扩展功能
文章信息
- 陈彩霞, 王泽昊, FENG Jie, 梁月
- CHEN Cai-Xia, WANG Ze-Hao, FENG Jie, LIANG Yue
- 植物病原真菌的菌核研究进展
- Sclerotia of plant pathogenic fungi
- 微生物学通报, 2018, 45(12): 2762-2768
- Microbiology China, 2018, 45(12): 2762-2768
- DOI: 10.13344/j.microbiol.china.180117
-
文章历史
- 收稿日期: 2018-02-06
- 接受日期: 2018-04-13
- 网络首发日期(www.cnki.net): 2018-04-23
2. The Alberta Plant Health Lab, Alberta Agriculture and Forestry, Edmonton, Alberta T5Y 6H3, Canada
2. The Alberta Plant Health Lab, Alberta Agriculture and Forestry, Edmonton, Alberta T5Y 6H3, Canada
真菌菌核是在遇到不良环境或其它外界刺激时营养菌丝体聚集形成的一种休眠结构,其形态多样,在适宜的环境条件下可萌发形成无性或有性繁殖结构。许多植物病原真菌能够形成菌核,如核盘菌、丝核菌、小核菌等[1],这些病原菌引起的病害普遍发生,并且严重影响农作物、经济作物、蔬菜及花卉等生产。因此,研究植物病原真菌的菌核生物学特性及发育分子机理具有重要的意义,并对能够形成菌核的真菌的防治具有重要的参考价值。
1 形成菌核的主要真菌种类形成菌核的真菌(Sclerotium-forming fungi)很多,包括菌根菌(11个属)、腐生菌(30个属)及植物病原菌(25个属)[2]。其中,植物病原真菌包括子囊菌门的核盘菌属(Sclerotinia)、葡萄孢属(Botrytis)或孢盘菌属(Botryotinia)和担子菌门的丝核菌属(Rhizoctonia)、小核菌属(Sclerotium)等[1-2]。其中,(1)核盘菌属于核盘菌科(Sclerotiniaceae),代表种核盘菌(Sclerotinia sclerotiorum)是一种死体营养型真菌。营养体菌丝透明、分隔,白色至棕褐色,成熟后形成菌核。菌核呈圆形或不规则形,似鼠粪状,初白色后变灰色,内部灰白色。菌核萌发后长出一至多个具长柄的肉质黄褐色子囊盘,其上着生一层子囊和侧丝。子囊无色,棍棒状,内含8个单胞无色子囊孢子,一般不产生无性分生孢子[3]。该病原菌寄主广泛,超过400种植物,包括经济作物(大豆、向日葵、油菜)、蔬菜(莴苣)、花卉(唐菖蒲、郁金香)等,引起的病害超过60种,如棉腐病、软腐病、茎腐病、猝倒病、冠腐病、花腐病和最常见的菌核病[4]。(2)葡萄孢菌属于核盘菌科,包括20余种真菌。代表种灰葡萄孢菌(Botrytis cinerea)作为一种死体营养型真菌,分布和寄主范围最广[5]。其分生孢子梗灰褐色,直立或稍微弯曲,丛生,单枝或树状分枝。分生孢子着生在孢子梗上,形似葡萄状。分生孢子单孢,多核,椭圆形,无色或淡灰色。该真菌形成不规则的黑色小菌核,能够侵染200多种植物,包括花、果、叶、芽和地下部分的贮藏器官(胡萝卜、甘薯),造成腐烂;对蔬菜(如卷心菜、西兰花)及浆果类植物(葡萄、草莓等)影响最为严重[6]。(3)丝核菌属于无孢科(Agonomycetaceae),以立枯丝核菌(Rhizoctonia solani)为典型种。菌丝初期无色、蛛网状,呈对角或直角分支,分支与主干相连处有缢缩并常具横隔,后期呈浅色至黄褐色。菌核形状不定,一般扁平,呈浅褐色至黑褐色,其表面粗糙、内外颜色一致,松散地分布在菌丝体中且彼此间有丝状体相连[7]。立枯丝核菌的无性世代不产孢子,有性世代为担子菌门亡革菌属(Pellicularia)。丝核菌寄主范围广泛,包括稻、麦、棉及多种蔬菜瓜果,主要病害有植物苗期的猝倒病、立枯病及禾谷类作物纹枯病等[8]。(4)小核菌属于无孢科,以齐整小核菌(Sclerotium rolfsii)作为代表种。无性世代无孢子形成,有性世代为担子菌门阿太菌属(Athelia)。菌丝多核且分枝,尖端具索状联合,不产孢子[9]。小菌核圆形或扁圆形,外皮层褐色,内部白色,菌核间无菌丝相连,表面光滑或粗糙[9]。该属寄主广泛,引起多种植物的病害,其中,齐整小核菌引起多种植物(如花生、韭菜)的白绢病而稻腐小核菌(S. oryzae)引起稻腐病[10]。
2 菌核的形成和发育真菌菌核是营养体菌丝交织和聚集构成的一种特殊休眠和存活结构,在真菌生活史及植物病原真菌引起的病害循环中具有重要的生物学和生态学意义。例如,在核盘菌的生活史和侵染过程中,菌核发挥着重要的作用,不但是长期存活结构而且是初侵染来源。菌核大小取决于寄主植物,如形成于向日葵种子表面的菌核直径比形成于菜豆上的大近百倍[3]。菌核主要细胞质储存物是多糖、蛋白质、多磷酸盐和脂质[1]。菌核的形成受各种条件的影响,特别是环境因素(如湿度、通风、温度等)[11]。例如,齐整小核菌的菌核形成与营养、光照、pH值等相关[12];灰葡萄孢菌的菌核形成需要黑暗条件[13];核盘菌的菌核形成还受到物理阻隔、次生代谢及活性氧(Reactive oxygen species,ROS)等方面影响[14]。
2.1 结构菌核结构比较复杂,由外向内依次分为外皮层(Rind)、皮质(Cortex)和髓质(Medulla)[1]。外皮层由含有黑色素的厚壁细胞组成,通常存在于菌核外表面,厚度是一到几个细胞。然而,不是所有真菌菌核都形成外皮层,如立枯丝核菌的菌核就没有外皮层结构[1]。外皮层的分化过程不同,核盘菌的菌核外皮层随着菌核膨大而开始分化;而小核盘菌(S. minor)的菌核生长至最大时外皮层才开始分化[15]。当菌核生长至最大时皮层可辨,但皮质的内外部并没有明显的分界。皮质最外层细胞具有与外皮层相似的特征(如色素沉积和液泡化),但皮质细胞更加紧实[1]。许多无隔膜不分枝菌丝交叉形成髓质,主要由薄壁细胞组成,其外层菌丝密度梯度增大且丝间含有胞外基质[1, 16]。
2.2 萌发菌核可以通过多种方式萌发,包括产孢萌发(Sporogenic germination)、产果萌发(Carpogenic germination)、产丝萌发(Myceliogenic germination)或上述方式的组合[1]。产丝萌发是在外源营养缺乏的条件下,菌核萌发产生菌丝,并侵染植物根部或茎基部造成苗期病害[17]。产孢萌发和产果萌发一般是以产生大菌核为特征,常见于由空气传播的病原真菌。例如,核盘菌主要以休眠体菌核的形式在土壤中越冬或越夏,在合适环境条件影响下,以产果或产丝的形式发生。核盘菌菌核在适宜条件下萌发形成子囊盘,子囊释放子囊孢子在空中分散并落在衰老植物组织上进行侵染,而一般不会直接侵染健康组织;产丝萌发的菌丝却可以直接侵入健康寄主植物[18]。灰葡萄孢菌菌核作为无性结构,可以直接进行产丝萌发并形成分生孢子梗或在具有不同交配型孢子情况下产生子囊盘[13]。此外,立枯丝核菌的菌核萌发活力受温度影响[19],核盘菌的菌核田间分布及萌发受温湿度影响,造成病害发生流行[20-21]。
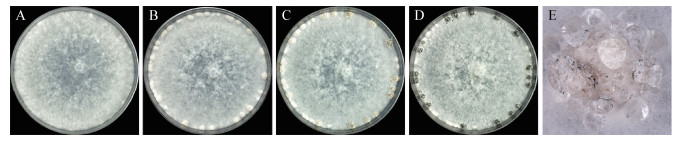
2.3 发育菌核发育通常根据形态学变化分为3个主要阶段(图 1),即初始阶段(Initiation,聚集形成独立初生体)、发展阶段(Development,体积增大并伴有液滴分泌)和成熟阶段(Maturation,表面定形且内部紧实并色素沉积)[14]。菌核的发育也可更精细地分为6个阶段,初始阶段(Initiation,营养菌丝开始聚集并形成气生菌丝簇)、缩聚阶段(Condensation,密度增大且气生菌丝表面有少量渗出液)、膨大阶段(Enlargement,体积快速增大并分泌大量渗出液,但表面依然呈白色)、固化阶段(Consolidation,颜色变成黄褐色且出现外表皮)、着色阶段(Pigmentation,黑色素聚集呈深黑色但表面渗出液无色或轻微着色)和成熟阶段(Maturation,菌核定形且表面黑色坚硬,但无渗出液,有时上覆薄层菌丝)[22]。另外,菌核初始阶段形成又具有3种形态类型:疏松型(Loose type)、终端型(Terminal type)、链状型(Strand type)[23]。其中,疏松型菌核没有明确的菌丝组织形式且结构松散,如丝核菌(R. solani);末端型菌核的菌丝尖分枝明确,如葱腐葡萄孢菌(B. allii);链状型菌核的链状菌丝具有大量的侧枝,如唐菖蒲核盘菌(S. gladioli)和齐整小核菌(S. rolfsii)[23]。菌核形成过程在显微水平上也被划分为以下4个亚阶段:分泌类粘性物质(Mucilage-like substance)、菌丝缠绕(Hyphal curling)、菌丝融合(Hyphal fusion)和晶体结构形成(Crystallization)[14]。菌核发育与成熟包括复杂的形态学和生物化学过程,如菌丝分枝与融合,营养物质和水分转移,表面渗出液形成等[12]。影响菌核发育的环境和营养因子广泛,如碳源、氮源、营养、pH等[24]。
|
| 图 1 菌核发育(以核盘菌为例) Figure 1 Sclerotial development (Sclerotinia sclerotiorum as an example) 注:A:营养生长阶段;B:初始阶段;C:发展阶段;D:成熟阶段;E:菌核渗出液. Note: A: Vegetative hypha; B: Initiation; C: Development; D: Maturation; E: Sclerotial exudate. |
|
|
黑色素(Melanin)广泛存在于动植物和微生物中,是一类具有相同物化性质的非均质疏水性大分子,由酚类或吲哚类化合物氧化聚合而成,存在亚基但确切排列方式未知[25]。不同生物的黑色素合成途径各异,真菌主要有2条合成途径。多数真菌合成黑色素通过1, 8-二羟基萘(1, 8- Dihydroxynaphthalene,DHN)途径,少量真菌通过L-3, 4-二羟基苯丙氨酸(L-3, 4-dihydroxyphenylalanine,DOPA)途径,后者与哺乳动物黑色素生物合成类似[25]。黑色素不溶于多数物质并具有酸化学降解抗性,但能溶解于碱性溶剂或被氧化分解。黑色素作为色素通常为深色,包括黑色或棕色,常与蛋白或碳水化合物复合[26]。黑色素疏水带负电荷结合能力广泛,具有结合毒性金属的功能,对于环境治理和生态修复具有重要意义[25]。黑色素在环境和医疗方面具有潜在应用价值,具有抗紫外线的能力,能够吸收电磁波光谱,防止光损伤[27]。黑色素中自由基群体稳定,可以清除辐射产生的自由基从而使生物体免受电离辐射[28]。真菌产生的黑色素能够抵抗外界环境中高温、裂解酶、有毒金属及微生物因子的影响,提高自身生存能力[29]。特别在菌核发育成熟阶段,黑色素的不断积累导致外皮层形成过程具有颜色渐变(由白色至浅黄色,最后至深褐色或黑色)特点[1],其中含有的大量黑色素具有降低细胞渗透性并保护菌核受到不利环境因素影响,如紫外线、活性氧及生物降解等[14]。此外,黑色素在植物病原菌的致病过程中也起到关键作用,如核盘菌附着胞产生黑色素可增加其机械强度有助于组织渗透[29]。
4 菌核形成的分子机制菌核形态建成(Sclerotiogenesis)及发育受到各种因素影响,包括环境感应(如光照、pH、次生代谢、活性氧等)、细胞传感与信号转导(如环腺苷酸cAMP、蛋白激酶PKA、丝裂原活蛋白激酶MAPK等)、蛋白磷酸化及其它相关因子调控等[12, 14]。(1)环境因素作为外源信号或通过调控相关信号转导机制,影响菌核的发育。例如,灰葡萄孢菌光照响应蛋白(BcLAE1)的缺乏能够导致菌核产生能力丧失[30],而其受光照调控的转录因子(BcLTF2)也能够影响菌核形成[31],说明光照对菌核形成的作用。环境pH水平也影响菌核发育,中性或碱性抑制菌核形成,而因草酸积累造成的酸性环境则利于菌核发育[4, 14]。次级代谢也与菌核发育相关,其中草酸作为非特异性毒素参与核盘菌侵染及菌核发育[4],而选择性毒素核盘菌素(Sclerin)与菌核形成及黑色素化相关[14]。此外,活性氧调控氧化还原动态平衡并作为信号分子参与菌核发育[14],其中NADPH氧化酶(SsNox)抑制核盘菌的菌核产生[32],其亚基(BcNoxA)及膜蛋白(BcNoxD)与灰葡萄孢菌的菌核形成及分生孢子产生有关[33]。过氧化氢酶(SCAT1)影响菌核的大小与数量以及黑色素产生[34],但超氧化物歧化酶(SsSOD1)却与菌核形成无关[35]。另外,谷胱甘肽转移酶(Ss-Ggt1)参与核盘菌的菌核及子囊盘发育,其缺失突变体产生异常菌核且无法萌发[36]。(2)菌核作为真菌形态建成的重要组成部分,其形成与发育与信息传导途径,特别是环腺苷酸(cAMP)途径相关。研究表明,cAMP对菌核发育表现2种不同影响,cAMP水平升高导致核盘菌的菌核无法形成[37],但却促进立枯丝核菌和齐整小核菌的菌核发育[38-39]。外界信号分子(如pH或活性氧等)能够激活腺苷酸环化酶并参与cAMP合成[14],其中腺苷酸环化酶(Sac1)调控核盘菌的菌核发育[40],而腺苷酸环化酶活性调控蛋白(BCG3)的缺失则抑制灰葡萄孢菌的菌核形成[41]。cAMP可以通过作为蛋白激酶(PKA)活性的调控因子参与核盘菌的菌核发育[14, 42],其催化亚基(Pka1)的缺失表明另一亚基(Pka2)可能具有重要的调控作用[43]。此外,cAMP也通过MAPK信号级联调控菌核发育。MAPK途径上游激活因子Ras抑制核盘菌的菌核发育并介导cAMP与MAPK的交互作用[44],MAPK下游转录因子(BcAtf1和Ste12)影响灰葡萄孢菌的菌核形成与致病性[45-46]。MAPK同源蛋白(Smk1)利用pH及cAMP作为信号调控菌核发育[47],同源蛋白(Smk3和Bmp3)则影响菌核形成及初侵染[48-49]。另外,MAPK激酶(BcSAK1)缺乏促进灰葡萄孢菌的菌核发育[50],而转录因子(Bcmads1)则影响菌核形成及分生孢子形态[51]。除了cAMP途径,蛋白质磷酸酶(PPs)作为磷酸化平衡关键因素与PKA和MAPK途径关联,在细胞调控及真菌形态变化(包括菌核形成)具有重要作用[14]。例如,核盘菌中丝/苏氨酸磷酸酶(钙调磷酸酶Calcineurin)参与菌核发育并可能与MAPK和PKA途径相关[52],灰葡萄孢菌丝/苏氨酸磷酸化激酶(BcATG1)则参与孢子形成及菌核发育[53]。综上所述,信息传导途径对菌核的发育调控可能依赖于上游信号与下游响应分子的协同活性及特定的激酶和磷酸酶[14]。(3)除了外界因素和信号调控途径,其它特异性基因或蛋白的表达与功能也对菌核的发育发挥作用[54]。蛋白质组学分析表明,不同功能蛋白(如碳水化合物代谢和细胞分化等)参与立枯丝核菌的菌核成熟[55]。核盘菌的菌核发育及渗出液的蛋白组成呈现丰富多样性[56-57],其中鉴定出的阿拉伯呋喃糖酶(Arabinofuranosidase)随后被发现参与核盘菌的致病过程[58]。黑色素对菌核具有重要意义[1],例如核盘菌黑色素合成蛋白(Scd1和Thr1)影响菌核发育及抗逆性[56, 59],而灰葡萄孢菌黑色素合成蛋白(BcSCD1和BcBRN)参与菌核的黑色素化[60-61]。此外,特异性蛋白(Ssp1和Ssp2)作为凝集素参与菌核及子囊盘发育[22, 54],而调控有性生殖的叉头框蛋白(SsFKH1)影响菌丝和菌核发育及致病性[62]。参与真菌侵染的分泌蛋白也影响菌核发育。例如核盘菌钙离子锚定相关分泌蛋白(Ss-Caf1)的缺失导致菌核数量减少但体积增大[63],细胞壁降解酶(SsXyl1)也影响菌核发育与萌发[64]。
5 展望本文从形成菌核的植物病原真菌的种类、菌核的生物学特性(包括结构、萌发、发育)、黑色素及菌核形成的分子生物学等方面入手,对真菌菌核研究的进展进行了综述。菌核作为一种特殊的休眠结构在真菌的生命过程及其引起的病害循环中发挥着重要的作用。尽管植物病原真菌的菌核形态与结构、萌发与形成、发育与调控及黑色素的功能等研究领域取得不断进展,但是在菌核的形态建成及发育调控的关键因子等方面仍亟待更加系统地研究。因此,未来研究需要关注以下几个方面:(1)扩大形成菌核的真菌种类及影响,特别是引起植物病害真菌;(2)以重要模式真菌(如核盘菌及灰葡萄孢菌)为材料进行多组学(基因组、蛋白组、转录组及代谢组等)高通量分析,为系统性探索关键因子及调控网络奠定大数据信息基础;(3)通过多样化分析方法(如同源重组、RNAi沉默、农杆菌转化等)及检测技术(如荧光定量、与荧光蛋白结合的基因定位及互作可视化),为靶标基因的功能与作用研究提供选择;(4)未来通过整合编码基因、调控转录、表达蛋白等方面进行系统生物学探索将成为菌核研究的主要趋势;(5)形成菌核的真菌引起的植物病害防控手段还存在认识不足及局限性,其中化学防治易造成环境污染及潜在抗药性,生物防治虽然环保但防效不稳定且难以定殖等。因此,通过探索真菌菌核的发育生物学与调控机理及致病机制等将为安全有效的防控植物病害奠定理论基础,也为其它药食用真菌的菌核研究提供参考借鉴。
| [1] |
Willetts HJ, Bullock S. Developmental biology of sclerotia[J]. Mycological Research, 1992, 96(10): 801-816. DOI:10.1016/S0953-7562(09)81027-7 |
| [2] |
Smith ME, Henkel TW, Rollins JA. How many fungi make sclerotia?[J]. Fungal Ecology, 2015, 13: 211-220. DOI:10.1016/j.funeco.2014.08.010 |
| [3] |
Bolton MD, Thomma BPHJ, Nelson BD. Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen[J]. Molecular Plant Pathology, 2006, 7(1): 1-16. DOI:10.1111/mpp.2006.7.issue-1 |
| [4] |
Boland GJ, Hall R. Index of plant hosts of Sclerotinia sclerotiorum[J]. Canadian Journal of Plant Pathology, 1994, 16(2): 93-108. DOI:10.1080/07060669409500766 |
| [5] |
Williamson B, Tudzynski B, Tudzynski P, et al. Botrytis cinerea: the cause of grey mould disease[J]. Molecular Plant Pathology, 2007, 8(5): 561-580. DOI:10.1111/mpp.2007.8.issue-5 |
| [6] |
Droby S, Lichter A. Post-harvest Botrytis infection: etiology, development and management[J]. Dordrecht: Springer, 2007, 349-367. |
| [7] |
Guo MY, Xie XW, Shi YX, et al. Pathogen identification and comprehensive control of Rhizoctonia solani in leaf rot and root rot of Brassica oleracea[J]. China Vegetables, 2015(2): 65-67. (in Chinese) 郭梦妍, 谢学文, 石延霞, 等. 甘蓝丝核菌根腐病与叶腐病的病原鉴定及综合防治技术[J]. 中国蔬菜, 2015(2): 65-67. DOI:10.3969/j.issn.1000-6346.2015.02.023 |
| [8] |
Ogoshi A. Introduction-the genus Rhizoctonia[J]. Dordrecht: Springer, 1996, 1-9. |
| [9] |
Punja ZK, Damiani A. Comparative growth, morphology, and physiology of three Sclerotium species[J]. Mycologia, 1996, 88(5): 694-706. DOI:10.1080/00275514.1996.12026706 |
| [10] |
Mehan VK, Mayee CD, McDonald D. Management of Sclerotium rolfsii caused stem and pod rots of groundnut a critical review[J]. International Journal of Pest Management, 1994, 40(4): 313-320. DOI:10.1080/09670879409371906 |
| [11] |
Coley-Smith JR, Cooke RC. Survival and germination of fungal sclerotia[J]. Annual Review of Phytopathology, 1971, 9(1): 65-92. DOI:10.1146/annurev.py.09.090171.000433 |
| [12] |
Chet I, Henis Y. Sclerotial morphogenesis in fungi[J]. Annual Review of Phytopathology, 1975, 13(1): 169-192. DOI:10.1146/annurev.py.13.090175.001125 |
| [13] |
Schumacher J. How light affects the life of Botrytis[J]. Fungal Genetics and Biology, 2017, 106: 26-41. DOI:10.1016/j.fgb.2017.06.002 |
| [14] |
Erental A, Dickman MB, Yarden O. Sclerotial development in Sclerotinia sclerotiorum: awakening molecular analysis of a "Dormant" structure[J]. Fungal Biology Reviews, 2008, 22(1): 6-16. DOI:10.1016/j.fbr.2007.10.001 |
| [15] |
Cooke RC. Physiology of sclerotia of Sclerotinia sclerotiorum during growth and maturation[J]. Transactions of the British Mycological Society, 1971, 56(1): 51-59. DOI:10.1016/S0007-1536(71)80107-9 |
| [16] |
Ordó ez-Valencia C, Ferrera-Cerrato R, Quintanar-Zú iga RE, et al. Morphological development of sclerotia by Sclerotinia sclerotiorum: a view from light and scanning electron microscopy[J]. Annals of Microbiology, 2015, 65(2): 765-770. DOI:10.1007/s13213-014-0916-x |
| [17] |
Mu HY, Zheng L, Liu H, et al. Myceliogenic germination of sclerotia of Sclerotinia sclerotiorum on rapeseed[J]. Chinese Journal of Oil Crop Sciences, 2017, 39(2): 228-233. (in Chinese) 母红岩, 郑露, 刘浩, 等. 油菜菌核病病原菌核的菌丝型萌发特性[J]. 中国油料作物学报, 2017, 39(2): 228-233. |
| [18] |
Hegedus DD, Rimmer SR. Sclerotinia sclerotiorum: when "to be or not to be" a pathogen?[J]. FEMS Microbiology Letters, 2005, 251(2): 177-184. DOI:10.1016/j.femsle.2005.07.040 |
| [19] |
Guo DJ, Guo D, Li DP, et al. Survial characteristics of sclerotia in rice sheath blight[J]. Guangxi Plant Protection, 2014, 27(1): 1-3. (in Chinese) 郭道君, 郭地, 李冬萍, 等. 稻纹枯病菌菌核的存活特性测定[J]. 广西植保, 2014, 27(1): 1-3. |
| [20] |
Yu QY, Fan X, Zhang J, et al. A comparision of the biological characteristics of autumn and spring-germination isolates of rape Sclerotinia sclerotiorum[J]. Journal of Huazhong Agricultural University, 2015, 34(5): 37-41. (in Chinese) 余秋玉, 范璇, 张静, 等. 油菜菌核病菌秋季与春季萌发菌株生物学特性的比较[J]. 华中农业大学学报, 2015, 34(5): 37-41. |
| [21] |
Zhang JC, Jiang S, Si H, et al. Distribution and germination of sclerotia of rape stem rot in field[J]. Shaanxi Journal of Agricultural Sciences, 2015, 61(4): 18-20. (in Chinese) 张吉昌, 江山, 司华, 等. 油菜菌核病田间菌核分布及萌发观察研究[J]. 陕西农业科学, 2015, 61(4): 18-20. DOI:10.3969/j.issn.0488-5368.2015.04.006 |
| [22] |
Li M, Rollins JA. The development-specific protein (Ssp1) from Sclerotinia sclerotiorum is encoded by a novel gene expressed exclusively in sclerotium tissues[J]. Mycologia, 2009, 101(1): 34-43. DOI:10.3852/08-114 |
| [23] |
Townsend BB, Willetts HJ. The development of sclerotia of certain fungi[J]. Transactions of the British Mycological Society, 1954, 37(3): 213-221. DOI:10.1016/S0007-1536(54)80003-9 |
| [24] |
Christias C, Lockwood JL. Conservation of mycelial constituents in four sclerotium-forming fungi in nutrient-deprived conditions[J]. Phytopathology, 1973, 63: 602-605. DOI:10.1094/Phyto-63-602 |
| [25] |
Eisenman HC, Casadevall A. Synthesis and assembly of fungal melanin[J]. Applied Microbiology and Biotechnology, 2012, 93(3): 931-940. DOI:10.1007/s00253-011-3777-2 |
| [26] |
Butler MJ, Day AW. Fungal melanins: a review[J]. Canadian Journal of Microbiology, 1998, 44(12): 1115-1136. DOI:10.1139/w98-119 |
| [27] |
Hill HZ. The function of melanin or six blind people examine an elephant[J]. Bioessays, 1992, 14(1): 49-56. DOI:10.1002/(ISSN)1521-1878 |
| [28] |
Turick CE, Ekechukwu AA, Milliken CE, et al. Gamma radiation interacts with melanin to alter its oxidation-reduction potential and results in electric current production[J]. Bioelectrochemistry, 2011, 82(1): 69-73. |
| [29] |
Butler MJ, Gardiner RB, Day AW. Melanin synthesis by Sclerotinia sclerotiorum[J]. Mycologia, 2009, 101(3): 296-304. DOI:10.3852/08-120 |
| [30] |
Schumacher J, Simon A, Cohrs KC, et al. The VELVET complex in the gray mold fungus Botrytis cinerea: impact of BcLAE1 on differentiation, secondary metabolism, and virulence[J]. Molecular Plant-Microbe Interactions, 2015, 28(6): 659-674. DOI:10.1094/MPMI-12-14-0411-R |
| [31] |
Cohrs KC, Simon A, Viaud M, et al. Light governs asexual differentiation in the grey mould fungus Botrytis cinerea via the putative transcription factor BcLTF2[J]. Environmental Microbiology, 2016, 18(11): 4068-4086. DOI:10.1111/1462-2920.13431 |
| [32] |
Kim HJ, Chen CB, Kabbage M, et al. Identification and characterization of Sclerotinia sclerotiorum NADPH oxidases[J]. Applied and Environmental Microbiology, 2011, 77(21): 7721-7729. DOI:10.1128/AEM.05472-11 |
| [33] |
Siegmund U, Marschall R, Tudzynski P. BcNoxD, a putative ER protein, is a new component of the NADPH oxidase complex in Botrytis cinerea[J]. Molecular Microbiology, 2015, 95(6): 988-1005. DOI:10.1111/mmi.12869 |
| [34] |
Yarden O, Veluchamy S, Dickman MB, et al. Sclerotinia sclerotiorum catalase SCAT1 affects oxidative stress tolerance, regulates ergosterol levels and controls pathogenic development[J]. Physiological and Molecular Plant Pathology, 2014, 85: 34-41. DOI:10.1016/j.pmpp.2013.12.001 |
| [35] |
Xu LS, Chen WD. Random T-DNA mutagenesis identifies a Cu/Zn superoxide dismutase gene as a virulence factor of Sclerotinia sclerotiorum[J]. Molecular Plant-Microbe Interactions, 2013, 26(4): 431-441. DOI:10.1094/MPMI-07-12-0177-R |
| [36] |
Li MY, Liang XF, Rollins JA. Sclerotinia sclerotiorum γ-glutamyl transpeptidase (Ss-Ggt1) is required for regulating glutathione accumulation and development of sclerotia and compound appressoria[J]. Molecular Plant-Microbe Interactions, 2012, 25(3): 412-420. DOI:10.1094/MPMI-06-11-0159 |
| [37] |
Rollins JA, Dickman MB. Increase in endogenous and exogenous cyclic AMP levels inhibits sclerotial development in Sclerotinia sclerotiorum[J]. Applied and Environmental Microbiology, 1998, 64(7): 2539-2544. |
| [38] |
Hashiba T, Ishikawa T. Effect of adenosine 3', 5'-cyclic monophosphate on induction of sclerotia in Rhizoctonia solani[J]. Phytopathology, 1978, 68(12): 1723-1727. DOI:10.1094/Phyto-68-1723 |
| [39] |
Hadar Y, Pines M, Chet I, et al. The regulation of sclerotium initiation in Sclerotium rolfsii by glucose and cyclic AMP[J]. Canadian Journal of Microbiology, 1983, 29(1): 21-26. DOI:10.1139/m83-004 |
| [40] |
Jurick WM Ⅱ, Rollins JA. Deletion of the adenylate cyclase (sac1) gene affects multiple developmental pathways and pathogenicity in Sclerotinia sclerotiorum[J]. Fungal Genetics and Biology, 2007, 44(6): 521-530. DOI:10.1016/j.fgb.2006.11.005 |
| [41] |
Doehlemann G, Berndt P, Hahn M. Different signalling pathways involving a Gα protein, cAMP and a MAP kinase control germination of Botrytis cinerea conidia[J]. Molecular Microbiology, 2006, 59(3): 821-835. DOI:10.1111/mmi.2006.59.issue-3 |
| [42] |
Harel A, Gorovits R, Yarden O. Changes in protein kinase A activity accompany sclerotial development in Sclerotinia sclerotiorum[J]. Phytopathology, 2005, 95(4): 397-404. DOI:10.1094/PHYTO-95-0397 |
| [43] |
Jurick WM Ⅱ, Dickman MB, Rollins JA. Characterization and functional analysis of a cAMP-dependent protein kinase A catalytic subunit gene (pka1) in Sclerotinia sclerotiorum[J]. Physiological and Molecular Plant Pathology, 2004, 64(3): 155-163. DOI:10.1016/j.pmpp.2004.07.004 |
| [44] |
Chen CB, Dickman MB. cAMP blocks MAPK activation and sclerotial development via Rap-1 in a PKA-independent manner in Sclerotinia sclerotiorum[J]. Molecular Microbiology, 2005, 55(1): 299-311. |
| [45] |
Temme N, Oeser B, Massaroli M, et al. BcAtf1, a global regulator, controls various differentiation processes and phytotoxin production in Botrytis cinerea[J]. Molecular Plant Pathology, 2012, 13(7): 704-718. DOI:10.1111/mpp.2012.13.issue-7 |
| [46] |
Schamber A, Leroch M, Diwo J, et al. The role of mitogen-activated protein (MAP) kinase signalling components and the Ste12 transcription factor in germination and pathogenicity of Botrytis cinerea[J]. Molecular Plant Pathology, 2010, 11(1): 105-119. DOI:10.1111/mpp.2010.11.issue-1 |
| [47] |
Chen CB, Harel A, Gorovoits R, et al. MAPK regulation of sclerotial development in Sclerotinia sclerotiorum is linked with pH and cAMP sensing[J]. Molecular Plant-Microbe Interactions, 2004, 17(4): 404-413. DOI:10.1094/MPMI.2004.17.4.404 |
| [48] |
Bashi ZD, Gyawali S, Bekkaoui D, et al. The Sclerotinia sclerotiorum Slt2 mitogen-activated protein kinase ortholog, SMK3, is required for infection initiation but not lesion expansion[J]. Canadian Journal of Microbiology, 2016, 62(10): 836-850. DOI:10.1139/cjm-2016-0091 |
| [49] |
Rui O, Hahn M. The Slt2-type MAP kinase Bmp3 of Botrytis cinerea is required for normal saprotrophic growth, conidiation, plant surface sensing and host tissue colonization[J]. Molecular Plant Pathology, 2007, 8(2): 173-184. DOI:10.1111/mpp.2007.8.issue-2 |
| [50] |
Segmüller N, Ellendorf U, Tudzynski B, et al. BcSAK1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea[J]. Eukaryotic Cell, 2007, 6(2): 211-221. DOI:10.1128/EC.00153-06 |
| [51] |
Zhang ZQ, Li H, Qin GZ, et al. The MADS-Box transcription factor Bcmads1 is required for growth, sclerotia production and pathogenicity of Botrytis cinerea[J]. Scientific Reports, 2016, 6: 33901. DOI:10.1038/srep33901 |
| [52] |
Harel A, Bercovich S, Yarden O. Calcineurin is required for sclerotial development and pathogenicity of Sclerotinia sclerotiorum in an oxalic acid-independent manner[J]. Molecular Plant-Microbe Interactions, 2006, 19(6): 682-693. DOI:10.1094/MPMI-19-0682 |
| [53] |
Sumita T, Izumitsu K, Tanaka C. A serine/threonine kinase gene BcATG1 is involved in conidiation and sclerotial development in Botrytis cinerea[J]. Mycoscience, 2016, 57(2): 107-117. DOI:10.1016/j.myc.2015.11.001 |
| [54] |
Li MY, Rollins JA. The development-specific ssp1 and ssp2 genes of Sclerotinia sclerotiorum encode lectins with distinct yet compensatory regulation[J]. Fungal Genetics and Biology, 2010, 47(6): 531-538. DOI:10.1016/j.fgb.2010.03.008 |
| [55] |
Kwon YS, Kim SG, Chung WS, et al. Proteomic analysis of Rhizoctonia solani AG-1 sclerotia maturation[J]. Fungal Biology, 2014, 118(5/6): 433-443. |
| [56] |
Liang Y, Rahman MH, Strelkov SE, et al. Developmentally induced changes in the sclerotial proteome of Sclerotinia sclerotiorum[J]. Fungal Biology, 2010, 114(8): 619-627. DOI:10.1016/j.funbio.2010.05.003 |
| [57] |
Liang Y, Strelkov SE, Kav NNV. The proteome of liquid sclerotial exudates from Sclerotinia sclerotiorum[J]. Journal of Proteome Research, 2010, 9(6): 3290-3298. DOI:10.1021/pr900942w |
| [58] |
Yajima W, Liang Y, Kav NNV. Gene disruption of an arabinofuranosidase/β-xylosidase precursor decreases Sclerotinia sclerotiorum virulence on canola tissue[J]. Molecular Plant-Microbe Interactions, 2009, 22(7): 783-789. DOI:10.1094/MPMI-22-7-0783 |
| [59] |
Liang Y, Xiong W, Steinkellner S, et al. Deficiency of the melanin biosynthesis genes SCD1 and THR1 affects sclerotial development and vegetative growth, but not pathogenicity, in Sclerotinia sclerotiorum[J]. Molecular Plant Pathology, 2018, 19(6): 1444-1453. DOI:10.1111/mpp.12627 |
| [60] |
Schumacher J. DHN melanin biosynthesis in the plant pathogenic fungus Botrytis cinerea is based on two developmentally regulated key enzyme (PKS)-encoding genes[J]. Molecular Microbiology, 2016, 99(4): 729-748. DOI:10.1111/mmi.2016.99.issue-4 |
| [61] |
Zhang CH, He YF, Zhu PK, et al. Loss of bcbrn1 and bcpks13 in Botrytis cinerea not only blocks melanization but also increases vegetative growth and virulence[J]. Molecular Plant-Microbe Interactions, 2015, 28(10): 1091-1101. DOI:10.1094/MPMI-04-15-0085-R |
| [62] |
Fan HD, Yu G, Liu YZ, et al. An atypical forkhead-containing transcription factor SsFKH1 is involved in sclerotial formation and is essential for pathogenicity in Sclerotinia sclerotiorum[J]. Molecular Plant Pathology, 2017, 18(7): 963-975. DOI:10.1111/mpp.2017.18.issue-7 |
| [63] |
Xiao XQ, Xie JT, Cheng JS, et al. Novel secretory protein Ss-Caf1 of the plant-pathogenic fungus Sclerotinia sclerotiorum is required for host penetration and normal sclerotial development[J]. Molecular Plant-Microbe Interactions, 2014, 27(1): 40-55. DOI:10.1094/MPMI-05-13-0145-R |
| [64] |
Yu Y, Xiao JF, Du J, et al. Disruption of the gene encoding endo-β-1, 4-xylanase affects the growth and virulence of Sclerotinia sclerotiorum[J]. Frontiers in Microbiology, 2016, 7: 1787. |
 2018, Vol. 45
2018, Vol. 45