扩展功能
文章信息
- 朱平, 吕均, 薛娟, 杨瑾, 孟昆, 李姗
- ZHU Ping, LÜ Jun, XUE Juan, YANG Jin, MENG Kun, LI Shan
- 肠道病原菌Ⅲ型分泌系统效应蛋白调控宿主细胞NF-κB和MAPK信号通路的研究进展
- Research progress in regulating host NF-κB and MAPK pathways by the type Ⅲ secretion system (T3SS) effectors of intestinal pathogenic bacteria
- 微生物学通报, 2019, 46(10): 2763-2771
- Microbiology China, 2019, 46(10): 2763-2771
- DOI: 10.13344/j.microbiol.china.180823
-
文章历史
- 收稿日期: 2018-10-19
- 接受日期: 2019-02-26
- 网络首发日期: 2019-04-16
2. 华中农业大学生命科学技术学院 湖北 武汉 430070;
3. 华中农业大学生物医学中心 湖北 武汉 430070
2. College of Life Science and Technology, Huazhong Agricultural University, Wuhan, Hubei 430070, China;
3. Bio-Medical Center, Huazhong Agricultural University, Wuhan, Hubei 430070, China
病原细菌感染对人类健康构成了严重的威胁,是医学界一直面临的重要课题。一类具有Ⅲ型分泌系统的肠道致病细菌,如具有YOP系统的耶尔森菌(Yersinia)[1]、具有Mxi-spa系统的志贺菌(Shigella)[2]、具有SPI-1和SPI-2系统的沙门菌(Salmonella)[3],以及具有LEE和Non-LEE毒力岛编码系统的肠道A/E致病菌等[4],均可以通过T3SS将效应蛋白“注射”到宿主细胞中,模拟和操纵宿主细胞的多种信号转导通路,包括细胞凋亡、细胞自噬和炎症反应等,从而有效地逃逸宿主细胞的防御反应,增强感染性和致病性[5-6]。病原菌的感染往往会引起宿主的炎症反应,为了维持在宿主细胞内的生存,病原菌已经进化出精细的途径来破坏宿主的先天性防御。胞外和胞内的模式识别受体(Pattern recognition receptors,PRRs)识别病原体相关分子模式(Pathogen-associated molecular patterns,PAMPs),进而刺激炎症信号级联反应[7]。病原菌感染宿主时,宿主会通过一系列的信号转导来激活丝裂原活化蛋白激酶(Mitogen-activated protein kinase,MAPK)以及核转录因子KappaB (Nuclear factor-κB,NF-κB)信号通路,从而启动下游抗感染相关基因和炎症因子的转录[5-6]。在病原菌与宿主细胞的相互作用过程中,大多数病原菌的效应蛋白都能通过这两条通路来干扰宿主炎症反应。本文就肠致病性大肠杆菌(Enteropathogenic Escherichia coli,EPEC)、肠出血性大肠杆菌(Enterohaemorrhagic E. coli,EHEC)、鼠类柠檬酸杆菌(Citrobacter rodentium)、志贺菌、耶尔森菌和沙门菌的T3SS效应蛋白在调控宿主细胞NF-κB和MAPK信号通路中的作用进行综述。
1 T3SS效应蛋白对宿主细胞NF-κB信号通路的影响NF-κB是真核细胞中重要的转录调节因子,通常以p50-p65异二聚体的形式与其抑制性蛋白IκB (Inhibitor kappaB)结合而呈非活化状态。当细胞受到刺激时,NF-κB的诱导剂通过细胞膜激活胞浆中的IKK (IκB kinase)使IκBα发生磷酸化,而磷酸化的IκB被泛素结合酶识别发生快速泛素化,继而迅速被蛋白酶体降解,胞浆中游离的NF-κB被转运至细胞核内,与包括细胞因子和趋化因子在内的靶基因启动子区域NF-κB结合位点相结合,从而启动靶基因转录和表达,进而造成炎症反应。IκB与NF-κB结合可使活化的NF-κB恢复到胞质中的非活性形式,从而抑制其介导的炎性基因转录[8]。NF-κB信号通路在宿主抵御病原菌感染的过程中起到了非常重要的作用。宿主细胞利用Toll样受体(Toll-like receptors,TLRs)识别PAMPs,激活NF-κB信号通路,从而启动炎症因子等抗感染基因的转录,拮抗病原菌的感染[8]。与此同时,很多病原菌感染宿主时,都会将效应蛋白或者毒素分泌到宿主细胞内来抑制宿主的信号通路,从而利于自身的繁殖和扩散。
EPEC、EHEC和C. rodentium属于引起黏附/抹平(Attaching/Effacing)损伤特点的A/E肠道致病菌,它们的T3SS效应蛋白NleE、NleF、NleB、NleC、NleH、Tir、EspL以及EspT可以协同靶向NF-κB信号途径中的不同蛋白。研究发现,A/E致病菌的效应蛋白NleE通过阻断p65转位到细胞核,从而抑制NF-κB激活[9-11]。深入研究显示,NleE是一类新的S-腺苷甲硫氨酸(S-adenosyl-L- methionine,SAM)依赖的甲基转移酶,能够特异性地修饰TAB 2/3锌指结构域中一个螯合锌离子的半胱氨酸残基。这一甲基化修饰使得锌指结构域失去锌离子,从而无法结合来自上游的泛素链信号,并最终导致NF-κB信号通路被抑制[12]。EPEC感染期间,NleF促进肿瘤坏死因子-α (Tumor necrosis factor-α,TNF-α)刺激引起的NF-κB核转位和IL-8分泌,NleF通过C末端影响IL-8的分泌,但具体机制有待进一步研究[13]。NleB也可以阻止NF-κB亚基p65转位到核[9],但与NleE不同的是,A/E致病菌的效应蛋白NleB转移N-乙酰葡萄糖胺(N-acetyl-D-glucosamine,GlcNAc)到TNF受体相关死亡结构域蛋白(TNF receptor-associated death domain,TRADD)、FAS相关死亡结构域蛋白(FAS-associated death domain protein,FADD)和受体相互作用蛋白激酶1 (Receptor-interacting protein kinase,RIPK1)死亡结构域的精氨酸残基上,干扰死亡结构域寡聚,阻止TNF死亡受体复合物组装,抑制TNF刺激引起的NF-κB激活[14-16],这种独特的翻译后修饰抑制NF-κB介导的下游细胞因子和趋化因子的转录激活。EHEC和C. rodentium的NleB也可以糖基化修饰3-磷酸甘油醛脱氢酶(Glyceraldehyde-3-phosphate,GAPDH),从而抑制TNF受体相关因子2 (TNF receptor- associated factor 2,TRAF2)的泛素化激活,进而抑制NF-κB激活,并且EHEC的NleB1糖基化修饰GAPDH的R197和R200位点[16]。NleC是一个锌金属蛋白酶,在C. rodentium和EPEC感染期间,NleC通过和p65的N末端20个氨基酸相互作用特异性靶向p65/RelA[17-18],直接切割NF-κB的p65亚基,阻断NF-κB激活,抑制IL-8分泌[19-24]。A/E致病菌的效应蛋白NleH1和NleH2也可以抑制NF-κB激活[25-27]。NleH1和NleH2结合但不磷酸化核糖体蛋白S3 (Ribosomal protein S3)。RPS3是细胞核中NF-κB复合物的一部分,可增强p65对NF-κB依赖的启动子的结合活性[28]。NleH1是一种丝氨酸/苏氨酸蛋白激酶,可以磷酸化v-Crk肉瘤病毒CT10致癌基因样蛋白(v-Crk sarcoma virus CT10 oncogene-like protein,CRKL),CRKL和IκB激酶β (IκB kinase-β,IKKβ)相互作用导致NleH1募集到IKKβ复合物[29],该募集可以抑制RPS3和p65的核转位,导致RPS3依赖的NF-κB激活被抑制[25, 28, 30]。A/E致病菌的效应蛋白Tir通过靶向TRAF2来抑制NF-κB激活[31],也可以和宿主细胞酪氨酸磷酸酶SHP-1相互作用,以促进SHP-1募集到接头蛋白TNF受体相关因子6 (TNF receptor- associated factor 6,TRAF6)并抑制TRAF6的泛素化,进而抑制炎症因子的产生[32]。EspL是半胱氨酸蛋白酶,在EPEC感染期间,EspL切割包含同型相互作用基序(Homotypic interaction motifs,RHIM)的蛋白包括RIPK1,而RIPK1在TNF刺激引起的NF-κB激活中发挥作用,因此EspL抑制NF-κB激活[33]。相反地,在EPEC感染期间,效应蛋白EspT通过激活巨噬细胞中的NF-κB、ERK1/2和JNK信号通路诱导促炎症因子COX-2、IL-8和IL-1β的表达[34]。
越来越多的研究报道了Shigella影响NF-κB通路的T3SS效应蛋白,包括OspZ、OspG、OspI、OspF、IpgB2、OspB以及IpaH。效应蛋白OspZ和OspG分别是NleE和NleH的同源物。与NleE相似,OspZ阻断p65转位到核[9],抑制NF-κB激活[9, 35],并且通过自身的49GITR52基序,OspZ结合并甲基化TAK1结合蛋白3 (TAK1-binding protein 3,TAB 3),抑制IL-8产生[35]。而OspG通过对E2-E3泛素化复合物的影响,抑制泛素依赖的IκBα蛋白酶体降解和NF-κB激活,抑制炎症反应[36-37],这与NleH相反。OspG也显示出激酶活性,具有自磷酸化活性,这对它的功能是必需的。OspI选择性脱去泛素结合酶(E2) UBC13里的谷氨酰胺残基的酰胺基,以消除它的泛素连接酶活性,而这对于激活TRAF6-NF-κB是必需的[38]。OspF的活性影响表观遗传调节因子的磷酸化状态,导致染色质上免疫相关基因启动子处的易接近性(Accessibility)降低,进而降低NF-κB结合到启动子上的水平,从而抑制了宿主免疫应答[39-40]。Shigella感染宿主后,效应蛋白IpgB2和OspB可以激活NF-κB,并且该激活需要鸟苷酸交换因子H1 (Guanine nucleotide exchange factor-H1,GEF-H1)以NOD1和RhoA激酶依赖的方式传递信号[41]。在Shigella中发现的IpaH晶体结构表明,它属于新的E3泛素连接酶家族,在抑制先天性免疫应答中发挥作用[42]。在上皮细胞中,Shigella的IpaH0722通过泛素化TRAF2抑制蛋白激酶C介导的NF-κB激活的抑制,进而抑制炎症反应[43],Shigella的IpaH4.5通过泛素化NF-κB的p65亚基抑制NF-κB的转录活性,从而下调炎症相关因子的表达[44],IpaH9.8通过NEMO/IKKγ复合物和ABIN-1相互作用,促进ABIN-1依赖的NEMO多聚泛素化,多聚泛素化的NEMO经历蛋白酶体依赖的降解,而NEMO对于NF-κB激活是必需的,因此NF-κB的激活被抑制[45]。由此可见,Shigella的IpaH蛋白家族的泛素化酶活性在抑制NF-κB激活的过程中发挥了重要作用。
类似地,小肠结肠炎耶尔森菌(Yersinia enterocolitica)的效应蛋白YopE、鼠疫耶尔森菌(Y. pestis)的YopP以及假结核耶尔森菌(Y. pseudotuberculosis) YopJ均可靶向NF-κB通路。Y. enterocolitica的YopE抑制NF-κB激活,阻止IL-8的产生[46]。Yersinia的效应蛋白YopP抑制转化生长因子-β-激活激酶(Transforming growth factor-β- activated kinase 1,TAK1)的活性,而TAK1的活性在感染引起的NF-κB激活过程中发挥重要作用[47]。效应蛋白YopJ是一种去泛素化酶,可以去掉TRAF2、TRAF6和IκBα的泛素基团,从而抑制NF-κB激活[48]。YopJ也可以乙酰化IKKα和IKKβ激活环上的丝氨酸和苏氨酸,抑制IKK复合物的活性,从而阻止IκB的磷酸化,从而抑制TNF-α刺激引起的NF-κB激活,抑制炎症信号通路[49]。
Salmonella利用众多效应蛋白如SopB、SipA、SopE、SopE2、AvrA、GogB、SpvD、SspH1、SseL、Sseks和PipA等效应蛋白来抑制NF-κB信号通路。效应蛋白SopB通过磷酸化修饰介导NF-κB信号通路的激活,引起宿主细胞的炎症反应[50]。效应蛋白SipA通过激活核苷酸结合寡聚结构域蛋白1/2 (Nucleotide-binding oligomerization domain 1/2,NOD1/NOD2)信号通路激活NF-κB,进而引起炎症反应[51]。S. typhimurium感染引起的先天性免疫需要SopE和SopE2激活Rho家族GTP酶,进而激活NF-κB信号通路[52]。SopE激活Rac1和Cdc42,引发Nod1信号通路和Rip2介导的NF-κB依赖的炎症反应[53]。效应蛋白AvrA可使IκBα和β-catenin去泛素化,从而阻止其降解,导致NF-κB信号通路的抑制和β-catenin通路的活化。抑制NF-κB通路下游调控的炎性因子如IL-6表达,可促进β-catenin通路相关的分子如c-myc和cyclin D1表达,而活化的β-catenin又进一步抑制NF-κB通路的活化[54]。S. enterica serovar Typhimurium利用细菌效应蛋白GogB通过与Skp1和FBXO22蛋白相互作用,靶向宿主SCF E3泛素连接酶,干扰IκBα泛素化来阻止NF-κB转位到核,进而抑制促炎基因的表达[55]。效应蛋白SpvD可以和宿主Xpo2蛋白相互作用来干扰核转运蛋白KPNA (Karyopherin-α,KPNA or importin-α)的核质循环,而p65的核转位需要KPNA,因此抑制了NF-κB的激活,最终抑制促炎免疫应答[56]。E3泛素连接酶效应蛋白SspH1可以结合并使丝/苏氨酸蛋白激酶N1泛素化,从而抑制NF-κB的活性[31]。Le Negrate等的研究表明,效应蛋白SseL具有去泛素化酶活性,可阻止IκBα的泛素化降解而抑制NF-κB信号通路[57],而Mesquita等的研究表明SseL并没有在下调宿主免疫应答以及参与NF-κB通路中发挥作用[58],可能是两者处理细胞的时间不同、感染的时间不同引起的。SseL可以结合并去泛素化RPS3,进而抑制RPS3核转位[59],而RPS3可以通过增加p65亚基对靶基因启动子的亲和力而引导NF-κB到特定的κB位点[28]。S. typhimurium的效应蛋白SseK1、SseK2和SseK3是EPEC的T3SS效应蛋白NleB的同源蛋白[60],可通过T3SS注入到细胞[61]。与NleB类似[14-15],SseK1和SseK3是N-乙酰葡萄糖胺(GlcNAc)转移酶,修饰TRADD并抑制NF-κB的激活,而SseK2只有在293ET细胞中过表达时才能抑制NF-κB激活[61],另一项研究发现SseK1糖基化修饰GAPDH,而SseK2糖基化修饰FADD[16]。最新研究进一步证实了SseK3糖基化修饰TRADD[62-63]。S. typhimurium的效应蛋白PipA、GtgA和GogA组成的蛋白酶家族切割RelA (p65)和RelB NF-κB转录因子,从而抑制宿主炎症反应[64]。
根据上述分析的肠道病原菌T3SS效应蛋白参与并调控NF-κB信号通路的最新研究进展并结合参考文献[5-6],将效应蛋白参与的信号通路总结如图 1所示。

|
| 图 1 T3SS效应蛋白调控宿主细胞NF-κB信号通路[5-6] Figure 1 Host NF-κB signaling pathway modulated by T3SS effectors[5-6] 注:T3SS效应蛋白通过靶向NF-κB信号通路的不同成分调节TRAF2/TRAF6-IKK complex-NF-κB信号通路来应答TNF-α和IL-1β刺激,从而影响NF-κB核转位,进而影响炎症因子的表达.黄色方框:效应蛋白;绿色椭圆:靶蛋白;黑色小圆圈:修饰. Notes: T3SS effectors modulate TRAF2/TRAF6-IKK complex-NF-κB pathway by targeting components of the NF-κB signaling cascade in response to TNF-α and IL-1β. Therefore, nuclear translocation of NF-κB was affected, which regulate the expression of inflammatory cytokines. Yellow rectangle: Effectors; Green oval: Target proteins; Small black circle: Modification. |
|
|
MAPK是一组能被不同的细胞外刺激激活的丝氨酸-苏氨酸蛋白激酶。MAPK通路是一种三级激酶模式,包括MAPK激酶激酶(MAP kinase kinase kinase,MKKK)、MAPK激酶(MAP kinase kinase,MKK)和MAPK,这3种激酶能依次激活,共同调节细胞的生长、分化、对环境的应激适应、炎症反应等多种重要的细胞生理/病理过程。MAPK包括p38、JNK和ERK,它们与其他信号通路共同调节炎症基因的表达[65]。
EPEC的效应蛋白NleD是一种特异性切割MAPK激酶JNK和p38而非ERK的锌依赖的锌金属蛋白酶,可以阻断AP-1转录因子的核转位,进而下调炎症反应[19, 66],并且NleD的R203位点对于切割p38和抑制IL-6产生起关键作用[66]。A/E致病菌的效应蛋白NleC可以抑制p38磷酸化和IL-8分泌,从而影响炎症反应[23]。此外,A/E致病菌的效应蛋白Tir以磷酸化免疫受体酪氨酸相关的抑制基序(Immunoreceptor tyrosine-based inhibition motifs,ITIMs)依赖的方式和宿主细胞包含SH2结构域的蛋白酪氨酸磷酸酶1 (SH2-containing protein tyrosine phosphatase 1,SHP-1)相互作用,促进SHP-1募集到接头蛋白TRAF6而抑制TRAF6泛素化。Tir以磷酸化ITIM基序依赖的方式抑制EPEC感染引起的TNF和IL-6的表达及ERK、JNK和p38的激活[32]。最新研究发现,体内外实验中,NleH1可以抑制ERK1/2和p38激活,而体内实验发现NleH2可以抑制p38激活[67]。相反地,C. rodentium和EPEC的效应蛋白EspT可以通过磷酸化ERK和JNK而促进IL-8和IL-1β的表达[34]。
Shigella flexneri的效应蛋白IpaH9.8通过它的泛素连接酶活性靶向酵母的MAPK Ste7,促进降解蛋白酶体依赖的Ste7来干扰信息素应答信号[68]。OspF具有磷酸苏氨酸裂合酶活性,可以转位到核,介导不可逆的脱磷酸化作用而失活MAPKs,消除ERK、JNK和p38激酶的激活[69],这也抑制了下游组蛋白H3在Ser10位点的磷酸化作用,从而导致上皮细胞中的NF-κB无法结合位于染色质中IL-8的启动子[39]。Shigella flexneri的OspB介导ERK1/2和p38激活,通过激活细胞质磷脂酶A2 (Cytosolic phospholipase,cPLA2)导致中性粒趋化因子释放的激活,中性粒细胞募集到感染的地方破坏上皮屏障的稳定,进而促进Shigella入侵结肠粘膜[70]。
用Yersinia感染HeLa细胞表明,YopE可以强烈抑制JNK和ERK激活,阻止IL-8产生,而YopT适度地抑制这些应答[46]。YopJ可以结合MAPK激酶家族,抑制它们的磷酸化和激活[71]。
在AvrA转基因果蝇和小鼠细胞中,Salmonella的AvrA通过乙酰转移酶活性在MAPK激酶MKK4和MKK7水平阻断JNK激活,这与在小鼠肠道中ΔavrA突变株诱导更高水平的JNK激活和炎症反应的发现一致[72]。随后的进一步研究表明,S. typhimurium感染后激活ERK通路,ERK通路对AvrA的磷酸化是必需的,磷酸化的AvrA通过与MKK7的相互作用进一步削弱JNK途径,而p38或NF-κB通路不受影响[73]。与Shigella的OspF类似,Salmonella的效应蛋白SpvC具有磷酸苏氨酸裂合酶活性,可以消除ERK、JNK和p38激酶的激活[69]。SpvC可以激活ERK和JNK,减少炎症因子的释放[74-75]。与此一致,对SpvC最新的动力学研究表明该酶对MAPK的活化环(Activation loop)具有特异性[76]。
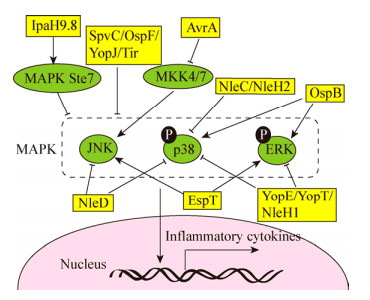
根据上述分析的肠道病原菌T3SS效应蛋白参与并调控MAPK信号通路的最新研究进展并结合参考文献[6],将效应蛋白参与的信号通路总结如图 2所示。
|
| 图 2 T3SS效应蛋白调控宿主细胞MAPK信号通路[6] Figure 2 Host MAPK signaling pathway modulated by T3SS effectors[6] 注:T3SS效应蛋白直接或间接调节MAPK的激活,MAPK包括JNK、p38和ERK,进而调节炎症因子的表达.黄色方框:效应蛋白;绿色椭圆:靶蛋白;黑色小圆圈:修饰. Notes: T3SS effectors directly or indirectly modulate the activation of MAPK, which include JNK, p38 and ERK. Therefore, the expression of inflammatory cytokines were regulated. Yellow rectangle: Effectors; Green oval: Target proteins; Small black circle: Modifation. |
|
|
病原菌在与宿主细胞的长期斗争中已进化出多种机制来逃避宿主的监视与防御,干扰宿主炎症反应,从而完成入侵并生存增殖的过程。MAPK和NF-κB两条通路都可以将细胞外的信号通过细胞内的激酶级联反应转化为细胞核内相应转录因子的激活,从而调控下游基因的转录,对抗病原微生物的感染。鉴于这两条信号通路在抗微生物信号途径中的枢纽作用,病原菌已经进化出许多机制来影响这些信号通路。不同病原菌的T3SS效应蛋白利用相似的途径来抑制炎症反应,并且同一种病原菌的效应蛋白之间又具有协同作用,但是不同病原菌的不同效应蛋白又利用了不同的分子机制作用于信号通路的不同节点。本文综述了T3SS效应蛋白对宿主细胞炎症反应MAPK和NF-κB信号通路的影响及相关分子机制的研究进展。研究这些病原体的效应蛋白与宿主反应的关系可以更好地理解它们的致病机理,从而能够帮助我们找到预防和治疗病原菌感染更基本、更有效的途径。
| [1] |
Cornelis GR, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells[J]. Molecular Microbiology, 1997, 23(5): 861-867. DOI:10.1046/j.1365-2958.1997.2731623.x |
| [2] |
Schuch R, Maurelli AT. The mxi-Spa type Ⅲ secretory pathway of Shigella flexneri requires an outer membrane lipoprotein, MxiM, for invasin translocation[J]. Infection and Immunity, 1999, 67(4): 1982-1991. |
| [3] |
Moest TP, Méresse S. Salmonella T3SSs: successful mission of the secret(ion) agents[J]. Current Opinion in Microbiology, 2013, 16(1): 38-44. DOI:10.1016/j.mib.2012.11.006 |
| [4] |
Wong ARC, Pearson JS, Bright MD, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements[J]. Molecular Microbiology, 2011, 80(6): 1420-1438. DOI:10.1111/j.1365-2958.2011.07661.x |
| [5] |
Raymond B, Young JC, Pallett M, et al. Subversion of trafficking, apoptosis, and innate immunity by type Ⅲ secretion system effectors[J]. Trends in Microbiology, 2013, 21(8): 430-441. DOI:10.1016/j.tim.2013.06.008 |
| [6] |
Pinaud L, Sansonetti PJ, Phalipon A. Host cell targeting by enteropathogenic bacteria T3SS effectors[J]. Trends in Microbiology, 2018, 26(4): 266-283. DOI:10.1016/j.tim.2018.01.010 |
| [7] |
Takeuchi O, Akira S. Pattern recognition receptors and inflammation[J]. Cell, 2010, 140(6): 805-820. DOI:10.1016/j.cell.2010.01.022 |
| [8] |
Hayden MS, Ghosh S. Signaling to NF-κB[J]. Genes & Development, 2004, 18(18): 2195-2224. |
| [9] |
Newton HJ, Pearson JS, Badea L, et al. The type Ⅲ effectors NleE and NleB from enteropathogenic E. coli and OspZ from Shigella block nuclear translocation of NF-κB p65[J]. PLoS Pathogens, 2010, 6(5): e1000898. DOI:10.1371/journal.ppat.1000898 |
| [10] |
Nadler C, Baruch K, Kobi S, et al. The type Ⅲ secretion effector NleE inhibits NF-κB activation[J]. PLoS Pathogens, 2010, 6(1): e1000743. DOI:10.1371/journal.ppat.1000743 |
| [11] |
Vossenkämper A, Marchès O, Fairclough PD, et al. Inhibition of NF-κB signaling in human dendritic cells by the enteropathogenic Escherichia coli effector protein NleE[J]. The Journal of Immunology, 2010, 185(7): 4118-4127. DOI:10.4049/jimmunol.1000500 |
| [12] |
Zhang L, Ding XJ, Cui JX, et al. Cysteine methylation disrupts ubiquitin-chain sensing in NF-κB activation[J]. Nature, 2012, 481(7380): 204-208. DOI:10.1038/nature10690 |
| [13] |
Pallett MA, Berger CN, Pearson JS, et al. The type Ⅲ secretion effector NleF of enteropathogenic Escherichia coli activates NF-κB early during infection[J]. Infection and Immunity, 2014, 82(11): 4878-4888. DOI:10.1128/IAI.02131-14 |
| [14] |
Li S, Zhang L, Yao Q, et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains[J]. Nature, 2013, 501(7466): 242-246. DOI:10.1038/nature12436 |
| [15] |
Pearson JS, Giogha C, Ong SY, et al. A type Ⅲ effector antagonizes death receptor signalling during bacterial gut infection[J]. Nature, 2013, 501(7466): 247-251. DOI:10.1038/nature12524 |
| [16] |
El Qaidi S, Chen KM, Halim A, et al. NleB/SseK effectors from Citrobacter rodentium, Escherichia coli, and Salmonella enterica display distinct differences in host substrate specificity[J]. Journal of Biological Chemistry, 2017, 292(27): 11423-11430. DOI:10.1074/jbc.M117.790675 |
| [17] |
Hodgson A, Wier EM, Fu K, et al. Metalloprotease NleC suppresses host NF-κB/inflammatory responses by cleaving p65 and interfering with the p65/RPS3 interaction[J]. PLoS Pathogens, 2015, 11(3): e1004705. DOI:10.1371/journal.ppat.1004705 |
| [18] |
Giogha C, Lung TWK, Mühlen S, et al. Substrate recognition by the zinc metalloprotease effector NleC from enteropathogenic Escherichia coli[J]. Cellular Microbiology, 2015, 17(12): 1766-1778. DOI:10.1111/cmi.12469 |
| [19] |
Baruch K, Gur-Arie L, Nadler C, et al. Metalloprotease type Ⅲ effectors that specifically cleave JNK and NF-κB[J]. The EMBO Journal, 2011, 30(1): 221-231. DOI:10.1038/emboj.2010.297 |
| [20] |
Mühlen S, Ruchaud-Sparagano MH, Kenny B. Proteasome- independent degradation of canonical NFκB complex components by the NleC protein of pathogenic Escherichia coli[J]. Journal of Biological Chemistry, 2011, 286(7): 5100-5107. DOI:10.1074/jbc.M110.172254 |
| [21] |
Yen H, Ooka T, Iguchi A, et al. NleC, a type Ⅲ secretion protease, compromises NF-κB activation by targeting p65/RelA[J]. PLoS Pathogens, 2010, 6(12): e1001231. DOI:10.1371/journal.ppat.1001231 |
| [22] |
Pearson JS, Riedmaier P, Marchès O, et al. A type Ⅲ effector protease NleC from enteropathogenic Escherichia coli targets NF-κB for degradation[J]. Molecular Microbiology, 2011, 80(1): 219-230. DOI:10.1111/j.1365-2958.2011.07568.x |
| [23] |
Sham HP, Shames SR, Croxen MA, et al. Attaching and effacing bacterial effector NleC suppresses epithelial inflammatory responses by inhibiting NF-κB and p38 mitogen-activated protein kinase activation[J]. Infection and Immunity, 2011, 79(9): 3552-3562. DOI:10.1128/IAI.05033-11 |
| [24] |
Li WQ, Liu YX, Sheng XL, et al. Structure and mechanism of a type Ⅲ secretion protease, NleC[J]. Acta Crystallographica Section D, 2014, D70: 40-47. |
| [25] |
Gao XF, Wan FY, Mateo K, et al. Bacterial effector binding to ribosomal protein S3 subverts NF-κB function[J]. PLoS Pathogens, 2009, 5(12): e1000708. DOI:10.1371/journal.ppat.1000708 |
| [26] |
Royan SV, Jones RM, Koutsouris A, et al. Enteropathogenic E. coli non-LEE encoded effectors NleH1 and NleH2 attenuate NF-κB activation[J]. Molecular Microbiology, 2010, 78(5): 1232-1245. DOI:10.1111/j.1365-2958.2010.07400.x |
| [27] |
Grishin AM, Cherney M, Anderson DH, et al. NleH defines a new family of bacterial effector kinases[J]. Structure, 2014, 22(2): 250-259. |
| [28] |
Wan FY, Anderson DE, Barnitz RA, et al. Ribosomal protein S3: a KH domain subunit in NF-κB complexes that mediates selective gene regulation[J]. Cell, 2007, 131(5): 927-939. DOI:10.1016/j.cell.2007.10.009 |
| [29] |
Pham TH, Gao XF, Singh G, et al. Escherichia coli virulence protein NleH1 interaction with the v-Crk sarcoma virus CT10 oncogene-like protein (CRKL) governs NleH1 inhibition of the ribosomal protein S3 (RPS3)/nuclear factor κB (NF-κB) pathway[J]. Journal of Biological Chemistry, 2013, 288(48): 34567-34574. DOI:10.1074/jbc.M113.512376 |
| [30] |
Wan FY, Weaver A, Gao XF, et al. IKKβ phosphorylation regulates RPS3 nuclear translocation and NF-κB function during infection with Escherichia coli strain O157:H7[J]. Nature Immunology, 2011, 12(4): 335-343. |
| [31] |
Ruchaud-Sparagano MH, Mühlen S, Dean P, et al. The enteropathogenic E. coli (EPEC) Tir effector inhibits NF-κB activity by targeting TNFα receptor-associated factors[J]. PLoS Pathogens, 2011, 7(12): e1002414. DOI:10.1371/journal.ppat.1002414 |
| [32] |
Yan DP, Wang XY, Luo LJ, et al. Inhibition of TLR signaling by a bacterial protein containing immunoreceptor tyrosine-based inhibitory motifs[J]. Nature Immunology, 2012, 13(11): 1063-1071. DOI:10.1038/ni.2417 |
| [33] |
Pearson JS, Giogha C, Mühlen S, et al. EspL is a bacterial cysteine protease effector that cleaves RHIM proteins to block necroptosis and inflammation[J]. Nature Microbiology, 2017, 2: 16258. DOI:10.1038/nmicrobiol.2016.258 |
| [34] |
Raymond B, Crepin VF, Collins JW, et al. The WxxxE effector EspT triggers expression of immune mediators in an Erk/JNK and NF-κB-dependent manner[J]. Cellular Microbiology, 2011, 13(12): 1881-1893. DOI:10.1111/j.1462-5822.2011.01666.x |
| [35] |
Zhang Y, Mühlen S, Oates CV, et al. Identification of a distinct substrate-binding domain in the bacterial cysteine methyltransferase effectors NleE and OspZ[J]. Journal of Biological Chemistry, 2016, 291(38): 20149-20162. DOI:10.1074/jbc.M116.734079 |
| [36] |
Kim DW, Lenzen G, Page AL, et al. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(39): 14046-14051. DOI:10.1073/pnas.0504466102 |
| [37] |
Zhou Y, Dong N, Hu LY, et al. The Shigella type three secretion system effector OspG directly and specifically binds to host ubiquitin for activation[J]. PLoS One, 2013, 8(2): e57558. DOI:10.1371/journal.pone.0057558 |
| [38] |
Sanada T, Kim M, Mimuro H, et al. The Shigella flexneri effector OspI deamidates UBC13 to dampen the inflammatory response[J]. Nature, 2012, 483(7391): 623-626. DOI:10.1038/nature10894 |
| [39] |
Arbibe L, Kim DW, Batsche E, et al. An injected bacterial effector targets chromatin access for transcription factor NF-κB to alter transcription of host genes involved in immune responses[J]. Nature Immunology, 2007, 8(1): 47-56. |
| [40] |
Harouz H, Rachez C, Meijer BM, et al. Shigella flexneri targets the HP1γ subcode through the phosphothreonine lyase OspF[J]. The EMBO Journal, 2014, 33(22): 2606-2622. DOI:10.15252/embj.201489244 |
| [41] |
Fukazawa A, Alonso C, Kurachi K, et al. GEF-H1 mediated control of NOD1 dependent NF-κB activation by Shigella effectors[J]. PLoS Pathogens, 2008, 4(11): e1000228. DOI:10.1371/journal.ppat.1000228 |
| [42] |
Singer AU, Rohde JR, Lam R, et al. Structure of the Shigella T3SS effector IpaH defines a new class of E3 ubiquitin ligases[J]. Nature Structural & Molecular Biology, 2008, 15(12): 1293-1301. |
| [43] |
Ashida H, Nakano H, Sasakawa C. Shigella IpaH0722 E3 ubiquitin ligase effector targets TRAF2 to inhibit PKC-NF-κB activity in invaded epithelial cells[J]. PLoS Pathogens, 2013, 9(6): e1003409. DOI:10.1371/journal.ppat.1003409 |
| [44] |
Wang F, Jiang Z, Li Y, et al. Shigella flexneri T3SS effector IpaH4.5 modulates the host inflammatory response via interaction with NF-κB p65 protein[J]. Cellular Microbiology, 2013, 15(3): 474-485. DOI:10.1111/cmi.12052 |
| [45] |
Ashida H, Kim M, Schmidt-Supprian M, et al. A bacterial E3 ubiquitin ligase IpaH9.8 targets NEMO/IKKγ to dampen the host NF-κB-mediated inflammatory response[J]. Nature Cell Biology, 2010, 12(1): 66-73. DOI:10.1038/ncb2006 |
| [46] |
Viboud GI, Mejía E, Bliska JB. Comparison of YopE and YopT activities in counteracting host signalling responses to Yersinia pseudotuberculosis infection[J]. Cellular Microbiology, 2006, 8(9): 1504-1515. DOI:10.1111/j.1462-5822.2006.00729.x |
| [47] |
Haase R, Richter K, Pfaffinger G, et al. Yersinia outer protein P suppresses TGF-β-activated kinase-1 activity to impair innate immune signaling in Yersinia enterocolitica-infected cells[J]. The Journal of Immunology, 2005, 175(12): 8209-8217. DOI:10.4049/jimmunol.175.12.8209 |
| [48] |
Zhou HL, Monack DM, Kayagaki N, et al. Yersinia virulence factor YopJ acts as a deubiquitinase to inhibit NF-κB activation[J]. The Journal of Experimental Medicine, 2005, 202(10): 1327-1332. DOI:10.1084/jem.20051194 |
| [49] |
Mittal R, Peak-Chew SY, McMahon HT. Acetylation of MEK2 and IκB kinase (IKK) activation loop residues by YopJ inhibits signaling[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(49): 18574-18579. DOI:10.1073/pnas.0608995103 |
| [50] |
Rogers LD, Brown NF, Fang Y, et al. Phosphoproteomic analysis of Salmonella-infected cells identifies key kinase regulators and SopB-dependent host phosphorylation events[J]. Science Signaling, 2011, 4(191): rs9. |
| [51] |
Keestra AM, Winter MG, Klein-Douwel D, et al. A Salmonella virulence factor activates the NOD1/NOD2 signaling pathway[J]. mBio, 2011, 2(6): e00266-11. |
| [52] |
Bruno VM, Hannemann S, Lara-Tejero M, et al. Salmonella Typhimurium type Ⅲ secretion effectors stimulate innate immune responses in cultured epithelial cells[J]. PLoS Pathogens, 2009, 5(8): e1000538. DOI:10.1371/journal.ppat.1000538 |
| [53] |
Keestra AM, Winter MG, Auburger JJ, et al. Manipulation of small Rho GTPases is a pathogen-induced process detected by NOD1[J]. Nature, 2013, 496(7444): 233-237. DOI:10.1038/nature12025 |
| [54] |
Ye ZD, Petrof EO, Boone D, et al. Salmonella effector AvrA regulation of colonic epithelial cell inflammation by deubiquitination[J]. The American Journal of Pathology, 2007, 171(3): 882-892. DOI:10.2353/ajpath.2007.070220 |
| [55] |
Pilar AVC, Reid-Yu SA, Cooper CA, et al. GogB is an anti-inflammatory effector that limits tissue damage during Salmonella infection through interaction with human FBXO22 and Skp1[J]. PLoS Pathogens, 2012, 8(6): e1002773. DOI:10.1371/journal.ppat.1002773 |
| [56] |
Rolhion N, Furniss RCD, Grabe G, et al. Inhibition of nuclear transport of NF-ĸB p65 by the Salmonella type Ⅲ secretion system effector SpvD[J]. PLoS Pathogens, 2016, 12(5): e1005653. DOI:10.1371/journal.ppat.1005653 |
| [57] |
Le Negrate G, Faustin B, Welsh K, et al. Salmonella secreted factor L deubiquitinase of Salmonella typhimurium inhibits NF-κB, suppresses IκBα ubiquitination and modulates innate immune responses[J]. The Journal of Immunology, 2008, 180(7): 5045-5056. DOI:10.4049/jimmunol.180.7.5045 |
| [58] |
Mesquita FS, Holden DW, Rolhion N. Lack of effect of the Salmonella deubiquitinase SseL on the NF-κB pathway[J]. PLoS One, 2013, 8(1): e53064. DOI:10.1371/journal.pone.0053064 |
| [59] |
Wu MM, El Qaidi S, Hardwidge PR. SseL deubiquitinates RPS3 to inhibit its nuclear translocation[J]. Pathogens, 2018, 7(4): 86. DOI:10.3390/pathogens7040086 |
| [60] |
Brown NF, Coombes BK, Bishop JL, et al. Salmonella phage ST64B encodes a member of the SseK/NleB effector family[J]. PLoS One, 2011, 6(3): e17824. DOI:10.1371/journal.pone.0017824 |
| [61] |
Günster RA, Matthews SA, Holden DW, et al. SseK1 and SseK3 type Ⅲ secretion system effectors inhibit NF-κB signaling and necroptotic cell death in Salmonella-infected macrophages[J]. Infection and Immunity, 2017, 85(3): e00010-17. |
| [62] |
Esposito D, Günster RA, Martino L, et al. Structural basis for the glycosyltransferase activity of the Salmonella effector SseK3[J]. Journal of Biological Chemistry, 2018, 293(14): 5064-5078. DOI:10.1074/jbc.RA118.001796 |
| [63] |
Park JB, Kim YH, Yoo Y, et al. Structural basis for arginine glycosylation of host substrates by bacterial effector proteins[J]. Nature Communications, 2018, 9(1): 4283. DOI:10.1038/s41467-018-06680-6 |
| [64] |
Sun H, Kamanova J, Lara-Tejero M, et al. A family of Salmonella type Ⅲ secretion effector proteins selectively targets the NF-κB signaling pathway to preserve host homeostasis[J]. PLoS Pathogens, 2016, 12(3): e1005484. DOI:10.1371/journal.ppat.1005484 |
| [65] |
Zhang YL, Dong C. MAP kinases in immune responses[J]. Cellular & Molecular Immunology, 2005, 2(1): 20-27. |
| [66] |
Creuzburg K, Giogha C, Wong Fok Lung T, et al. The type Ⅲ effector NleD from Enteropathogenic Escherichia coli differentiates between host substrates p38 and JNK[J]. Infection and Immunity, 2017, 85(2): e00620-16. |
| [67] |
Kralicek SE, Nguyen M, Rhee KJ, et al. EPEC NleH1 is significantly more effective in reversing colitis and reducing mortality than NleH2 via differential effects on host signaling pathways[J]. Laboratory Investigation, 2018, 98(4): 477-488. DOI:10.1038/s41374-017-0016-1 |
| [68] |
Rohde JR, Breitkreutz A, Chenal A, et al. Type Ⅲ secretion effectors of the IpaH family are E3 ubiquitin ligases[J]. Cell Host & Microbe, 2007, 1(1): 77-83. |
| [69] |
Li HT, Xu H, Zhou Y, et al. The phosphothreonine lyase activity of a bacterial type Ⅲ effector family[J]. Science, 2007, 315(5814): 1000-1003. DOI:10.1126/science.1138960 |
| [70] |
Ambrosi C, Pompili M, Scribano D, et al. The Shigella flexneri OspB effector: an early immunomodulator[J]. International Journal of Medical Microbiology, 2015, 305(1): 75-84. DOI:10.1016/j.ijmm.2014.11.004 |
| [71] |
Orth K, Palmer LE, Bao ZQ, et al. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector[J]. Science, 1999, 285(5435): 1920-1923. DOI:10.1126/science.285.5435.1920 |
| [72] |
Jones RM, Wu HX, Wentworth C, et al. Salmonella AvrA coordinates suppression of host immune and apoptotic defenses via JNK pathway blockade[J]. Cell Host & Microbe, 2008, 3(4): 233-244. |
| [73] |
Du FY, Galán JE. Selective inhibition of type Ⅲ secretion activated signaling by the Salmonella effector AvrA[J]. PLoS Pathogens, 2009, 5(9): e1000595. DOI:10.1371/journal.ppat.1000595 |
| [74] |
Mazurkiewicz P, Thomas J, Thompson JA, et al. SpvC is a Salmonella effector with phosphothreonine lyase activity on host mitogen-activated protein kinases[J]. Molecular Microbiology, 2008, 67(6): 1371-1383. DOI:10.1111/j.1365-2958.2008.06134.x |
| [75] |
Haneda T, Ishii Y, Shimizu H, et al. Salmonella type Ⅲ effector SpvC, a phosphothreonine lyase, contributes to reduction in inflammatory response during intestinal phase of infection[J]. Cellular Microbiology, 2012, 14(4): 485-499. DOI:10.1111/j.1462-5822.2011.01733.x |
| [76] |
Chambers KA, Abularrage NS, Scheck RA. Selectivity within a family of bacterial phosphothreonine lyases[J]. Biochemistry, 2018, 57(26): 3790-3796. DOI:10.1021/acs.biochem.8b00534 |
 2019, Vol. 46
2019, Vol. 46