J]. Bioresource Technology, 2019, 275: 192-199. DOI:10.1016/j.biortech.2018.12.053 |
| [32] |
Xu M, Cao JS, Li C, et al. Operational and biological analyses of branched water-adjustment and combined treatment of wastewater from a chemical industrial park[J]. Environmental Technology, 2018, 39(2): 253-263. DOI:10.1080/09593330.2017.1298676 |
| [33] |
Wang BH. Study on the influence factors and the dynamic changes of microbial community structure of activated sludge during the treatment of fluoride wastewater[J]. Hangzhou: Master's Thesis of Hangzhou Normal University, 2017 (in Chinese) 王彬浩.活性污泥降解氟化工废水的影响因素及微生物群落结构的动态变化研究[D].杭州: 杭州师范大学硕士学位论文, 2017 http://cdmd.cnki.com.cn/Article/CDMD-10346-1017195990.htm |
| [34] |
Zhao FZ, Ju F, Huang KL, et al. Comprehensive insights into the key components of bacterial assemblages in pharmaceutical wastewater treatment plants[J]. Science of the Total Environment, 2019, 651: 2148-2157. DOI:10.1016/j.scitotenv.2018.10.101 |
| [35] |
Hu J, Zhang LL, Chen JM, et al. Performance and microbial analysis of a biotrickling filter inoculated by a specific bacteria consortium for removal of a simulated mixture of pharmaceutical volatile organic compounds[J]. Chemical Engineering Journal, 2016, 304: 757-765. DOI:10.1016/j.cej.2016.06.078 |
| [36] |
Zhao X, Chen ZL, Wang XC, et al. Remediation of pharmaceuticals and personal care products using an aerobic granular sludge sequencing bioreactor and microbial community profiling using Solexa sequencing technology analysis[J]. Bioresource Technology, 2015, 179: 104-112. DOI:10.1016/j.biortech.2014.12.002 |
| [37] |
Muter O, Perkons I, Selga T, et al. Removal of pharmaceuticals from municipal wastewaters at laboratory scale by treatment with activated sludge and biostimulation[J]. Science of the Total Environment, 2017, 584-585: 402-413. DOI:10.1016/j.scitotenv.2017.01.023 |
| [38] |
Kim S, Rossmassler K, Broeckling CD, et al. Impact of inoculum sources on biotransformation of pharmaceuticals and personal care products[J]. Water Research, 2017, 125: 227-236. DOI:10.1016/j.watres.2017.08.041 |
| [39] |
Davids M, Gudra D, Radovica-Spalvina I, et al. The effects of ibuprofen on activated sludge: shift in bacterial community structure and resistance to ciprofloxacin[J]. Journal of Hazardous Materials, 2017, 340: 291-299. DOI:10.1016/j.jhazmat.2017.06.065 |
| [40] |
Kong Q, He X, Feng Y, et al. Pollutant removal and microorganism evolution of activated sludge under ofloxacin selection pressure[J]. Bioresource Technology, 2017, 241: 849-856. DOI:10.1016/j.biortech.2017.06.019 |
| [41] |
Liao XB, Li BX, Zou RS, et al. Biodegradation of antibiotic ciprofloxacin: pathways, influential factors, and bacterial community structure[J]. Environmental Science and Pollution Research, 2016, 23(8): 7911-7918. DOI:10.1007/s11356-016-6054-1 |
| [42] |
Wang XC, Shen JM, Chen ZL, et al. Removal of pharmaceuticals from synthetic wastewater in an aerobic granular sludge membrane bioreactor and determination of the bioreactor microbial diversity[J]. Applied Microbiology and Biotechnology, 2016, 100(18): 8213-8223. DOI:10.1007/s00253-016-7577-6 |
| [43] |
Ramos C, Amorim CL, Mesquita DP, et al. Simultaneous partial nitrification and 2-fluorophenol biodegradation with aerobic granular biomass: reactor performance and microbial communities[J]. Bioresource Technology, 2017, 238: 232-240. DOI:10.1016/j.biortech.2017.03.173 |
| [44] |
Duque AF, Bessa VS, Castro PML. Bacterial community dynamics in a rotating biological contactor treating 2-fluorophenol-containing wastewater[J]. Journal of Industrial Microbiology & Biotechnology, 2014, 41(1): 97-104. |
| [45] |
Amorim CL, Duque AF, Afonso CMM, et al. Bioaugmentation for treating transient 4-fluorocinnamic acid shock loads in a rotating biological contactor[J]. Bioresource Technology, 2013, 144: 554-562. DOI:10.1016/j.biortech.2013.07.010 |
| [46] |
Zhao ZQ, Tian BH, Zhang X, et al. Aerobic degradation study of three fluoroanilines and microbial community analysis: the effects of increased fluorine substitution[J]. Biodegradation, 2015, 26(1): 1-14. DOI:10.1007/s10532-014-9704-3 |
| [47] |
Duque AF, Bessa VS, Castro PML. Characterization of the bacterial communities of aerobic granules in a 2-fluorophenol degrading process[J]. Biotechnology Reports, 2015, 5: 98-104. DOI:10.1016/j.btre.2014.12.007 |
| [48] |
Mejia-Avendaño S, Duy SV, Sauvé S, et al. Generation of perfluoroalkyl acids from aerobic biotransformation of quaternary ammonium polyfluoroalkyl surfactants[J]. Environmental Science & Technology, 2016, 50(18): 9923-9932. |
| [49] |
Harding-Marjanovic KC, Houtz EF, Yi S, et al. Aerobic biotransformation of fluorotelomer thioether amido sulfonate (Lodyne) in AFFF-amended microcosms[J]. Environmental Science & Technology, 2015, 49(13): 7666-7674. |
| [50] |
Lewis M, Kim MH, Wang N, et al. Engineering artificial communities for enhanced FTOH degradation[J]. Science of the Total Environment, 2016, 572: 935-942. DOI:10.1016/j.scitotenv.2016.07.223 |
| [51] |
Lewis M, Kim MH, Liu EJ, et al. Biotransformation of 6:2 polyfluoroalkyl phosphates (6:2 PAPs): effects of degradative bacteria and co-substrates[J]. Journal of Hazardous Materials, 2016, 320: 479-486. DOI:10.1016/j.jhazmat.2016.08.036 |
| [52] |
Figueroa M, Del Río AV, Campos JL, et al. Filamentous bacteria existence in aerobic granular reactors[J]. Bioprocess and Biosystems Engineering, 2015, 38(5): 841-851. DOI:10.1007/s00449-014-1327-x |
| [53] |
Li YC, Hao W, Lv JP, et al. The role of N-acyl homoserine lactones in maintaining the stability of aerobic granules[J]. Bioresource Technology, 2014, 159: 305-310. DOI:10.1016/j.biortech.2014.02.090 |
| [54] |
Werner JJ, Knights D, Garcia ML, et al. Bacterial community structures are unique and resilient in full-scale bioenergy systems[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(10): 4158-4163. DOI:10.1073/pnas.1015676108 |
| [55] |
Ju F, Zhang T. Bacterial assembly and temporal dynamics in activated sludge of a full-scale municipal wastewater treatment plant[J]. The ISME Journal, 2015, 9(3): 683-695. DOI:10.1038/ismej.2014.162 |
| [56] |
Liu F, Hu XM, Zhao X, et al. Microbial community structures' response to seasonal variation in a full-scale municipal wastewater treatment plant[J]. Environmental Engineering Science, 2019, 36(2): 172-179. DOI:10.1089/ees.2018.0280 |
| [57] |
Pala-Ozkok I, Rehman A, Kor-Bicakci G, et al. Effect of sludge age on population dynamics and acetate utilization kinetics under aerobic conditions[J]. Bioresource Technology, 2013, 143: 68-75. DOI:10.1016/j.biortech.2013.05.095 |
| [58] |
Zhang B, Yu QW, Yan GQ, et al. Seasonal bacterial community succession in four typical wastewater treatment plants: correlations between core microbes and process performance[J]. Scientific Reports, 2018, 8(1): 4566. DOI:10.1038/s41598-018-22683-1 |
| [59] |
Xue J, Schmitz BW, Caton K, et al. Assessing the spatial and temporal variability of bacterial communities in two Bardenpho wastewater treatment systems via Illumina MiSeq sequencing[J]. Science of the Total Environment, 2019, 657: 1543-1552. DOI:10.1016/j.scitotenv.2018.12.141 |
| [60] |
Purser S, Moore PR, Swallow S, et al. Fluorine in medicinal chemistry[J]. Chemical Society Reviews, 2008, 37(2): 320-330. DOI:10.1039/B610213C |
| [61] |
Liu JX, Wang N, Buck RC, et al. Aerobic biodegradation of[14C] 6:2 fluorotelomer alcohol in a flow-through soil incubation system[J]. Chemosphere, 2010, 80(7): 716-723. DOI:10.1016/j.chemosphere.2010.05.027 |
| [62] |
Longstaffe JG, Simpson MJ, Maas W, et al. Identifying components in dissolved humic acid that bind organofluorine contaminants using 1H{19F} reverse heteronuclear saturation transfer difference NMR spectroscopy[J]. Environmental Science & Technology, 2010, 44(14): 5476-5482. |
| [63] |
Mardani G, Mahvi AH, Hashemzadeh-Chaleshtori M, et al. Application of genetically engineered dioxygenase producing Pseudomonas putida on decomposition of oil from spiked soil[J]. Journal of Natural Pharmaceutical Products, 2017, 12(S3): e64313. |
| [64] |
Zhang R, Xu XJ, Chen WL, et al. Genetically engineered Pseudomonas putida X3 strain and its potential ability to bioremediate soil microcosms contaminated with methyl parathion and cadmium[J]. Applied Microbiology and Biotechnology, 2016, 100(4): 1987-1997. DOI:10.1007/s00253-015-7099-7 |
| [65] |
Zhang S, Lu XX, Wang N, et al. Biotransformation potential of 6: 2 fluorotelomer sulfonate (6:2 FTSA) in aerobic and anaerobic sediment[J]. Chemosphere, 2016, 154: 224-230. DOI:10.1016/j.chemosphere.2016.03.062 |
| [66] |
Dasu K, Liu JX, Lee LS. Aerobic soil biodegradation of 8:2 fluorotelomer stearate monoester[J]. Environmental Science & Technology, 2012, 46(7): 3831-3836. |
| [67] |
Tu QC, He ZL, Wu LY, et al. Metagenomic reconstruction of nitrogen cycling pathways in a CO2-enriched grassland ecosystem[J]. Soil Biology and Biochemistry, 2017, 106: 99-108. DOI:10.1016/j.soilbio.2016.12.017 |
| [68] |
Feng JJ, Penton CR, He ZL, et al. Long-term warming in Alaska enlarges the diazotrophic community in deep soils[J]. mBio, 2019, 10(1): e02521-18. DOI:10.1128/mBio.02521-18 |
| [69] |
He ZL, Zhang P, Wu LW, et al. Microbial functional gene diversity predicts groundwater contamination and ecosystem functioning[J]. mBio, 2018, 9(1): e02435-17. DOI:10.1128/mBio.02435-17 |
| [70] |
Chai ZY, He ZL, Deng YY, et al. Cultivation of seaweed Gracilaria lemaneiformis enhanced biodiversity in a eukaryotic plankton community as revealed via metagenomic analyses[J]. Molecular Ecology, 2018, 27(4): 1081-1093. DOI:10.1111/mec.14496 |
| [71] |
Li B, Wu WM, Watson DB, et al. Bacterial community shift and coexisting/coexcluding patterns revealed by network analysis in a uranium-contaminated site after bioreduction followed by reoxidation[J]. Applied and Environmental Microbiology, 2018, 84(9): e02885-17. |
| [72] |
Shu DT, Yue H, He YL, et al. Divergent assemblage patterns of abundant and rare microbial sub-communities in response to inorganic carbon stresses in a simultaneous anammox and denitrification (SAD) system[J]. Bioresource Technology, 2018, 257: 249-259. DOI:10.1016/j.biortech.2018.02.111 |
| [73] |
Zhang ZM, Yu ZD, Wang ZH, et al. Understanding of aerobic sludge granulation enhanced by sludge retention time in the aspect of quorum sensing[J]. Bioresource Technology, 2019, 272: 226-234. DOI:10.1016/j.biortech.2018.10.027 |
| [74] |
Xia Y, Wang XH, Wen XH, et al. Overall functional gene diversity of microbial communities in three full-scale activated sludge bioreactors[J]. Applied Microbiology and Biotechnology, 2014, 98(16): 7233-7242. DOI:10.1007/s00253-014-5791-7 |
| [75] |
Zhang Y, Xie JP, Liu MM, et al. Microbial community functional structure in response to antibiotics in pharmaceutical wastewater treatment systems[J]. Water Research, 2013, 47(16): 6298-6308. DOI:10.1016/j.watres.2013.08.003 |
| [76] |
Wu LW, Ning DL, Zhang B, et al. Global diversity and biogeography of bacterial communities in wastewater treatment plants[J]. Nature Microbiology, 2019, 4. |
| [77] |
Lu HJ, Chandran K, Stensel D. Microbial ecology of denitrification in biological wastewater treatment[J]. Water Research, 2014, 64: 237-254. DOI:10.1016/j.watres.2014.06.042 |
| [78] |
Wang P, Yu ZS, Zhao JH, et al. Seasonal changes in bacterial communities cause foaming in a wastewater treatment plant[J]. Microbial Ecology, 2016, 71(3): 660-671. DOI:10.1007/s00248-015-0700-x |
| [79] |
Ramola B, Singh A. Heavy metal concentrations in pharmaceutical effluents of industrial area of Dehradun (Uttarakhand), India[J]. Journal of Environmental & Analytical Toxicology, 2013, 3(3): 173. |
| [80] |
Salinas MB, Fardeau ML, Cayol JL, et al. Petrobacter succinatimandens gen. nov., sp. nov., a moderately thermophilic, nitrate-reducing bacterium isolated from an Australian oil well[J]. International Journal of Systematic and Evolutionary Microbiology, 2004, 54(3): 645-649. DOI:10.1099/ijs.0.02732-0 |
| [81] |
Ju F, Xia Y, Guo F, et al. Taxonomic relatedness shapes bacterial assembly in activated sludge of globally distributed wastewater treatment plants[J]. Environmental Microbiology, 2014, 16(8): 2421-2432. DOI:10.1111/1462-2920.12355 |
| [82] |
Kalyuhznaya MG, Martens-Habbena W, Wang TS, et al. Methylophilaceae link methanol oxidation to denitrification in freshwater lake sediment as suggested by stable isotope probing and pure culture analysis[J]. Environmental Microbiology Reports, 2009, 1(5): 385-392. DOI:10.1111/j.1758-2229.2009.00046.x |
| [83] |
Kaparullina EN, Doronina NV, Ezhov VA, et al. EDTA degradation by cells of Chelativorans oligotrophicus immobilized on a biofilter[J]. Applied Biochemistry and Microbiology, 2012, 48(4): 396-400. DOI:10.1134/S0003683812040096 |
| [84] |
Jayamani I, Cupples AM. Stable isotope probing and high-throughput sequencing implicate Xanthomonadaceae and Rhodocyclaceae in ethylbenzene degradation[J]. Environmental Engineering Science, 2015, 32(3): 240-249. DOI:10.1089/ees.2014.0456 |
| [85] |
Pérez-Pantoja D, Donoso R, Agulló L, et al. Genomic analysis of the potential for aromatic compounds biodegradation in Burkholderiales[J]. Environmental Microbiology, 2012, 14(5): 1091-1117. DOI:10.1111/j.1462-2920.2011.02613.x |
| [86] |
Ji JY, Xing YJ, Ma ZT, et al. Toxicity assessment of anaerobic digestion intermediates and antibiotics in pharmaceutical wastewater by luminescent bacterium[J]. Journal of Hazardous Materials, 2013, 246-247: 319-323. DOI:10.1016/j.jhazmat.2012.12.025 |
| [87] |
Boente RF, Ferreira LQ, Falcão LS, et al. Detection of resistance genes and susceptibility patterns in Bacteroides and Parabacteroides strains[J]. Anaerobe, 2010, 16(3): 190-194. DOI:10.1016/j.anaerobe.2010.02.003 |
| [88] |
Colombo S, Arioli S, Guglielmetti S, et al. Virome-associated antibiotic-resistance genes in an experimental aquaculture facility[J]. FEMS Microbiology Ecology, 2016, 92(3): fiw003. DOI:10.1093/femsec/fiw003 |
| [89] |
Li B, Zhang XX, Guo F, et al. Characterization of tetracycline resistant bacterial community in saline activated sludge using batch stress incubation with high-throughput sequencing analysis[J]. Water Research, 2013, 47(13): 4207-4216. DOI:10.1016/j.watres.2013.04.021 |
| [90] |
Huang KL, Tang JY, Zhang XX, et al. A comprehensive insight into tetracycline resistant bacteria and antibiotic resistance genes in activated sludge using next-generation sequencing[J]. International Journal of Molecular Sciences, 2014, 15(6): 10083-10100. DOI:10.3390/ijms150610083 |
| [91] |
Qiu JG, Liu B, Zhao LL, et al. A novel degradation mechanism for pyridine derivatives in Alcaligenes faecalis JQ135[J]. Applied and Environmental Microbiology, 2018, 84(15): e00910-18. DOI:10.1128/AEM.00910-18 |
| [92] |
Zhang HH, Wang Y, Zhao C, et al. Biodegradation of ochratoxin A by Alcaligenes faecalis isolated from soil[J]. Journal of Applied Microbiology, 2017, 123(3): 661-668. DOI:10.1111/jam.13537 |
| [93] |
Li T, Deng XP, Wang JJ, et al. Biodegradation of 3, 4-dichloroaniline by a novel Myroides odoratimimus strain LWD09 with moderate salinity tolerance[J]. Water, Air, & Soil Pollution, 2012, 223(6): 3271-3279. |
| [94] |
Zhou YQ, Wang TY, Jiang ZZ, et al. Ecological effect and risk towards aquatic plants induced by perfluoroalkyl substances: bridging natural to culturing flora[J]. Chemosphere, 2017, 167: 98-106. DOI:10.1016/j.chemosphere.2016.09.146 |
| [95] |
Chen SQ, Zhou YQ, Meng J, et al. Seasonal and annual variations in removal efficiency of perfluoroalkyl substances by different wastewater treatment processes[J]. Environmental Pollution, 2018, 242: 2059-2067. DOI:10.1016/j.envpol.2018.06.078 |
| [96] |
Kim MH, Wang N, McDonald T, et al. Biodefluorination and biotransformation of fluorotelomer alcohols by two alkane-degrading Pseudomonas strains[J]. Biotechnology and Bioengineering, 2012, 109(12): 3041-3048. DOI:10.1002/bit.24561 |
| [97] |
Kim MH, Wang N, Chu KH. 6:2 Fluorotelomer alcohol (6:2 FTOH) biodegradation by multiple microbial species under different physiological conditions[J]. Applied Microbiology and Biotechnology, 2014, 98(4): 1831-1840. DOI:10.1007/s00253-013-5131-3 |
| [98] |
Zhang S, Merino N, Wang N, et al. Impact of 6:2 fluorotelomer alcohol aerobic biotransformation on a sediment microbial community[J]. Science of the Total Environment, 2017, 575: 1361-1368. DOI:10.1016/j.scitotenv.2016.09.214 |
| [99] |
Nguyen LN, Nghiem LD, Oh S. Aerobic biotransformation of the antibiotic ciprofloxacin by Bradyrhizobium sp. isolated from activated sludge[J]. Chemosphere, 2018, 211: 600-607. DOI:10.1016/j.chemosphere.2018.08.004 |
| [100] |
Pan LJ, Li J, Li CX, et al. Study of ciprofloxacin biodegradation by a Thermus sp. isolated from pharmaceutical sludge[J]. Journal of Hazardous Materials, 2018, 343: 59-67. DOI:10.1016/j.jhazmat.2017.09.009 |
| [101] |
Caracciolo AB, Grenni P, Rauseo J, et al. Degradation of a fluoroquinolone antibiotic in an urbanized stretch of the River Tiber[J]. Microchemical Journal, 2018, 136: 43-48. DOI:10.1016/j.microc.2016.12.008 |
| [102] |
Moreira IS, Ribeiro AR, Afonso CM, et al. Enantioselective biodegradation of fluoxetine by the bacterial strain Labrys portucalensis F11[J]. Chemosphere, 2014, 111: 103-111. DOI:10.1016/j.chemosphere.2014.03.022 |
| [103] |
Gao N, Liu CX, Xu QM, et al. Simultaneous removal of ciprofloxacin, norfloxacin, sulfamethoxazole by co-producing oxidative enzymes system of Phanerochaete chrysosporium and Pycnoporus sanguineus[J]. Chemosphere, 2018, 195: 146-155. DOI:10.1016/j.chemosphere.2017.12.062 |
| [104] |
Santos F, Mucha AP, Alexandrino DAM, et al. Biodegradation of enrofloxacin by microbial consortia obtained from rhizosediments of two estuarine plants[J]. Journal of Environmental Management, 2019, 231: 1145-1153. DOI:10.1016/j.jenvman.2018.11.022 |
| [105] |
Bright TV, Clark BR, O'Brien E, et al. Bacterial production of hydroxylated and amidated metabolites of flurbiprofen[J]. Journal of Molecular Catalysis B: Enzymatic, 2011, 72(3/4): 116-121. |
| [106] |
Kim DW, Heinze TM, Kim BS, et al. Modification of norfloxacin by a Microbacterium sp. strain isolated from a wastewater treatment plant[J]. Applied and Environmental Microbiology, 2011, 77(17): 6100-6108. DOI:10.1128/AEM.00545-11 |
| [107] |
Rusch M, Kauschat A, Spielmeyer A, et al. Biotransformation of the antibiotic danofloxacin by Xylaria longipes leads to an efficient reduction of its antibacterial activity[J]. Journal of Agricultural and Food Chemistry, 2015, 63(31): 6897-6904. DOI:10.1021/acs.jafc.5b02343 |
| [108] |
Saccomanno M, Hussain S, O'Connor NK, et al. Biodegradation of pentafluorosulfanyl-substituted aminophenol in Pseudomonas spp.[J]. Biodegradation, 2018, 29(3): 259-270. DOI:10.1007/s10532-018-9827-z |
| [109] |
Hasan SA, Ferreira MIM, Koetsier MJ, et al. Complete Biodegradation of 4-fluorocinnamic acid by a consortium comprising Arthrobacter sp. strain G1 and Ralstonia sp. strain H1[J]. Applied and Environmental Microbiology, 2011, 77(2): 572-579. DOI:10.1128/AEM.00393-10 |
| [110] |
Song EX, Wang MZ, Shen DS. Isolation, identification and characterization of a novel Ralstonia sp. FD-1, capable of degrading 4-fluoroaniline[J]. Biodegradation, 2014, 25(1): 85-94. DOI:10.1007/s10532-013-9642-5 |
| [111] |
Cao WL, Song EX, Shen DS, et al. Cometabolism of fluoroanilines in the presence of 4-fluoroaniline by Ralstonia sp. FD-1[J]. Journal of Chemistry, 2015, 2015: 206150. |
| [112] |
Misiak K, Casey E, Murphy CD. Factors influencing 4-fluorobenzoate degradation in biofilm cultures of Pseudomonas knackmussii B13[J]. Water Research, 2011, 45(11): 3512-3520. DOI:10.1016/j.watres.2011.04.020 |
| [113] |
Amorim CL, Ferreira ACS, Carvalho MF, et al. Mineralization of 4-fluorocinnamic acid by a Rhodococcus strain[J]. Applied Microbiology and Biotechnology, 2014, 98(4): 1893-1905. DOI:10.1007/s00253-013-5149-6 |
| [114] |
Eppinger E, Bürger S, Stolz A. Spontaneous release of fluoride during the dioxygenolytic cleavage of 5-fluorosalicylate by the salicylate 1, 2-dioxygenase from Pseudaminobacter salicylatoxidans BN12[J]. FEMS Microbiology Letters, 2016, 363(1): fnv211. DOI:10.1093/femsle/fnv211 |
| [115] |
Tiedt O, Mergelsberg M, Eisenreich W, et al. Promiscuous defluorinating enoyl-CoA hydratases/hydrolases allow for complete anaerobic degradation of 2-fluorobenzoate[J]. Frontiers in Microbiology, 2017, 8: 2579. DOI:10.3389/fmicb.2017.02579 |
| [116] |
Kwon BG, Lim HJ, Na SH, et al. Biodegradation of perfluorooctanesulfonate (PFOS) as an emerging contaminant[J]. Chemosphere, 2014, 109: 221-225. DOI:10.1016/j.chemosphere.2014.01.072 |
| [117] |
Liu JX, Lee LS, Nies LF, et al. Biotransformation of 8:2 fluorotelomer alcohol in soil and by soil bacteria isolates[J]. Environmental Science & Technology, 2007, 41(23): 8024-8030. |
| [118] |
Shaw DMJ, Munoz G, Bottos EM, et al. Degradation and defluorination of 6:2 fluorotelomer sulfonamidoalkyl betaine and 6:2 fluorotelomer sulfonate by Gordonia sp. strain NB4-1Y under sulfur-limiting conditions[J]. Science of the Total Environment, 2019, 647: 690-698. DOI:10.1016/j.scitotenv.2018.08.012 |
| [119] |
Van Hamme JD, Bottos EM, Bilbey NJ, et al. Genomic and proteomic characterization of Gordonia sp. NB4-1Y in relation to 6:2 fluorotelomer sulfonate biodegradation[J]. Microbiology, 2013, 159(8): 1618-1628. |
| [120] |
Merino N, Wang M, Ambrocio R, et al. Fungal biotransformation of 6:2 fluorotelomer alcohol[J]. Remediation Journal, 2018, 28(2): 59-70. DOI:10.1002/rem.21550 |
| [121] |
Tseng N, Wang N, Szostek B, et al. Biotransformation of 6:2 fluorotelomer alcohol (6:2 FTOH) by a wood-rotting fungus[J]. Environmental Science & Technology, 2014, 48(7): 4012-4020. |
| [122] |
Amorim CL, Moreira IS, Maia AS, et al. Biodegradation of ofloxacin, norfloxacin, and ciprofloxacin as single and mixed substrates by Labrys portucalensis F11[J]. Applied Microbiology and Biotechnology, 2014, 98(7): 3181-3190. DOI:10.1007/s00253-013-5333-8 |
| [123] |
Kavanagh E, Winn M, Gabhann CN, et al. Microbial biotransformation of aryl sulfanylpentafluorides[J]. Environmental Science and Pollution Research, 2014, 21(1): 753-758. DOI:10.1007/s11356-013-1985-2 |
| [124] |
Krah D, Ghattas AK, Wick A, et al. Micropollutant degradation via extracted native enzymes from activated sludge[J]. Water Research, 2016, 95: 348-360. DOI:10.1016/j.watres.2016.03.037 |
| [125] |
Tiedt O, Mergelsberg M, Boll K, et al. ATP-dependent C-F bond cleavage allows the complete degradation of 4-fluoroaromatics without oxygen[J]. mBio, 2016, 7(4): e00990-16. DOI:10.1128/mBio.00990-16 |
| [126] |
Zhou JZ, He ZL, Yang YF, et al. High-throughput metagenomic technologies for complex microbial community analysis: open and closed formats[J]. mBio, 2015, 6(1): e02288-14. DOI:10.1128/mBio.02288-14 |
| [127] |
Tu QC, Yu H, He ZL, et al. GeoChip 4: a functional gene-array-based high-throughput environmental technology for microbial community analysis[J]. Molecular Ecology Resources, 2014, 14(5): 914-928. |
| [128] |
Yin XL, Jiang XT, Chai BL, et al. ARGs-OAP v2.0 with an expanded SARG database and Hidden Markov Models for enhancement characterization and quantification of antibiotic resistance genes in environmental metagenomes[J]. Bioinformatics, 2018, 34(13): 2263-2270. DOI:10.1093/bioinformatics/bty053 |
| [129] |
Pope PB, Smith W, Denman SE, et al. Isolation of Succinivibrionaceae implicated in low methane emissions from Tammar wallabies[J]. Science, 2011, 333(6042): 646-648. DOI:10.1126/science.1205760 |
| [130] |
Prasse C, Stalter D, Schulte-Oehlmann U, et al. Spoilt for choice: a critical review on the chemical and biological assessment of current wastewater treatment technologies[J]. Water Research, 2015, 87: 237-270. DOI:10.1016/j.watres.2015.09.023 |
| [131] |
Pomiès M, Choubert JM, Wisniewski C, et al. Modelling of micropollutant removal in biological wastewater treatments: a review[J]. Science of the Total Environment, 2013, 443: 733-748. DOI:10.1016/j.scitotenv.2012.11.037 |
| [132] |
Liu D, Hoynes-O'Connor A, Zhang FZ. Bridging the gap between systems biology and synthetic biology[J]. Frontiers in Microbiology, 2013, 4: 211. |
 2019, Vol. 46
2019, Vol. 46