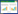扩展功能
文章信息
- 苏琴, 谢玲, 陈艳露, 廖仕同, 张艳, 农倩
- SU Qin, XIE Ling, CHEN Yan-Lu, LIAO Shi-Tong, ZHAGN Yan, NONG Qian
- 香蕉枯萎病生防菌绿头枝孢菌LS1的筛选鉴定
- Screen and identification of biocontrol strain Cladosporium chlorocephalum LS1 against banana Fusarium wilt
- 微生物学通报, 2019, 46(12): 3248-3256
- Microbiology China, 2019, 46(12): 3248-3256
- DOI: 10.13344/j.microbiol.china.190033
-
文章历史
- 收稿日期: 2019-01-10
- 接受日期: 2019-04-28
- 网络首发日期: 2019-05-15
2. 广西大学农学院 广西 南宁 530004
2. College of Agriculture, Guangxi University, Nanning, Guangxi 530004, China
香蕉枯萎病是由尖孢镰刀菌古巴专化型(Fusarium oxysporum f. sp. cubense)侵染引起的破坏维管束、导致植株死亡的一种真菌土传毁灭性病害,防控难度极大,被称为“香蕉的癌症”[1-2]。广西是我国重要的香蕉产地,2016年产量达到319.9万t,占全国总产量的24.6%[3],但近年由于规模化连作种植,导致香蕉枯萎病(Fusarium oxysporum f. sp. cubense)的多发危害,已严重制约广西香蕉产业的发展[4]。目前,香蕉枯萎病主要防控措施有选育抗病品种[5]、化学药剂[1-2]、作物轮作[6-7]、生物防治[8-9]、分子遗传改良[10-11]等。基于抗病品种产量普遍不佳,化学药剂防治效果不理想的制约,安全、高效、环保的生物防治技术逐渐成为香蕉枯萎病防控的研究热点。深色有隔内生真菌(Dark septate endophyte,DSE)是一类菌丝深色有分隔,可形成“微菌核”的土壤子囊菌或半知菌,分布广泛,主要定殖在植物根组织细胞内或细胞间隙,宿主具有非专一性且对宿主无致病性[12-13]。研究表明DSE能与宿主互惠共生,在促进植物生长和提高抗逆、抗病性等方面都发挥着重要作用[14-16]。张晓蓉等[17]通过接种DSE真菌Phialophora mustea K36与Z48显著缓解了尖孢镰刀菌对番茄生长的抑制作用;农倩等[18]筛选获得对香蕉枯萎病有较强防治能力的DSE菌株Schizothecium sp. L-14,并揭示该菌主要通过增强香蕉的抗氧化防护系统而提高其对香蕉枯萎病的抗性。Surono等[19]发现接种DSE真菌Phialocepahala fortinii CKG.I.11能促进芦笋的生长并对枯萎病有显著抑制作用。目前利用DSE防治香蕉枯萎病的研究报道较少,可利用的菌株数量不多。本研究拟对分离到的DSE菌株的促生及生防作用进行评价,对优势菌株进行分类鉴定,为利用DSE防治香蕉枯萎病提供候选菌种资源。
1 材料与方法 1.1 材料 1.1.1 菌株来源供试DSE菌株LS1、LC8、B2、MS38、TD1由本实验室分离保存;桂蕉6号香蕉组培苗由广西植物组培苗有限公司提供;香蕉枯萎病病原菌为镰刀菌4号生理小种的强致病力菌株(FOC4)[20],由广西农业科学院植物保护研究所付岗博士惠赠。
1.1.2 主要试剂和仪器所用试剂均为国产分析纯。PCR仪,苏州东胜兴业科学仪器有限公司;智能人工气候箱,宁波江南仪器厂。
1.1.3 培养基燕麦培养基:燕麦粉10 g,琼脂18 g,MgSO4·7H2O 1 g,KH2PO4 1.5 g,NaNO3 1 g,加水至1 L,自然pH值。PDA培养基:新鲜马铃薯200 g煮沸30 min后过滤取澄清滤液,葡萄糖20 g,琼脂15 g,加水至1 L,自然pH值。PDB培养基配方同PDA培养基,但不加入琼脂。以上培养基均经过1×105 Pa灭菌20 min。
1.2 方法 1.2.1 菌株对香蕉生长影响的测定取活化后的DSE菌块接种于燕麦培养基平板上,每个平板3个菌块,28 ℃培养10 d。取株高粗壮相当的香蕉组培苗插种于菌落上,根系平铺菌落表面,每菌落移栽一株。将培养皿移入组培瓶,于28 ℃、光照度18 000 lx、光/暗周期16 h/8 h、湿度70%的人工气候箱中培养40 d。每处理3个重复,每重复10皿,以不接种DSE菌株处理为对照。实验结束后测定香蕉苗的株高、茎宽、鲜重和干重。
1.2.2 菌株的定殖检测与再分离用自来水冲洗新鲜香蕉根并剪成约1 cm的小段置于灭菌50 mL离心管中,加入10% KOH溶液完全浸没根段,90 ℃恒温水浴锅中解离15−20 min后,用墨水醋染色30 min (墨水:米醋的体积比为5:95),清水漂洗3次并浸泡于清水中12 h进行脱色,根系置光学显微镜下观察DSE的定殖情况,并计算定殖率:定殖率=(定殖根段数/被镜检根段总数)×100%。DSE菌株的再分离参照张艳等[21]的方法进行。
1.2.3 菌株对香蕉枯萎病的防治效果测定平皿测定参照农倩等[18]的方法进行:将DSE-香蕉共生体(连同燕麦培养基)移至长有镰刀菌的水琼脂培养基上,未接菌的对照香蕉组培苗直接接入带有镰刀菌的水琼脂平板上,每个处理重复10皿,观察记录病级[18]、计算病情指数和防治效果。
盆栽试验取4叶期大小的香蕉幼苗,参照农倩等[18]的方法进行。制备5×105 CFU/mL的DSE菌液进行灌根处理,每株香蕉苗浇灌50 mL菌液,每隔15 d浇灌1次,共浇灌3次。浇灌结束15 d后,对香蕉苗进行伤根并接种镰刀菌孢子液(1×106 CFU/mL),对照(Control)以清水灌根并接种病原菌,每处理3个重复,每重复8株苗。接种病原菌25 d后解剖香蕉球茎,观察记录发病情况并计算防效。病情分级标准参照农倩等[18]。
1.2.4 生防菌株的分类鉴定形态观察:观察菌株在PDA培养基上的菌落特征,并通过盖玻片斜插法,使菌丝着生在盖玻片上,光学显微镜下观察盖玻片上的菌丝形态,放置4 ℃培养2−4周,进行诱导产孢观察。
分子鉴定:将菌株接种于PDB培养基中,于28 ℃、150 r/min振荡培养15 d后,过滤收集菌丝进行总DNA提取。ITS基因的PCR扩增采用通用引物ITS1[22] (5′-TCCGTAGGTGAACCTGCGG-3′)和ITS4 (5′-TCCTCCGCTTATTGATATGC-3′)。50 μL PCR反应体系:MasterMix 25 μL,正、反向引物(10 μmol/L)各2 μL,DNA模板1 μL,ddH2O 20 μL。PCR反应条件:94 ℃ 2 min;94 ℃ 40 s,50 ℃ 30 s,68 ℃ 50 s,35个循环;72 ℃ 10 min。回收扩增产物后进行测序,通过NCBI BLASTn对序列进行比对分析,使用MEGA 4.0构建系统发育树。
2 结果与分析 2.1 接种菌株对香蕉生长的影响由表 1可知,接种DSE菌株可有效促进香蕉植株的生长,植株的株高、茎宽、鲜重和干重的增长幅度分别为4.49%−21.91%、3.66%−30.37%、18.42%−47.36%、35.9%−42.40%,其中接种LS1后香蕉植株生长健壮,未出现各种病害症状,该处理株高、茎宽、鲜重和干重均比对照显著增加,其中鲜重与干重分别比对照增加47.36%与42.40%,效果明显(表 1,图 1)。
| Strains | Growth vigor |
Stem height (cm) |
Growth rate (%) |
Stem width (cm) |
Growth rate (%) |
Weight (g) |
Growth rate (%) |
Dry weight (mg) |
Growth rate (%) |
| LS1 | +++ | 6.51±0.44a | 21.91 | 2.49±0.13a | 30.37 | 1.12±0.22a | 47.36 | 5.91±0.82a | 42.40 |
| B2 | ++ | 5.85±0.57ab | 9.55 | 2.01±0.11b | 5.23 | 0.92±0.14ab | 21.05 | 5.79±0.47a | 39.52 |
| LC8 | ++ | 6.08±0.43ab | 13.86 | 1.98±0.39b | 3.66 | 1.00±0.17a | 31.58 | 5.87±0.31a | 41.45 |
| MS38 | ++ | 5.64±0.44b | 5.62 | 2.08±0.09b | 8.90 | 0.96±0.05ab | 26.32 | 5.68±0.48a | 36.87 |
| TD1 | ++ | 5.58±0.19b | 4.49 | 2.10±0.15b | 9.95 | 0.91±0.14ab | 18.42 | 5.64±0.76a | 35.90 |
| CK | + | 5.34±0.87b | 0.00 | 1.91±0.25b | 0.00 | 0.76±0.21b | 0.00 | 4.15±0.95b | 0.00 |
| 注:越多数量的“+”表示香蕉植株的促生效果更明显,不同小写字母表示各处理在0.05水平上达差异显著. Note: More “+” indicate more obvious the growth promoting effect; Different small letters indicated significant difference at 0.05 level. |
|||||||||

|
| 图 1 DSE菌株LS1对香蕉植株的促生效果 Figure 1 Growth promotion ability of LS1 on banana |
|
|
香蕉组培苗接种DSE菌株40 d后,对香蕉根系解离染色进行显微镜观察。通过计算,5株DSE菌株的定殖率在30%−45%之间,其中LS1、TD1、B2的定殖率较高,但相互间差异不显著,菌株LC8的定殖率最低(图 2A)。接种LS1菌株的香蕉根段中发现了深色、有横隔的菌丝以及“微菌核”等DSE典型结构,空白对照中未见菌丝体定殖(图 2B、C)。从根系中分离到与供试DSE性状、形态结构一致的内生真菌,表明接种40 d后“DSE-香蕉”共生体系已经建立。
|
| 图 2 不同DSE菌株的定殖率(A)、香蕉根段中的LS1菌株(B)以及香蕉根段空白对照(C) Figure 2 Colonization rate (A) and colonization of LS1 in banana roots (B) and control (C) 注:不同小写字母表示各处理在0.05水平上差异显著. Note: Different small letters indicated significant difference at 0.05 level. |
|
|
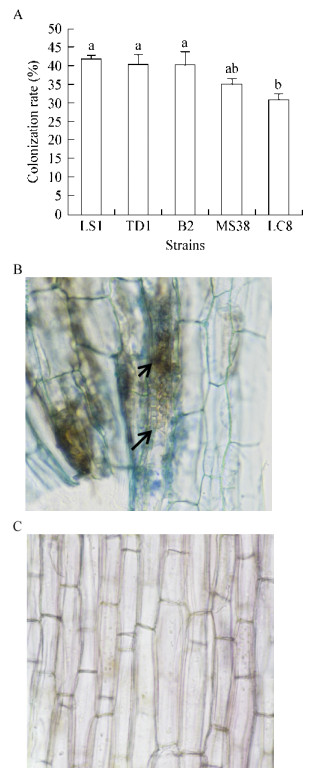
香蕉接种DSE处理对香蕉枯萎病产生了强弱不一的防治效果,平皿防效为57.76%−86.19%,盆栽防效为12.69%−63.19% (图 3A)。其中菌株LS1处理的香蕉植株表现的防治效果最佳(图 3B−E),平皿及盆栽防效均显著高于其它处理,具有进一步研究利用的价值,将对其进行进一步的分类鉴定。
|
| 图 3 不同DSE菌株对香蕉枯萎病的防治效果(A)和菌株LS1对香蕉枯萎病的防治效果(B–E) Figure 3 Biocontrol effect against banana Fusarium wilt of different DSE strains (A) and biocontrol effect of LS1 (B−E) 注:不同小写字母表示各处理在0.05水平上达差异显著. Note: Small letters indicated significant difference at 0.05 level. |
|
|
菌株LS1在PDA培养基上生长7 d的生长速率约为4.97 mm/d,28 ℃培养2周后,菌落直径约70 mm。菌落圆形,褐色至褐绿色,绒毡状,边缘平整,有浅放射状沟纹,背面深褐色。显微镜下观察可见LS1菌丝分枝有隔,黄褐色至褐色,光滑,厚壁,直径1.4 μm−4.7 μm。分生孢子梗在菌丝的顶端或侧枝上直立产生,不分枝,褐色,光滑,有隔,大小为(22.4−386.7) μm×(2.7−5.2) μm。分生孢子梗顶端及中部膨大形成产孢细胞,膨大部位3.7 μm−6.3 μm。枝孢多为圆柱形,部分中部稍有收缩,有0−2个分隔,其上分生孢子链生,枝孢大小为(6.4−28.5) μm×(2.0−5.6) μm。分生孢子近圆形、椭圆形、柠檬形或纺锤形,黄褐色,无分隔,部分孢子两端或一端有孢脐,(3.4−8.4) μm×(2.2−4.7) μm;近圆形孢子直径为2.4 μm−7.6 μm (图 4)。

|
| 图 4 菌株LS1形态鉴定 Figure 4 Morphological characteristics of strain LS1 注:A:在PDA培养基上的菌落形态;B:产孢梗和分生孢子,标尺=10 μm;C:分生孢子,标尺=10 μm. Note: A: Colony on PDA; B: Conidiophores and conidia of strain LS1, Bars=10 μm; C: Conidia, Bars=10 μm. |
|
|
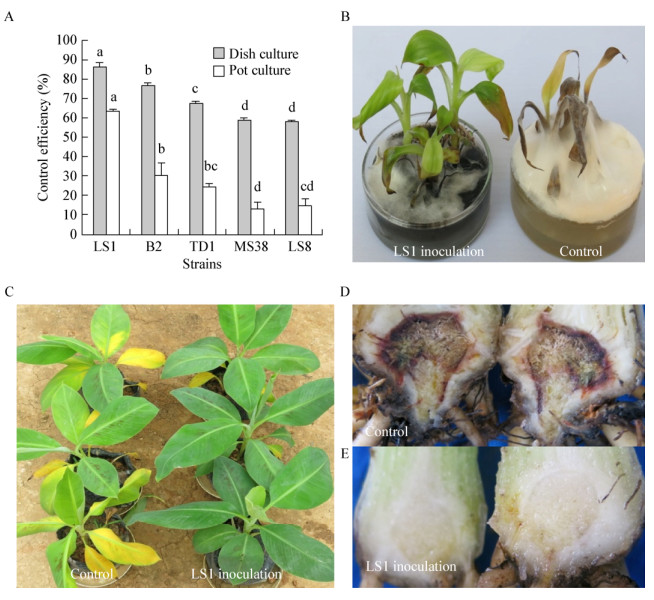
利用rDNA-ITS引物对菌株LS1的总DNA扩增后得到一条长553 bp的PCR产物,经回收纯化测序后提交GenBank获得登录号为MH376857。该序列在NCBI中进行BLAST分析,发现LS1与枝孢属(Cladosporium)中的C. chlorocephalum (AF393686)、C. tenuissimum (MK311278)、C. colocasiae (NR119840)、C. oxysporum (MH863870)、C. anthropophilum (MK111492)、C. cladosporioides (MH397132)等6个种的菌株相似率均达到100%。选取这些序列及枝孢属其他序列构建基于rDNA-ITS序列的系统发育树(图 5),结果显示LS1与上述6株真菌聚为一支,支持率为87%。将菌株LS1形态学特征分别与上述6株真菌进行比较(表 2),发现菌株LS1的形态特征与C. chlorocephalum基本一致,将菌株LS1鉴定为绿头枝孢菌C. chlorocephalum。
|
| 图 5 菌株LS1与相关菌株基于ITS片段的系统发育树 Figure 5 Phylogenetic tree derived from analysis of the ITS sequences of strain LS1 and relative species |
|
|
| Species | Colony color | Conidiophores | Conidiogenous cell | Conidia | Reference |
| LS1 | Olivaceous to brown | Brown, smooth, septate, branched, occasionally once unbranched with swelling base | 0−2 septate, cylindrical, slightly depressed in the middle | Ellipsoid to fusiform, aseptate, with 1-2 distal hila | |
| C. chlorocephalum | Olivaceous | Brown, smooth, septate, branched, occasionally once unbranched with swelling base | 0−2 septate, cylindrical, slightly depressed in middle | Ellipsoid to fusiform, aseptate | [23-24] |
| C. tenuissimum | Light green, later gray | Unbranched, geniculate-sinuous to the top, 2−7 septate | 0−2 septate, cylindrical- crescent | Ellipsoid, obovoid, smooth, 0−1 septate | [23, 25] |
| C. colocasiae | Brown | Unbranched, septate with swellings top | 0−2 septate, cylindrical- fusiform | Ellipsoid, obovoid, 1−3 septate, occasionally aseptate | [23] |
| C. oxysporum | Olivaceous | Unbranched, obvious swellings | 0−1 septate, cylindrical | Obovoid, occasionally ellipsoid | [23] |
| C. anthropophilum | Greyish-green, cover with gray hyphae | Branched, sometimes verruculose towards the base, walls slightly thickened | Cylindrical, slightly swellings at top | Ellipsoid, asperulate, with 1-5 distal hila | [26] |
| C. cladosporioides | Olivaceous | Unbranched, not swelling, verruculose, conidial scars | 0−1 septate, fusiform | Ellipsoid, obovoid, 0−1septate, verruculose | [23-24] |
研究发现,地球上几乎任何生境都有DSE的分布,而且越是极端的地区,DSE的分布越为广泛,表明DSE具有重要的生态地位[12-13]。研究显示,接种DSE菌株能有效促进香蕉[18]、番茄[27]、野生稻[28]、甜高粱[29]、甘蔗[30]、铁皮石斛[31]等作物的生长。本研究用蔗地土壤分离到的DSE菌株接种香蕉,共生40 d后定殖率在40%左右,香蕉生长情况良好,证明了DSE的宿主非专一性。另外,通过接种DSE有效提高了香蕉的株高、茎宽、鲜重和干重,不同程度地促进了香蕉的生长,与前人的研究结果一致。通过镜检,我们发现香蕉根部组织中的DSE菌丝网络,推测DSE可能通过这些贯通的菌丝体加速根系对水分和养分的吸收利用,以实现植株的快速生长。研究表明,DSE能通过分泌纤维素酶、淀粉酶、漆酶等多种胞外水解酶,促进植株对养分的吸收利用[32],本研究中的DSE菌株能否分泌上述植物生长的有益酶还需进一步研究揭示。
生防菌株由于受外界环境的影响,在田间的防治效果不稳定,极大限制了其在生产上的应用。由于“DSE-植物”共生体中DSE定殖在植物的根系内部而非根系表面,受各种环境因子的影响相对较小,因此其防效相对稳定。本研究利用筛选到的5株DSE菌,通过室内平皿和盆栽实验评价其对香蕉枯萎病的防治效果,结果显示香蕉接种DSE处理对香蕉枯萎病产生了强弱不一的防治效果,其中菌株LS1处理的香蕉植株表现的防治效果显著优于其它菌株,平皿防治效率高达86.19%,盆栽防治效率为63.19%,防效相对稳定,但田间的防治效果仍需进一步的田间评价。农倩等[18]发现香蕉接种DSE菌株Schizothecium sp.后能增强香蕉的抗氧化防护系统,提高其对香蕉枯萎病的抗性,本研究中的DSE菌株LS1是否具有类似的机理还需进一步的实验证实。
DSE的分类是目前DSE研究热点之一。由于多数DSE在可培养条件下均以无性态存在,有性态很少出现,给形态学分类带来了困难,而分子鉴定技术给DSE的分类提供了重要的技术支持。基于形态学特征描述已被鉴定出来的DSE包括10多个属约30个种[33],呈现多样性。本研究结合形态学和分子鉴定技术将菌株LS1鉴定为绿头枝孢菌(Cladosporium chlorocephalum),丰富了DSE分类内容。目前该菌株已申请中国普通微生物菌种保藏管理中心的菌种专利保藏,编号为CGMCC 16498。据报道Cladosporium chlorocephalum在牡丹上能引起叶斑病[34],而本文发现该菌对香蕉枯萎病具有生防作用,证明了该菌生态功能的多样性,但是否因为不同寄主下,植物-微生物互作机理的差异造成了生态功能的差异,有待进一步的探索研究。
| [1] |
Siamak SB, Zheng SJ. Banana Fusarium wilt (Fusarium oxysporum f. sp. cubense) control and resistance, in the context of developing wilt-resistant bananas within sustainable production systems[J]. Horticultural Plant Journal, 2018, 4(5): 208-218. DOI:10.1016/j.hpj.2018.08.001 |
| [2] |
Maryani N, Lombard L, Poerba YS, et al. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin[J]. Studies in Mycology, 2019, 92: 155-194. DOI:10.1016/j.simyco.2018.06.003 |
| [3] |
National Statistics Bureau. China Statistical Yearbook-2017[M]. Beijing: China Statistics Press, 2017: 195. (in Chinese) 中国统计局. 中国统计年鉴-2017[M]. 北京: 中国统计出版社, 2017: 195. |
| [4] |
Ling RJ. Research on present situation and countermeasures of banana industry development in Guangxi[D]. Nanning: Master's Thesis of Guangxi University, 2018 (in Chinese) 凌荣娟.广西香蕉产业发展现状与对策研究[D].南宁: 广西大学硕士学位论文, 2018 http://cdmd.cnki.com.cn/Article/CDMD-10593-1018132339.htm |
| [5] |
Ghag SB, Shekhawat UKS, Ganapathi TR. Fusarium wilt of banana: biology, epidemiology and management[J]. International Journal of Pest Management, 2015, 61(3): 250-263. DOI:10.1080/09670874.2015.1043972 |
| [6] |
Wang BB, Li R, Ruan YZ, et al. Pineapple-banana rotation reduced the amount of Fusarium oxysporum more than maize-banana rotation mainly through modulating fungal communities[J]. Soil Biology and Biochemistry, 2015, 86: 77-86. DOI:10.1016/j.soilbio.2015.02.021 |
| [7] |
Deltour P, Fran a SC, Pereira OL, et al. Disease suppressiveness to Fusarium wilt of banana in an agroforestry system: Influence of soil characteristics and plant community[J]. Agriculture, Ecosystems & Environment, 2017, 239: 173-181. |
| [8] |
Raza W, Ling N, Zhang RF, et al. Success evaluation of the biological control of Fusarium wilts of cucumber, banana, and tomato since 2000 and future research strategies[J]. Critical Reviews in Biotechnology, 2017, 37(2): 202-212. |
| [9] |
Fu L, Penton CR, Ruan YZ, et al. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease[J]. Soil Biology and Biochemistry, 2017, 104: 39-48. DOI:10.1016/j.soilbio.2016.10.008 |
| [10] |
Ghag SB, Shekhawat UKS, Ganapathi TR. Transgenic banana plants expressing a Stellaria media defensin gene (Sm-AMP-D1) demonstrate improved resistance to Fusarium oxysporum[J]. Plant Cell, Tissue and Organ Culture, 2014, 119(2): 247-255. |
| [11] |
Dale J, James A, Paul JY, et al. Transgenic Cavendish bananas with resistance to Fusarium wilt tropical race 4[J]. Nature Communications, 2017, 8(1): 1496. DOI:10.1038/s41467-017-01670-6 |
| [12] |
Zhang Y. Studies on dark septate endophytes (DSE) in special ecosystems of Yunnan[D]. Kunming: Doctoral Dissertation of Yunnan University, 2012 (in Chinese) 张燕.云南几种特殊生境中深色有隔内生真菌(DSE)研究[D].昆明: 云南大学博士学位论文, 2012 http://d.wanfangdata.com.cn/Thesis/Y2192666 |
| [13] |
Xie L. The species diversity and ecological function of dark septate endophytes in two ecosytems of Guangxi[D]. Nanning: Doctoral Dissertation of Guangxi University, 2018 (in Chinese) 谢玲.广西两种生境深色有隔内生真菌多样性与生态功能研究[D].南宁: 广西大学博士学位论文, 2018 |
| [14] |
Mandyam K, Jumpponen A. Seeking the elusive function of the root-colonising dark septate endophytic fungi[J]. Studies in Mycology, 2005, 53: 173-189. DOI:10.3114/sim.53.1.173 |
| [15] |
Deng X, Song XS, Yin DC, et al. Research advances in improving host plant resistance by dark septate endophytes[J]. Journal of Anhui Agricultural Sciences, 2015, 43(31): 10-11, 17. (in Chinese) 邓勋, 宋小双, 尹大川, 等. 深色有隔内生真菌提高宿主植物抗逆性的研究进展[J]. 安徽农业科学, 2015, 43(31): 10-11, 17. DOI:10.3969/j.issn.0517-6611.2015.31.004 |
| [16] |
Knapp DG, Kovács GM, Zajta E, et al. Dark septate endophytic pleosporalean genera from semiarid areas[J]. Persoonia, 2015, 35: 87-100. DOI:10.3767/003158515X687669 |
| [17] |
Zhang XR, Li T, Wang CJ, et al. Enhanced tolerance of tomatoes against Fusarium oxysporum by inoculation with dark septate endophyte[J]. Chinese Journal of Biological Control, 2017, 33(3): 394-400. (in Chinese) 张晓蓉, 李涛, 王超君, 等. 深色有隔内生真菌甘瓶霉对番茄抗枯萎病的作用[J]. 中国生物防治学报, 2017, 33(3): 394-400. |
| [18] |
Nong Q, Zhang WL, Lan TJ, et al. Screening and identification of dark septate endophyte strain L-14 and its mechanism of banana Fusarium wilt disease resistance[J]. Chinese Journal of Tropical Crops, 2017, 38(3): 559-564. (in Chinese) 农倩, 张雯龙, 蓝桃菊, 等. 一株抗香蕉枯萎病DSE菌株的筛选鉴定及抗病机理初探[J]. 热带作物学报, 2017, 38(3): 559-564. DOI:10.3969/j.issn.1000-2561.2017.03.027 |
| [19] |
Surono, Narisawa K. The inhibitory role of dark septate endophytic fungus Phialocephala fortinii against Fusarium disease on the Asparagus officinalis growth in organic source conditions[J]. Biological Control, 2018, 121: 159-167. DOI:10.1016/j.biocontrol.2018.02.017 |
| [20] |
Ploetz RC. Management of Fusarium wilt of banana: A review with special reference to tropical race 4[J]. Crop Protection, 2015, 73: 7-15. DOI:10.1016/j.cropro.2015.01.007 |
| [21] |
Zhang Y, Lan TJ, Liao ST, et al. Diversity of endophytic fungi in mangrove plants of Beibu Gulf, Guangxi[J]. Microbiology China, 2017, 44(4): 783-794. (in Chinese) 张艳, 蓝桃菊, 廖仕同, 等. 广西北部湾红树植物内生真菌多样性[J]. 微生物学通报, 2017, 44(4): 783-794. |
| [22] |
Zeng Q, Zhong WM, Xiang Y, et al. Isolation, identification and evaluation of 52 fungi from the deep-sea sediments of South China Sea[J]. Microbiology China, 2018, 45(9): 1904-1915. (in Chinese) 曾奇, 仲伟茂, 向瑶, 等. 南海深海沉积物中52株真菌的初步分离鉴定及其代谢产物活性[J]. 微生物学通报, 2018, 45(9): 1904-1915. |
| [23] |
Zhang ZY. Chinese Flora (Cladosporium Link; Fusicladium Bonorden; Pyricularia Saccardo)[M]. Beijing: Science Press, 2003: 67-68. (in Chinese) 张中义. 中国真菌志(枝孢属黑星孢属梨孢属)[M]. 北京: 科学出版社, 2003: 67-68. |
| [24] |
Geng YH. A survey on soil dematiaceous hyphomycetes at species level from Tibetan Plateau[D]. Tai'an: Master's Thesis of Shandong Agricultural University, 2008 (in Chinese) 耿月华.西藏高原地区土壤中的暗色丝孢菌物种多样性考察[D].泰安: 山东农业大学硕士学位论文, 2008 http://d.wanfangdata.com.cn/Thesis_Y1374858.aspx |
| [25] |
He PX, Zhao LS. Preliminary report on Cladosporium tenuissimum cooke[J]. Jilin Forestry Science and Technology, 1987(1): 25-28. (in Chinese) 何平勋, 赵连书. 极细枝孢(Cladosporium tenuiussimum cooke)研究初报[J]. 吉林林业科技, 1987(1): 25-28. |
| [26] |
Sandoval-Denis M, Gené J, Sutton DA, et al. New species of Cladosporium associated with human and animal infections[J]. Persoonia, 2016, 36: 281-298. DOI:10.3767/003158516X691951 |
| [27] |
Andrade-Linares DR, Grosch R, Restrepo S, et al. Effects of dark septate endophytes on tomato plant performance[J]. Mycorrhiza, 2011, 21(5): 413-422. DOI:10.1007/s00572-010-0351-1 |
| [28] |
Yuan ZL, Zhang CL, Lin FC, et al. Identity, diversity, and molecular phylogeny of the endophytic mycobiota in the roots of rare wild rice (Oryza granulate) from a nature reserve in Yunnan, China[J]. Applied and Environmental Microbiology, 2010, 76(5): 1642-1652. DOI:10.1128/AEM.01911-09 |
| [29] |
Diene O, Takahashi T, Yonekura A, et al. A new fungal endophyte, Helminthosporium velutinum, promoting growth of a bioalcohol plant, sweet sorghum[J]. Microbes and Environments, 2010, 25(3): 216-219. DOI:10.1264/jsme2.ME09165 |
| [30] |
Qin LP, Su Q, Zhang WL, et al. Selection of excellent sugarcane growth-promoting DSE strains and their inoculation effects in fields[J]. Chinese Journal of Tropical Crops, 2015, 36(10): 1861-1865. (in Chinese) 覃丽萍, 苏琴, 张雯龙, 等. 甘蔗优良DSE促生菌株的筛选及田间接种效果[J]. 热带作物学报, 2015, 36(10): 1861-1865. DOI:10.3969/j.issn.1000-2561.2015.10.023 |
| [31] |
Xie L, Zhang WL, Qin LP, et al. Inoculation effects of dark septate endophytes (DSE) on the growth of Dendrobium candidum seedling[J]. Journal of Southern Agriculture, 2014, 45(6): 1010-1014. (in Chinese) 谢玲, 张雯龙, 覃丽萍, 等. 深色有隔内生真菌(DSE)引进菌株对铁皮石斛的接种效应[J]. 南方农业学报, 2014, 45(6): 1010-1014. DOI:10.3969/j:issn.2095-1191.2014.6.1010 |
| [32] |
Upson R, Read DJ, Newsham KK. Nitrogen form influences the response of Deschampsia antarctica to dark septate root endophytes[J]. Mycorrhiza, 2009, 20(1): 1-11. |
| [33] |
Addy HD, Piercey MM, Currah RS. Microfungal endophytes in root[J]. Canadian Journal of Botany, 2005, 83(1): 1-13. |
| [34] |
Duan YB. Study on the identification and biological characteristics of pathogenic fungi of peony leaf spot[D]. Luoyang: Master's Thesis of Henan University of Science and Technology, 2009 (in Chinese) 段亚冰.牡丹叶斑病病原真菌鉴定及生物学特性研究[D].洛阳: 河南科技大学硕士学位论文, 2009 http://cdmd.cnki.com.cn/Article/CDMD-10464-1011257999.htm |
 2019, Vol. 46
2019, Vol. 46