扩展功能
文章信息
- GONG Xiao-Wei, CHEN Qi-Wei, Ferguson-Noel Naola, ZHENG Fu-Ying, Stipkovits Laszlo, Szathmary Susan, LIU Yong-Sheng
- 宫晓炜, 陈启伟, Ferguson-Noel Naola, 郑福英, Stipkovits Laszlo, Szathmary Susan, 刘永生
- Comparison the pathogenicity of three Mycoplasma synoviae isolates from broiler flocks
- 3株禽滑液囊支原体分离株致病性的比较和评价
- Microbiology China, 2019, 46(4): 790-797
- 微生物学通报, 2019, 46(4): 790-797
- DOI: 10.13344/j.microbiol.china.180339
-
文章历史
- Received: April 28, 2018
- Accepted: August 02, 2018
- Published online: September 30, 2018
2. Department of Population Health, Poultry Diagnostic and Research Center, College of Veterinary Medicine, University of Georgia, Athens 30602, USA;
3. RT-Europe Non-profit Research Center, Mosonmagyarovar 9200, Hungary
2. 美国佐治亚大学 人口健康部禽病诊断与研究中心 美国 雅典城 30602 ;
3. 生物防护和传染病防控欧洲三角中心 匈牙利 莫逊马雅罗瓦 9200
Mycoplasma synoviae (MS) is an important pathogen that causes economic losses to poultry industry[1]. Infection can also be associate with upper respiratory disease, airsacculitis, synovitis, tenosynovitis and bursitis, which lead to lameness, pale comb and retarded growth of chickens and turkeys. Severe swelling of joints and feet may be observed in infected poultry and usually noticed as disease progresses[2]. Besides, eggshell apex abnormality described as a novel presentation of MS infection. Disease severity has been influenced by other respiratory pathogens, such as newcastle disease virus (NDV), infectious bronchitis virus (IBV) and avian influenza virus (AIV). A number of studies have demonstrated a synergistic role of viruses and E. coli in avian mycoplasma disease in increasing the pathogenicity of mycoplasma and causing serious clinical manifestations and mortality rates in poultry worldwide[3-5].
Infectious synovitis was first described and associated with mycoplasma infection in the early 1950's[6], before the organism, MS, was identified[7]. Subsequently, since the expansion of the poultry industry, MS appeared to have worldwide distribution and its importance has been highlighted in several studies and there is an increased consciousness to generate MS-free poultry[8-9]. In China, the prevalence of MS infection was investigated from 2013 to 2014, and 110 MS strains which belong to K group through vlhA gene sequencing were isolated from 16 Chinese provinces. While MS constitutes a single serotype, there are significant differences in the virulence and pathogenicity of strains. Some MS strains appeared subclinical or inapparent infection of the upper respiratory tract and exhibited lower morbidity rate, and some MS strains can cause synovitis and result in a decrease of egg production rate, hatchability rates and high morbidity rates[10-11].
The evaluation of pathogenicity of MS strains is based on clinical signs, the pathological changes of tracheal mucosal thickness, analysis of foot-pad lesion, specific serology, isolation and molecular diagnosis[12]. As data are lacking in China, the aim of this study was to compare and characterize the pathogenicity of three MS isolates (CHN-WF224-2016, CHN-BZJ2-2015, CHN-JNB19-2016) from Shandong province and the attenuated live vaccine strain (MS-H).
2 Materials and Methods 2.1 MS isolatesThree field strains (CHN-WF224-2016, CHN-BZJ2-2015, CHN-JNB19-2016) were isolated from MS-infected Chinese native broiler chickens from different farms located in 3 cities (one isolate per city) of Shandong province between 2015 to 2016 according to the procedures as described previously[13-14]. Three isolates were subject to 16S rRNA gene (from PDRC, UGA, unpublished) and portion of vlhA gene PCR[1]. The nucleotide sequences of 16S rRNA and vlhA gene were determined and have been deposited in GenBank under following accession numbers: KY750311-KY750313 and MF073022-MF073024, and BLAST analysis of these sequences showed that three field isolates belong to MS. MS-H live vaccine strain was deposited in our laboratory at Lanzhou veterinary research institute. Approximately 1 mL of growing culture was sprayed per chicken, 100 μL of broth culture was inoculated into the left foot pad, and NDV/IBV live nasal vaccine which was purchased from QiLu DOBIO Co., Ltd. was inoculated 30 μL. The MS titer of each inoculum (color changing units (CCU)/mL) was determined as previously described[15].
2.2 Experimental birds and designsAll animal procedures in this experiment were approved by the Institutional Animal Care and Use Committee of the Lanzhou veterinary research institute, CAAS. Ninety-six 1-day-old broiler chicks were purchased from Lanzhou Zhengda Chicken Co., Ltd. and randomly divided into six groups with each group of 16 at 11 days age. All chickens in this study were provide with feed and water ad libitum and were euthanized by carbon dioxide before necropsy. At 21 days of age, 15 chickens were randomly selected and tested by ELISA and culture of the trachea swabs to confirm that chicks were Mycoplasma negative, and same time, four groups of 16 chicks each were inoculated (aerosol and foot pad) with broth culture of CHN-WF224-2016 (2.2×108 CCU/mL), CHN-BZJ2- 2015 (2.65×108 CCU/mL), CHN-JNB19-2016 (3.36× 108 CCU/mL) and MS-H (7.2×108 CCU/mL). The live attenuated temperature-sensitive MS-H vaccine strain used in this experiment was cultured at 33 ℃, other isolates at 37 ℃. These MS-inoculated groups and a control group of 16 chickens were also infected with Newcastle disease (La Sota strain) and infectious bronchitis virus (H120 strain) by nasal drop. A sixth group of 16 chickens served as negative control. The chickens were observed by air sac and foot pad lesion scoring, bled for serology, tracheal swabs and laryngeal wash were obtained for culture and real-time quantitative PCR (qPCR), and tracheal sections for histopathology at 10 days and 21 days post infection (DPI).
2.3 Isolation and identification of mycoplasmaCotton swabs from tracheal and air sacs of chickens were inoculated onto modified Frey's agar and broth at 37 ℃. Colonies were confirmed as MS using portion of vlhA gene PCR and sequencing[1].
2.4 SerologyThe commercial mycoplasma synoviae or gallisepticum antibody test kit (IDEXX, Westbrook, Maine) was used to detect antibody titers following the manufacture's instruction.
2.5 qPCRAt 10 days and 21 days post challenge, the larynxes of individual birds were collected in 5 mL sterile PBS. qPCR was carried out using primers and a procedure described by Raviv[16]. Genomic DNA was extracted from 200 μL of the laryngeal wash using the QIAamp cador Pathogen Mini Kit. Real-time PCR was performed using a Agilent Technologies Stratagene Mx3000P and a cycle threshold (Ct) value≤36 was considered positive, and the standard plasmid was constructed containing MS genome target (16S-23S rDNA ISR).
2.6 Evaluation of lesionsThe lesions in chickens necropsied during the study were evaluated grossly by foot-pad lesions scoring on a scale from 0 to 4[17]. The tracheal lesions were evaluated microscopically by measuring the width of the tracheal mucosa. A section was collected from the upper third of trachea and fixed in 4% paraformaldehyde. The tracheal mucosa thickness was measured at four equidistant points on histological slides of cross sections of tracheas[18].
2.7 Statistical analysisThe air sac lesion scores, foot-pad lesion scores and MS isolation were analyzed using the Kruskal-Wallis Rank Sums test. The mean tracheal thickness and mean copy numbers (MCNs) Log10 were analyzed using the Tukey-kramer honest significant difference test. These analyses were performed using SPSS software (version 19.0) and R software, respectively.
3 Results and Analysis 3.1 SerologyThe serological results for this trial are presented in Table 1. All chickens tested pre-inoculation and the groups that were uninfected with MS strain were negative for mycoplasma gallisepticum (MG) and MS antibodies. The group inoculated with MS-H and CHN-JNB19-2016 did not seroconvert strongly, with the exception of the chickens that were infected with CHN- WF224-2016 and CHN-BZJ2-2015 at 10 or 21DPI.
| MS strain | NDV/IBV | DPI | ELISA2 & 3 |
| None | No | 10 | 0/8 (0.28)a |
| None | Yes | 10 | 0/8 (0.29)a |
| MS-H | Yes | 10 | 1/8 (0.39)ab |
| CHN-WF224-2016 | Yes | 10 | 8/8 (0.92)bc |
| CHN-BZJ2-2015 | Yes | 10 | 8/8 (1.05)c |
| CHN-JNB19-2016 | Yes | 10 | 3/8 (0.43)ab |
| None | No | 21 | 0/8 (0.26)a |
| None | Yes | 21 | 0/8 (0.31)a |
| MS-H | Yes | 21 | 5/8(0.55)ab |
| CHN-WF224-2016 | Yes | 21 | 8/8 (1.75)bc |
| CHN-BZJ2-2015 | Yes | 21 | 8/8 (2.1)c |
| CHN-JNB19-2016 | Yes | 21 | 7/8(0.94)ab |
| Note: 1: Values within a column with a different lower case superscript are significantly different (P < 0.05); 2: No. of positive samples/No. of tested samples (ELISA: S/P > 0.5); 3: S/P ratio. 注:1:最后一列数字的上标, 小写字母不同代表有显著性差异(P < 0.05);2:阳性样本数量/检测样本总数(ELISA检测结果, S/P > 0.5为阳性); 3:S/P的平均值. | |||
As presented in Table 2, foot pad lesions were observed in all of the infected groups at 10 or 21 DPI. The most severe lesions were observed in the group inoculated with CHN-BZJ2-2015. Mean footpad lesion scores were significantly greater compare with CHN-JNB19-2016 and MS-H group (P < 0.05). There were no footpad lesions in the control groups (Figure 1).
| MS strain | NDV/IBV | DPI | Air sac lesion score2 & 3 | Foot pad lesion score2 & 3 | Tracheal nucosal thickness4 | MS isolation2 | qPCR2 & 5 |
| None | No | 10 | 0/8 (0±0)a | 0/8 (0±0)a | 116±38a | 0/8a | 0/8 (0.0)a |
| None | Yes | 10 | 0/8(0±0)a | 0/8 (0±0)a | 128±52a | 0/8a | 0/8 (0.0)a |
| MS-H | Yes | 10 | 2/8 (0.3±0.5)ab | 2/8 (0.2±0.4)a | 159±35ab | 0/8a | 6/8 (4.7±1.9)b |
| CHN-WF224-2016 | Yes | 10 | 6/8 (1.1±0.8)b | 7/8 (1.1±0.6)bc | 183±71ab | 6/8b | 8/8 (6.7±0.5)c |
| CHN-BZJ2-2015 | Yes | 10 | 7/8 (1.4±0.9)b | 8/8 (1.6±0.7)c | 242±58b | 8/8b | 8/8 (6.9±0.4)c |
| CHN-JNB19-2016 | Yes | 10 | 4/8 (0.6±0.7)ab | 3/8 (0.4±0.5)ab | 164±92ab | 3/8ab | 8/8 (7.1±0.6)c |
| None | No | 21 | 0/8 (0±0)a | 0/8(0±0)a | 111±43a | 0/8a | 0/8 (0.0)a |
| None | Yes | 21 | 0/8(0±0)a | 0/8 (0±0)a | 146±60a | 0/8a | 0/8 (0.0)a |
| MS-H | Yes | 21 | 0/8 (0±0)a | 4/8 (0.5±0.5)ab | 163±53ab | 2/8a | 6/8 (4.6±1.9)b |
| CHN-WF224-2016 | Yes | 21 | 5/8 (1.0±0.9)ab | 8/8 (1.8±0.7)bc | 200±61ab | 8/8b | 8/8 (6.0±0.7)b |
| CHN-BZJ2-2015 | Yes | 21 | 7/8 (1.5±0.7)b | 8/8 (2.6±0.7)c | 304±76c | 8/8b | 8/8 (6.1±0.6)b |
| CHN-JNB19-2016 | Yes | 21 | 4/8 (0.6±0.7)ab | 5/8 (0.6±0.5)ab | 148±62a | 5/8ab | 6/8 (4.7±2.9)b |
| Note: 1: Values within a column with a different lower case superscript are significantly different (P < 0.05); 2: No. of positive samples/No. of tested samples; 3: Mean score±SD; 4: Mean thickness for the group in micrometers±SD; 5: Mean DNA copy number (MCN) Log10±SD. 注:1:每列数字的上标, 小写字母不同代表有显著性差异(P < 0.05);2:阳性样本数量/检测样本总数; 3:均数±标准差; 4:每组的平均气管粘膜厚度±标准差; 5:DNA平均拷贝数(log10)±标准差. | |||||||

|
| Figure 1 Foot pad lesions in MS strains challenged and non-challenged chicks 图 1 MS攻毒组和未攻毒组的足垫损伤 Note: A: Lesion score 0, No foot-pad lesion was observed; B: Lesion score 1, Foot-pad slightly inflamed, with a slightly increased amount of cloudy joint fluid; C: Lesion score 3, Foot-pad markedly inflamed, with thickened and hyperemic footpad tissue. 注:A:0分,正常足垫;B:1分,足垫有轻微的肿胀,伴有少量的炎性渗出;C:3分,足垫明显肿胀,有明显的炎性渗出和充血. |
|
|
The mean air sac lesion scores are summarized in Table 2. Although the air sac lesion were more severe in the CHN-WF224-2016 and CHN-BZJ2-2015 group, the mean lesion scores of infected groups were not significant difference after pairwise comparison (P > 0.05), but were significantly higher than control groups (P < 0.05).
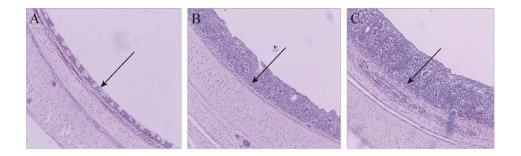
3.4 Tracheal lesionsAs shown in Table 2, the Mean tracheal mucosal thickness measurements of the infected groups were not significantly different from each other at 10 DPI (P > 0.05). The CHN-BZJ2-2015 group had a significantly higher measurement than other infected groups and control groups (P < 0.05) at 21 DPI (Figure 2).
|
| Figure 2 The tracheal mucosa thickness observation in MS strains challenged and non-challenged chicks 图 2 MS攻毒组和未攻毒组的气管粘膜厚度观察 Note: A: Normal tracheal mucosa; B: Tracheal mucosa of low pathogenicity MS strains challenged chicks, showing normal ciliated columnar epithelial lining and goblet cell with a single focus of leucocyte infiltration; C: Tracheal mucosa of high pathogenicity MS strain challenged chicks, showing squamous to cuboidal epithelium and marked thickening of mucosa associated with severe diffuse leucocyte infiltration. 注:A:正常粘膜;B:低致病力MS攻毒组,支气管粘膜可见较正常的假复层纤毛柱状上皮细胞和杯状细胞,有少量白细胞浸入;C:高致病力MS攻毒组,可见气管黏膜表面假复层纤毛柱状上皮结构丧失,出现鳞状上皮化生,有大量炎性细胞浸入,气管粘膜明显增厚. |
|
|
MS was isolated from the joint fluid of 75% (6/8), 100% (8/8) and 37.5% (3/8) of the chickens in the CHN-WF224-2016, CHN-BZJ2-2015 and CHN- JNB19-2016 groups at 10 DPI, respectively. MS isolated from MS-H group were significantly fewer compared to CHN-WF224-2016 or CHN-BZJ2-2015 groups (P < 0.05) at 21 DPI.
3.6 qPCRMS was detected in the all chicken laryngeal wash of CHN-WF224-2016 and CHN-BZJ2-2015 groups by Real-time PCR at 10 and 21 DPI, while the 87.5% (7/8) of MS-H group was positive at 10 and 21 DPI. CHN-JNB19-2015 group was tested 100% (8/8) and 75% (6/8) positive at 10 and 21 DPI, respectively. Mean DNA copy numbers in the CHN-WF224-2016, CHN-BZJ2-2015 and CHN- JNB19-2015 were significantly higher than the MS-H group at 10 DPI (P < 0.05).
4 Disscussion and ConclusionOver the last decade, China has become the second largest poultry production country in the world. Almost 22% of meat in China is from chickens, behind pork. Based on literature data [11, 19], the number of MS infection in veterinary clinic has risen since 2008 in China, with an increasing trend on the year by year. MS can result in a variety of different signs such as depression, decreased feed intake, breathing difficulty, nasal discharge, diarrhea, weight loss and lameness. Between 2013 and 2014, MS infection was circulating in Chinese native-type chickens, affecting their productivity and causing the loss of millions of chickens in Chinese poultry farms. Unlike USA, UK and Neherlands[20], China Lacks eradication progammes to establish MS-free chicken flock, so antimicrobial drugs, such as tiamulin, tylosin, enrofloxacin and florfenicol were used for treatment of MS infection in chickens. The live, attenuated, temperature-sensitive MS vaccine strain MS-H is used to control virulent MS infection in commercial chicken flocks, but rarely used in China. Research shows that although MS-H vaccination may help to reduce or prevent clinical signs, it does not prevent infection with field MS strain[21-22].
Presently, MS outbreaks majorly in poultry industry and causes enormous economic loss in China. However, no efficient vaccine is successfully developed and put into use in China. In order to screen candidate for further studying the live attenuated or inactivated vaccine, the virulence of the epidemic MS strains were evaluated in this study. The systematic analysis of pathogenicity of Chinese MS isolates can provide experimental data and materials to develop more effective MS vaccine.
Obtained results showed that three isolates (CHN-WF224-2016, CHN-BZJ2-2015, CHN-JNB19- 2016) originally obtained from Chinese native chickens are significant differences in virulence. Although these isolates belonged to same genotype, K group, there was no correlation between pathogenicity and genotype for MS, which is consistent with the findings of previous study[11].
In this study, chickens were infected systemically by a combination of the left footpad and aerosol routes to evaluate respiratory and synovitis virulence. Besides, MS strains challenge with live NDV/IBV vaccination was employed in this trial, and a NDV/IBV control group was incorporated. This challenge method has been demonstrated to increase the incidence and severity of mycoplasma lesions[23]. All groups challenged with MS in this study showed mild respiratory signs, but joint and footpad swelling were more severe in CHN-WF224-2016 and CHN-BZJ2-2015 group. The chickens from CHN-BZJ2-2015 group showed lameness and had significantly lower weight gains than other group at 21DPI, suggesting relatively high virulence.
Serological titers (ELISA), footpad lesion scores, tracheal mucosal thickness measurements, MS isolation and qPCR copy numbers was highest in the CHN-BZJ2-2015 group, compare with MS-H and control groups. While air sac lesion scores were numerically greatest in the CHN-BZJ2-2015 group, this difference lacked statistical significance, which may be related with the number of birds and wide variation in scores in each of the respiratory challenge groups.
Footpad inoculation is an accepted method to determing the ability of MS to cause synovitis. Lesions of synovitis were reproduced in groups challenged with the MS isolates, but footpad lesion scores were greatest in the CHN-BZJ2-2015 group, which was consistent with the clinical symptom when we sampled in the field.
MS was readily recovered from the tracheas from MS-infected groups. In this study, MS isolated earlier from the trachea of chickens in CHN-BZJ2-2015 and CHN-WF224-2016 groups at 10 or 21 DPI, compared with the MS-H or CHN-JNB19-2015 group, which was the same as the results of serological test, chickens were serologically positive earlier and s/p ratio were higher in the CHN-BZJ2-2015 and CHN-WF224-2016 group. The "Hot" field strains of MS elicited stronger serological reactions than less virulent MS strains[24], so these trends likely reflect that CHN-BZJ2-2015 and CHN-WF224-2016 had more invasiveness and fecundity, compared with CHN-JNB19-2015 or MS-H.
The qPCR conducted on laryngeal wash from each group was positive in CHN-BZJ2-2015 and CHN-WF224-2016 groups at 10 and 21 DPI except for MS-H or CHN-JNB19-2015 group at 10 or 21DPI, even though MS isolation, which may suggesting that CHN-JNB19-2015 strain similar to MS-H strain had low virulence and did not colonize the upper respiratory system completely. The previous study has shown that the difference in ability of a temperature-sensitive mycoplasma vaccine to to colonize the respiratory system was related to the different administered routes which may result from variation in the number of organisms deposited in the respiratory system[25]. MS-H vaccine can colonize the respiratory system of chickens after eyedrop innoculation[26].
In conclusion, the recent MS isolates had strong virulence, and especially CHN-BZJ2-2015 isolate was clearly capable of inducing synovitis and weight loss. Although these isolates had a limited ability to induce lesion and changes in air sac, this result may not be representative of field condition. Further work will be required to fully elucidate the transmission of these isolate which may spread into susceptible chickens through horizontal transmission and influence egg production through vertical transmission. In the meantime, it is an important that MS control and eradication programme should be enforced in China based on early detection of infected flocks by regular monitoring and a series of control measures.
| [1] |
Hong Y, García M, Leiting V, et al. Specific detection and typing of Mycoplasma synoviae strains in poultry with PCR and DNA sequence analysis targeting the hemagglutinin encoding gene vlhA[J]. Avian Diseases, 2004, 48(3): 606-616. DOI:10.1637/7156-011504R |
| [2] |
May M, Kleven SH, Brown DR. Sialidase activity in Mycoplasma synoviae[J]. Avian Diseases, 2007, 51(4): 829-833. DOI:10.1637/7806-120106-REGR.1 |
| [3] |
Sid H, Hartmann S, Petersen H, et al. Mycoplasma gallisepticum modifies the pathogenesis of influenza A virus in the avian tracheal epithelium[J]. International Journal of Medical Microbiology, 2016, 306(3): 174-186. DOI:10.1016/j.ijmm.2016.04.001 |
| [4] |
Stipkovits L, Glavits R, Palfi V, et al. Pathologic lesions caused by coinfection of Mycoplasma gallisepticum and H3N8 low pathogenic avian influenza virus in chickens[J]. Veterinary Pathology, 2012, 49(2): 273-283. DOI:10.1177/0300985811415702 |
| [5] |
Raviv Z, Ferguson-Noel N, Laibinis V, et al. Role of Mycoplasma synoviae in commercial layer Escherichia coli peritonitis syndrome[J]. Avian Diseases, 2007, 51(3): 685-690. DOI:10.1637/0005-2086(2007)51[685:ROMSIC]2.0.CO;2 |
| [6] |
Anderson GC, Bletner JK, Munro DA, et al. Studies of infectious synovitis in chickens[J]. American Journal of Veterinary Research, 1956, 17(65): 747-754. |
| [7] |
Jordan FTW. Avian mycoplasma and Pathogenicity-a review[J]. Avian Pathology, 1975, 4(3): 165-174. |
| [8] |
Feberwee A, de Vries TS, Landman WJM. Seroprevalence of Mycoplasma synoviae in Dutch commercial poultry farms[J]. Avian Pathology, 2008, 37(6): 629-633. DOI:10.1080/03079450802484987 |
| [9] |
Landman WJM. Is Mycoplasma synoviae outrunning Mycoplasma gallisepticum? A viewpoint from the Netherlands[J]. Avian Pathology, 2014, 43(1): 2-8. DOI:10.1080/03079457.2014.881049 |
| [10] |
Kreizinger Z, Grózner D, Sulyok KM, et al. Antibiotic susceptibility profiles of Mycoplasma synoviae strains originating from Central and Eastern Europe[J]. BMC Veterinary Research, 2017, 13(1): 342. DOI:10.1186/s12917-017-1266-2 |
| [11] |
Sun SK, Lin X, Liu JM, et al. Phylogenetic and pathogenic analysis of Mycoplasma synoviae isolated from native chicken breeds in China[J]. Poultry Science, 2017, 96(7): 2057-2063. |
| [12] |
El-Gazzar M, Ghanem M, McDonald K, et al. Development of Multilocus Sequence Typing (MLST) for Mycoplasma synoviae[J]. Avian Diseases, 2017, 61(1): 25-32. DOI:10.1637/11417-040516-Reg |
| [13] |
Dušanić D, Bečič RL, Cizelj I, et al. Mycoplasma synoviae invades non-phagocytic chicken cells in vitro[J]. Veterinary Microbiology, 2009, 138(1/2): 114-119. |
| [14] |
Ghaniei A. Molecular characterization of Mycoplasma synoviae isolated from broiler chickens of West Azarbaijan province by PCR of vlhA gene[J]. Veterinary Research Forum, 2016, 7(3): 197-202. |
| [15] |
Razin S, Tully JG. Methods in Mycoplasmology[M]. New York: Academic Press, 1983: 185-196.
|
| [16] |
Raviv Z, Kleven SH. The development of diagnostic real-time taqman PCRs for the four pathogenic avian mycoplasmas[J]. Avian Diseases, 2009, 53(1): 103-107. DOI:10.1637/8469-091508-Reg.1 |
| [17] |
Kleven SH, Fletcher OJ, Davis RB. Influence of strain of Mycoplasma synoviae and route of infection on development of synovitis or airsacculitis in broilers[J]. Avian Diseases, 1975, 19(1): 126-135. DOI:10.2307/1588963 |
| [18] |
Whithear KG. Control of avian mycoplasmoses by vaccination[J]. Revue Scientifique et Technique, 1996, 15(4): 1527-1553. DOI:10.20506/rst.issue.15.4.2493 |
| [19] |
Xue J, Xu MY, Ma ZJ, et al. Serological investigation of Mycoplasma synoviae infection in China from 2010 to 2015[J]. Poultry Science, 2017, 96(9): 3109-3112. DOI:10.3382/ps/pex134 |
| [20] |
Dijkman R, Feberwee A, Landman WJM. Development, validation and field evaluation of a quantitative real-time PCR able to differentiate between field Mycoplasma synoviae and the MS-H-live vaccine strain[J]. Avian Pathology, 2017, 46(4): 403-415. DOI:10.1080/03079457.2017.1296105 |
| [21] |
Feberwee A, Morrow CJ, Ghorashi SA, et al. Effect of a live Mycoplasma synoviae vaccine on the production of eggshell apex abnormalities induced by a M. synoviae infection preceded by an infection with infectious bronchitis virus D1466[J]. Avian Pathology, 2009, 38(5): 333-340
|
| [22] |
Feberwee A, Dijkman R, Klinkenberg D, et al. Quantification of the horizontal transmission of Mycoplasma synoviae in non-vaccinated and MS-H-vaccinated layers[J]. Avian Pathology, 2017, 46(4): 346-358. DOI:10.1080/03079457.2017.1282602 |
| [23] |
Kleven SH, King DD, Anderson DP. Airsacculitis in broilers from Mycoplasma synoviae: effect on air-sac lesions of vaccinating with infectious bronchitis and Newcastle virus[J]. Avian Diseases, 1972, 16(4): 915-924. DOI:10.2307/1588772 |
| [24] |
Ewing ML, Cookson KC, Phillips RA, et al. Experimental infection and transmissibility of Mycoplasma synoviae with delayed serologic response in chickens[J]. Avian Diseases, 1998, 42(2): 230-238. DOI:10.2307/1592472 |
| [25] |
Noormohammadi AH, Jones JF, Harrigan KE, et al. Evaluation of the non-temperature-sensitive field clonal isolates of the Mycoplasma synoviae vaccine strain MS-H[J]. Avian Diseases, 2003, 47(2): 355-360. DOI:10.1637/0005-2086(2003)047[0355:EOTNFC]2.0.CO;2 |
| [26] |
Morrow CJ, Markham JF, Whithear KG. Production of temperature-sensitive clones of Mycoplasma synoviae for evaluation as live vaccines[J]. Avian Diseases, 1998, 42(4): 667-670. DOI:10.2307/1592700 |
 2019, Vol. 46
2019, Vol. 46