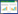扩展功能
文章信息
- 赵娟, 刘霆, 刘伟成, 刘德文, 张殿朋, 卢彩鸽
- ZHAO Juan, LIU Ting, LIU Wei-Cheng, LIU De-Wen, ZHANG Dian-Peng, LU Cai-Ge
- 番茄灰霉病生防链霉菌筛选及鉴定
- Screening and identification of the biocontrol Streptomyces against tomato Botrytis cinerea
- 微生物学通报, 2019, 46(10): 2548-2558
- Microbiology China, 2019, 46(10): 2548-2558
- DOI: 10.13344/j.microbiol.china.180788
-
文章历史
- 收稿日期: 2018-10-12
- 接受日期: 2018-12-26
- 网络首发日期: 2019-01-09
由半知菌亚门真菌灰葡萄孢(Botrytis cinerea)侵染所致的番茄灰霉病是一类重要的真菌病害,该病害可危害番茄的茎、叶、花和果实,在潮湿多雨及温室大棚环境中的发生尤为严重[1-2]。果蔬灰霉病目前主要依靠化学防治,但是长期大量使用化学药剂存在农药残留、病原菌容易产生抗药性以及环境污染等问题[3]。探索新型高效、广谱、低毒、环保的植物病害防治技术是当今农业生产研究的难点和热点。生物防治具有安全无污染、病原菌不易产生抗药性等特点,是果蔬病害绿色防控的有效途径。目前国内外有关枯草芽孢杆菌(Bacillus subtilis)、多粘芽孢杆菌(Bacillus polymyxa)、木霉菌(Trichoderma spp.)等微生物对果蔬作物灰霉病防治效果的研究较多[2, 4-6]。
放线菌(Actinomycete)广泛存在于土壤、海洋等不同的自然生态环境中,具有种类繁多、代谢功能各异等特点[7-8]。从微生物中发现的生物活性物质中有70%来自放线菌,其中约50%是链霉菌属的代谢产物[9]。放线菌产生的抗生素和酶等生物活性物质,在植物病害的生物防治方面具有巨大的应用价值。本研究从青海特殊生境土壤中分离筛选到多株对番茄灰霉病菌具有拮抗作用的放线菌,对其中拮抗效果明显且具有广谱抑菌活性的链霉菌T22进行防病促生效果检测和种级分类地位明确,以期为果蔬作物灰霉病绿色防控提供菌株资源和理论依据。
1 材料与方法 1.1 材料 1.1.1 病原菌番茄灰霉病菌(Botrytis cinerea)、桃褐腐病菌(Monilinia fructicola)、辣椒炭疽病菌(Colletotrichum capsici)、小麦纹枯病菌(Rhizoctonia cereali)、黄瓜枯萎病菌(Fusarium oxysporum f. sp. cucumerinum)、棉花枯萎病菌(Fusarium oxysporum f. sp. vasinfectum)、甘兰枯萎病菌(Fusarium oxysporum f. sp. conglutinans)由本研究室保存。马铃薯叶斑病菌(Alternaria alternata)由中国农业大学植物病理实验室提供。番茄品种为佳粉十六来自北京市农林科学院蔬菜中心。
1.1.2 培养基高氏一号培养基[10]、液体高氏一号培养基[10]用于放线菌分离培养,马铃薯葡萄糖琼脂培养基[10]用于病原真菌培养。放线菌形态观察采用蔗糖硝酸盐琼脂培养基、葡萄糖天门冬素琼脂培养基、马铃薯琼脂培养基、酵母膏麦芽膏琼脂培养基(ISP2)、燕麦粉琼脂培养基(ISP3)、无机盐淀粉琼脂培养基(ISP4)[11]。羧甲基纤维素钠培养基[12]、胶体几丁质培养基[13]分别用于菌株产纤维素酶、几丁质酶活性平板定性检测。茯苓粉液体培养基[14]、胶体几丁质液体培养基[13]分别用于菌株产葡聚糖酶、几丁质酶活性摇瓶定量检测。
1.1.3 主要试剂和仪器50%咯菌腈可湿性粉剂,先正达作物保护有限公司;细菌基因组DNA提取试剂盒、DNA纯化试剂盒、DNA marker DL2000、DNA扩增引物,北京博迈德生物技术有限公司;EasyTaq® DNA Polymerase,北京全式金生物技术有限公司。SPX智能型生化培养箱,宁波江南仪器厂;全温振荡培养箱,常州恒隆仪器有限公司;全自动凝胶成像系统,Bio-Rad公司;PCR仪,Eppendorf公司;双光速分光光度计,日立高新技术国际贸易有限公司。
1.2 方法 1.2.1 拮抗放线菌筛选采用琼脂块法[7]从分离自青藏高原地区的放线菌中筛选对番茄灰霉病菌具有拮抗活性的菌株。拮抗效果显著的菌株,采用对峙培养法[15]和生长速率法[16]测定其对桃褐腐病菌(Monilinia fructicola)、黄瓜枯萎病菌(Fusarium oxysporum f. sp. cucumerinum)等多种植物病原真菌的抑菌谱。
1.2.2 菌株T22产胞外酶活性定性检测对番茄灰霉病菌具有拮抗活性的放线菌菌株分别接种于羧甲基纤维素钠培养基上,28 ℃培养5 d,采用1 mg/mL刚果红溶液染色1 h,1 mol/L NaCl溶液脱色1 h[17],观察菌落周围是否有水解圈,测量菌落直径d与水解圈直径D,对其产纤维素酶活性进行定性检测。同时将各拮抗放线菌接种于胶体几丁质培养基上28 ℃培养5 d,直接观察菌落周围是否有透明水解圈,测量菌落直径d与水解圈直径D,对其产几丁质酶活性进行定性检测。
1.2.3 菌株T22产胞外酶活性定量检测将具广谱拮抗性且产胞外酶活性强的菌株T22接入装有100 mL高氏一号液体培养基的250 mL三角瓶中,28 ℃、180 r/min摇床培养3 d。将其作为种子液按照5%接种量分别接入装有100 mL茯苓粉液体培养基和胶体几丁质液体培养基的250 mL三角瓶中,28 ℃、180 r/min培养7 d。培养液4 ℃、12 000 r/min离心10 min获得葡聚糖酶和几丁质酶粗酶液。
葡聚糖酶活性测定方法[18]:取0.05 mol/L、pH 5.5的乙酸钠-乙酸缓冲液配制的0.1%底物昆布多糖1 mL,37 ℃水浴预热5 min,加入1 mL茯苓粉液体培养基上获得的粗酶液,混匀后置37 ℃水浴30 min。采用DNS法[19]测定反应液在540 nm的吸光度,计算其葡聚糖酶活性。葡聚糖酶活性单位定义为:在40 ℃下1 mL酶液1 min水解昆布多糖生成1 μg葡萄糖时所需酶量为1 U。
几丁质酶活性测定方法[20]:取1 mL 10 g/L胶体几丁质加入1 mL胶体几丁质液体培养基上获得的粗酶液,混匀后置37 ℃水浴30 min。采用DNS法[19]测定反应液在540 nm的吸光度,计算其几丁质酶活性。几丁质酶活性定义为:在37 ℃下1 mL酶液1 min生成1 μg N-乙酰氨基葡萄糖时所需酶量为1 U。
1.2.4 菌株T22对番茄灰霉病的离体防效采用离体叶片法检测供试菌株T22对番茄灰霉病的离体防效,试验设3组处理,每组处理10片叶片。选取大小一致的番茄植株(佳粉十六)叶片,2% (质量体积比)次氯酸钠消毒3 min,无菌水冲洗干净。采用菌株T22无菌发酵滤液对番茄叶片进行喷施,直到布满叶片。24 h后采用灭菌打孔器取直径7 mm的番茄灰霉病菌(Botrytis cinerea)菌饼接种于处理叶片中央,未接种液体发酵培养基替换T22无菌发酵滤液作为空白对照,50%咯菌腈可湿性粉剂1 000倍液替换T22无菌发酵滤液作为化学药剂对照。病原菌接种7 d后测量番茄叶片病斑直径,计算菌株T22发酵液对番茄灰霉病离体防效的公式:防效%=(空白对照平均病斑直径-处理平均病斑直径)/空白对照平均病斑直径×100%。
1.2.5 菌株T22对番茄种子发芽的影响选取颗粒饱满、大小一致的番茄(佳粉十六)种子,采用菌株T22无菌发酵滤液10、100、200和500倍稀释液浸泡种子,用未接种液体发酵培养基作为对照。每个处理设3次重复,每次重复设10粒种子。28 ℃培养24 h后收集种子,无菌水冲洗干净后,将其放在铺有两层无菌水浸湿滤纸的培养皿中,28 ℃保湿培养7 d,统计发芽率,测量胚轴、胚根长度,计算种子活力指数:种子活力指数(Vigor index)=(胚轴长度+胚根长度)×发芽率。
1.2.6 菌株T22种类鉴定(1) 形态学观察和生理生化特性检测
将菌株T22接种于放线菌形态观察培养基[21-22]上,28 ℃培养7 d观察菌株生长情况,记录气生菌丝颜色、基内菌丝颜色以及可溶性色素的有无及颜色。将菌株T22接种至高氏一号培养基上,将灭菌盖玻片以45度角斜插入高氏一号培养基内,28 ℃培养5 d后取出盖玻片,在10×40倍光学显微镜下观察菌丝、孢子形态。参照东秀珠等[22]方法对菌株T22进行碳源利用和生理生化特性检测。参考《链霉菌鉴定手册》[11],结合形态学特征和生理生化特性对菌株T22进行初步鉴定。
(2) 分子生物学鉴定
将菌株T22接种于高氏一号培养基上,28 ℃培养7 d收集菌体。采用细菌基因组提取试剂盒提取全基因组DNA后,利用细菌通用引物27f (5′-AG AGTTTGATCCTGGCTCAG-3′)和1492r (5′-TACGG CTACCTTGTTACGACTT-3′)对其16S rRNA基因序列进行PCR扩增。PCR反应体系(25 μL):上、下游引物(10 pmol/L)各1 μL,dNTP mixture (10 mmol/L) 1 μL,2.5 U/μL Taq DNA聚合酶0.5 μL,10×PCR buffer 2.5 μL,基因组DNA模板1 μL,ddH2O 18 μL。PCR反应条件:94 ℃ 5 min;94 ℃ 1 min,56 ℃ 1 min,72 ℃ 2 min,35次循环;72 ℃ 10 min。
同时分别采用引物对gyrB PF/gyrB PR、recA PF/recA PR、rpoB PF/rpoB PR对菌株T22的3个看家基因gyrB、recA和rpoB进行PCR扩增[23-24]。扩增产物经连接、转化、鉴定后,阳性克隆送北京博迈德生物技术公司测序。所得序列于NCBI进行BLAST相似性搜索,Clustal X软件进行多序列比对。将菌株T22的16S rRNA基因与上述3个看家基因采用BioEdit软件拼接后,采用PAUP软件(PAUP* version 4.02 beta)中的最大似然(Maximum likelihood,ML)法进行聚类分析[25]。
2 结果与分析 2.1 拮抗菌株筛选从青藏高原特殊生境土壤中分离的56株放线菌纯培养菌株中,有14株对番茄灰霉病菌(Botrytis cinerea)表现出不同程度的拮抗效果,其中菌株T22拮抗效果最为明显(图 1)。抑菌谱测定表明拮抗菌T22对所有供试病原真菌均有不同程度的抑制作用(图 2),其中对番茄灰霉病菌、桃褐腐病菌、辣椒炭疽病菌拮抗圈直径分别为20、26和23 mm;其无菌发酵滤液对番茄灰霉病菌、桃褐腐病菌、黄瓜枯萎病菌的抑制率分别为84.6%、81.5%和79.1% (表 1)。

|
| 图 1 拮抗菌T22及其无菌发酵滤液对番茄灰霉病菌的抑制作用 Figure 1 Inhibition effect of antagonistic Streptomyce strain T22 and its cell free culture filtrate on Botrytis cinerea 注:A:琼脂块法;B:对峙培养法;C和D:生长速率法. Note: A: Agar block method; B: Confrontation culture assay; C and D: Mycelia growth assay. |
|
|
|
| 图 2 拮抗菌T22无菌发酵滤液对供试病原真菌的抑制作用 Figure 2 Inhibition effect of cell free culture filtrate of antagonistic Streptomyce strain T22 against the test pathogenic fungi 注:A:桃褐腐病菌;B:黄瓜枯萎病菌;C:交链格孢菌;D:棉花枯萎病菌;CK:PDA培养基;T22:添加有20%菌株T22无菌发酵滤液的PDA培养基. Note: A: Monilinia fructicola; B: Fusarium oxysporum f. sp. cucumerinum; C: Alternaria alternata; D: Fusarium oxysporum f. sp. vasinfectum; CK: PDA media; T22: PDA media amended with 20% cell free culture filtrate of strain T22. |
|
|
| Pathogenic fungi | Inhibition zone (mm) | Inhibition rate (%) |
| Botrytis cinerea | 20 | 84.6 |
| Colletotrichum capsici | 23 | 73.7 |
| Fusarium oxysporum f. sp. cucumerinum | 16 | 79.1 |
| Fusarium oxysporum f. sp. conglutinans | 14 | 34.2 |
| Moniliniafructicola | 26 | 81.5 |
| Rhizoctonia cereali | 14 | 23.7 |
| Alternaria alternata | 18 | 52.6 |
| Fusarium oxysporum f. sp. vasinfectum | 14 | 39.5 |
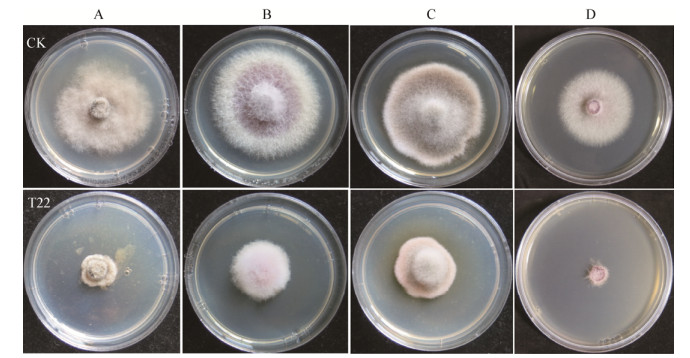
平板产胞外酶活性检测结果表明,经刚果红染色、NaCl脱色,菌株T22在羧甲基纤维素钠培养基上能够产生明显的水解圈(图 3A),其直径大小为34.7±1.5 mm,产酶水解圈直径与菌落直径大小比值(D/d)为2.74。菌株T22在胶体几丁质培养基上能产生明显的透明水解圈(图 3B),其直径大小为33.7±2.1 mm,产酶水解圈直径与菌落直径大小比值(D/d)为3.06。与其他供试拮抗菌株相比,拮抗菌T22具有较强产纤维素酶、几丁质酶活性。产胞外酶活性定量检测结果表明,菌株T22在茯苓粉液体培养生长5 d时葡聚糖酶活性为5.42±0.61 U;在胶体几丁质液体培养基生长5 d时几丁质酶活性为4.63±0.74 U。

|
| 图 3 拮抗菌T22产胞外酶活性平板检测 Figure 3 Extracellular hydrolase production of antagonistic Streptomyce strain T22 in Petridish 注:A:纤维素酶;B:几丁质酶. Note: A: Cellulase; B: Chitinase. |
|
|
拮抗菌T22无菌发酵滤液处理番茄离体叶片后接种病原菌,能够明显降低番茄灰霉病病斑直径(图 4)。T22无菌发酵滤液对番茄灰霉病的离体防效达到55.1%,与50%咯菌腈1 000倍液喷施处理防效无显著差异(表 2)。

|
| 图 4 拮抗菌T22对番茄灰霉病的离体防效 Figure 4 In vitro biocontrol effect of antagonistic Streptomyce strain T22 on tomato grey mould 注:A:未接种液体培养基喷施+灰霉病菌;B:菌株T22无菌发酵滤液喷施+灰霉病菌;C:50%咯菌腈1 000倍液喷施+灰霉病菌. Note: A: Uninoculated liquid medium spray+Botrytis cinerea; B: Cell free culture filtrate of strain T22 spray+Botrytis cinerea; C: 1 000 fold dilution of 50% fludioxonil spray+Botrytis cinerea. |
|
|
| Treatments | Average diameters of lesions (cm) |
Biocontrol effect (%) |
| M+Bc | 2.16±0.23 a | - |
| T22+Bc | 0.97±0.21 b | 55.1 |
| F+Bc | 0.84±0.09 b | 61.1 |
| 注:M+Bc:未接种液体培养基喷施+灰霉病菌;T22+Bc:菌株T22无菌发酵滤液喷施+灰霉病菌;F+Bc:50%咯菌腈1 000倍液喷施+灰霉病菌;同列数值后不同字母表示差异显著(P<0.05). Note: M+Bc: Uninoculated liquid medium spray+Botrytis cinerea; T22+Bc: Cell free culture filtrate of strain T22 spray+Botrytis cinerea; F+Bc: 1 000 fold dilution of 50% fludioxonil spray+Botrytis cinerea; Different letters in same column represent significant differences (P<0.05). |
||
拮抗菌T22无菌发酵滤液在适宜的稀释倍数下对番茄种子发芽具有不同程度促进作用。拮抗菌T22无菌发酵滤液100倍稀释液处理,番茄胚轴、胚根长度分别较对照增加15.1%、29.7%,种子活力指数增加43.9%;其200倍稀释液处理的番茄种子,胚轴、胚根长度和种子活力指数较对照分别增加8.8%、18.1%和32.3% (表 3)。
| Treatments | Hypocotyl | Radicle | Vigor index | |||||
| Length (cm) | Increase (%) | Length (cm) | Increase (%) | Value | Increase (%) | |||
| CK | 3.06±0.94 | - | 9.10±1.28 | - | 851.20 | - | ||
| 10 | 2.71±0.49 | -11.3 | 8.13±1.52 | -10.7 | 759.01 | -10.8 | ||
| 100 | 3.52±0.87 | 15.1 | 11.80±1.65 | 29.7 | 1 225.60 | 43.9 | ||
| 200 | 3.33±0.71 | 8.8 | 10.75±1.03 | 18.1 | 1 126.00 | 32.3 | ||
| 500 | 3.11±0.58 | 1.7 | 9.68±0.84 | 6.3 | 1 023.04 | 20.2 | ||
| 注:CK为未接种液体发酵培养基;10、100、200、500依次为拮抗菌T22无菌发酵滤液10、100、200和500倍稀释液. Note: CK represents uninoculated liquid medium; 10, 100, 200, 500 represents the 10, 100, 200 and 500 fold dilutions of the cell free culture filtrate of antagonistic Streptomyce strain T22. |
||||||||
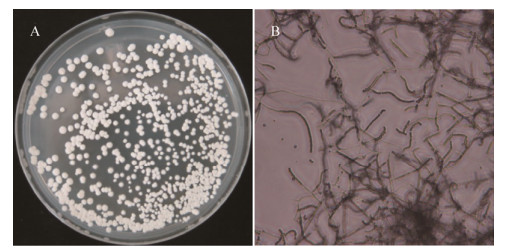
菌株T22在高氏一号培养基上生长良好,气生菌丝茂盛,菌落白色至浅灰色;10×40倍光学显微镜下可以观察到菌株T22气生菌丝直至柔曲,孢子成链,卵圆形(图 5)。菌株T22在国际链霉菌计划推荐的大多数培养基上气生菌丝白色至灰白色或淡橄榄黄色,基内菌丝为白色至黄色,可产生可溶性色素(表 4)。菌株T22能够利用D-葡萄糖、L-阿拉伯糖、D-甘露醇、肌醇、麦芽糖、棉子糖,不能利用L-鼠李糖、D-蔗糖、D-木糖、D-果糖。菌株T22牛奶胨化、淀粉水解、明胶液化阳性,硝酸盐还原阴性,不产生黑色素、硫化氢(表 5)。
|
| 图 5 菌株T22在高氏一号培养基上的形态学特征 Figure 5 Morphological characters of antagonistic Streptomyce strain T22 on Gause media 注:A:菌落形态;B:显微形态(10×40). Note: A: Colony morphology; B: Micromorphology (10×40). |
|
|
| Media | Aerial mycelium | Substrate mycelium | Soluble pigment |
| Gause medium | White | Yellow, Tawny | - |
| Sucrose nitrate agar | White, Pale gray | White | - |
| Glucose asparagus agar | White | Black gray | Black gray |
| Potato dextrose agar | White | Yellow | Dark gray |
| Yeast malt agar (ISP2) | White | Yellow | Dark yellow |
| Oatmeal agar (ISP3) | White | Yellow | Dark yellow |
| Inorganic salt starch agar (ISP4) | Light olive yellow | Yellow | Dark green |
| Items | Results | Items | Results |
| D-Glucose | + | Milk coagulation | + |
| L-Arabinose | + | Amylolysis | + |
| D-Mannitol | + | Gelatin liquefaction | + |
| Inositol | + | Nitrate reductase | - |
| Maltose | + | Black pigment | - |
| Raffinose | + | Hydrogen sulfide | - |
| L-Rhamnose | - | ||
| D-Sucrose | - | ||
| D-Xylose | - | ||
| D-Fructose | - | ||
| 注:+:阳性;-:阴性. Note: +: Positive; -: Negative. |
|||
对菌株T22的16S rRNA、gyrB、recA和rpoB基因序列进行PCR扩增,分别得到大小约为1 300、180、430和530 bp的片段。BLAST比对分析发现与菌株T22相应序列相似性较高的菌株均属于链霉菌属,且该菌株上述基因序列与白黑链霉菌Streptomyces alboniger相应序列(NR043228、KT384804、KT385152、KT388774)相似性均高达99%-100%。将菌株T22的16S rRNA、gyrB、recA和rpoB基因序列均提交GenBank,登录号分别为MK054273、MK054274、MK054275和MK070496。
将该菌株的16S rRNA基因与3个看家基因(gyrB、recA和rpoB)序列拼接后,以Streptomyces seoulensis作为外群构建多基因系统进化树(图 6)。结果表明,该菌株与白黑链霉菌(Streptomyces alboniger NRRL B-1832)聚在同一系统分支上,自举值为100%,结合形态学特征和培养特征,将菌株T22鉴定为白黑链霉菌(Streptomyces alboniger)。

|
| 图 6 基于拮抗菌T22多基因聚类分析构建的系统进化树 Figure 6 Phylogenetic tree constructed based on the multiple sequences analyses of antagonistic Streptomyce strain T22 注:分支上的数字表示构建系统进化树时1 000次计算形成该节点的百分比;括号内数值为相应菌株16S rRNA、gyrB、recA和rpoB基因GenBank登录号;标尺10表示遗传距离. Note: The bootstrap values (%) presented at the branches were calculated from 1 000 replications; Numbers in the parentheses are GenBank accession numbers of the 16S rRNA, gyrB, recA and rpoB genes of the corresponding strains; The scale bar 10 indicates genetic distance. |
|
|
灰霉病是温室番茄种植过程中一种重要的真菌病害,常造成严重的产量和质量损失。化学防治存在农药残留、病原菌容易产生抗药性及环境污染等问题,生物防治逐步成为番茄灰霉病绿色防控的有效措施。生物防治的关键在于具有优良拮抗活性生防潜力菌株的分离筛选和防效验证,目前已有较多学者开展番茄灰霉病生防菌株评价的相关工作。马超等[5]研究表明,分离自植物的解淀粉芽孢杆菌和地衣芽孢杆菌对番茄灰霉病菌具有良好的抑制效果,其发酵液对番茄灰霉病菌的抑制率分别可达86.6%和83.0%。董晓军等[26]筛选的木霉菌株T-CD01、T-CD02、T-ZG01对灰霉菌菌丝生长的抑制率为69.54%-74.62%,其固态发酵制剂在田间对番茄灰霉病的相对防效为62.4%-70.8%。张春慧[27]研究表明链霉菌TD-1产生的挥发性物质能够抑制灰霉病菌的产孢能力、孢子萌发和菌丝生长。
由于灰霉病菌种内变异大、病原菌容易产生抗性[28],合理开发现有微生物资源、增添新型微生物农药种类对于果蔬灰霉病绿色防控具有重要意义。本研究筛选到一株对番茄灰霉病菌具有明显拮抗活性的链霉菌T22,并结合形态学、生理生化特性、多基因聚类分析等将链霉菌T22鉴定为白黑链霉菌(Streptomyces alboniger)。目前国内外关于白黑链霉菌(Streptomyces alboniger)的报道主要是分离自其代谢产物中的嘌呤霉素,该抗生素属于氨基糖苷类,能有效抑制革兰氏阳性菌生长[29]。但至今未见有关该菌抑制真菌或产生抗真菌活性物质的相关研究。本研究表明筛选到的该菌株及其发酵液中的代谢产物能够有效抑制多种植物病原真菌菌丝生长,对番茄灰霉病具有良好离体防效,且能够促进番茄种子发芽。
除了抗真菌活性物质,链霉菌T22对病原真菌的有效抑制还与其分泌的胞外水解酶有关。病原真菌的细胞壁以几丁质、纤维素为骨架,以β-1, 3-葡聚糖为主要填充物所组成,容易被几丁质酶(Chitinase)、纤维素酶(Cellulase)等胞外水解酶分解,造成病原菌菌丝崩解[30]。于妍华等[20]研究表明,病原真菌细胞壁中的几丁质、纤维素能够诱导放线菌合成几丁质酶和纤维素酶,从而溶解真菌菌丝,导致病原真菌死亡。本研究发现,链霉菌T22具有较强的产纤维素酶、葡聚糖酶和几丁质酶活性,我们推测这些胞外水解酶在该菌株对病原真菌菌丝生长的抑制中也发挥重要作用。本研究表明,链霉菌T22具有较强的抑菌、产酶、防病和促生活性,在番茄灰霉病生物防治中具有较好的开发应用潜力。该菌株的相关生防特性与其次生代谢产物密切相关,本研究仅对白黑链霉菌的防病促生效果和种类鉴定进行了研究,对于该菌次级代谢产物中的抗真菌活性物质分离解析有待后续研究。
| [1] |
Zhao JJ, Lu F, Wu J, et al. Sensitivity of Botrytis cinerea from greenhouses in Hebei province to boscalid and fludioxonil[J]. Acta Phytopathologica Sinica, 2018, 48(6): 817-821. (in Chinese) 赵建江, 路粉, 吴杰, 等. 河北省设施番茄灰霉病菌对啶酰菌胺和咯菌腈的敏感性[J]. 植物病理学报, 2018, 48(6): 817-821. |
| [2] |
Zhao WS, Lu XY, Guo QG, et al. A preparation of Bacillus subtitles BAB-1 DPC against tomato gray mold[J]. Chinese Journal of Biological Control, 2018, 34(1): 99-108. (in Chinese) 赵卫松, 鹿秀云, 郭庆港, 等. 防治番茄灰霉病的枯草芽胞杆菌BAB-1粉尘剂研制[J]. 中国生物防治学报, 2018, 34(1): 99-108. |
| [3] |
Ji MS, Liu Y, Zhu H, et al. Detection of the resistance of Botrytis cinerea on tomato in Liaoning province to fungicides and cross-resistance[J]. Agrochemicals, 2017, 56(9): 676-678. (in Chinese) 纪明山, 刘妍, 朱赫, 等. 辽宁省番茄灰霉病菌对常用杀菌剂的抗药性监测与交互抗药性[J]. 农药, 2017, 56(9): 676-678. |
| [4] |
Zhao XB, Shangguan NN, Li YF, et al. Fermentation condition optimization and control effects of antagonistic bacterial strain 18BS-12 against Botrytis cinerea[J]. Journal of Northwest A&F University (Natural Science Edition), 2016, 44(6): 116-124. (in Chinese) 赵新贝, 上官妮妮, 李月飞, 等. 番茄灰霉病拮抗细菌18BS-12发酵条件的优化及防治效果[J]. 西北农林科技大学学报:自然科学版, 2016, 44(6): 116-124. |
| [5] |
Ma C, Zhu HL, Huang TW, et al. Screening and identification of endophytic antagonistic bacteria to Botrytis cinerea in tomato[J]. Journal of Shanxi Agricultural Sciences, 2018, 46(3): 437-440, 452. (in Chinese) 马超, 朱洪磊, 黄太伟, 等. 番茄灰霉病菌内生拮抗细菌的筛选及鉴定[J]. 山西农业科学, 2018, 46(3): 437-440, 452. DOI:10.3969/j.issn.1002-2481.2018.03.30 |
| [6] |
Yang CL, Xi YD, Xie HR, et al. Chitinase production conditions of Trichoderma harzianum Th-30 and its antagonistic activity against Botrytis cinerea[J]. Acta Phytophylacica Sinica, 2009, 36(4): 295-300. (in Chinese) 杨春林, 席亚东, 谢华蓉, 等. 哈茨木霉Th-30几丁质酶的生产条件及对灰霉病菌的拮抗作用[J]. 植物保护学报, 2009, 36(4): 295-300. DOI:10.3321/j.issn:0577-7518.2009.04.002 |
| [7] |
Zhu WJ, Xue QH, Cao YR, et al. Distribution and characteristics of soil antagonistic actinomycetes on northern slope of Taibai mountain, Qinling[J]. Chinese Journal of Applied Ecology, 2011, 22(11): 3003-3010. (in Chinese) 朱文杰, 薛泉宏, 曹艳茹, 等. 秦岭太白山北坡土壤拮抗性放线菌分布及特性[J]. 应用生态学报, 2011, 22(11): 3003-3010. |
| [8] |
Ge YL, Liu ZL, Chen L, et al. Screening and identification of marine actinomycetes for biocontrol on Rhizoctonia cerealis[J]. Agrochemicals, 2018, 57(2): 111-113, 136. (in Chinese) 葛玉力, 刘兆良, 陈玲, 等. 小麦纹枯病生防海洋放线菌的筛选与鉴定[J]. 农药, 2018, 57(2): 111-113, 136. |
| [9] |
Huang XH, Li S, Tan ZJ, et al. Progress of study on endophytic actinomycetes in plant[J]. Biotechnology Bulletin, 2008(1): 42-46. (in Chinese) 黄晓辉, 李珊, 谭周进, 等. 植物内生放线菌研究进展[J]. 生物技术通报, 2008(1): 42-46. |
| [10] |
Cheng LJ, Xue QH, Lai HX, et al. Experimental Techniques of Microbiology[M]. Xi'an: World Publishing Corporation, 2000. (in Chinese) 程丽娟, 薛泉宏, 来航线, 等. 微生物学实验技术[M]. 西安: 世界图书出版社, 2000. |
| [11] |
Actinomycetes Classification Group, Institute of Microbiology, Chinese Academy of Sciences. Identification Mannual of Streptomyces[M]. Beijing: Science Press, 1975. (in Chinese) 中国科学院微生物研究所放线菌分类组. 链霉菌鉴定手册[M]. 北京: 科学出版社, 1975. |
| [12] |
Jiao XF, Sheng XL, Shan JY, et al. Screening of a bacterium degrading cellulose and its mutation by UV irradiation[J]. Chemistry & Bioengineering, 2010, 27(1): 52-54. (in Chinese) 焦秀凤, 盛晓莉, 单继阳, 等. 纤维素分解菌的筛选及其紫外诱变[J]. 化学与生物工程, 2010, 27(1): 52-54. DOI:10.3969/j.issn.1672-5425.2010.01.015 |
| [13] |
Zhang C. Screening and identification of chitinase-producing marine microbe and the characterization of its chitinase[D]. Dalian: Master's Thesis of Dalian Polytechnic University, 2012 (in Chinese) 张灿.海洋产几丁质酶菌株的筛选、鉴定及其酶学性质研究[D].大连: 大连工业大学硕士学位论文, 2012 http://cdmd.cnki.com.cn/Article/CDMD-10152-1015574747.htm |
| [14] |
Sock J, Rohringer R, Kang ZS. Extracellular β-1, 3-glucanases in stem rust-affected and abiotically stressed wheat leaves[J]. Plant Physiology, 1990, 94(3): 1376-1389. DOI:10.1104/pp.94.3.1376 |
| [15] |
Liu CY, Ji HL, Wang R, et al. Antagonistic effects against several plant pathogenic fungi and the growth adaptability of a Chaetomium subaffine biocontrol strain LB-1[J]. Journal of Plant Protection, 2018, 45(2): 332-339. (in Chinese) 刘彩云, 季洪亮, 王瑞, 等. 近缘毛壳菌生防菌株LB-1对几种常见植物病原真菌的拮抗作用及其生长适应性分析[J]. 植物保护学报, 2018, 45(2): 332-339. |
| [16] |
Fang ZD. Plant Disease Research Methods[M]. 3rd ed. Beijing: China Agriculture Press. (in Chinese) 方中达. 植病研究方法[M]. 3版. 北京: 中国农业出版社. |
| [17] |
Dong XY, Zhan PY, Ren XQ, et al. Isolation, screening and identification of cellulase producing bacteria from rabbit manure[J]. Journal of Hebei Agricultural Sciences, 2017, 21(5): 56-59, 80. (in Chinese) 董小英, 詹沛运, 任晓强, 等. 兔粪中产纤维素酶菌的分离筛选与初步鉴定[J]. 河北农业科学, 2017, 21(5): 56-59, 80. |
| [18] |
Gao XN, Tu X, Huang LL, et al. The screen of plant endophytic actinomycetes producing β-1, 3-glucanase and antifungal activity of β-1, 3-glucanase[J]. Microbiology China, 2009, 36(8): 1189-1194. (in Chinese) 高小宁, 涂璇, 黄丽丽, 等. 产β-1, 3-葡聚糖酶植物内生放线菌的筛选及抑菌活性研究[J]. 微生物学通报, 2009, 36(8): 1189-1194. |
| [19] |
Chen YQ. Biochemical Experimental Methods and Techniques[M]. Beijing: Science Press, 2002. (in Chinese) 陈毓荃. 生物化学实验方法和技术[M]. 北京: 科学出版社, 2002. |
| [20] |
Yu YH, Xue QH, Tang M. Inductive effects of fungal pathogens of American Ginseng and Ginseng on chitinase and cellulase of antigonistic actinomycetes[J]. Journal of Northwest A&F University (Natural Science Edition), 2011, 39(7): 110-116. (in Chinese) 于妍华, 薛泉宏, 唐明. 西洋参和人参病原真菌菌体对放线菌2种水解酶的诱导[J]. 西北农林科技大学学报:自然科学版, 2011, 39(7): 110-116. |
| [21] |
Shirling EB, Gottlieb D. Methods for characterization of Streptomyces species[J]. International Journal of Systematic Bacteriology, 1996, 16(3): 313-340. |
| [22] |
Dong XZ, Cai MY. Systematic Identification Mannual of Common Bacteria[M]. Beijing: Science Press, 2001. (in Chinese) 东秀珠, 蔡妙英. 常见细菌系统鉴定手册[M]. 北京: 科学出版社, 2001. |
| [23] |
Guo YP, Zheng W, Rong XY, et al. A multilocus phylogeny of the Streptomyces griseus 16S rRNA gene clade: use of multilocus sequence analysis for streptomycete systematics[J]. International Journal of Systematic and Evolutionary Microbiology, 2008, 58(1): 149-159. DOI:10.1099/ijs.0.65224-0 |
| [24] |
Rong XY, Guo YP, Huang Y. Proposal to reclassify the Streptomyces albidoflavus clade on the basis of multilocus sequence analysis and DNA-DNA hybridization, and taxonomic elucidation of Streptomyces griseus subsp. solvifaciens[J]. Systematic and Applied Microbiology, 2009, 32(5): 314-322. DOI:10.1016/j.syapm.2009.05.003 |
| [25] |
Swofford D. PAUP*: Phylogenetic analysis using parsimony (*and Other Methods), Version 4.0b10. Sunderland[R]. MA: Sinauer Associates, 2002
|
| [26] |
Dong XJ, Jia RF. Primary study of Trichoderma biocontrol on tomato grey mould[J]. Primary Agricultural Technology Extension, 2018, 6(1): 33-35. (in Chinese) 董晓军, 贾瑞芬. 木霉菌防治番茄灰霉病的初步研究[J]. 基层农技推广, 2018, 6(1): 33-35. |
| [27] |
Zhang CH. Study on the inhibiting of volatiles produced by Streptomyces alboflavus TD-1 on Botrytis cinerea[D]. Tianjin: Master's Thesis of Tianjin University of Science & Technology, 2015 (in Chinese) 张春慧.链霉菌TD-1产挥发性物质抑制番茄灰霉病菌的研究[D].天津: 天津科技大学硕士学位论文, 2015 http://cdmd.cnki.com.cn/Article/CDMD-10057-1016104569.htm |
| [28] |
Elad Y, Williamson B, Tudzynski P, et al. Botrytis spp. and diseases they cause in agricultural systems-an introduction[A]//Elad Y, Williamson B, Tudzynski P, et al. Botrytis: Biology, Pathology and Control[M]. Dordrecht: Springer, 2007: 1-8
|
| [29] |
Cuyàs E, Martin-Castillo B, Corominas-Faja B, et al. Anti-protozoal and anti-bacterial antibiotics that inhibit protein synthesis kill cancer subtypes enriched for stem cell-like properties[J]. Cell Cycle, 2015, 14(22): 3527-3532. DOI:10.1080/15384101.2015.1044173 |
| [30] |
Zhao AN, Ding WL. Progress on the control of medicinal plants soil-borne disease by anti-microorganism[J]. China Journal of Chinese Materia Medica, 2005, 30(7): 485-487. (in Chinese) 赵阿娜, 丁万隆. 利用拮抗微生物防治中药材土传病害研究进展[J]. 中国中药杂志, 2005, 30(7): 485-487. DOI:10.3321/j.issn:1001-5302.2005.07.001 |
 2019, Vol. 46
2019, Vol. 46