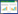扩展功能
文章信息
- 孙清扬, 张静静, 李冰清, 王岩, 张晓华
- SUN Qing-Yang, ZHANG Jing-Jing, LI Bing-Qing, WANG Yan, ZHANG Xiao-Hua
- 金属离子对重组深渊滕黄单胞菌低温α-淀粉酶LamA的活性和稳定性影响
- Effect of metal ions on the activity and stability of recombinant cold-adapted α-amylase LamA from Luteimonas abyssi
- 微生物学通报, 2019, 46(11): 2848-2856
- Microbiology China, 2019, 46(11): 2848-2856
- DOI: 10.13344/j.microbiol.china.180998
-
文章历史
- 收稿日期: 2018-12-06
- 接受日期: 2019-04-19
- 网络首发日期: 2019-05-30
2. 山东省医学科学院基础医学研究所 山东 济南 250031
2. Institute of Basic Medicine, Shandong Academy of Medical Science, Jinan, Shandong 250031, China
α-淀粉酶可催化淀粉内部α-1, 4糖苷键的水解[1-3],是目前用途最广泛、需求量最大的工业用酶之一,在食品、纺织、饲料等领域都有大量应用[4-5]。α-淀粉酶的热稳定性与其空间结构具有重要关系[2, 6],主要受氢键、疏水相互作用、静电相互作用等影响[7],金属离子等填充物也是重要的影响因素[8-10]。对Bacillus amyloliquefaciens的α-淀粉酶研究发现,Asp233结合Ca2+可影响酶的热稳定性,为α-淀粉酶热稳定性改造提供了思路[11]。在研究最为透彻的B. licheniformis的α-淀粉酶中,结构域A、B之间嵌入的Ca2+也对其稳定性和活性起到重要作用[12-13]。然而,对低温α-淀粉酶在此方面的研究却较为欠缺。α-淀粉酶的热稳定性是影响其应用的重要因素,目前使用较广泛的中高温α-淀粉酶难以用于低温处理的加工环节,较低的热稳定性又是低温α-淀粉酶在工业应用中的瓶颈[14]。而开发温度耐受范围较宽的α-淀粉酶,则可在具有温度变化的生产过程中,节省转换酶类的成本和时间,宽松加工条件,具有较好的应用前景。
当前绝大多数的α-淀粉酶来源于陆地生物[15-17],海洋来源的α-淀粉酶则处于高度未开发状态。本实验的深渊藤黄单胞菌XH031 (Luteimonas abyssi XH031)是从深海分离到的一株细菌[18],在前期研究中已经完成了该菌株的全基因组测序,克隆表达了一个α-淀粉酶基因,纯化了α-淀粉酶LamA,并对其酶学特性进行了初步研究。LamA蛋白序列较新颖,其比活力可达到8 881 U/mg,在同类α-淀粉酶中比活力较高,且在低温环境下仍能保持较高酶活力[19]。本课题拟在前期研究的基础上,寻找提升低温α-淀粉酶LamA热稳定性的途径,开发其在较高温度下的应用价值,使其具有更宽的温度耐受范围;并初步明确影响这一特性的关键氨基酸位点,为更多α-淀粉酶向宽温度耐受方向改造提供参考依据。
1 材料与方法 1.1 材料 1.1.1 菌株和质粒用于实验的菌株和质粒如表 1所示。
| Strains or plasmids | Usage | Source |
| Luteimonas abyssi XH031 | Target strain | The South Pacific circulation[18] |
| E. coli JM109 | Amplification of recombinant plasmid | New England Biolaboratories (Beverly, MA) |
| E. coli BL21(DE3) | As an expression host, to construct expression engineering bacteria | Novagen (Madison, WI) |
| pET-24a(+) | As an expression vector, to construct an α-amylase recombinant expression plasmid | Novagen (Madison, WI) |
| pET-24a/LamA | Site-directed mutation template | This study |
酵母膏5.0,蛋白胨10.0,NaCl 10.0;调节pH至7.0,固体培养基需加入2%琼脂,1×105 Pa灭菌20 min。
1.1.3 主要试剂和仪器Prime STAR GXL DNA Polymerase,TaKaRa公司;质粒提取试剂盒,北京博迈德基因技术有限公司;琼脂糖凝胶DNA回收试剂盒,天根生化科技有限公司。高速冷冻离心机,Thermo公司;PCR仪,Applied Biosystems公司;酶标仪,Fecan公司。
1.2 方法 1.2.1 金属离子对LamA热稳定性的影响测定在等浓度的LamA中分别加入终浓度1 mmol/L和5 mmol/L的Na+、K+、Ca2+、Fe3+、Mn2+、Al3+、Zn2+、Mg2+,并设置空白对照。吸取10 μL酶液,加入90 μL用50 mmol/L Tris-HCl缓冲液(pH 8.0)配制的1%可溶性淀粉溶液,于65 ℃反应10 min。加入100 μL DNS试剂,沸水浴5 min,冷却,定容至1 mL。吸取150 μL反应液,用酶标仪测定其OD540。参考麦芽糖标准曲线,计算待测蛋白的酶活力[19]。再于LamA中分别加入浓度逐渐升高的Ca2+,与空白对照一同于30、55和65 ℃温浴30 min,测定剩余酶活力。
1.2.2 LamA关键位点预测及突变对LamA的蛋白序列进行BLASTp和CD-Search分析,并将其与相似性较高且经过实验验证的α-淀粉酶序列P22630 [Aeromonas hydrophila]、O18420 [Drosophila subobscura]、O18408 [Drosophila melanogaster]、P22998 [Streptomyces violaceus]、P27350 [Streptomyces thermoviolaceus]进行多序列比对,参考研究最为透彻的B. licheniformis的α-淀粉酶(UniProtKB:Q208A7)的钙结合区域关键位点[20],预测可能影响LamA热稳定性及Ca2+结合的氨基酸位点。
以pET-24a/LamA为模板,以突变位点为中心设计表 2所示引物,使Asn120、Gln185、Asp200、His233、Met234和Thr224突变为性质相反的氨基酸。以pET-24a/LamA为模板,将含有突变位点的引物作为扩增引物,PCR扩增体系(50 μL):5×Prime STAR GXL Buffer 10 μL,dNTP Mixture (2.5 mmol/L) 4 μL,Primer 1 (20 μmol/L) 1 μL,Primer 2 (20 μmol/L) 1 μL,Template 0.5 μL,Prime STAR GXL DNA polymerase 2 μL,ddH2O 31.5 μL。PCR反应条件:98 ℃ 2 min;98 ℃ 10 s,60 ℃ 15 s,68 ℃ 10 s/1 kb,共30个循环;72 ℃ 2 min。扩增产物进行琼脂糖凝胶电泳分析,并将目的片段切胶回收纯化,得到含有突变位点的线性片段;5′-磷酸化处理使其自连为环状质粒,转化进感受态细胞BL21中;挑取阳性克隆,提取质粒,进行酶切和测序验证。
| Primers | Sequences (5′→3′) |
| N120D-F | GTGGTGCTCGACCACATGGCG |
| N120D-R | GTCGGCATAGGTCTCGACGCC |
| H233D/M234C-F | GCTGCGAAAGACTGTCCGGCC |
| H233D/M234C-R | ATCCACCCGGAAGCCGGTCAC |
| Q185E-F | CAGGTCGAGCAGTGGCGCCTG |
| Q185E-R | GCTCACGTCGGTGTAGTCGAC |
| D200R-F | CCGCGCCTGGTCGCCAACGAT |
| D200R-R | AAGGCCGGGATCGTCGCCGCC |
| T224D-F | GTGGACGGCTTCCGGGTGGAT |
| T224D-R | GCCGAGCGCCTTCAGTGCGAG |
| 注:下划线为突变的氨基酸,红色为定点突变的核苷酸位点. Note: Mutated amino acids are underlined and site-directed mutant nucleotides are marked in red. |
|
将活化的重组表达菌株和空载对照接种至LB液体培养基(卡那霉素终浓度100 μg/mL)中,37 ℃、170 r/min振荡培养;待OD600达到0.4-0.6之间,加入终浓度0.1 mmol/L的IPTG,16 ℃、170 r/min诱导表达12-20 h。于4 ℃、12 000 r/min离心10 min收集菌体,重悬于过滤除菌的Binding buffer中,置于冰上超声破碎(小探头200 V,超声5 s,间隙10 s,50次);于4 ℃、12 000 r/min离心30 min,收集上清液,用0.2 μm滤器过滤,4 ℃保存备用。将上清液加入镍层析柱中上样,依次用咪唑浓度逐渐提高(10、20、50、75、250 mmol/L)的缓冲液(20 mmol/L Tris-HCl,pH 8.0;0.5 mmol/L NaCl)洗涤蛋白层析柱,最后用咪唑浓度500 mmol/L的缓冲液洗脱层析柱。将收集到的蛋白液于4 ℃透析,除盐复性。配置12% (质量体积比)的分离胶,用SDS-PAGE法检测表达蛋白的纯化结果[19]。
吸取2 μL酶液,以透析液作为空白对照,使用NanoDrop 2000/2000c测定蛋白浓度,并将酶液稀释为相同浓度。各取10 μL酶液加入可溶性淀粉平板,37 ℃过夜;使用卢戈氏碘液检验,观察是否有透明圈。利用麦芽糖标准溶液绘制的标准曲线,计算LamA和各突变蛋白的比活力。
1.2.4 温度对LamA及突变蛋白的活性和稳定性的影响测定参照1.2.1的方法,在90 μL的1%可溶性淀粉溶液中分别加入10 μL等浓度的LamA和突变蛋白,于不同温度(4、10、16、30、37、50、60、70、80、90、100 ℃)下反应10 min,测定和计算LamA和各突变蛋白在不同反应温度下的相对酶活。再将等浓度的LamA和突变蛋白于不同温度(0、10、20、30、37、50、60、70、80、90、100 ℃)下温浴20 min,在相同反应体系下,于最适温度反应10 min,测定LamA和各突变蛋白的剩余酶活力。
1.2.5 LamA和突变蛋白在钙离子条件下的热稳定性测定在等浓度的LamA和突变蛋白中,加入终浓度5 mmol/L的Ca2+,分别于37 ℃和65 ℃下温浴1 h。参照1.2.1的方法,在1%可溶性淀粉溶液中加入10 μL酶液,于最适温度(LamA和T224D为50 ℃;N120D和Q185E为37 ℃)反应10 min,测定其剩余酶活力。
1.2.6 钙离子结合位点的蛋白结构分析对LamA的蛋白序列进行BLAST分析,在PDB数据库中选取相似度高的蛋白,利用软件PyMOL和Adobe Illustrator模拟LamA的Ca2+结合区域的蛋白结构。
2 结果与分析 2.1 金属离子对LamA热稳定性的影响在LamA中分别添加不同浓度的Na+、K+、Ca2+、Fe3+、Mn2+、Al3+、Zn2+、Mg2+,检测其在65 ℃下的酶活力(图 1)。Na+、K+、Al3+对LamA在65 ℃的酶活力几乎没有影响;Fe3+、Mn2+、Zn2+、Mg2+有轻微抑制作用,但不具显著差异;Ca2+则明显提高了酶活力。
|
| 图 1 不同金属离子对LamA热稳定性的影响 Figure 1 Effects of different metal ions on thermo-stability of LamA |
|
|
进一步实验发现,未添加Ca2+时,65 ℃处理30 min完全失活(5 min即失去90%活性),55 ℃处理30 min相对酶活为5%;而在添加1 mmol/L和5 mmol/L的Ca2+后,相对酶活显著上升(表 3)。此外,当Ca2+浓度继续升高时(5-50 mmol/L),相对酶活没有随之增强,说明5 mmol/L的Ca2+足够使LamA保持在65 ℃和55 ℃的酶活力及稳定性。根据上述结果,初步推测LamA具有影响热稳定性的Ca2+结合位点。
| Temperature (℃) | Time of treatment (min) | Calcium ion concentration (mmol/L) | Relative activity (%) |
| 55 | 30 | 0 | 5 |
| 1 | 35 | ||
| 5 | 55 | ||
| 65 | 30 | 0 | 0 |
| 1 | 25 | ||
| 5 | 36 | ||
| 注:将30 ℃处理后的酶活力计为100%. Note: The enzymatic activity after treatment at 30 ℃ was counted as 100%. |
|||
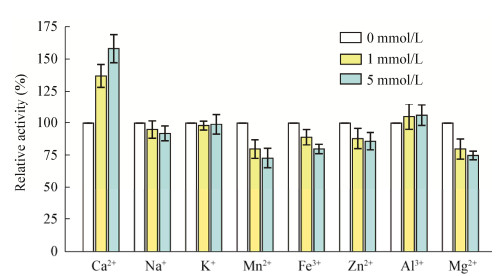
α-淀粉酶LamA的基因序列全长为1 428 bp,编码510个氨基酸,预测其信号肽长度为35个氨基酸,BLASTp结果分析其为钙结合蛋白。选取与LamA相似性较高且经过实验验证的α-淀粉酶序列,与LamA的蛋白序列进行多序列比对(图 2),并参考B. licheniformis的α-淀粉酶(UniProtKB:Q208A7)钙结合区域关键位点[20],预测了5个可能影响LamA耐热性及Ca2+结合的氨基酸位点:Asn120、Gln185、Asp200、His233和Met234。通过NCBI的CD-Search预测的Ca2+结合位点Thr224也于此处一并研究。
|
| 图 2 LamA与其他α-淀粉酶的蛋白序列比对 Figure 2 The protein sequence alignment of LamA and other α-amylases using the DNAMAN 注:*:主要研究的氨基酸位点,其中Thr224是通过NCBI的CD-Search预测的钙离子结合位点;图中其他蛋白序列的UniProtKB:P22630 [Aeromonas hydrophila]、O18420 [Drosophila subobscura]、O18408 [Drosophila melanogaster]、P22998 [Streptomyces violaceus]、P27350 [Streptomyces thermoviolaceus]. Note: The sites marked with * represent the amino acid sites. Thr224 is a calcium ion binding site predicted by NCBI's CD-Search. The UniProtKB of other sequences are as follows: P22630 [Aeromonas hydrophila], O18420 [Drosophila subobscura], O18408 [Drosophila melanogaster], P22998 [Streptomyces violaceus], P27350 [Streptomyces thermoviolaceus]. |
|
|
由于LamA序列较新颖,与PDB数据库中其他蛋白序列相似性较低,不适合进行同源建模。选取与LamA相似度相对高且同样结合Ca2+的蛋白进行比对(表 4),发现Ca2+活性中心比较保守,可用这些蛋白来模拟LamA的Ca2+结合区域(图 3)。综合模拟结果,判断Asn120、Asp200和His233为Ca2+结合区域的保守位点,Gln185也可能在该区域中起到关键作用。Asp具有负电性,Asn和His为极性氨基酸,均可与Ca2+产生相互作用。进一步推测,在未添加Ca2+时,区域中多个羰基氧可产生静电排斥;而当羰基氧与Ca2+结合后,则会使结构的稳定性增加。此外,Asn120与Asp200之间的氢键也可能对蛋白结构的稳定性起到作用。
| Accession | Max score | Total score | Query cover (%) | E value | Perc. Ident (%) |
| 1VIW_A | 94.7 | 94.7 | 89 | 1e-20 | 25.69 |
| 1TMQ_A | 94.7 | 94.7 | 89 | 1e-20 | 25.86 |
| 1G94_A | 90.1 | 90.1 | 76 | 4e-19 | 28.20 |
| 1JXK_A | 87.0 | 87.0 | 87 | 5e-18 | 25.06 |
| 1XGZ_A | 83.6 | 83.6 | 66 | 8e-17 | 26.67 |

|
| 图 3 黄粉虫幼虫α-淀粉酶钙离子结合区域的蛋白结构模拟 Figure 3 Protein structure simulation of calcium ion binding region of Tenebrio molitor larval α-amylase 注:图中所使用蛋白为黄粉虫幼虫α-淀粉酶(PDB ID: 1TMQ_A),其Asn98、Asp155、His189与LamA的Asn120、Asp200和His233对应. Note: The protein used in the figure is Tenebrio molitor larval α-amylase (PDB ID: 1TMQ_A), and Asn98, Asp155 and His189 in the figure correspond to Asn120, Asp200 and His233 of LamA respectively. |
|
|
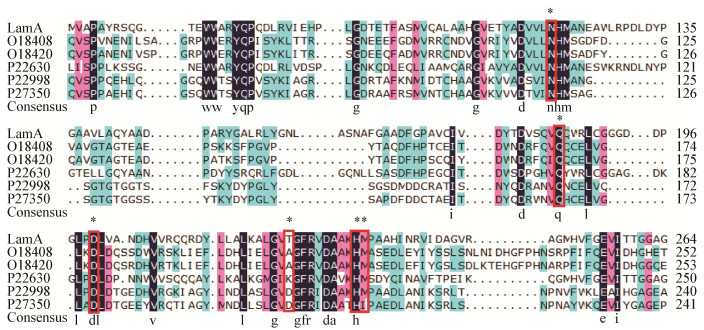
对预测的关键位点进行突变,诱导表达和纯化了重组突变蛋白,并使用可溶性淀粉平板检验各突变蛋白的酶活力:D200R和H233D/M234C完全失活;N120D、Q185E、T224D出现透明圈,但活性均较LamA有所降低(图 4)。利用麦芽糖标准曲线计算比活力,突变蛋白的比活力均低于LamA (表 5)。
|
| 图 4 可溶性淀粉平板检验LamA及突变蛋白酶活力 Figure 4 Enzyme activity assay of LamA and mutant proteins by soluble starch plate medium |
|
|
| Protein | Specific activity (U/mg) |
| LamA | 2 706.8 |
| N120D | 1 574.1 |
| Q185E | 1 595.1 |
| T224D | 1 587.6 |
于pH 8.0的反应体系中进行酶活力测定实验,结果如图 5所示,突变蛋白T224D的最适反应温度与LamA相同,均为50 ℃;而N120D与Q185E的最适反应温度为37 ℃。温度升高后,3种突变蛋白相对酶活的降低速率与LamA无显著差别。此外,对Thr224的突变影响了其低温活性。
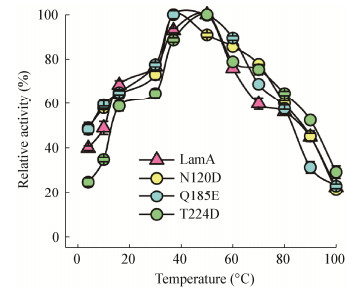
|
| 图 5 温度对LamA及突变蛋白活性的影响 Figure 5 Effects of temperature on the activity of LamA and mutant proteins 注:将最适反应温度下的酶活力计为100%. Note: The enzyme activity at the optimum reaction temperature was counted as 100%. |
|
|
温度稳定性实验的结果如图 6所示,LamA和3种突变蛋白在30-37 ℃之间稳定性最高,且稳定性受高温的影响程度差别不大。综合两个实验结果,初步判断Asn120、Gln185和Thr224并非直接决定热稳定性的位点,可能还需其他条件才能实现对LamA热稳定性的影响。
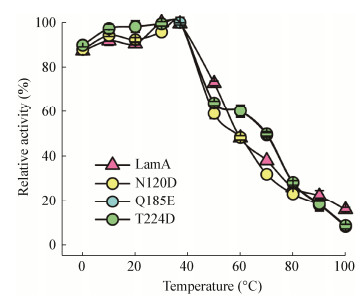
|
| 图 6 不同温度对LamA及突变蛋白稳定性的影响 Figure 6 Effects of temperature on the stability of LamA and mutant proteins 注:将最高的残余酶活力计为100%. Note: The highest residual enzyme activity was counted as 100%. |
|
|
在5 mmol/L的Ca2+条件下,测定LamA和突变蛋白在65 ℃的稳定性,发现N120D的实验结果较有意义(图 7)。常温下N120D与LamA的酶活力差别不大,说明Asn120与Ca2+的结合不会直接增强酶活力;但当温度升高至65 ℃时,LamA的相对酶活降为36%,而N120D的相对酶活则骤降为10%,仅为同温下LamA酶活力的27.8%。说明当Ca2+存在时,Asn120位点对LamA的热稳定性作用显著。
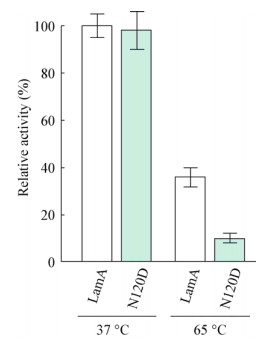
|
| 图 7 5 mmol/L钙离子条件下N120与LamA的热稳定性 Figure 7 Comparison of thermal stability of N120 and LamA under 5 mmol/L calcium ion condition 注:将LamA在37 ℃的酶活力计为100%. Note: The enzymatic activity of LamA at 37 ℃ was counted as 100%. |
|
|
目前已有许多研究探讨了钙结合对高温α-淀粉酶热稳定性的关键作用[8, 11, 21-23],但对低温α-淀粉酶的相关研究却较为欠缺。LamA作为低温α-淀粉酶,却可通过添加Ca2+来提高热稳定性,因而具有较好的研究价值。本实验初步明确了Asn120为此过程的关键位点,推测Asn120与Ca2+的结合不会直接增强酶活力,但能帮助LamA在较高的温度下稳定结构。此后计划将Asn120突变为更多其他氨基酸,来进一步明确其作用机制。此外,根据与B. licheniformis的α-淀粉酶的比对分析[13, 20],推测Asn120、Asp200和His233还可能是LamA中Ca-Na-Ca结构的关键位点,对钠离子结合的相关研究也将成为下一步的探讨方向。
α-淀粉酶的热稳定性影响了其在实际应用中的经济可行性[24]。目前发现的低温酶通常具有较高的结构灵活性[25],表现出更低的活化焓和活化熵[26],因此可以在生产过程中节省能源、提高效率[27-31]。但低温酶较弱的温度耐受性一直是其应用中的瓶颈[32-33]。解决这一问题的思路通常是通过定点突变来改造低温酶[34-35],例如最近对来自Euplotes focardii的低温α-淀粉酶的突变改造[36]。而LamA则为这一问题的解决提供了另一种方向:通过直接添加离子等方式,使低温α-淀粉酶获得更宽的温度耐受范围,可使其在食品、饲料加工等具有温度变化的生产过程中具有更好的应用前景。
本实验中的LamA在同类α-淀粉酶中比活力较高,且在低温环境下仍能保持较高酶活力[19],添加Ca2+后热稳定性的增强,则赋予了它更高的潜在应用价值。在本次研究中,已初步明确了与这一特性相关的关键位点。通过研究LamA的热稳定性与Ca2+的相关性,也有望为更多α-淀粉酶向宽温度耐受方向改造提供参考依据。
| [1] |
Janeček Š, Svensson B, Macgregor EA. α-Amylase: an enzyme specificity found in various families of glycoside hydrolases[J]. Cellular and Molecular Life Sciences, 2014, 71(7): 1149-1170. DOI:10.1007/s00018-013-1388-z |
| [2] |
Nielsen JE, Borchert TV. Protein engineering of bacterial α-amylases[J]. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology, 2000, 1543(2): 253-274. DOI:10.1016/S0167-4838(00)00240-5 |
| [3] |
Dhital S, Warren FJ, Butterworth PJ, et al. Mechanisms of starch digestion by α-amylase—structural basis for kinetic properties[J]. Critical Reviews in Food Science and Nutrition, 2017, 57(5): 875-892. DOI:10.1080/10408398.2014.922043 |
| [4] |
Khemakhem B, Ali MB, Aghajari N, et al. Engineering of the α-amylase from Geobacillus stearothermophilus US100 for detergent incorporation[J]. Biotechnology and Bioengineering, 2009, 102(2): 380-389. DOI:10.1002/bit.22083 |
| [5] |
van der Maarel JEC, van der Veen B, Uitdehaag JCM, et al. Properties and applications of starch-converting enzymes of the α-amylase family[J]. Journal of Biotechnology, 2002, 94(2): 137-155. DOI:10.1016/S0168-1656(01)00407-2 |
| [6] |
Tomazic SJ, Klibanov AM. Mechanisms of irreversible thermal inactivation of Bacillus alpha-amylases[J]. Journal of Biological Chemistry, 1988, 263(7): 3086-3091. |
| [7] |
Zonouzi R, Khajeh K, Monajjemi M, et al. Role of the salt bridge between Arg176 and Glu126 in the thermal stability of the Bacillus amyloliquefaciens α-amylase (BAA)[J]. Journal of Microbiology and Biotechnology, 2013, 23(1): 7-14. |
| [8] |
Tang SY, Le QT, Shim JH, et al. Enhancing thermostability of maltogenic amylase from Bacillus thermoalkalophilus ET2 by DNA shuffling[J]. FEBS Journal, 2010, 273(14): 3335-3345. |
| [9] |
Hiteshi K, Gupta R. Thermal adaptation of α-amylases: a review[J]. Extremophiles, 2014, 18(6): 937-944. DOI:10.1007/s00792-014-0674-5 |
| [10] |
Violet M, Meunier JC. Kinetic study of the irreversible thermal denaturation of Bacillus licheniformis α-amylase[J]. Biochemical Journal, 1989, 263(3): 665-670. DOI:10.1042/bj2630665 |
| [11] |
Lee S, Mouri Y, Minoda M, et al. Comparison of the wild-type α-amylase and its variant enzymes in Bacillus amyloliquefaciens in activity and thermal stability, and insights into engineering the thermal stability of Bacillus α-amylase[J]. Journal of Biochemistry, 2006, 139(6): 1007-1015. DOI:10.1093/jb/mvj107 |
| [12] |
Ghollasi M, Khajeh K, Naderi-Manesh H, et al. Engineering of a Bacillus α-amylase with improved thermostability and calcium independency[J]. Applied Biochemistry and Biotechnology, 2010, 162(2): 444-459. DOI:10.1007/s12010-009-8879-2 |
| [13] |
Li LZ, Yang J, Li J, et al. Role of two amino acid residues' insertion on thermal stability of thermophilic α-amylase AMY121 from a deep sea bacterium Bacillus sp. SCSIO 15121[J]. Bioprocess and Biosystems Engineering, 2015, 38(5): 871-879. DOI:10.1007/s00449-014-1330-2 |
| [14] |
Gerday C, Aittaleb M, Bentahir M, et al. Cold-adapted enzymes: from fundamentals to biotechnology[J]. Trends in Biotechnology, 2000, 18(3): 103-107. |
| [15] |
Holligan S, Wang JX, Cant JP, et al. A proteomics approach to detect tissue-wide adaptive changes in the pancreas associated with increased pancreatic α-amylase activity in domestic cattle (Bos taurus)[J]. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 2013, 8(1): 65-71. DOI:10.1016/j.cbd.2012.12.002 |
| [16] |
Behringer V, Borchers C, Deschner T, et al. Measurements of salivary alpha amylase and salivary cortisol in hominoid primates reveal within-species consistency and between-species differences[J]. PLoS One, 2013, 8(4): e60773. DOI:10.1371/journal.pone.0060773 |
| [17] |
Blesák K, Janeček Š. Two potentially novel amylolytic enzyme specificities in the prokaryotic glycoside hydrolase α-amylase family GH57[J]. Microbiology, 2013, 159: 2584-2593. DOI:10.1099/mic.0.071084-0 |
| [18] |
Fan XY, Yu T, Li Z, et al. Luteimonas abyssi sp. nov., isolated from deep-sea sediment[J]. International Journal of Systematic and Evolutionary Microbiology, 2014, 64: 668-674. DOI:10.1099/ijs.0.056010-0 |
| [19] |
Song QH, Wang Y, Yin C, et al. LaaA, a novel high-active alkalophilic alpha-amylase from deep-sea bacterium Luteimonas abyssi XH031T[J]. Enzyme and Microbial Technology, 2016, 90: 83-92. DOI:10.1016/j.enzmictec.2016.05.003 |
| [20] |
Machius M, Declerck N, Huber R, et al. Activation of Bacillus licheniformis α-amylase through a disorder→order transition of the substrate-binding site mediated by a calcium–sodium–calcium metal triad[J]. Structure, 1998, 6(3): 281-292. DOI:10.1016/S0969-2126(98)00032-X |
| [21] |
Suzuki Y, Ito N, Yuuki T, et al. Amino acid residues stabilizing a Bacillus alpha-amylase against irreversible thermoinactivation[J]. Journal of Biological Chemistry, 1989, 264(32): 18933-18938. |
| [22] |
Khemakhem B, Ali MB, Aghajari N, et al. The importance of an extra loop in the B-domain of an α-amylase from B. stearothermophilus US100[J]. Biochemical and Biophysical Research Communications, 2009, 385(1): 78-83. DOI:10.1016/j.bbrc.2009.04.137 |
| [23] |
Ali MB, Khemakhem B, Robert X, et al. Thermostability enhancement and change in starch hydrolysis profile of the maltohexaose-forming amylase of Bacillus stearothermophilus US100 strain[J]. Biochemical Journal, 2006, 394(1): 51-56. DOI:10.1042/BJ20050726 |
| [24] |
Haki GD, Rakshit SK. Developments in industrially important thermostable enzymes: a review[J]. Bioresource Technology, 2003, 89(1): 17-34. DOI:10.1016/S0960-8524(03)00033-6 |
| [25] |
Sanchez AC, Ravanal MC, Andrews BA, et al. Heterologous expression and biochemical characterization of a novel cold-active α-amylase from the Antarctic bacteria Pseudoalteromonas sp. 2-3[J]. Protein Expression and Purification, 2019, 155: 78-85. DOI:10.1016/j.pep.2018.11.009 |
| [26] |
Cavicchioli R, Charlton T, Ertan H, et al. Biotechnological uses of enzymes from psychrophiles[J]. Microbial Biotechnology, 2011, 4(4): 449-460. DOI:10.1111/j.1751-7915.2011.00258.x |
| [27] |
Margesin R, Feller G. Biotechnological applications of psychrophiles[J]. Environmental Technology, 2010, 31(8/9): 835-844. |
| [28] |
Groudieva T, Kambourova M, Yusef H, et al. Diversity and cold-active hydrolytic enzymes of culturable bacteria associated with Arctic sea ice, Spitzbergen[J]. Extremophiles, 2004, 8(6): 475-488. DOI:10.1007/s00792-004-0409-0 |
| [29] |
Sarmiento F, Peralta R, Blamey JM. Cold and hot extremozymes: industrial relevance and current trends[J]. Frontiers in Bioengineering and Biotechnology, 2015, 3: 148. |
| [30] |
Arabacı N, Arıkan B. Isolation and characterization of a cold-active, alkaline, detergent stable α-amylase from a novel bacterium Bacillus subtilis N8[J]. Preparative Biochemistry and Biotechnology, 2018, 48(5): 419-426. DOI:10.1080/10826068.2018.1452256 |
| [31] |
Wang XF, Kan GF, Ren XL, et al. Molecular cloning and characterization of a novel α-amylase from antarctic sea ice bacterium Pseudoalteromonas sp. M175 and its primary application in detergent[J]. BioMed Research International, 2018, 2018: 3258383. |
| [32] |
Place SP, Hofmann GE. Constitutive expression of a stress-inducible heat shock protein gene, hsp70, in phylogenetically distant Antarctic fish[J]. Polar Biology, 2005, 28(4): 261-267. DOI:10.1007/s00300-004-0697-y |
| [33] |
Cavicchioli R, Siddiqui KS, Andrews D, et al. Low-temperature extremophiles and their applications[J]. Current Opinion in Biotechnology, 2002, 13(3): 253-261. DOI:10.1016/S0958-1669(02)00317-8 |
| [34] |
Mabrouk SB, Aghajari N, Ali MB, et al. Enhancement of the thermostability of the maltogenic amylase MAUS149 by Gly312Ala and Lys436Arg substitutions[J]. Bioresource Technology, 2011, 102(2): 1740-1746. DOI:10.1016/j.biortech.2010.08.082 |
| [35] |
Cesarini S, Bofill C, Pastor FIJ, et al. A thermostable variant of P. aeruginosa cold-adapted LipC obtained by rational design and saturation mutagenesis[J]. Process Biochemistry, 2012, 47(12): 2064-2071. DOI:10.1016/j.procbio.2012.07.023 |
| [36] |
Yang G, Yao H, Mozzicafreddo M, et al. Rational engineering of a cold-adapted α-amylase from the Antarctic ciliate Euplotes focardii for simultaneous improvement of thermostability and catalytic activity[J]. Applied and Environmental Microbiology, 2017, 83(13): e00449-17. |
 2019, Vol. 46
2019, Vol. 46