扩展功能
文章信息
- 纪南南, 杨广, 梁如冰
- JI Nan-Nan, YANG Guang, LIANG Ru-Bing
- MvaT调控铜绿假单胞菌吩嗪的合成机制
- Regulation of MvaT on phenazine synthesis in Pseudomonas aeruginosa
- 微生物学通报, 2019, 46(7): 1662-1671
- Microbiology China, 2019, 46(7): 1662-1671
- DOI: 10.13344/j.microbiol.china.180646
-
文章历史
- 收稿日期: 2018-08-20
- 接受日期: 2018-10-17
- 网络首发日期: 2018-12-26
吩嗪类化合物是假单胞菌产生的一类重要的小分子次级代谢产物,属于多氮稠杂环化合物,主要包括绿脓菌素(pyocyanin,PYO)、吩嗪-1-酰胺(penazine-1-carboxamide,PCN)、2-羟基吩嗪(2-hydroxyphenazine,2-HPCA)和吩嗪-1-羧酸(phenazine-1-carboxylic acid,PCA)等,参与了假单胞菌的杀菌抑菌、信号转导、基因水平转移、药物外排和生物膜形成等许多生理过程,具有重要的生理生化作用[1-5]。绿脓菌素是条件性致病菌铜绿假单胞菌分泌的多种毒性因子之一,在其侵染过程中起重要作用[6]。吩嗪化合物可在病原菌感染人和动物时直接干扰被侵染细胞的信号通路如IL-8和NF-KB途径,促进细菌侵染进程;同时吩嗪化合物可降低丝氨酸蛋白酶活性,导致肺的囊性纤维化[7]。
研究认为铜绿假单胞菌中吩嗪类化合物合成是通过莽草酸代谢途径(shikimic acid pathway),以分支酸为中间产物合成吩嗪的基本三环骨架,产生的吩嗪-1-羧酸可作为前体进一步形成其它复杂结构的吩嗪类化合物[4]。铜绿假单胞菌中已发现2个独立的吩嗪合成基因簇,各自包括7个基因(phzA1–G1:phzA1/B1/C1/D1/E1/F1/G1)与(phzA2–G2:phzA2/B2/C2/D2/E2/F2/G2),均可实现莽草酸向吩嗪-1-羧酸(PCA)的转化[5]。此外,由phzH、phzS和phzM 3个基因编码的特定酶,可将吩嗪-1-羧酸分别转化为绿脓菌素、吩嗪-1-酰胺和2-羟基吩嗪[5]。
转录因子MvaT属于H-NS蛋白家族,已被证实参与了甲羟戊酸代谢、细菌毒性因子表达、生物膜形成、信号分子合成、鞭毛组装及基因水平转移等多个过程的调控,有助于细菌适应复杂多变的环境[8-12]。有研究结果显示,敲除铜绿假单胞菌mvaT基因可提升菌株的吩嗪类化合物产量,相关合成基因簇的转录表达水平有所提升,推测该转录因子参与了吩嗪类化合物的代谢过程,但具体机制仍不十分明确[13]。
铜绿假单胞菌SJTD-1是本课题组分离获得的一株石油高效降解菌株,前期比较蛋白组结果显示,在以C18为唯一碳源培养时,MvaT蛋白的表达量较其在以葡萄糖为唯一碳源培养时上调了2.45倍[14-15]。mvaT基因敲除菌株SJTD-1(ΔmvaT)的吩嗪化合物产量较野生型菌株有2−3倍的提升[16]。因此,本研究以铜绿假单胞菌SJTD-1与SJTD-1(ΔmvaT)为研究对象,检测其在不同培养基中的吩嗪类化合物产量;同时通过体外克隆与表达重组蛋白MvaT,并结合体外凝胶阻滞实验,检测确定MvaT蛋白对吩嗪合成相关基因是否具有直接地调控作用,这将有助于解析MvaT蛋白对铜绿假单胞菌吩嗪合成过程的调控模式,也可为后续构建高产吩嗪类化合物的工程菌株提供思路。
1 材料与方法 1.1 材料 1.1.1 菌株和质粒及引物| 菌株/质粒 Strains/Plamids |
基因型/抗性/描述 Description of genotypes and resistances |
来源 Sources/References |
| 菌株Strains | ||
| Escherichia coli DH5α |
F′/endA1 hsdR17 (rK–mK–) glnV44 thi–1 recA1 gyrA (NalR) relA1 Δ (lacIZYA-argF) U169 deoR (Φ80lacΔ (lacZ)M15) |
Invitrogen |
| E. coli BL21(DE3) | 蛋白异源表达菌株 Protein allogeneic expression strain |
Novagen |
| P. aeruginosa SJTD-1 |
野生型烷烃降解铜绿假单胞菌菌株 Strain with the alkane-degradation capability isolated from oil-contaminated soil, wild type |
[13] |
| P. aeruginosa SJTD-1(ΔmvaT) |
mvaT敲除突变株 mvaT mutant of P. aeruginosa SJTD-1 |
[16] |
| 质粒Plasmids | ||
| pET28a | 大肠杆菌表达载体, oriT, 卡那霉素抗性 E. coli expression plasmid, Kmr |
Novagen |
| pET28a-mvaT | MvaT表达载体, oriT, 卡那霉素抗性 Plasmid pET28a inserted with mvaT gene at Nde Ⅰ/Hind Ⅲ sites, Kmr |
This study |
| 引物名称 Primers name |
引物序列 Primers sequence (5′→3′) |
描述 Description |
| mvaT-orf-F | ggggggCATATGtccctgatcaacgaatatcgc | 扩增mvaT编码基因片段 Amplification of mvaT gene fragment |
| mvaT-orf-R | ggggggGGATCCctggtttagccgagcagggt | |
| phzA1G1-U200-F | ccaaaagaaacccgggctg | 扩增phzA1G1操纵子上游450 bp和 200 bp片段 Amplification of 450 bp and 200 bp promoter region of phzA1G1 operon |
| phzA1G1-U450-F | tagcaatcccgctccct | |
| phzA1G1-U200/450-FAM-R | FAM-gcgccgcctccgagaggg | |
| phzA2G2-U200-F | cctgtcaaatctggttacaa | 扩增phzA2G2操纵子上游450 bp和 200 bp片段 Amplification of 450 bp and 200 bp promoter region of phzA2G2 operon |
| phzA2G2-U450-F | cgcaagcgtccggccattca | |
| phzA2G2-U200/450-FAM-R | FAM-ggtgcgaatctccgccagtt | |
| phzS-U237-F | cgacaccgctgcgccggc | 扩增phzS基因上游237 bp和100 bp 片段 Amplification of 237 bp and 100 bp promoter region of phzS gene |
| phzS-U100-F | cggcgttatccgccgctgc | |
| phzS-U100/237-FAM-R | FAM-gggtgcttccttttctcgagtgttc | |
| phzH-U200-F | cacgttttttcctgttctatcattg | 扩增phzH基因上游200 bp和100 bp 片段 Amplification of 200 bp and 100 bp promoter region of phzH gene |
| phzH-U100-F | gcgaagcattcgtacgcc | |
| phzH-U100/200-FAM-R | FAM-agggaaactcctataattgatgttt | |
| phzM-U200-F | caggcagttggaaagttcca | 扩增phzM基因上游200 bp和100 bp 片段 Amplification of 200 bp and 100 bp promoter region of phzM gene |
| phzM-U100-F | ggtgttctgcggtatttctc | |
| phzM-U100/200-FAM-R | FAM-cttttattctctctcgttacacatttcc | |
| 注:下划线标示限制性酶切位点序列,FAM表示FAM荧光集团标记. Note: The underline represented the sequences of the restriction sites, and FAM represented the flureoscence group of FAM. |
||
蛋白胨和酵母粉,Oxoid公司;所有无机化学试剂,国药集团化学试剂有限公司(上海);十二烷基磺酸钠(SDS)、丙烯酰胺、琼脂粉、乙二胺四乙酸(EDTA)、N, N, N′, N′-四甲基乙二胺(TEMED)、尿素等缓存液试剂,生工生物工程(上海)股份有限公司;rTaq酶,TaKaRa公司;PCR产物纯化试剂盒和细菌基因组提取试剂盒,天根生物技术有限公司;限制性内切酶,Thermo Fisher公司;亲和层析介质Ni-TA,Bio-Rad公司。
低温台式离心机、梯度PCR仪,Eppendorf公司;紫外分光光度计与数显式稳压稳流电泳仪,上海精科实业有限公司;Nanodrop核酸分析仪,Nanodrop Technologies公司;蛋白电泳仪与凝胶成像仪,Bio-Rad公司。
1.1.3 培养基和培养条件LB液体培养基(g/L):蛋白胨10.0,酵母提取物5.0,NaCl 8.0 (pH 7.0−7.2)。LB固体培养基:在LB液体培养基中加入1.5%−2%琼脂粉。KB培养基(g/L):蛋白胨20.0,甘油15 ml,K2HPO4 0.392,无水MgSO4 0.732 (pH 7.5)。PB培养基(g/L):蛋白胨20.0,MgCl2 1.4,K2SO4 10.0 (pH 7.0−7.2)。PPM培养基(g/L):蛋白胨20.0,葡萄糖18.0,NaCl 1.0,KNO3 5.0 (pH 7.0−7.2)。所有培养基均100 kPa灭菌20 min后备用。所有大肠杆菌和铜绿假单胞菌的菌株均在37 ℃、200 r/min振荡培养。
1.2 方法 1.2.1 吩嗪化合物含量的测定吩嗪化合物含量测定方法参考文献[17]。具体步骤如下:先将铜绿假单胞菌菌株SJTD-1和SJTD-1(ΔmvaT)在LB固体培养基活化后,挑取单菌落接种到LB培养基中培养至OD600为0.5。收集细胞,无菌水洗3次后重悬菌体。以初始OD600为0.05时转接入500 ml的PPM、PB、KB、LB培养基中培养,在不同时间点取样2 mL;加入等体积氯仿,振荡混匀,静置30−60 min。8 000 r/min离心5 min后吸取有机相,加入0.2 mol/L盐酸2 ml,振荡混匀混合,静置30 min。取上层盐酸相测定OD520值,吩嗪含量(mg/L)=OD520×17.072,取3次独立实验的平均值计算。
1.2.2 MvaT蛋白异源表达菌株的构建利用引物mvaT-orf-F/R PCR扩增mvaT基因编码区片段。利用Nde Ⅰ和Hind Ⅲ酶切连接后,将其插入同样酶切处理后的表达载体pET28a中,化学法转入E. coli DH5α感受态中以获得阳性克隆。提取质粒转化E. coli BL21(DE3)细胞,获得MvaT蛋白表达菌株。
1.2.3 蛋白异源表达与亲和纯化蛋白异源表达与亲和纯化方法参照文献[18]。将MvaT蛋白表达菌株接种到500 ml LB液体培养基中培养至OD600为0.6,以1:1 000比例加入1 mg/mL IPTG诱导培养3−5 h。8 000 r/min离心10 min后收集菌体,超声破碎细胞。4 ℃、12 000 r/min离心30 min后收集上清。利用镍亲和层析纯化获得重组MvaT蛋白,SDS-PAGE电泳检测纯化的重组MvaT蛋白,BCA法测定蛋白质浓度。
1.2.4 凝胶阻滞实验(Electrophoretic mobility shift assay, EMSA)PCR扩增带FAM标记的phzA1G1、phzA2G2、phzH、phzS和phzM基因上游启动子区片段,引物见表 2;纯化PCR产物并测定浓度。20 μl结合反应体系:dsDNA片段(0.3 μmol/L) 1 μL,MvaT蛋白1−5 μL,10×Binding buffer 2 μL,去离子H2O补足20 μL。8%非变性聚丙烯酰胺凝胶50 mL:30%丙烯酰胺13.3 mL,10×TBE 5 mL,H2O 31.4 mL,10% AP 350 μL,TEMED 33 μL。将纯化蛋白与目标DNA片段在室温结合30 min后,通过8%非变性聚丙烯酰胺凝胶进行检测,利用凝胶成像系统拍照。
2 结果与分析 2.1 mvaT基因缺失可显著提高铜绿假单胞菌SJTD-1的吩嗪类化合物产量检测了野生菌株SJTD-1和mvaT基因敲除突变株SJTD-1(ΔmvaT)在4种不同培养基中培养24 h和72 h后的吩嗪化合物产量,结果(图 1)显示,与野生型菌株相比,mvaT基因敲除突变株SJTD-1(ΔmvaT)的吩嗪类化合物产量均有显著提升,不同培养基中的提升倍数不同,可达3−8倍;在PB培养基中该突变株的吩嗪化合物产量可达9 mg/L,而野生菌株SJTD-1在4种培养基中的吩嗪化合物产量均低于3 mg/L。该结果与已有的报道一致,铜绿假单胞菌PAO1的mvaT敲除菌株所产生的绿脓菌素是野生菌株的2倍以上[13]。说明mvaT基因敲除可有效提升菌株SJTD-1中吩嗪化合物的产量。
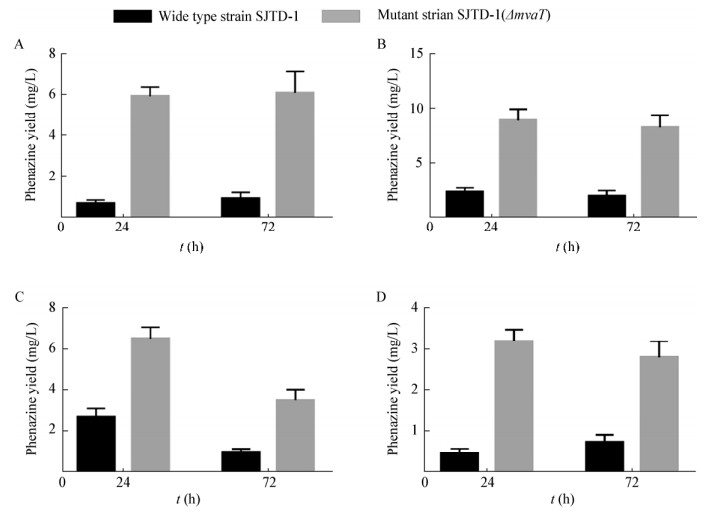
|
| 图 1 两个菌株在PPM (A)、PB (B)、KB (C)、LB (D)培养基中的吩嗪化合物产量 Figure 1 Phanazine compounds yield of two strains cultured in PPM (A), PB (B), KB (C), and LB (D) media |
|
|
为了研究MvaT蛋白是否可以直接调控菌株SJTD-1的吩嗪合成基因,对其编码基因进行了序列比对、异源表达与亲和层析纯化。氨基酸序列比对结果显示该MvaT蛋白与不同来源H-NS家族中的MvaT亚家族蛋白有较高的一致性,序列保守性较高(图 2A),这与之前报道一致[8]。利用含有mvaT基因的表达质粒,在大肠杆菌BL21(DE3)中实现了基因的异源表达与亲和纯化,重组MvaT蛋白分子量大小约为14 kD,产量约为30 mg/L (图 2B、2C)。

|
| 图 2 MvaT蛋白的氨基酸序列比对与重组质粒鉴定及重组蛋白纯化图 Figure 2 The amino acid sequence alignment, the recombinant plasmids analysis and the protein purification of MvaT protein 注:A:不同来源MvaT蛋白的氨基酸序列比对结果. B:pET28a-mvaT质粒酶切鉴定图,M:Marker;1:pET28a-mvaT质粒;2:酶切后的pET28a-mvaT质粒. C:重组MvaT蛋白的SDS-PAGE电泳图谱,U:超声后上清;L:上柱流出液;W:洗杂液;E1和E2:目标蛋白洗脱液. Notes: A: the mutiple sequences alignment of MvaT proteins from different organisms. B: the restriction enzyme analysis of plasmid pET28a-mvaT, M: marker; 1: plasmid pET28a-mvaT; 2: enzyme-treated plasmid pET28a-mvaT. C: SDS-PAGE analysis of the recombinant MvaT protein, U: supernant solution of sonication; L: solution after loading on the column; W: solution after wash buffer; E1 and E2: solution after elution. |
|
|
为确定MvaT蛋白是否直接调控吩嗪合成的基因,将体外纯化的重组MvaT蛋白分别与2个吩嗪-1-羧酸合成基因簇(phzA1G1和phzA2G2)和3个吩嗪-1-羧酸转化基因(phzH、phzS和phzM)的上游启动子区进行结合,用体外凝胶阻滞实验来检测此蛋白与不同DNA片段的结合情况。
2.3.1 MvaT蛋白可与phzA1G1和phzA2G2基因簇的启动子区结合分别以5′端带有FAM标记的phzA1G1和phzA2G2基因簇上游的450 bp片段和200 bp片段作为目标DNA片段,检测其与重组MvaT蛋白的结合情况。凝胶阻滞实验结果显示,MvaT蛋白加入会产生明显的阻滞条带,且该阻滞现象随蛋白浓度增加而加强;空白DNA片段对照和加入牛血清白蛋白(Bovine serum albumin,BSA)的对照均无阻滞条带出现(图 3)。这表明MvaT蛋白与phzA1G1和phzA2G2基因簇的上游区域存在特异性结合。因不同长度DNA片段与MvaT蛋白结合时均只出现一条结合条带,说明该MvaT蛋白与DNA的结合区域是位于基因簇上游的200 bp以内。MvaT蛋白可能直接调控了phzA1G1和phzA2G2基因簇的转录。

|
| 图 3 MvaT蛋白与phzA1G1和phzA2G2基因簇上游片段结合的EMSA图谱 Figure 3 The EMSA results of MvaT protein binding with the upstream regions of phzA1G1 and phzA2G2 clusters 注:A–D的目标DNA片段依次为phzA1G1-450 bp、phzA1G1-200 bp、phzA2G2-450 bp、phzA2G2-200 bp;DNA投入量均为3 pmol;−:空白对照;N:BSA阴性对照;MvaT蛋白/DNA摩尔比依次为:50/1,100/1,200/1. Notes: Target DNA fragments of A–D were phzA1G1-450 bp, phzA1G1-200 bp, phzA2G2-450 bp and phzA2G2-200 bp, respectively; the input of all the DNA fragments were 3 pmol; −: the blank control; N: The negative control added with BSA protein; The molar ratio of MvaT/DNA was 50/1, 100/1, and 200/1, respectively. |
|
|
以5′端带有FAM标记的phzH、phzS和phzM上游200 bp片段和100 bp片段为目标DNA片段,检测其与MvaT蛋白的结合情况。结果显示,MvaT蛋白也可与phzH、phzS和phzM的上游片段发生特异性结合,出现阻滞条带,同样具有浓度依赖性(图 4)。跟MvaT蛋白与phzA1G1和phzA2G2基因簇上游区域的结合不同的是,MvaT蛋白与phzH、phzS和phzM的结合区域位于其上游100 bp以内,MvaT蛋白也可能直接调控了这3个基因的转录。

|
| 图 4 MvaT蛋白与phzH、phzS和phzM基因上游片段结合的EMSA图谱 Figure 4 The EMSA results of MvaT protein with the upstream fragments of phzH, phzS and phzM genes 注:A–F的目标DNA片段依次为phzH-200 bp、phzS-200 bp、phzM-200 bp、phzH-100 bp、phzS-100 bp、phzM-100 bp;DNA投入量均为3 pmol;−:空白对照;N:BSA阴性对照;MvaT蛋白/DNA摩尔比依次为:50/1,100/1,200/1. Notes: Target DNA fragments of A–F were phzH-200 bp, phzS-200 bp, phzM-200 bp, phzH-100 bp, phzS-100 bp and phzM-100 bp, respectively; The input of all the DNA fragments were 3 pmol; −: the blank control, N: the negative control added with BSA; the molar ratio of MvaT/DNA was 50/1, 100/1, and 200/1, respectively. |
|
|
吩嗪类化合物作为假单胞菌尤其是铜绿假单胞菌的一种重要的毒力因子,其生物合成受群体感应系统(quorum sensing,QS)和GacA/GacS双组分调控系统(two-component regulatory systems,TCSs)等多个系统调控;吩嗪类化合物也可作为信号分子对多种基因,包括生物膜合成、细菌运动、细菌致病和生物发光等基因加以调控,并调控自身生物合成,提高菌株的环境适应能力[19-23]。如铜绿假单胞菌的吩嗪-1-羧酸合成基因簇phzA1G1上游具有QS感应元件las-box,可通过与PqsR和RhlR结合正调控PCA的合成代谢;而在phzA2G2上游则存在负调控因子QscR的结合区域,负调控因子QscR可通过抑制基因las和rhl的表达对吩嗪的合成起到负调控的效果[21]。GacA/GacS双组分调控系统可通过活化小RNA RsmY和RsmZ,实现吩嗪合成代谢的抑制系统去阻遏,促进HCN、PYO等吩嗪类化合物的生成[22],而转录调节因子σS则会抑制绿脓菌素的合成[23-25]。研究显示,铜绿假单胞菌PAO1中存在的2个典型H-NS家族转录因子蛋白MvaT和MvaU可调控绿脓菌素合成。单敲除mvaT或mvaU基因,绿脓菌素产量均有2−3倍提高,这与本研究的结果一致,但如果双敲除mvaT和mvaU基因,则未能检测到绿脓菌素的合成[13],说明MvaT蛋白在吩嗪的合成过程中起到重要作用。同时,群体感应信号因子PQS [2-heptyl-3-hydroxy-4(1H)-quinolone]可促进绿脓菌素的合成,其基因缺失则可阻断绿脓菌素合成,它可通过负调控MvaT和RsmA来实现促进PA-IL凝集素合成基因lecA的转录[9, 26-27]。由于本文结果显示MvaT可与吩嗪合成基因的上游启动子区域发生直接结合,因而推测PQS对吩嗪合成过程的作用很可能是通过抑制mvaT基因的转录,减少MvaT与吩嗪合成基因上游启动子的结合进而启动吩嗪合成基因转录来实现的。因此,MvaT蛋白可直接调控吩嗪化合物的合成,同时也可以介导其它因子对吩嗪合成的间接调控作用。另一方面,由于绿脓菌素或吩嗪-1-羧酸等可实现高效的电子传递,介导微生物燃料电池中阳极胞外电子的传递过程[28-29],通过基因改造提升绿脓菌素的产量可提高细胞外电子传递效率和电流的输出功率,增加产电量[16, 30]。mvaT基因敲除突变株SJTD-1(ΔmvaT)的吩嗪化合物产量增加,细胞存活时间延长,因此放电时间更持久,总放电量更多[16]。此外,有研究发现吩嗪类化合物浓度可影响假单胞菌NY3对十六烷烃的降解效率,两者呈正相关,这是由于吩嗪类化合物可与NADH或GSH反应产生超氧负离子自由基和/或羟基自由基,产生的羟基自由基进攻碳原子,形成碳自由基,促进十六烷的降解氧化[31]。因此,深入解析调控吩嗪类化合物合成的分子机制,并基于此来构建特定的工程菌株,有助于推进其理论研究与应用进程。
目前,在假单胞菌属中发现了多种H-NS家族的类MvaT家族蛋白,在铜绿假单胞菌存在MvaT和MvaU 2个蛋白,荧光假单胞菌中有3个,丁香假单胞菌中有4个,恶臭假单胞菌中则存在5个,在棕色固氮菌中也发现了一个MvaT家族蛋白。所有MvaT蛋白的氨基酸序列一致性可达47 %[8]。转录因子MvaT在微生物许多重要生命活动中发挥重要作用。研究发现,MvaT蛋白可调控包括细菌鞭毛组装、生物膜形成及信号分子的合成等重要的生理生化过程。在假单孢菌株Y1000中,过表达mvaT基因可显著降低细菌在半固体培养基上的运动能力,MvaT可直接影响细菌Ⅳ型纤毛的合成与组装[10]。在铜绿假单胞菌中mvaT基因突变会导致信号分子N-乙酰高丝氨酸内酯(N-acyl-homoserine lactones,AHL)和PA-IL凝集素的含量上升[9]。此外,MvaT可通过调控QS系统来影响下游的生物膜合成基因,抑制生物膜的形成[11]。在生防假单胞菌2p24中,MvaT蛋白与其同源蛋白MvaV互相作用,影响生物膜形成与抗生素2, 4-二乙酰基间苯三酚(2, 4-diacetylphloroglucinol,2, 4-DAPG)的生物合成过程[12]。尽管前期研究发现铜绿假单胞菌的mvaT基因缺失菌株的吩嗪类化合物产量增加,且相关合成基因簇的转录水平均有所提升,推测该转录因子参与了吩嗪类化合物的代谢过程,但是并未有证据表明MvaT蛋白是否直接调控了吩嗪化合物的合成或者通过其它因子来实现。本文首次通过体外实验证实MvaT蛋白对吩嗪合成相关基因上游启动子区存在结合作用,说明MvaT蛋白对吩嗪化合物的合成具有直接的转录调控作用。
本文初步研究了石油降解铜绿假单胞菌SJTD-1中MvaT蛋白是否调控了吩嗪类化合物合成代谢过程。体内外实验结果显示,MvaT蛋白与5个吩嗪化合物合成基因(phzA1G1、phzA2G2、phzM、phzS和phzH)的上游启动子区均有特异性结合,说明MvaT蛋白极有可能直接参与了这5个基因的转录调控过程。后续我们将继续对MvaT与DNA结合的具体位点和调控模式进行深入研究。本文结果不仅可推进MvaT调控吩嗪合成的分子机制研究,同时结合吩嗪类化合物可作为电子介质进行电子传递的特性,及其产生菌即石油降解菌SJTD-1的特点,有助于开发通过降解烷烃污染物、高产吩嗪化合物进而搭建微生物燃料电池平台,实现环境污染物的高效处理与有效转化。
| [1] |
Dietrich Lars EP, Price-Whelan A, Petersen A, et al. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa[J]. Molecular Microbiology, 2006, 61(5): 1308-1321. DOI:10.1111/mmi.2006.61.issue-5 |
| [2] |
Mavrodi DV, Blankenfeldt W, Thomashow LS. Phenazine compounds in fluorescent Pseudomonas spp. biosynthesis and regulation[J]. Annual Review of Phytopathology, 2006, 44: 417-445. DOI:10.1146/annurev.phyto.44.013106.145710 |
| [3] |
Haas D, Défago G. Biological control of soil-borne pathogens by Fluorescent pseudomonads[J]. Nature Reviews Microbiology, 2005, 3(4): 307-319. DOI:10.1038/nrmicro1129 |
| [4] |
Pierson LS, Pierson EA. Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes[J]. Applied Microbiology and Biotechnology, 2010, 86(6): 1659-1670. DOI:10.1007/s00253-010-2509-3 |
| [5] |
Mavrodi DV, Bonsall RF, Delaney SM, et al. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1[J]. Journal of Bacteriology, 2001, 183(21): 6454-6465. DOI:10.1128/JB.183.21.6454-6465.2001 |
| [6] |
Hunter RC, Klepac-Ceraj V, Lorenzi MM, et al. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity[J]. American Journal of Respiratory Cell and Molecular Biology, 2012, 47(6): 738-745. DOI:10.1165/rcmb.2012-0088OC |
| [7] |
Britigan EB, Railsback MA, Cox CD. The Pseudomonas aeruginosa secretory product pyocyanin inactivates α1 protease inhibitor: implications for the pathogenesis of cystic fibrosis lung disease[J]. Infection and Immunity, 1999, 67(3): 1207-1212. |
| [8] |
Tendeng C, Soutourina OA, Danchin A, et al. MvaT proteins in Pseudomonas spp.: a novel class of H-NS-like proteins[J]. Microbiology, 2003, 149(Pt11): 3047-3050. |
| [9] |
Diggle SP, Winzer K, Lazdunski A, et al. Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression[J]. Journal of Bacteriology, 2002, 184(10): 2576-2586. DOI:10.1128/JB.184.10.2576-2586.2002 |
| [10] |
Vallet I, Diggle SP, Stacey RE, et al. Biofilm formation in Pseudomonas aeruginosa: fimbrial cup gene clusters are controlled by the transcriptional regulator MvaT[J]. Journal of Bacteriology, 2004, 186(9): 2880-2890. DOI:10.1128/JB.186.9.2880-2890.2004 |
| [11] |
Diggle SP, Stacey RE, Dodd C, et al. The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa[J]. Environmental Microbiology, 2006, 8(6): 1095-1104. DOI:10.1111/emi.2006.8.issue-6 |
| [12] |
Wu XG, Wei YR, Liu JC, et al. MvaT and MvaV transcriptionally regulate PcoI/PcoR quorum-sensing system in Pseudomonas fluorescens 2P24[J]. Acta Microbiologica Sinica, 2012, 52(6): 710-717. (in Chinese) 吴小刚, 魏亚蕊, 刘九成, 等. 生防假单胞菌2P24中mvaT和mvaV基因对PcoI/PcoR群体感应系统的调控作用[J]. 微生物学报, 2012, 52(6): 710-717. |
| [13] |
Li CR, Wally H, Miller SJ, et al. The multifaceted proteins MvaT and MvaU, members of the H-NS family, control arginine metabolism, pyocyanin synthesis, and prophage activation in Pseudomonas aeruginosa PAO1[J]. Journal of Bacteriology, 2009, 191(20): 6211-6218. DOI:10.1128/JB.00888-09 |
| [14] |
Liu H, Liang RB, Tao F, et al. Genome sequence of Pseudomonas aeruginosa strain SJTD-1, a bacterium capable of degrading long-chain alkanes and crude oil[J]. Journal of Bacteriology, 2012, 194(17): 4783-4784. DOI:10.1128/JB.01061-12 |
| [15] |
Liu H, Sun WB, Liang RB, et al. iTRAQ-based quantitative proteomic analysis of Pseudomonas aeruginosa SJTD-1: A global response to n-octadecane induced stress[J]. Journal of Proteomics, 2015, 123: 14-28. DOI:10.1016/j.jprot.2015.03.034 |
| [16] |
You T, Liu JH, Liang RB, et al. Survival elongation of Pseudomonas aeruginosa improves power output of microbial fuel cells[J]. Chinese Journal of Biotechnology, 2017, 33(4): 601-608. (in Chinese) 游婷, 刘季华, 梁如冰, 等. 铜绿假单胞菌存活时间延长可提高生物燃料电池的产电量[J]. 生物工程学报, 2017, 33(4): 601-608. |
| [17] |
Liang HH, Li LL, Dong ZL, et al. The YebC family protein PA0964 negatively regulates the Pseudomonas aeruginosa quinolone signal system and pyocyanin production[J]. Journal of Bacteriology, 2008, 190(18): 6217-6227. DOI:10.1128/JB.00428-08 |
| [18] |
Lu Z, Liang R, Liu X, et al. RNase HIII from Chlamydophila pneumoniae can efficiently cleave double-stranded DNA carrying a chimeric ribonucleotide in the presence of manganese[J]. Molecular Microbiology, 2012, 83(5): 1080-1093. DOI:10.1111/j.1365-2958.2012.07990.x |
| [19] |
Withers H, Swift S, Williams P. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria[J]. Current Opinion in Microbiology, 2001, 4(2): 186-193. DOI:10.1016/S1369-5274(00)00187-9 |
| [20] |
Passador L, Cook JM, Gambello MJ, et al. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication[J]. Science, 1993, 260(5111): 1127-1130. DOI:10.1126/science.8493556 |
| [21] |
Bertani I, Ševo M, Kojic M, et al. Role of GacA, LasI, RhlI, Ppk, PsrA, Vfr and ClpXP in the regulation of the stationary-phase sigma factor rpoS/RpoS in Pseudomonas[J]. Archives of Microbiology, 2003, 180(4): 264-271. DOI:10.1007/s00203-003-0586-8 |
| [22] |
Baehler E, Maurhofer M, Keel C. Henolic signal metabolites from bacteria and plants affect production of antifungal compounds in the biocontrol agent Pseudomonas fluorescens CHA0 in Abstr[A]//The Proceedings of the 9th International Sympoium Microbiology Ecology[C]. Amsterdam, 2001: 266
|
| [23] |
Sarniguet A, Kraus J, Henkels MD, et al. The sigma factor σS affects antibiotic production and biological control activity of Pseudomonas fluorescens Pf-5[J]. Proceedings of the National Academy of Sciences of the United States of America, 1995, 92(26): 12255-12259. DOI:10.1073/pnas.92.26.12255 |
| [24] |
Zhou JF, Ge YH, Liu T, et al. Effect of rpoS mutation on two gene cluster of phenazine in Pseudomonas aeruginosa PAO1[J]. Acta Microbiologica Sinica, 2010, 50(3): 411-417. (in Chinese) 周金凤, 葛宜和, 刘婷, 等. rpoS基因插入突变对铜绿假单胞菌两个吩嗪合成基因簇的调控[J]. 微生物学报, 2010, 50(3): 411-417. |
| [25] |
Miao J, Chi XY, Wang YH, et al. Regulation of pyocyanin biosynthesis by transcriptional factor sigma38 in Pseudomonas aeruginosa PAO1[J]. Acta Microbiologica Sinica, 2017, 57(2): 229-239. (in Chinese) 缪静, 迟晓艳, 王艳华, 等. 转录调节因子σ38介导铜绿假单胞菌绿脓菌素合成代谢调控[J]. 微生物学报, 2017, 57(2): 229-239. |
| [26] |
Diggle SP, Winzer K, Chhabra SR, et al. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR[J]. Molecular Microbiology, 2003, 50(1): 29-43. DOI:10.1046/j.1365-2958.2003.03672.x |
| [27] |
McKnight SL, Iglewski BH, Pesci EC. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa[J]. Journal of Bacteriology, 2000, 182(10): 2702-2708. DOI:10.1128/JB.182.10.2702-2708.2000 |
| [28] |
Rabaey K, Boon N, Siciliano SD, et al. Biofuel cells select for microbial consortia that self-mediate electron transfer[J]. Applied and Environmental Microbiology, 2004, 70(9): 5373-5382. DOI:10.1128/AEM.70.9.5373-5382.2004 |
| [29] |
Pham TH, Boon N, Aelterman P, et al. Metabolites produced by Pseudomonas sp. enable a Gram-positive bacterium to achieve extracellular electron transfer[J]. Applied Microbiology and Biotechnology, 2008, 77(5): 1119-1129. DOI:10.1007/s00253-007-1248-6 |
| [30] |
Yong XY, Shi DY, Chen YL, et al. Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells[J]. Bioresource Technology, 2014, 152: 220-224. DOI:10.1016/j.biortech.2013.10.086 |
| [31] |
Nie HY. Studies on characteristics of the secretion of extracellular small active compounds by P. aeruginosa NY3 and their promotion mechanisms to the efficiency of biodegradation of hydrocarbon[D]. Xi'an: Doctoral Dissertation of Xi'an University of Architecture and Technology, 2017 (in Chinese) 聂红云.铜绿假单胞菌NY3胞外小分子活性物及其促进烃类降解的作用机制研究[D].西安: 西安建筑科技大学博士学位论文, 2017 http://cdmd.cnki.com.cn/Article/CDMD-10703-1017737334.htm |
 2019, Vol. 46
2019, Vol. 46




