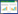扩展功能
文章信息
- 张佳鑫, 蒋一凡, 雷昕诺, 秦艺文, 湛洋, 王乃东
- ZHANG Jia-Xin, JIANG Yi-Fan, LEI Xin-Nuo, QIN Yi-Wen, ZHAN Yang, WANG Nai-Don
- Src和Abl酪氨酸蛋白激酶家族参与病原微生物感染的研究进展
- Research advances in Src and Abl tyrosine protein kinase family involved in pathogenic microbial infection
- 微生物学通报, 2019, 46(10): 2781-2786
- Microbiology China, 2019, 46(10): 2781-2786
- DOI: 10.13344/j.microbiol.china.180793
-
文章历史
- 收稿日期: 2018-10-14
- 接受日期: 2019-02-13
- 网络首发日期: 2019-04-11
2. 宜春学院数学与计算机科学学院 江西 宜春 336000;
3. 兽用蛋白质工程疫苗湖南省重点实验室 湖南 长沙 410128;
4. 兽用疫苗逆向创制湖南省工程研究中心 湖南 长沙 410128
2. College of Mathematics and Computer Science, Yichun University, Yichun, Jiangxi 336000, China;
3. Hunan Provincial Key Laboratory of Protein Engineering in Animal Vaccines, Changsha, Hunan 410128, China;
4. Research & Development Center for Animal Reverse Vaccinology of Hunan Province, Changsha, Hunan 410128, China
酪氨酸激酶(Tyrosine kinase,TK)作为一种酪氨酸特异性蛋白激酶的家族,在介导细胞外信号向胞内传导过程中起重要作用,它通过ATP上的磷酸基团作用于靶蛋白的酪氨酸残基,并将靶蛋白的酪氨酸残基磷酸化,从而将信号从细胞表面传递至细胞质和细胞核,调节细胞生理过程[1]。根据是否存在细胞膜受体可将其分成受体型和非受体型两大类:受体酪氨酸激酶(Receptor tyrosine kinases,RTKs)为一类多功能的细胞表面跨膜蛋白,具有调节细胞增殖、分化以及死亡等作用;非受体酪氨酸激酶(Nonreceptor tyrosine kinase,NRTK)是一个较大的激酶家族,具有催化蛋白的酪氨酸磷酸化功能,而Src和Abl家族激酶是NRTK的重要成员[2]。在细胞应答刺激(外环境或内环境)时,Src和Abl家族激酶能被激活,并在细胞的免疫应答、粘附、分裂、凋亡、迁移、骨架重塑、基因表达和转录等多种复杂的生理过程中发挥重要作用[3-4]。
Src及Abl激酶家族在参与病原微生物感染过程中涉及病原和细胞蛋白组成结构、细胞信号通路以及相关作用机制等,其扮演的角色比较复杂。Src及Abl激酶家族可参与病原微生物的入侵。研究发现柯萨奇病毒(Coxsackie virus,CV)在入侵上皮细胞过程中通过激活Abl和Fyn激酶来完成入侵[5];Abl家族激酶可通过调节病毒附着所需的神经节苷脂,促进多瘤病毒的细胞入侵[6]等。Src及Abl激酶家族也可参与多种病原微生物的增殖。研究发现人免疫缺陷病毒1型(Human immunodeficiency virus type 1,HIV-1) Nef蛋白通过PXXP基序(P为脯氨酸,X为任意氨基酸)与Src家族激酶成员(Hck、Lyn、Fyn和Src)的SH3结构域结合,诱导T细胞信号传递途径的改变,从而促进HIV-1增殖[7];而丙型肝炎病毒(Hepatitis C virus,HCV)非结构蛋白5A (NS5A)通过与含有SH3结构域的蛋白(包括Grb2、两性蛋白Ⅱ、PI3K和Src家族的成员)相互作用调节免疫功能,促进HCV病毒复制[8];肠道病毒71型的感染可以因Src家族激酶成员Fyn的下调被明显抑制[9];此外,也有研究发现Src和Abl激酶协同作用于幽门螺杆菌(Helicobacter pylori)的感染过程、Src和Syk激酶参与登革热病毒(Dengue virus,DENV)的复制等。充分了解Src及Abl激酶家族参与病原微生物感染的重要生物学作用,有助于揭示细胞信号传导机制,并对病原微生物感染防治起到积极作用。
1 Src和Abl家族激酶的组成与结构特点Src家族激酶包含一个或若干个Src同源结构域(SH1、SH2、SH3和SH4)(图 1),包含9个具有相似结构和功能的成员(c-Src、Lck、Hck、Fyn、Blk、Lyn、Fgr、Yes和Yrk)[10]。其中SH1是酪氨酸激酶蛋白的典型结构功能域,是ATP结合区和自身磷酸化位点;SH2是激活Ras基因和传导促有丝分裂信号的重要结构域;SH3是与鸟氨酸释放因子和GTP酶活化蛋白相互作用的重要结构域;SH4由15−17个氨基酸残基组成,具有修饰脂肪酸的作用[11]。

|
| 图 1 Src家族激酶结构特点 Figure 1 The structure characteristics of Src family kinase |
|
|
Abl激酶家族由Abl (Abelson酪氨酸激酶,也称c-Abl或Abl1)和Abl2组成。Abl1和Abl2激酶具有位于中央的SH3-SH2-SH1结构域(图 2),它们之间具有超过90%的序列相似性,它们都具有氨基末端“帽子”区域、独特的长羧基末端尾以及与蛋白质(如p53,ATM等)的相互作用位点[12]。Abl1具有3个PXXP基序,可与含SH3结构域的衔接蛋白(Adaptor protein,如Crk和Nck)相互作用,同时该基序具有核定位信号,能调节其在细胞核和细胞质之间穿梭,而Abl2基因由于缺乏核定位信号,因此主要定位于细胞质和细胞器内富含F-肌动蛋白的区域[13]。

|
| 图 2 Abl家族激酶结构特点 Figure 2 The structure characteristics of Abl family kinase |
|
|
目前已证实Src和Abl家族激酶参与细胞的肌动蛋白蠕动、肌动蛋白基座形成和细胞扩散等生理过程[14],而从Src和Abl家族激酶角度出发,探究病原微生物感染机制的研究报道日渐增多(表 1)。
| 病原 Pathogen |
相关疾病 Associated diseases |
致病过程 Pathogenic process |
参与的酪氨酸激酶 Tyrosine kinases involved |
参考文献 References |
| 柯萨奇病毒 Coxsackie virus |
脑膜炎;心肌炎 Meningitis; Myocarditis |
入侵 Invasion |
Abl; Fyn | [5] |
| 多瘤病毒 Polyomavirus |
肉瘤;癌症 Sarcoma; Cancer |
病毒内化;复制 Virus internalization; Replication |
Arg; Abl | [6] |
| 丙型肝炎病毒 Hepatitis C virus |
肝炎;肝硬化 Hepatitis; Hepatocirrhosis |
病毒复制 Viral replication |
Hck; Lck; Lyn; Fyn | [8] |
| 痘苗病毒 Vaccinia virus |
天花 Smallpox |
肌动蛋白尾部形成 Actin-tail formation |
Abl; Src | [15] |
| 埃博拉病毒 Ebola virus |
埃博拉出血热 Ebola hemorrhagic fever |
入侵 Invasion |
Src | [16] |
| 无形体 Anaplasma |
粒细胞无形体病 Granulocytic anaplasmosis |
入侵 Invasion |
Abl | [17] |
| 幽门螺杆菌 Helicobacter pylori |
胃炎;胃溃疡;胃癌 Gastritis; Ulcer; Cancer |
细胞扩散;入侵 Cell scattering; Invasion |
Src; Abl; Arg | [18-19] |
| 肠致病性大肠杆菌 Enteropathogenic Escherichia coli (EPEC) |
腹泻 Diarrhoea |
基座形成 Pedestal formation |
Abl | [20-23] |
| 登革热病毒 Dengue virus (DENV) |
登革热 Dengue |
病毒复制 Viral replication |
Src; Syk | [24-27] |
| 猪繁殖与呼吸综合征病毒 Porcine reproductive and respiratory syndrome virus (PRRSV) |
猪繁殖与呼吸综合征 Porcine reproductive and respiratory syndrome |
入侵 Invasion |
Fyn; Hck | [28] |
H. pylori是引起胃十二指肠疾病的一种重要病原菌。细胞毒素相关蛋白A (CagA)是H. pylori蛋白编码的一种重要毒力因子[29]。研究表明,CagA阳性菌编码的Ⅳ型分泌系统(T4SS)将CagA运输到胃上皮细胞质。而CagA蛋白羧基端在结构上有氨基酸序列基序(Glu-Pro-Ile-Tyr-Ala,EPIYA基序),该基序中的酪氨酸位点会在Src和Abl激酶作用下发生磷酸化[18]。
在H. pylori感染的初始阶段,c-Src磷酸化EPIYA-C或EPIYA-D基序(EPIYA基序可分为EPIYA-A、EPIYA-B、EPIYA-C和EPIYA-D) 0.5−2.0 h后,CagA在c-Src的作用下发生磷酸化,进而磷酸化的CagA (CagAPY)通过经典的“负反馈环”机制抑制其自身的酪氨酸激酶,导致c-Src失活,从而诱导细胞骨架重排;在感染晚期(2−8 h),CagAPY在Abl激酶的作用下持续磷酸化,诱导严重的胃疾病(如胃炎、胃溃疡、胃癌或粘膜相关淋巴组织淋巴瘤)[19]。
2.2 Abl激酶与EPECEPEC是一种能诱导肌动蛋白细胞骨架重组并在肠上皮细胞上形成肌动蛋白基座的病原体,可引起腹泻病和其他并发症等病变。肌动蛋白基座结构的形成是EPEC感染的标志,该基座的形成取决于针筒状Ⅲ型分泌系统(T3SS)介导的细菌蛋白Tir嵌入质膜,而Tir蛋白在与细胞内Src和Abl家族酪氨酸激酶相互作用时磷酸化[20]。有研究表明,Tir通过富含脯氨酸的区域(PRR)与Tec和Abl激酶SH3结构域结合,促使Tir在Y474位点磷酸化(TirPY474),随后磷酸化TirP再与激酶SH2结构域相互作用[21-22]。此外,Abl底物能与衔接蛋白CrkII、Grb2及p130Cas (也是EPEC基座形成中的主要蛋白质)相互作用,这种相互作用提示Abl参与由感染肠道病原启动的肌动蛋白-细胞骨架结构的动态变化[23]。
2.3 Src激酶和脾酪氨酸激酶(Syk)与DENVDENV是一种蚊虫传播的黄病毒,研究表明C端Src激酶(Csk)是参与DENV复制的激酶之一,当Csk缺乏时,DENV在细胞中的感染率急剧减少[24]。已知Csk活性受自身不同结构域间的分子内相互作用调节,Csk的SH3结构域与磷酸化激酶结构域结合能增强Csk激酶活性,但Csk过表达可通过阻断Src激酶家族信号传导而负反馈调控DENV感染[25]。
此外,属于非受体酪氨酸激酶家族的脾酪氨酸激酶(Syk)也参与DENV感染,研究表明急性DENV感染期间,登革2型病毒(DENV-2)免疫复合物能在1 h内激活Syk,激活后的Syk进一步诱导细胞因子IL-1β、肿瘤坏死因子和IL-6 mRNA水平升高,接种4 h后则能促进单核细胞分泌成熟的IL-1[26]。同时Syk激活ERK1/2通路促进IL-1分泌,从而促进DENV感染[27]。因此,Src和Syk激酶能增强DENV感染,同时有望作为干扰急性DENV感染的新治疗靶标。
2.4 Fyn、Hck激酶和PRRSVPRRSV能引起一种高度传染性疾病,俗称“蓝耳病”,通常导致Th2免疫应答降低和持久性病毒感染[30]。PRRSV核衣壳蛋白中含有由脯氨酸、谷氨酸、赖氨酸、脯氨酸、组氨酸、苯丙氨酸和脯氨酸组成的氨基酸基序(PEKPHFP),该基序能与宿主细胞酪氨酸激酶蛋白[蛋白酪氨酸激酶(Fyn)和造血细胞激酶(Hck)]的Src同源结构域(SH3)结合[31],促进PRRSV的侵染。而PRRSV核衣壳蛋白中的第56位氨基酸残基(P56)对于完成该病毒的生命周期至关重要,其突变会破坏基序与SH3的相互作用,导致PRRSV丧失感染性[28]。
3 Src和Abl家族激酶与病原微生物的脯氨酸基序(PXXP)互作多种病原微生物蛋白含有PXXP基序,如上文已经提到的HIV-1、HCV、痘苗病毒和PRRSV。此外,还有猪圆环病毒2型(Porcine circovirus type 2,PCV2)[32]、乙型肝炎病毒[33]、戊型肝炎病毒(Hepatitis E virus,HEV)[34]和禽呼肠孤病毒[35]等。而PXXP基序能与激酶SH3结构域互作,这种互作有助于调节细胞信号转导、膜运输和细胞骨架重排[36],病毒也可以操纵细胞蛋白复合物与宿主细胞中含SH3的靶蛋白相互作用,以完成病毒生命周期。基于此,许多学者从病原微生物的结构入手对PXXP基序与激酶SH3结构域互作展开研究,比如:Wang等[32]基于对PCV2核衣壳(Capsid)蛋白结构分析发现,致病性PCV2 loop CT中PXXP基序和Loop HI中IYD基序有磷酸化修饰,而非致病性猪圆环病毒1型缺少这两个对应结构,从而预测该基序能与酪氨酸激酶SH3结构域互作;Yang等[34]研究发现HEV基因1型表面的线性VP13表位中PXXP基序能与SH3结构域结合,该发现有助于HEV的深入生物学研究;Martínez-Bonet等[37]发现HIV-1 Nef蛋白PXXP基序能与Src家族酪氨酸激酶(尤其是Hck和Lyn激酶) SH3结构域相互作用,从而促进HIV-1体外复制。探索酪氨酸蛋白激酶家族与病原微生物之间的机制有望成为研究病原微生物致病机理的新切入点。
4 小结与展望综上所述,Src和Abl家族激酶具有独特的结构域,能与多种病原微生物的效应蛋白相互作用诱发细胞信号转导,从而参与微生物的入侵、病毒释放、肌动蛋白运动性和细胞扩散等。终止由该类激酶引发的异常信号转导是否能成为防控病原微生物感染的新靶点?尤其针对一些病毒性感染,从激酶角度入手能否抑制或阻断病毒复制和释放?这些问题的解决,仍需对Src和Abl家族激酶参与微生物感染机制展开后续深入研究,比如使用激酶抑制剂或基因干扰方法调控酪氨酸激酶的表达,从而扩展病原微生物的致病机制研究和防控的应用研发。
| [1] |
Mao LM, Geosling R, Penman B, et al. Local substrates of non-receptor tyrosine kinases at synaptic sites in neurons[J]. Acta Physiologica Sinica, 2017, 69(5): 657-665. |
| [2] |
Siveen KS, Prabhu KS, Achkar IW, et al. Role of non receptor tyrosine kinases in hematological malignances and its targeting by natural products[J]. Molecular Cancer, 2018, 17: 31. DOI:10.1186/s12943-018-0788-y |
| [3] |
Khatri A, Wang J, Pendergast AM. Multifunctional Abl kinases in health and disease[J]. Journal of Cell Science, 2016, 129(1): 9-16. |
| [4] |
Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk[J]. Cold Spring Harbor Perspectives in Biology, 2011, 3(3): a002352. |
| [5] |
Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions[J]. Cell, 2006, 124(1): 119-131. DOI:10.1016/j.cell.2005.10.035 |
| [6] |
Swimm A, Bornmann WG, Jiang MX, et al. Abl family tyrosine kinases regulate sialylated ganglioside receptors for polyomavirus[J]. Journal of Virology, 2010, 84(9): 4243-4251. DOI:10.1128/JVI.00129-10 |
| [7] |
Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4[J]. The EMBO Journal, 1995, 14(3): 484-491. DOI:10.1002/j.1460-2075.1995.tb07024.x |
| [8] |
Macdonald A, Mazaleyrat S, McCormick C, et al. Further studies on hepatitis C virus NS5A-SH3 domain interactions: identification of residues critical for binding and implications for viral RNA replication and modulation of cell signalling[J]. Journal of General Virology, 2005, 86(4): 1035-1044. DOI:10.1099/vir.0.80734-0 |
| [9] |
Zhu YZ, Qi ZT, Xu QQ, et al. Application of protein tyrosine kinase FYN proto-oncogene in controlling enterovirus 71 infections: CN, 105288654A[P]. 2016-02-03 (in Chinese) 朱勇喆, 戚中田, 徐庆强, 等.蛋白酪氨酸激酶FYN癌基因在防治肠道病毒71型感染中的应用: 中国, 105288654A[P]. 2016-02-03 |
| [10] |
Liu ST, Pham H, Pandol SJ, et al. Src as the link between inflammation and cancer[J]. Frontiers in Physiology, 2014, 4: 416. |
| [11] |
Arbesú M, Maffei M, Cordeiro TN, et al. The unique domain forms a fuzzy intramolecular complex in Src family kinases[J]. Structure, 2017, 25(4): 630-640.e4. DOI:10.1016/j.str.2017.02.011 |
| [12] |
Wang J, Pendergast AM. The emerging role of ABL kinases in solid tumors[J]. Trends in Cancer, 2015, 1(2): 110-123. DOI:10.1016/j.trecan.2015.07.004 |
| [13] |
Antoku S, Saksela K, Rivera GM, et al. A crucial role in cell spreading for the interaction of Abl PxxP motifs with Crk and Nck adaptors[J]. Journal of Cell Science, 2008, 121(18): 3071-3082. DOI:10.1242/jcs.031575 |
| [14] |
Hojjat-Farsangi M. Targeting non-receptor tyrosine kinases using small molecule inhibitors: an overview of recent advances[J]. Journal of Drug Targeting, 2016, 24(3): 192-211. DOI:10.3109/1061186X.2015.1068319 |
| [15] |
Humphries AC, Donnelly SK, Way M. Cdc42 and the Rho GEF intersectin-1 collaborate with Nck to promote N-WASP-dependent actin polymerisation[J]. Journal of Cell Science, 2014, 127(3): 673-685. DOI:10.1242/jcs.141366 |
| [16] |
Furuyama W, Marzi A, Carmody AB, et al. Fcγ-receptor Ⅱa-mediated Src signaling pathway is essential for the antibody-dependent enhancement of Ebola virus infection[J]. PLoS Pathogens, 2016, 12(12): e1006139. DOI:10.1371/journal.ppat.1006139 |
| [17] |
Lin MQ, Den Dulk-Ras A, Hooykaas PJJ, et al. Anaplasma phagocytophilum AnkA secreted by type Ⅳ secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection[J]. Cellular Microbiology, 2007, 9(11): 2644-2657. DOI:10.1111/j.1462-5822.2007.00985.x |
| [18] |
Egtmeyer N, Neddermann M, Asche CI, et al. Subversion of host kinases: a key network in cellular signaling hijacked by Helicobacter pylori CagA[J]. Molecular Microbiology, 2017, 105(3): 358-372. DOI:10.1111/mmi.13707 |
| [19] |
Lind J, Backert S, Hoffmann R, et al. Systematic analysis of phosphotyrosine antibodies recognizing single phosphorylated EPIYA-motifs in CagA of East Asian-type Helicobacter pylori strains[J]. BMC Microbiology, 2016, 16: 201. DOI:10.1186/s12866-016-0820-6 |
| [20] |
Campellone KG. Cytoskeleton-modulating effectors of enteropathogenic and enterohaemorrhagic Escherichia coli: Tir, EspFU and actin pedestal assembly[J]. FEBS Journal, 2010, 277(11): 2390-2402. DOI:10.1111/j.1742-4658.2010.07653.x |
| [21] |
Young JC, Clements A, Lang AE, et al. The Escherichia coli effector EspJ blocks Src kinase activity via amidation and ADP ribosylation[J]. Nature Communications, 2014, 5: 5887. DOI:10.1038/ncomms6887 |
| [22] |
Gruenheid S, DeVinney R, Bladt F, et al. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells[J]. Nature Cell Biology, 2001, 3(9): 856-859. DOI:10.1038/ncb0901-856 |
| [23] |
Wong ARC, Raymond B, Collins JW, et al. The enteropathogenic E. coli effector EspH promotes actin pedestal formation and elongation via WASP-interacting protein (WIP)[J]. Cellular Microbiology, 2012, 14(7): 1051-1070. DOI:10.1111/j.1462-5822.2012.01778.x |
| [24] |
Kumar R, Agrawal T, Khan NA, et al. Identification and characterization of the role of c-terminal Src kinase in dengue virus replication[J]. Scientific Reports, 2016, 6: 30490. DOI:10.1038/srep30490 |
| [25] |
Chu JJH, Yang PL. c-Src protein kinase inhibitors block assembly and maturation of dengue virus[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(9): 3520-3525. DOI:10.1073/pnas.0611681104 |
| [26] |
Callaway JB, Smith SA, McKinnon KP, et al. Spleen tyrosine kinase (Syk) mediates IL-1β induction by primary human monocytes during antibody-enhanced dengue virus infection[J]. Journal of Biological Chemistry, 2015, 290(28): 17306-17320. DOI:10.1074/jbc.M115.664136 |
| [27] |
Acosta EG, Bartenschlager R. Paradoxical role of antibodies in dengue virus infections: considerations for prophylactic vaccine development[J]. Expert Review of Vaccines, 2015, 15(4): 467-482. |
| [28] |
Kenney SP, Meng XJ. An SH3 binding motif within the nucleocapsid protein of porcine reproductive and respiratory syndrome virus interacts with the host cellular signaling proteins STAMI, TXK, Fyn, Hck, and cortactin[J]. Virus Research, 2015, 204: 31-39. DOI:10.1016/j.virusres.2015.04.004 |
| [29] |
Krisch LM, Posselt G, Hammerl P, et al. CagA phosphorylation in Helicobacter pylori-infected B cells is mediated by the nonreceptor tyrosine kinases of the Src and Abl families[J]. Infection and Immunity, 2016, 84(9): 2671-2680. DOI:10.1128/IAI.00349-16 |
| [30] |
Hu Y, Cong XY, Chen L, et al. Synergy of TLR3 and 7 ligands significantly enhances function of DCs to present inactivated PRRSV antigen through TRIF/MyD88-NF-κB signaling pathway[J]. Scientific Reports, 2016, 6: 23977. DOI:10.1038/srep23977 |
| [31] |
Mayer BJ, Eck MJ. SH3 domains: Minding your p's and q's[J]. Current Biology, 1995, 5(4): 364-367. DOI:10.1016/S0960-9822(95)00073-X |
| [32] |
Wang ND, Zhan Y, Wang AB, et al. In silico analysis of surface structure variation of PCV2 capsid resulting from loop mutations of its capsid protein (Cap)[J]. Journal of General Virology, 2016, 97(12): 3331-3344. DOI:10.1099/jgv.0.000634 |
| [33] |
Feng HX, Tan TL, Niu DD, et al. HBV X protein interacts with cytoskeletal signaling proteins through SH3 binding[J]. Frontiers in Bioscience (Elite edition), 2010, 2: 143-150. |
| [34] |
Yang YL, Lin SL, Nan YC, et al. A linear surface epitope in a proline-rich region of ORF3 product of genotype 1 hepatitis E virus[J]. Viruses, 2016, 8(8): 227. DOI:10.3390/v8080227 |
| [35] |
Xie LJ, Xie ZX, Huang L, et al. The study of the activation of PI3K/Akt pathway by the σA and σNS protein of avian reovirus[J]. Chinese Journal of Animal and Veterinary Sciences, 2016, 47(7): 1451-1458. (in Chinese) 谢丽基, 谢芝勋, 黄莉, 等. 禽呼肠孤病毒σA和σNS蛋白激活PI3K/Akt信号通路的研究[J]. 畜牧兽医学报, 2016, 47(7): 1451-1458. |
| [36] |
Pagano MA, Tibaldi E, Palù G, et al. Viral proteins and Src family kinases: mechanisms of pathogenicity from a "liaison dangereuse"[J]. World Journal of Virology, 2013, 2(2): 71-78. DOI:10.5501/wjv.v2.i2.71 |
| [37] |
Martínez-Bonet M, Palladino C, Briz V, et al. A conserved GPG-Motif in the HIV-1 nef core is required for principal nef-activities[J]. PLoS One, 2015, 10(12): e0145239. DOI:10.1371/journal.pone.0145239 |
 2019, Vol. 46
2019, Vol. 46