扩展功能
文章信息
- 杨淼, 王海涛, 薛松
- YANG Miao, WANG Hai-Tao, XUE Song
- 三角褐指藻甘油酯对氮胁迫的响应
- Glycerolipid remodeling of Phaeodactylum tricornutum in response to nitrogen stress
- 微生物学通报, 2018, 45(5): 996-1006
- Microbiology China, 2018, 45(5): 996-1006
- DOI: 10.13344/j.microbiol.china.170594
-
文章历史
- 收稿日期: 2017-07-31
- 接受日期: 2017-12-24
- 网络首发日期(www.cnki.net): 2017-12-27
2. 中国科学院大学 北京 100049;
3. 大连理工大学生命科学与技术学院 辽宁 大连 116024
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. School of Life Science and Biotechnology, Dalian University of Technology, Dalian, Liaoning 116024, China
硅藻广泛分布于各种海洋与淡水环境中,包括250个以上的属,种类多达10万种,这使得硅藻成为继被子植物之后生物多样性最高的光合生物[1]。作为最重要的单细胞光合真核生物,硅藻固碳能力高,对自然环境适应能力强,其生物量相当于40%的海洋初级生产力,同时相当于20%的全球初级生产力,具有重要的生态学作用[2]。硅藻起源于十亿年前发生于红藻及异养真核生物之间的二次内共生[3]。硅藻基因组表明其具有完整的尿素循环,而尿素循环只存在于后生动物和硅藻这两类真核生物中[4-5]。尿素循环参与氮的再利用并将细胞代谢产生的碳返回至中心代谢[6-7]。氮元素还能参与生物大分子如蛋白质、核酸及含氮脂质的合成,从而促进生物的生长发育[7]。研究氮代谢将有利于认识海洋硅藻碳代谢的调节过程,并为硅藻对海洋环境的适应机制研究提供理论基础[8]。
硅藻的模式生物三角褐指藻(Phaeodactylum tricornutum Bohlin)具有3种形态,包括梭形、卵圆形及三叉形[9],其基因组已完成测序[5]。三角褐指藻富含二十碳五烯酸(EPA)[10]及岩藻黄素[11],是重要的水产饵料[12]。在胁迫条件下三角褐指藻油脂含量高,是潜在的生物燃料原料生产者[13],因此具有重要的研究价值。在适宜生长条件下,真核微藻光合固碳的40%用于合成蛋白质[14],其碳骨架主要由TCA循环供应。氮有效性的降低会减少TCA循环的代谢物,从而使TCA循环的碳来源——乙酰辅酶A转向脂肪酸的合成[15]。氮胁迫能降低藻细胞蛋白质含量并促进以甘油三酯(TAG)为主的储存脂质的增加[16]。最近关于三角褐指藻的研究表明,氮胁迫[17]、碱性pH胁迫、碳酸氢盐添加[18]及有机酸添加[19]对其脂质代谢具有非常显著且复杂的影响,近些年来备受关注[20]。
微藻脂质组以甘油酯为主[21],而在正常生长条件下微藻甘油酯以极性甘油酯为主,包括糖脂、磷脂及甜菜碱脂[22]。糖脂是叶绿体膜结构的主要组分,主要分布于叶绿体膜结构如类囊体及被膜上,包括单半乳糖甘油二酯(MGDG)、双半乳糖甘油二酯(DGDG)及硫代异鼠李糖甘油二酯(SQDG)。构成多种膜结构组分及作为脂质代谢中间产物的极性脂,主要分布于叶绿体外的膜结构如线粒体、微粒体(Microbody)、内质网上,包括磷脂酰胆碱(PC)、磷脂酰乙醇胺(PE)及甜菜碱脂[1, 2-二酰基甘油-O-4′-(N, N, N-三甲基)高丝氨酸(DGTS)、二酰甘油羟甲基三甲基-β-丙氨酸(DGTA)与1, 2-二酰基甘油-3-O-羧羟甲基胆碱(DGCC)],此外磷脂酰甘油(PG)主要分布于光合膜结构中[23]。在正常富营养条件下,微藻只含有微量中性脂,这些中性脂以TAG为主,TAG是能量及碳储存化合物[24]。
在胁迫诱导条件下,微藻脂质的主要特征通常表现为TAG的大量积累,同时伴随着极性脂的分解或转化,其酰基组成会发生很大的变化,这些特征表明微藻的甘油酯组发生了重组,从而适应其外部环境条件的变化[25-26]。目前关于微藻甘油酯组对胁迫条件尤其是营养盐胁迫的响应机制研究主要集中于莱茵衣藻、微拟球藻及小球藻等藻种[27-28],而关于三角褐指藻对氮胁迫的响应多集中于转录组及蛋白质组水平上的TAG积累及其调控机制研究[7, 29-31],还包括TAG积累的动态变化研究[32-33],但是目前关于三角褐指藻甘油酯尤其是极性脂对氮胁迫的响应研究报道还很少[9, 34]。
高效薄层色谱作为高效分离脂质的经典方法能同时分离多种组分,分离后可结合质谱或气相色谱进行进一步定性定量分析[35-36]。本文利用高效薄层色谱结合气相色谱法比较分析了三角褐指藻在正常及氮胁迫条件下的脂肪酸及甘油酯组分的变化规律,阐明了三角褐指藻所积累TAG脂肪酸的主要来源及在胁迫前生成的各极性甘油酯脂肪酸的去向,从而为进一步认识三角褐指藻对氮胁迫的响应机制提供新信息。
1 材料与方法 1.1 主要试剂和仪器氯仿、甲醇(CH3OH)、磷酸(H3PO4)、展开剂正己烷、乙醚、乙酸、丙酮和甲苯均为分析纯试剂,天津市科密欧化学试剂有限公司;萃取剂正己烷为色谱纯试剂,德国默克集团;十七烷酸甘油三酯(Glyceryl triheptadecanoate)和樱草黄(Primuline),美国西格玛奥德里奇公司;玻璃纤维滤膜(直径47 mm),Whatman GF/C公司;薄层色谱硅胶板(TLC,Silica gel 60 F254,Alumium sheets 0.20 mm),德国默克集团。照度计,德国Gigahertz Optik;分光光度计,日本Jasco公司;叶绿素荧光仪,德国WALZ公司;气相色谱,美国安捷伦科技有限公司。
1.2 藻种及培养条件三角褐指藻获赠于辽宁省海洋水产科学研究院刘卫东研究员,培养基为3×f/2[37],海水经0.22 μm滤膜过滤后高温高压灭菌(1×105 Pa,15 min),冷却至室温后待用。三角褐指藻在3 L摇瓶中静置培养,每天手动摇瓶3次,光周期设置为14 h光照:10 h黑暗,通过照度计测定光照强度为100 μmol/(m2·s)。藻细胞经3×f/2富氮培养4 d至指数生长期,4 000 r/min离心5 min收集藻细胞,并用无氮3×f/2培养基重悬清洗藻细胞,重复离心(4 000 r/min,5 min)后用无氮3×f/2培养基将藻细胞再次重悬,利用分光光度计测定藻液在波长680 nm下的光密度值,将初始接种光密度值调节为0.245,接种体积为1.5 L,在上述培养条件下继续缺氮培养3 d后收获。根据Maxwell等[38]方法,通过叶绿素荧光仪测定藻细胞的最大PS Ⅱ光量子产率Fv/Fm及实际PS Ⅱ光量子产率φPSⅡ。
在缺氮培养的第0天和第3天分别通过离心(4 000 r/min,5 min)收集一定体积的新鲜藻细胞,用液氮冷冻后保存于-80 ℃待用;同时将20 mL藻液滤过预称重的玻璃纤维滤膜,于60 ℃下烘干至恒重,根据该滤膜在抽滤藻细胞前后的质量差来计算上述一定体积藻细胞的干重。
1.3 脂质提取将保存于-80 ℃的新鲜藻泥(细胞干重约20 mg)取出后立即加入400 μL的氯仿:甲醇:甲酸(2:1:0.1,体积比),振荡30 s,加入200 μL 0.2 mol/L H3PO4和1 mol/L KCl,再振荡30 s后离心(12 000 r/min,2 min)分层[39],共提取3次。
1.4 高效薄层色谱分离脂质将下层有机相中已溶解的脂质均匀点分布在10 cm×10 cm薄层色谱硅胶板上,待氯仿挥干后在展析缸中展开,中性脂(以TAG为主)展开剂为正己烷:乙醚:乙酸(85:15:1,体积比),极性脂(糖脂及磷脂)展开剂为丙酮:甲苯:水(91:30:7,体积比),待展开至距离上端约1 cm时取出TLC板,待TLC板溶剂挥干后进行显色。显色剂为0.05%樱草黄(溶剂为20%水和80%丙酮),利用薄层色谱显色喷雾瓶喷板,紫外灯365 nm下观察,并将相应脂质条带刮下。
1.5 转酯化和气相色谱分析将脂质转移至10 mL圆底烧瓶中,加入5 mL 2% H2SO4-CH3OH (体积百分数),于70 ℃油浴中混合磁力搅拌1 h,待冷却至室温后加入2 mL正己烷及0.75 mL去离子水,混合30 s后将上层含有脂肪酸甲酯的正己烷转至2 mL色谱瓶中进行气相色谱分析,其中十七烷酸甘油三酯为内标[40],此外可根据需要用高纯N2对正己烷萃取液进行浓缩。各类甘油酯或各种脂肪酸含量表示为每克藻细胞干重中所含脂酰基的毫克数。
2 结果与分析 2.1 氮胁迫对三角褐指藻生长及光合活性的影响如表 1所示,氮胁迫3 d后三角褐指藻的光密度值OD680较富氮条件降低29%,最大PS Ⅱ光量子产率Fv/Fm降低9%,而实际PS Ⅱ光量子产率φPSⅡ则降低27%。由此表明,三角褐指藻经氮胁迫诱导后细胞分裂速率显著降低,其光合活性也显著下降。此外Fv/Fm的低幅度降低也表明了三角褐指藻仍处于早期氮胁迫阶段。
| Growth condition | OD680 | Fv/Fm | φPSⅡ |
| N+ | 0.637±0.047* | 0.768±0.006* | 0.465±0.010* |
| N- | 0.451±0.008 | 0.697±0.004 | 0.341±0.007 |
| 注:N+:富营养培养条件;N-:缺氮培养条件.所有数值表示为3个生物学重复的平均值±标准偏差. *:同一列数据之间差异显著(P < 0.05). Note: N+: The nitrogen repletion condition; N-: The nitrogen depletion condition. Data were all expressed as average of 3 biological replicates±SD. *: Statistical significant differences—one-way analysis of variance (ANOVA), P-value < 0.05. | |||
氮胁迫后总甘油酯含量不变,但各甘油酯的含量变化显著。糖脂含量减少61%,其中MGDG、DGDG及SQDG含量分别减少78%、28%、43%,磷脂(PL)含量减少63%,TAG含量增加6倍(图 1)。

|
| 图 1 氮胁迫对三角褐指藻甘油酯含量的影响 Figure 1 The glycerolipid phenotypes of P. tricornutum in response to nitrogen starvation 注:A:氮胁迫对三角褐指藻糖脂、极性脂及甘油酯含量的影响;B:氮胁迫对各种甘油酯含量的影响. N+:富营养培养条件;N-:缺氮培养条件.所有数值表示为3个生物学重复的平均值±标准偏差. *:同一列数据之间差异显著(P < 0.05). PL:磷脂. Note: A: Effects of nitrogen stress on contents of glycolipid, polar lipid and glycerolipid in P. tricornutum; B: Effects of nitrogen stress on contents of the individual glycerolipid in P. tricornutum. N+: Nitrogen repletion condition; N-: Nitrogen depletion condition. Data were all expressed as average of 3 biological replicates±SD. *: Statistical significant differences—one-way analysis of variance (ANOVA), P-value < 0.05. PL: Phospholipid. |
|
|
氮胁迫后各类甘油酯的脂肪酸含量变化差异非常大。如图 2所示,MGDG的主要脂肪酸为16:1n7、16:3n4及EPA,占MGDG总脂肪酸含量的80%以上,各脂肪酸在氮胁迫后变化差异显著。MGDG的饱和脂肪酸(SFA)及单不饱和脂肪酸(MUFA)中16:0及16:1n7分别下降了39%及84%,多不饱和脂肪酸(PUFA)中16:2n4、16:3n4及EPA分别下降了91%、72%、78%。DGDG的主要脂肪酸为16:1n7及EPA,占DGDG总脂肪酸含量的70%以上。DGDG中16:2n4、EPA及二十二碳六烯酸(DHA)含量变化差异显著,16:2n4及EPA含量均下降40%以上,DHA几乎下降100%。SQDG的脂肪酸以SFA及MUFA为主,包括14:0、16:0及16:1n7,占SQDG总脂肪酸含量的80%以上,氮胁迫后3种脂肪酸含量变化显著,分别下降41%、33%、53%。PL的主要脂肪酸为16:0、16:1n7及EPA,占PL总脂肪酸含量的65%以上,氮胁迫后3种脂肪酸含量变化显著,分别下降50%、82%、50%。如图 3所示,TAG的主要脂肪酸为16:0及16:1n7,占TAG总脂肪酸比例由57%提高至81%,氮胁迫后二者含量均增加了9倍。其中TAG的PUFA包括16:2n4及EPA,16:2n4含量几乎不变,而EPA的含量增加了5倍,但二者的组成比例之和却由18%下降至6%。
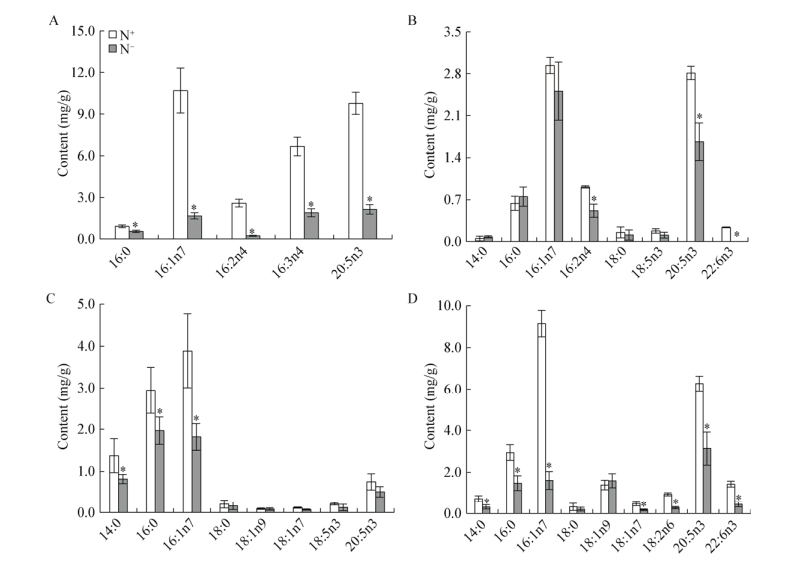
|
| 图 2 氮胁迫对三角褐指藻各极性脂脂肪酸含量的影响 Figure 2 The polar lipid phenotypes of P. tricornutum in response to nitrogen stress 注:A:氮胁迫对三角褐指藻MGDG脂肪酸含量的影响;B:氮胁迫对三角褐指藻DGDG脂肪酸含量的影响;C:氮胁迫对三角褐指藻SQDG脂肪酸含量的影响;D:氮胁迫对三角褐指藻PL脂肪酸含量的影响. N+:富营养培养条件;N-:缺氮培养条件.所有数值表示为3个生物学重复的平均值±标准偏差. *:同一列数据之间差异显著(P < 0.05). Note: A: Effects of nitrogen starvation on the fatty acid contents of MGDG in P. cornutum; B: Effects of nitrogen stress on the fatty acid contents of DGDG in P. cornutum; C: Effects of nitrogen stress on the fatty acid contents of SQDG in P. cornutum; D: Effects of nitrogen stress on the fatty acid contents of phospholipid in P. cornutum. N+: Nitrogen repletion condition; N-: Nitrogen depletion condition. Data were all expressed as average of 3 biological replicates±SD. *: Statistical significant differences—one-way analysis of variance (ANOVA), P-value < 0.05. |
|
|
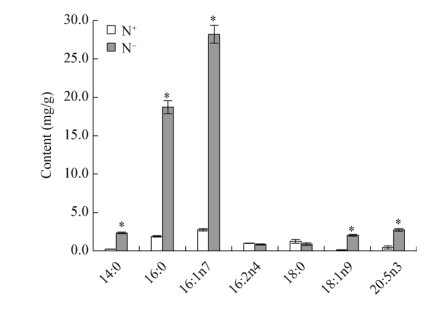
|
| 图 3 氮胁迫对三角褐指藻TAG含量的影响 Figure 3 The TAG phenotypes of P. tricornutum in response to nitrogen stress 注:N+:富营养培养条件;N−:缺氮培养条件.所有数值表示为3个生物学重复的平均值±标准偏差. *:同一列数据之间差异显著(P < 0.05). Note: N+: Nitrogen repletion condition; N−: Nitrogen depletion condition. Data were all expressed as average of 3 biological replicates±SD. *: Statistical significant differences—one-way analysis of variance (ANOVA), P-value < 0.05. |
|
|
氮胁迫后总脂肪酸含量几乎不变,但各脂肪酸含量在氮胁迫前后变化差异很大,如图 4所示,表现为SFA及MUFA含量的显著增加,而PUFA含量的显著降低。其中除18:0和18:1n7外,SFA和MUFA包括14:0、16:0、16:1n7和18:1n9含量均显著增加,14:0和16:1n7含量分别增加44%和22%,16:0和18:1n9含量分别增加1.5倍和1.0倍。此外,PUFA中16:2n4、16:3n4、18:2n6、EPA和DHA含量均显著下降,分别下降66%、67%、56%、49%和65%。

|
| 图 4 氮胁迫对三角褐指藻各脂肪酸含量的影响 Figure 4 The fatty acid phenotypes of P. tricornutum in response to nitrogen stress 注:N+:富营养培养条件;N−:缺氮培养条件.所有数值表示为3个生物学重复的平均值±标准偏差. *:同一列数据之间差异显著(P < 0.05). Note: N+: Nitrogen repletion condition; N−: Nitrogen depletion condition. Data were all expressed as average of 3 biological replicates±SD. *: Statistical significant differences—one-way analysis of variance (ANOVA), P-value < 0.05. |
|
|
氮胁迫后,藻细胞中16:0总含量增加了14.15 mg/g,而TAG中16:0增加了16.83 mg/g,所有极性脂的16:0含量只减少了2.68 mg/g,其中除DGDG中16:0含量变化不显著外,MGDG、SQDG和PL的16:0含量变化均显著,分别减少0.35、0.98和1.46 mg/g。氮胁迫后,藻细胞中16:1n7总含量增加了6.4 mg/g,而TAG中16:1n7增加了25.47 mg/g,所有极性脂的16:1n7含量减少了19.07 mg/g,其中除DGDG中16:1n7含量变化不显著外,MGDG、SQDG和PL中16:1n7含量分别减少9.03、2.07和7.54 mg/g,这些结果与16:0类似。氮胁迫后,藻细胞中EPA总含量减少了9.86 mg/g,而TAG中EPA增加了2.3 mg/g,极性脂中EPA含量减少了12.16 mg/g,其中除SQDG中EPA含量变化不显著外,MGDG、DGDG和PL中EPA含量均显著减少,分别减少7.63、1.15和3.14 mg/g,这与上述16:0及16:1n7均不同。氮胁迫后,藻细胞中DHA总含量减少了1.24 mg/g,只有极性脂中DGDG和PL的DHA含量显著降低,分别减少0.24 mg/g和0.99 mg/g,而TAG中并无DHA的积累。
3 讨论藻类脂质代谢包括从头合成途径、转化途径和亚细胞区室之间的脂质运输途径,这些途径在环境条件发生改变时需要进行严格调控才能达到脂质内稳态并保证亚细胞膜结构的稳定性[41]。不同种类微藻的脂质代谢在很多方面都不同,这也表明了藻类种属在进化上的高度多样性。
目前关于环境条件改变对微藻脂肪酸组成影响的研究已有大量报道,这些研究表明氮、磷、硅、硫、温度、盐度、光照强度等条件发生改变时脂肪酸的组成会发生改变[9, 25, 27, 42-44]。研究表明环境条件改变也能使微藻每种脂质的含量发生改变,通常极性脂含量会大幅下降,同时伴随着储存脂质TAG的大量积累。长期以来,TAG被认为是藻类、植物、酵母及动物中主要的碳源及能源[45]。但是最近酵母及哺乳动物细胞的研究表明,TAG代谢可能在脂质内稳态中起重要作用,能为细胞周期进程提供重要代谢产物[46]。此外,在莱茵衣藻中,氮胁迫诱导的TAG强化积累来源包括糖脂的转化[47]和从头合成途径[43],但目前三角褐指藻甘油酯对氮胁迫的具体响应研究并不多。
Abida等[9]的研究表明在氮胁迫条件下三角褐指藻的MGDG和PG含量显著下降,其他极性脂含量保持不变,TAG含量显著增加。而本文中三角褐指藻的所有极性脂含量均显著下降,与Abida等[9]研究结果不同,可能是由于藻株自然特性及培养条件不同所致,包括培养体积、培养方式、光照强度、培养基等,可见三角褐指藻的甘油酯尤其是极性脂在不同的培养条件下对氮胁迫的响应是不同的。在本文中,氮胁迫诱导的三角褐指藻并无脂质净积累,但其脂质组分之间已发生重组,具体表现为TAG显著积累而极性脂水平显著降低。极性膜脂对氮胁迫的响应主要表现为以MGDG为主的选择性分解,使得DGDG/MGDG的比率从0.3增加至0.8 (由图 1计算得出),这与莱茵衣藻的膜脂适应结果一致,表现为非双层形成脂质DGDG与双层形成脂质MGDG的比率在胁迫诱导后增加[25]。因此,甘油酯头部基团的交换是三角褐指藻适应氮胁迫的机制之一。
3.1 氮胁迫后三角褐指藻TAG的酰基来源——从头合成和极性脂转化氮胁迫期间脂肪酸从头合成对TAG积累非常重要[48],研究表明将脂肪酸合成抑制剂浅蓝菌素(一种β-酮类-酰基-ACP合成酶的特异性抑制剂),添加至莱茵衣藻细胞培养液中能抑制TAG积累,TAG含量较氮胁迫2 d的对照组细胞少合成80%,因此表明了脂肪酸从头合成对TAG合成的贡献[43]。外源供应的乙酸盐能进一步促进氮胁迫处理藻细胞的脂质积累,这也证明了脂肪酸合成对TAG合成的重要作用[49]。从膜脂循环的酰基链也被认为对TAG合成有贡献,因为主要膜脂组分减少与TAG积累同时发生[50]。酰基链从一种脂质转移至另一种脂质能通过两种方式实现:通过酰基转移酶实现如CrPDAT和CrDGAT,或者是通过脂酶(如PGD)水解一种脂质(如MGDG)释放出游离脂肪酸后再活化成酰基-CoA或酰基-ACP。转酰基途径对脂质合成的贡献作用已通过CrPDAT和CrDGAT的研究得到证实[51-52]。最近通过从莱茵衣藻中分离一株半乳糖脂酶合成缺陷诱变株pgd1证明了酯酶PGD1能特异性水解脂膜MGDG的sn-1位脂酰基18:1n9,同时释放的酰基以18:1n9-CoA或18:1n9-ACP形式进一步参与TAG的合成[49]。总之,这些证据表明氮胁迫下合成的TAG大部分来源于从头合成途径,部分来源于膜脂酰基链的转化。但是这两种途径各自的贡献率通常取决于培养条件(自养或兼养)或胁迫类型(光照、温度或营养盐等)。
在植物和微藻中,脂肪酸的合成发生在叶绿体内,新合成的脂肪酸大多是饱和或单不饱和的,如16:0、18:0、18:1等[48]。这些脂肪酸能通过生成极性脂在去饱和酶作用下生成PUFA,而去饱和酶不会以TAG为底物,说明TAG中积累的PUFA来自于极性脂的转化[44],因此可以通过TAG中积累的SFA和MUFA含量来估计氮胁迫后从头合成途径对TAG合成的贡献度,而TAG中积累的PUFA含量则可以用来估计氮胁迫后各极性脂的酰基转化作用对TAG合成所做的贡献。在本文中,氮胁迫后藻细胞TAG增加的16:0大部分为从头合成,还有部分来自原有极性脂(表 2),通过原有各极性脂中16:0的减少量推算出其对TAG转化作用的贡献度大小为PL > SQDG > MGDG。由图 4计算出TAG积累的16:0从头合成比例为84%,只有16%为原有极性脂的转化作用。同样地,原有各极性脂中16:1n7对TAG转化作用的贡献度大小为MGDG > PL > SQDG,TAG中积累的16:1n7只有25%来自从头合成作用,而极性脂的转化贡献度则高达75%。另外,TAG中增加的EPA全部来自于极性脂的转化,而藻细胞中所有极性脂减少的总EPA含量中只有19%用于转化成TAG (由图 4计算得出),这部分EPA向TAG的流动可用于消耗藻细胞因氮胁迫诱导积累的多余电子、NAD(P)H及ATP,从而保护细胞免受氧化损伤,而原有极性脂中减少的其余EPA则发生了分解作用。TAG中EPA占藻细胞总EPA的百分比由2%升高至27%,这也说明了极性脂中EPA向TAG的流动或转化作用。有趣的是,藻细胞TAG中并无DHA积累,这与Popko等[34]研究结果一致,说明藻细胞中减少的DHA全部发生了分解。DHA及EPA在应对环境胁迫因子氮胁迫时的响应结果不同,说明这两种PUFA对胁迫条件的响应机制也不同,而这也有待于进一步研究。
| 脂肪酸 Fatty acid |
从头合成 De novo synthesis |
极性脂转化 Polar lipid recycling |
|||
| 含量 Content (mg/g) |
合成贡献度 Contribution (%) |
含量 Content (mg/g) |
合成贡献度 Contribution (%) |
||
| 14:0 | 1.11 | 52 | 1.01 | 48 | |
| 16:0 | 14.15 | 84 | 2.68 | 16 | |
| 16:1n7 | 6.40 | 25 | 19.07 | 75 | |
| 18:1n9 | 1.90 | 100 | 0.00 | 0 | |
| EPA | 0.00 | 0 | 2.30 | 100 | |
| Total | 23.56 | 48 | 25.06 | 52 | |
综上所述,氮胁迫后三角褐指藻的SFA (主要是16:0)在藻细胞总脂肪酸及TAG组成中显著增加,而在极性脂中保持不变或显著减少,这些结果支持了脂肪酸的从头合成及其向TAG合成的流动,这与莱茵衣藻和微拟球藻的情况类似[44, 53]。此外,MUFA 16:1n7在藻细胞总脂肪酸组成中的增加量要显著低于TAG中的显著增加量,与之对应的是极性脂中该脂肪酸含量的大幅下降,这说明了极性脂中16:1n7向TAG中的流动,即极性脂的酰基转化对TAG合成具有重要作用,这与莱茵衣藻中情况类似[49]。由此可见,氮胁迫后三角褐指藻TAG合成来源为从头合成及极性脂转化(表 2),其各自贡献度分别为48%和52%,约为1:1,其中从头合成部分主要来自于16:0,极性脂转化部分主要来自MGDG和PL的16:1n7,还包括一部分来自极性脂的EPA。
3.2 氮胁迫后极性脂中PUFA的去向微藻在多种胁迫条件下的一个重要响应特征是极性脂含量降低,尤其是构成糖脂的PUFA水平大幅下降[28, 54]。研究表明这些降低的PUFA在胁迫诱导下共有两个重要去向,一是作为酰基供体或是甘油二酯骨架用于合成TAG,发生膜脂转化作用[25];二是作为氧化作用的底物,发生了分解作用[28]。
本文中,氮胁迫3 d使极性脂含量减少了46.5 mg/g (由图 1计算得出),其中54%转化成了TAG,46%进行了分解(由图 2及图 3计算得出)。其中极性脂的PUFA含量减少了59%,除少量EPA作为酰基供体转化成TAG外,大部分极性脂尤其是糖脂MGDG中的PUFA如16:2n4、16:3n4及EPA在氮胁迫条件下都发生了分解作用。
Légeret等[25]研究表明,莱茵衣藻在短期热胁迫内特异性多不饱和MGDG sn1-18:3/sn2-16:4能通过一种未经鉴定的酶直接转化成甘油二酯(DAG sn1-18:3/sn2-16:4),再转化成多不饱和TAG sn1-18:3/sn2-16:4/sn3-18:3,TAG的第3个脂肪酸来源于PE或DGTS,这些研究结果为多不饱和极性脂通过甘油骨架或酰基向TAG的转化提供了证据。本文中极性脂MGDG、DGDG和PL的酰基组分EPA也在氮胁迫诱导后通过这种酰基转化途径合成了TAG,这与Popko等[34]研究结果一致。因此在氮胁迫下三角褐指藻中以EPA为主的PUFA从膜脂到TAG的流动也能成为其避免细胞自身发生脂质过氧化的一个策略。
此外本文中三角褐指藻以16:2n4、16:3n4和EPA为主的PUFA还发生了分解作用,三角褐指藻这种PUFA种类的选择性分解导致氮胁迫下SFA的组分增加,膜结构中PUFA/SFA的比率减小,说明膜脂组成变化的适应性能在新环境条件下维持正确的膜流动性,该现象称为内粘性适应(Homeoviscous adaptation)[25],本研究结果表明这也是三角褐指藻甘油酯应对氮胁迫的一种保守机制。
微藻在氮胁迫下的甘油酯响应是一个动态变化过程[32-33],TAG在积累的同时伴随着极性脂的合成、分解与转化[9, 33],因此TAG的积累与极性脂的变化密切相关;依据藻细胞脂肪酸相对丰度的变化,TAG的积累分为不同阶段[55],表明极性脂对TAG积累的贡献度也逐渐变化。本文中三角褐指藻氮胁迫2 d后最大PS Ⅱ光量子产率Fv/Fm只降低了9%,TAG含量只有57.8 mg/g,表明其处于早期氮胁迫阶段,本研究结果突出了原有极性脂在早期氮胁迫阶段中对TAG积累的重要贡献作用,其贡献度达52%。本文通过三角褐指藻甘油酯及其脂肪酸含量的变化,定量研究了氮胁迫早期从头合成和原有极性脂转化途径对TAG积累的各自贡献度,还定量研究了氮胁迫早期大量减少的极性脂在TAG转化及分解途径中的分配去向,这些结果表明了氮胁迫早期极性脂对TAG积累的重要性以及甘油酯响应在维持脂质内稳态过程中所发挥的重要作用,而目前的研究大多集中于高含量TAG的氮胁迫阶段[31]或其积累的动态变化过程[32-33],关于早期氮胁迫时期内TAG合成来源及极性脂的响应研究并不多,这将为氮胁迫不同时期内微藻甘油酯响应机制的研究奠定基础。
4 结论综上所述,当氮胁迫诱导的三角褐指藻TAG含量为57.8 mg/g时,积累的TAG酰基中有48%来自从头合成,52%来自极性脂转化;而氮胁迫诱导所减少的极性脂酰基中有54%转化成TAG,46%发生了分解。三角褐指藻极性脂发生的重组作用,一方面能促进TAG的合成,另一方面可通过保持一定水平的PUFA来维持藻细胞的脂质内稳态,这些对于生物柴油工业化生产及富含PUFA的营养食品开发具有重要的生物学启示作用。此外,高效薄层色谱结合气相色谱技术能为微藻甘油酯对环境变化响应提供准确的定性定量信息,从而促进微藻脂质代谢研究。
| [1] |
Nelson DM, Tréguer P, Brzezinski MA, et al. Production and dissolution of biogenic silica in the ocean: revised global estimates, comparison with regional data and relationship to biogenic sedimentation[J]. Global Biogeochemical Cycles, 1995, 9(3): 359-372. DOI:10.1029/95GB01070 |
| [2] |
van Den Hoek C, Mann D, Jahns HM. Algae: An Introduction to Phycology[M]. Cambridge: Cambridge University Press, 1996.
|
| [3] |
Bhattacharya D, Archibald JM, Weber APM, et al. How do endosymbionts become organelles? Understanding early events in plastid evolution[J]. Bioessays, 2007, 29(12): 1239-1246. DOI:10.1002/(ISSN)1521-1878 |
| [4] |
Armbrust EV, Berges JA, Bowler C, et al. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism[J]. Science, 2004, 306(5693): 79-86. DOI:10.1126/science.1101156 |
| [5] |
Bowler C, Allen AE, Badger JH, et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes[J]. Nature, 2008, 456(7219): 239-244. DOI:10.1038/nature07410 |
| [6] |
Allen AE, Vardi A, Bowler C. An ecological and evolutionary context for integrated nitrogen metabolism and related signaling pathways in marine diatoms[J]. Current Opinion in Plant Biology, 2006, 9(3): 264-273. DOI:10.1016/j.pbi.2006.03.013 |
| [7] |
Zhao PP, Gu WH, Wu SC, et al. Changes in central carbon metabolism of Phaeodactylum tricornutum are beneficial for lipid accumulation under nitrogen starvation conditions[J]. Chinese Science Bulletin, 2015, 60(23): 2196-2208. 赵佩佩, 顾文辉, 伍松翠, 等. 氮限制有利于三角褐指藻脂质积累[J]. 科学通报, 2015, 60(23): 2196-2208. |
| [8] |
Hockin NL, Mock T, Mulholland F, et al. The response of diatom central carbon metabolism to nitrogen starvation is different from that of green algae and higher plants[J]. Plant Physiology, 2012, 158(1): 299-312. DOI:10.1104/pp.111.184333 |
| [9] |
Abida H, Dolch LJ, Meï C, et al. Membrane glycerolipid remodeling triggered by nitrogen and phosphorus starvation in Phaeodactylum tricornutum[J]. Plant Physiology, 2015, 167(1): 118-136. DOI:10.1104/pp.114.252395 |
| [10] |
Shen PL, Wang HT, Pan YF, et al. Identification of characteristic fatty acids to quantify triacylglycerols in microalgae[J]. Frontiers in Plant Science, 2016, 7: 162. |
| [11] |
Dambek M, Eilers U, Breitenbach J, et al. Biosynthesis of fucoxanthin and diadinoxanthin and function of initial pathway genes in Phaeodactylum tricornutum[J]. Journal of Experimental Botany, 2012, 63(15): 5607-5612. DOI:10.1093/jxb/ers211 |
| [12] |
Patil V, Källqvist T, Olsen E, et al. Fatty acid composition of 12 microalgae for possible use in aquaculture feed[J]. Aquaculture International, 2007, 15(1): 1-9. DOI:10.1007/s10499-006-9060-3 |
| [13] |
Xue J, Niu YF, Huang T, et al. Genetic improvement of the microalga Phaeodactylum tricornutum for boosting neutral lipid accumulation[J]. Metabolic Engineering, 2015, 27: 1-9. DOI:10.1016/j.ymben.2014.10.002 |
| [14] |
Berges JA, Harrison PJ. Nitrate reductase activity quantitatively predicts the rate of nitrate incorporation under steady state light limitation: a revised assay and characterization of the enzyme in three species of marine phytoplankton[J]. Limnology and Oceanography, 1995, 40(1): 82-93. DOI:10.4319/lo.1995.40.1.0082 |
| [15] |
Levitan O, Dinamarca J, Zelzion E, et al. Remodeling of intermediate metabolism in the diatom Phaeodactylum tricornutum under nitrogen stress[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(2): 412-417. DOI:10.1073/pnas.1419818112 |
| [16] |
Guerra LT, Levitan O, Frada MJ, et al. Regulatory branch points affecting protein and lipid biosynthesis in the diatom Phaeodactylum tricornutum[J]. Biomass and Bioenergy, 2013, 59: 306-315. DOI:10.1016/j.biombioe.2013.10.007 |
| [17] |
Alipanah L, Rohloff J, Winge P, et al. Whole-cell response to nitrogen deprivation in the diatom Phaeodactylum tricornutum[J]. Journal of Experimental Botany, 2015, 66(20): 6281-6296. DOI:10.1093/jxb/erv340 |
| [18] |
Mus F, Toussaint JP, Cooksey KE, et al. Physiological and molecular analysis of carbon source supplementation and pH stress-induced lipid accumulation in the marine diatom Phaeodactylum tricornutum[J]. Applied Microbiology and Biotechnology, 2013, 97(8): 3625-3642. DOI:10.1007/s00253-013-4747-7 |
| [19] |
Wu SC, Huang AY, Zhang BY, et al. Enzyme activity highlights the importance of the oxidative pentose phosphate pathway in lipid accumulation and growth of Phaeodactylum tricornutum under CO2 concentration[J]. Biotechnology for Biofuels, 2015, 8: 78. DOI:10.1186/s13068-015-0262-7 |
| [20] |
Zienkiewicz K, Du ZY, Ma W, et al. Stress-induced neutral lipid biosynthesis in microalgae—Molecular, cellular and physiological insights[J]. Biochimica et Biophysica Acta, 2016, 1861(9): 1269-1281. DOI:10.1016/j.bbalip.2016.02.008 |
| [21] |
Horn PJ, Benning C. The plant lipidome in human and environmental health[J]. Science, 2016, 353(6305): 1228-1232. DOI:10.1126/science.aaf6206 |
| [22] |
Boudière L, Michaud M, Petroutsos D, et al. Glycerolipids in photosynthesis: composition, synthesis and trafficking[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2014, 1837(4): 470-480. DOI:10.1016/j.bbabio.2013.09.007 |
| [23] |
Li-Beisson Y, Nakamura Y, Harwood J. Lipids: from chemical structures, biosynthesis, and analyses to industrial applications[A]//Nakamura Y, Li-Beisson Y. Lipids in Plant and Algae Development[M]. Cham: Springer International Publishing, 2016: 1-18 https://link.springer.com/chapter/10.1007/978-3-319-25979-6_1
|
| [24] |
Bréhélin C, Kessler F, van Wijk KJ. Plastoglobules: versatile lipoprotein particles in plastids[J]. Trends in Plant Science, 2007, 12(6): 260-266. DOI:10.1016/j.tplants.2007.04.003 |
| [25] |
Légeret B, Schulz-Raffelt M, Nguyen HM, et al. Lipidomic and transcriptomic analyses of Chlamydomonas reinhardtii under heat stress unveil a direct route for the conversion of membrane lipids into storage lipids[J]. Plant, Cell & Environment, 2016, 39(4): 834-847. |
| [26] |
Alboresi A, Perin G, Vitulo N, et al. Light remodels lipid biosynthesis in Nannochloropsis gaditana by modulating carbon partitioning between organelles[J]. Plant Physiology, 2016, 171(4): 2468-2482. |
| [27] |
Yang DW, Song DH, Kind T, et al. Lipidomic analysis of Chlamydomonas reinhardtii under nitrogen and sulfur deprivation[J]. PLoS One, 2015, 10(9): e0137948. DOI:10.1371/journal.pone.0137948 |
| [28] |
Martin GJO, Hill DRA, Olmstead ILD, et al. Lipid profile remodeling in response to nitrogen deprivation in the microalgae Chlorella sp. (Trebouxiophyceae) and Nannochloropsis sp. (Eustigmatophyceae)[J]. PLoS One, 2014, 9(8): e103389. DOI:10.1371/journal.pone.0103389 |
| [29] |
Longworth J, Wu DY, Huete-Ortega M, et al. Proteome response of Phaeodactylum tricornutum, during lipid accumulation induced by nitrogen depletion[J]. Algal Research, 2016, 18: 213-224. DOI:10.1016/j.algal.2016.06.015 |
| [30] |
Ge F, Huang WC, Chen Z, et al. Methylcrotonyl-CoA carboxylase regulates triacylglycerol accumulation in the model diatom Phaeodactylum tricornutum[J]. The Plant Cell, 2014, 26(4): 1681-1697. DOI:10.1105/tpc.114.124982 |
| [31] |
Yang ZK, Niu YF, Ma YH, et al. Molecular and cellular mechanisms of neutral lipid accumulation in diatom following nitrogen deprivation[J]. Biotechnology for Biofuels, 2013, 6(1): 67. DOI:10.1186/1754-6834-6-67 |
| [32] |
Remmers IM, Martens DE, Wijffels RH, et al. Dynamics of triacylglycerol and EPA production in Phaeodactylum tricornutum under nitrogen starvation at different light intensities[J]. PLoS One, 2017, 12(4): e0175630. DOI:10.1371/journal.pone.0175630 |
| [33] |
Natunen K, Seppälä J, Koivula RJ, et al. Monitoring cell-specific neutral lipid accumulation in Phaeodactylum tricornutum (Bacillariophyceae) with Nile Red staining-a new method for FlowCAM[J]. Journal of Phycology, 2017, 53(2): 396-404. DOI:10.1111/jpy.2017.53.issue-2 |
| [34] |
Popko J, Herrfurth C, Feussner K, et al. Metabolome analysis reveals betaine lipids as major source for triglyceride formation, and the accumulation of sedoheptulose during nitrogen-starvation of Phaeodactylum tricornutum[J]. PLoS One, 2016, 11(10): e0164673. DOI:10.1371/journal.pone.0164673 |
| [35] |
Wang Z, Benning C. Arabidopsis thaliana polar glycerolipid profiling by thin layer chromatography (TLC) coupled with gas-liquid chromatography (GLC)[J]. Journal of Visualized Experiments, 2011(49): e2518. |
| [36] |
Schröter J, Griesinger H, Reuÿ E, et al. Unexpected products of the hypochlorous acid-induced oxidation of oleic acid: a study using high performance thin-layer chromatography–electrospray ionization mass spectrometry[J]. Journal of Chromatography A, 2016, 1439: 89-96. DOI:10.1016/j.chroma.2015.11.059 |
| [37] |
Feng DN, Chen ZA, Xue S, et al. Increased lipid production of the marine oleaginous microalgae Isochrysis zhangjiangensis (Chrysophyta) by nitrogen supplement[J]. Bioresource Technology, 2011, 102(12): 6710-6716. DOI:10.1016/j.biortech.2011.04.006 |
| [38] |
Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide[J]. Journal of Experimental Botany, 2000, 51(345): 659-668. DOI:10.1093/jexbot/51.345.659 |
| [39] |
Meng YY, Jiang JP, Wang HT, et al. The characteristics of TAG and EPA accumulation in Nannochloropsis oceanica IMET1 under different nitrogen supply regimes[J]. Bioresource Technology, 2015, 179: 483-489. DOI:10.1016/j.biortech.2014.12.012 |
| [40] |
Liu J, Liu YN, Wang HT, et al. Direct transesterification of fresh microalgal cells[J]. Bioresource Technology, 2015, 176: 284-287. DOI:10.1016/j.biortech.2014.10.094 |
| [41] |
Liu B, Benning C. Lipid metabolism in microalgae distinguishes itself[J]. Current Opinion in Biotechnology, 2013, 24(2): 300-309. DOI:10.1016/j.copbio.2012.08.008 |
| [42] |
Yu ET, Zendejas FJ, Lane PD, et al. Triacylglycerol accumulation and profiling in the model diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum (Baccilariophyceae) during starvation[J]. Journal of Applied Phycology, 2009, 21(6): 669-681. DOI:10.1007/s10811-008-9400-y |
| [43] |
Fan JL, Andre C, Xu CC. A chloroplast pathway for the de novo biosynthesis of triacylglycerol in Chlamydomonas reinhardtii[J]. FEBS Letters, 2011, 585(12): 1985-1991. DOI:10.1016/j.febslet.2011.05.018 |
| [44] |
Goold HD, Cuiné S, Légeret B, et al. Saturating light induces sustained accumulation of oil in plastidal lipid droplets in Chlamydomonas reinhardtii[J]. Plant Physiology, 2016, 171(4): 2406-2417. |
| [45] |
Li YT, Han DX, Yoon K, et al. Molecular and cellular mechanisms for lipid synthesis and accumulation in microalgae: biotechnological implications[A]//Richmond A, Hu Q. Handbook of Microalgal Culture: Applied Phycology and Biotechnology[M]. Chichester: John Wiley & Sons, Ltd., 2013: 545-565
|
| [46] |
Kohlwein SD. Triacylglycerol homeostasis: insights from yeast[J]. Journal of Biological Chemistry, 2010, 285(21): 15663-15667. DOI:10.1074/jbc.R110.118356 |
| [47] |
Li XB, Moellering ER, Liu BS, et al. A galactoglycerolipid lipase is required for triacylglycerol accumulation and survival following nitrogen deprivation in Chlamydomonas reinhardtii[J]. The Plant Cell, 2012, 24(11): 4670-4686. DOI:10.1105/tpc.112.105106 |
| [48] |
Moellering ER, Benning C. RNA interference silencing of a major lipid droplet protein affects lipid droplet size in Chlamydomonas reinhardtii[J]. Eukaryotic Cell, 2010, 9(1): 97-106. DOI:10.1128/EC.00203-09 |
| [49] |
Goodson C, Roth R, Wang ZT, et al. Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid body production with acetate boost[J]. Eukaryotic Cell, 2011, 10(12): 1592-1606. DOI:10.1128/EC.05242-11 |
| [50] |
Siaut M, Cuiné S, Cagnon C, et al. Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves[J]. BMC Biotechnology, 2011, 11: 7. DOI:10.1186/1472-6750-11-7 |
| [51] |
Yoon K, Han DX, Li YT, et al. Phospholipid: diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga Chlamydomonas reinhardtii[J]. The Plant Cell, 2012, 24(9): 3708-3724. DOI:10.1105/tpc.112.100701 |
| [52] |
Liu J, Han DX, Yoon K, et al. Characterization of type 2 diacylglycerol acyltransferases in Chlamydomonas reinhardtii reveals their distinct substrate specificities and functions in triacylglycerol biosynthesis[J]. The Plant Journal, 2016, 86(1): 3-19. DOI:10.1111/tpj.2016.86.issue-1 |
| [53] |
Dong HP, Williams E, Wang DZ, et al. Responses of Nannochloropsis oceanica IMET1 to long-term nitrogen starvation and recovery[J]. Plant Physiology, 2013, 162(2): 1110-1126. DOI:10.1104/pp.113.214320 |
| [54] |
Ma XN, Liu J, Liu B, et al. Physiological and biochemical changes reveal stress-associated photosynthetic carbon partitioning into triacylglycerol in the oleaginous marine alga Nannochloropsis oculata[J]. Algal Research, 2016, 16: 28-35. DOI:10.1016/j.algal.2016.03.005 |
| [55] |
Breuer G, Lamers PP, Martens DE, et al. The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains[J]. Bioresource Technology, 2012, 124: 217-226. DOI:10.1016/j.biortech.2012.08.003 |
 2018, Vol. 45
2018, Vol. 45




