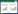扩展功能
文章信息
- 李志乾, 胡颖嵩, 张常建, 刘彦希, 韩雪琳, 韩黎
- LI Zhi-Qian, HU Ying-Song, ZHANG Chang-Jian, LIU Yan-Xi, HAN Xue-Lin, HAN Li
- 在大肠杆菌中高效表达可用于检测人源Rap1活性RapBD蛋白方法的建立
- Expression of RapBD in Escherichia coli for detecting human Rap1 activity
- 微生物学通报, 2018, 45(11): 2488-2493
- Microbiology China, 2018, 45(11): 2488-2493
- DOI: 10.13344/j.microbiol.china.180191
-
文章历史
- 收稿日期: 2018-03-13
- 接受日期: 2018-05-03
- 网络首发日期(www.cnki.net): 2018-06-04
2. 中国人民解放军疾病预防控制所医院感染监控中心 北京 100071
2. Department of Hospital Infection Control and Research, Institute of Disease Control and Prevention of Chinese People's Liberation Army, Beijing 100071, China
Rap是一种小GTP酶,属于Ras超家族。Rap分为2个亚类:Rap1和Rap2,具有Rap1A、Rap1B、Rap2A、Rap2B和Rap2C 5个家族成员[1]。其中,Rap1被研究最多,它通过各种信号通路参与不同的细胞活动,包括细胞的粘附和增殖以及基因的激活[2]。Rap1不仅可以调控整联蛋白,也可以调控细胞骨架[3]。作为小GTP酶,Rap1通过活性变化来调控这些过程。当Rap1以GDP结合的形式存在时是没有活性的,而以GTP结合的形式存在时是有活性的。GTP酶激活蛋白(GAPs)和鸟嘌呤核苷酸交换因子(GEFs)参与调控小GTP酶Rap1的活性,GAPs促进GTP酶以GDP结合形式存在,而GEFs促进其以GTP结合形式存在[4]。Rap1在细胞中起着分子开关的作用,对于高效的信号转导过程至关重要[5]。鉴于此,Rap1活性的检测显得尤为重要。目前常用的检测Rap1活性的成熟方法是RapBD pulldown实验,其原理是利用Rap1下游效应蛋白RalGDS的RapBD结构域能特异性结合GTP-Rap1[6]。市面上现有的Rap1活性检测试剂盒,如Abcam公司的Rap1 Activation Assay Kit,Cell Signaling Technology公司的Active Rap1 Detection Kit #8818,以及Thermo Fisher Scientific公司的Active Rap1 Pull-Down and Detection Kit也都是利用RapBD pulldown的原理[7-10],它们提供可以直接利用的结合有RapBD的琼脂糖珠子。试剂盒虽然使用方便,但是价格昂贵且生产厂家少。本文将人RalGDS蛋白的RapBD结构域的基因序列优化后构建在pGEX-4T-1质粒载体上,使融合蛋白GST-RapBD在大肠杆菌中大量表达后,固化在谷胱甘肽亲和树脂上[11],再利用融合蛋白Pulldown GTP-Rap1,满足了实验室大批量检测Rap1活性的需求。
1 材料与方法 1.1 细胞和质粒人肺泡上皮细胞A549、人支气管上皮细胞BEAS-2B、pGEX-4T-1质粒为本实验室保存;大肠杆菌BL21感受态细胞购于北京全式金生物技术有限公司。
1.2 培养基、主要试剂和仪器LB培养基(g/L):胰蛋白胨10.0,酵母提取物5.0,氯化钠10.0,琼脂15.0−20.0,pH 7.2。
细胞裂解液:25 mmol/L Tris (pH 7.2),150 mmol/L NaCl,5 mmol/L MgCl2,5% Glycerol,1% NP-40。
RPMI medium 1640 basic、Fetal bovine serum购于Gibco公司;谷胱甘肽亲和树脂珠子、异丙基硫代β-D-半乳糖苷(IPTG)购于Promega公司;Rap1抗体购于Cell Signaling Technology公司。
台式高速冷冻离心机,Eppendorf公司;恒温振荡培养箱,天津市欧诺仪器仪表有限公司;脱色摇床,北京六一生物科技有限公司;DNA混合仪,宁波新芝生物科技股份有限公司。
1.3 方法 1.3.1 构建表达GST-RapBD的载体提交需要合成的人源RalGDS的RapBD氨基酸序列和载体pGEX-4T-1,酶切位点为BamHⅠ和XhoⅠ,华大基因公司合成序列并利用pGEX-4T-1载体构建能表达GST-RapBD的重组质粒,并将重组质粒导入大肠杆菌DH5α感受态细胞中得到亚克隆。
1.3.2 原核表达融合蛋白GST-RapBD将转化的感受态细胞涂布到含有100 mg/L氨苄青霉素的LB固体培养基上,37 ℃培养过夜。随机挑取单菌落接种至含有100 mg/L氨苄青霉素的100 mL LB液体培养基中,37 ℃、200 r/min培养至OD600为0.5左右,加入终浓度为1 mmol/L的IPTG,16 ℃诱导20 h,对照不加IPTG诱导。将菌液转移至50 mL离心管中,3 600 r/min离心30 min,将得到的菌体沉淀加入PBS缓冲液,在冰水混合物上静置,利用超声破碎仪破碎菌体,每次超声2 s,间歇2 s,共10 min。随后将裂解液转移至1.5 mL离心管,4 ℃、12 000 r/min离心10 min,取上清与600 μL经过预平衡的GST beads在4 ℃进行孵育1 h后,3 600 r/min离心1 min收集Beads,加入预冷的PBS缓冲液冲洗3次,4 ℃保存。
1.3.3 GST-RapBD pulldown实验用100 mm培养皿培养A549或BEAS-2B细胞,约1×107个细胞/皿。弃去细胞培养基,用PBS缓冲液冲洗3遍后,每皿加入1 mL含有蛋白酶抑制剂的细胞裂解液冰上裂解细胞20 min后收集细胞。4 ℃、14 000 r/min离心15 min,取上清,将上清平均分为2份,一份加入30 μL空载的GST-beads,另一份加入30 μL与细菌裂解液孵育的GST-RapBD beads,在4 ℃进行孵育1 h后,3 600 r/min离心1 min收集Beads。然后加入预冷的500 μL细胞裂解液洗涤Beads,重复3次后加入30 μL 2×Loading buffer,100 ℃金属浴10 min。蛋白样品经SDS-PAGE电泳后,检测GTP-Rap1。
2 结果与分析 2.1 RapBD的氨基酸序列及其原核表达系统中优化后的核苷酸序列查询NCBI得到人源RapBD的氨基酸序列如图 1所示,共127个氨基酸;原核表达系统中优化后的核苷酸序列见图 2。核苷酸序列两侧的酶切位点为BamHⅠ和XhoⅠ。DNA测序结果表明RapBD序列已正确插入载体,GST-RapBD重组质粒构建成功,质粒图谱见图 3。

|
| 图 1 人源RapBD的氨基酸序列 Figure 1 The amino acid sequence of human RapBD |
|
|

|
| 图 2 原核表达系统中优化后的核苷酸序列 Figure 2 Nucleotide sequence optimized in prokaryotic expression system |
|
|
|
| 图 3 插入RapBD的pGEX-4T-1质粒图谱 Figure 3 Plasmid profile of inserted RapBD fragment |
|
|
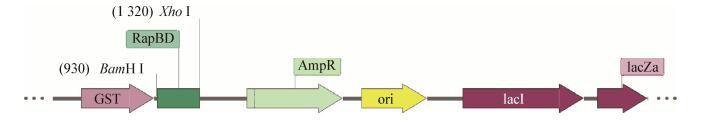
如图 4所示,蛋白样品经SDS-PAGE电泳分离后,考马斯亮蓝染色结果显示在分子量40 kD可见融合蛋白条带。融合蛋白GST-RapBD在16 ℃、IPTG诱导条件下培养20 h后可在大肠杆菌中大量表达,见第2泳道,而且用GST beads纯化后在相同位置可见融合蛋白条带,见第5、6泳道。蛋白大小符合预期。
|
| 图 4 GST-RapBD融合蛋白表达和纯化及其与A549细胞裂解物的结合电泳图 Figure 4 The electropherogram of expression and purification of GST-RapBD fusion protein and its combination with A549 cell lysates 注:M:预染蛋白Marker;1:未经IPTG诱导大肠杆菌,菌体超声破碎后的裂解物;2:IPTG诱导后的大肠杆菌裂解物;3:空的GST beads;4:A549细胞裂解物孵育空的GST beads;5:IPTG诱导后的大肠杆菌裂解物处理后的GST beads;6:A549细胞裂解物孵育IPTG诱导后的大肠杆菌裂解物处理后的GST beads. Note: M: Pre-stained protein marker; 1: Lysates of E. coli without IPTG induction; 2: Lysates of E. coli induced by IPTG; 3: Empty GST beads; 4: Empty GST beads incubated with lysates of A549 cells; 5: GST beads treated with IPTG-induced E. coli lysates; 6: GST beads treated with IPTG-induced E. coli lysates and then incubated with lysates of A549 cells. |
|
|
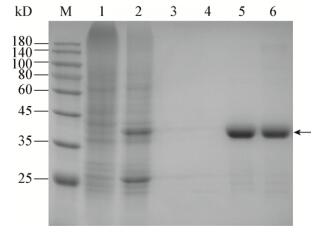
除了A549细胞,在BEAS-2B细胞中进行验证的考马斯亮蓝染色结果见图 5。
|
| 图 5 GST-RapBD融合蛋白表达和纯化及其与BEAS-2B细胞裂解物的结合电泳图 Figure 5 The electropherogram of expression and purification of GST-RapBD fusion protein and its combination with BEAS-2B cell lysates 注:M:预染蛋白Marker;1:IPTG诱导后的大肠杆菌裂解物;2:空的GST beads;3:BEAS-2B细胞裂解物孵育空的GST beads;4:IPTG诱导后的大肠杆菌裂解物处理后的GST beads;5:BEAS-2B细胞裂解物孵育IPTG诱导后的大肠杆菌裂解物处理后的GST beads. Note: M: Pre-stained protein marker; 1: Lysates of E. coli induced by IPTG; 2: Empty GST beads; 3: Empty GST beads incubated with lysates of BEAS-2B cells; 4: GST beads treated with IPTG-induced E. coli lysates; 5: GST beads treated with IPTG-induced E. coli lysates and then incubated with lysates of BEAS-2B cells. |
|
|
采用GST pulldown实验检测GST-RapBD融合蛋白能否特异性结合GTP-Rap1。图 6结果显示,第1泳道中A549细胞总蛋白中有Rap1的表达,第2泳道中空的GST beads不能结合GTP-Rap1,而第5泳道中构建的重组质粒表达出来的GST-RapBD融合蛋白能够特异性结合GTP-Rap1。

|
| 图 6 GST-RapBD融合蛋白与A549细胞中GTP-Rap1的特异性结合 Figure 6 The binding of GST-RapBD fusion protein and GTP-Rap1 in A549 cells 注:1:A549细胞总蛋白;2:空的GST beads;3:A549细胞裂解物孵育空的GST beads;4:IPTG诱导后的大肠杆菌,菌体超声破碎后的裂解物处理后的GST beads;5:A549细胞裂解物孵育IPTG诱导后的大肠杆菌裂解物处理后的GST beads. Note: 1: Total protein of A549 cells; 2: Empty GST beads; 3: Empty GST beads incubated with lysates of A549 cells; 4: GST beads treated with IPTG-induced E. coli lysates; 5: GST beads treated with IPTG-induced E. coli lysates and then incubated with Lysates of A549 cells. |
|
|
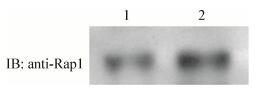
同样地,构建的重组质粒表达出来的GST-RapBD融合蛋白能够特异性结合BEAS-2B细胞中的GTP-Rap1,如图 7所示。
|
| 图 7 GST-RapBD融合蛋白与BEAS-2B细胞中GTP-Rap1的特异性结合 Figure 7 The binding of GST-RapBD fusion protein and GTP-Rap1 in BEAS-2B cells 注:1:BEAS-2B细胞总蛋白;2:BEAS-2B细胞裂解物孵育IPTG诱导后的大肠杆菌裂解物处理后的GST beads. Note: 1: Total protein of BEAS-2B cells; 2: GST beads treated with IPTG-induced E. coli lysates and then incubated with lysates of BEAS-2B cells. |
|
|
近年来,大量研究表明Rap可以调节细胞粘附和吞噬。在宿主与微生物相互作用的过程中,Rap1不仅可以调控补体受体3介导的吞噬[12],还可以介导Fcγ受体依赖的吞噬[13]。在乙型肝炎病毒的研究中,Rap调控肝部炎症,影响肝硬化和肝癌的发生[14]。此外,Rap也参与人类癌细胞的侵袭和转移,与许多恶性肿瘤的研究密切相关[15-16]。Rap作为小GTP酶,其活性受到RapGEFs和RapGAPs的调控。RapGEFs导致GDP解离,促使GTP与Rap结合,进而激活Rap;而RapGAPs加快Rap上GTP的水解,促进Rap失活[4]。已知的RapGEFs主要有4类,分别是C3G (CrkSH3-domain binding guanine-nucleotide releasing factor)、CD-GEFs (CalDAG-GEF)、PDZ-GEFs和Epacs (Exchange protein directly activated by cAMP);已知的RapGAPs主要包括RapGAPs和SPAR (Spine-associated RapGAP)两类[17]。小GTP酶Rap1的活性变化对于RapGEFs和RapGAPs以及其他上下游蛋白的研究非常重要,这就使得Rap1的活性检测成为了这些研究的关键。
我们研究了在烟曲霉和肺上皮细胞相互作用过程中第二信使cAMP的效应蛋白Epac发挥的功能。Epac是环腺苷酸鸟嘌呤交换因子,可以激活下游小G蛋白酶Rap1和Rap2[18-19]。Epac的活性检测是技术难题,目前主要是通过检测Rap的活性来间接反映。我们构建了能表达人RapBD的重组质粒,操作简单、成本低廉,使Rap1的活性检测不再依赖试剂盒,可以更加方便、经济地检测Rap1活性,为Rap1蛋白功能和作用机制及其相关信号通路的研究提供了便利。
| [1] |
Minato N. Rap G protein signal in normal and disordered lymphohematopoiesis[J]. Experimental Cell Research, 2013, 319(15): 2323-2328. DOI:10.1016/j.yexcr.2013.04.009 |
| [2] |
Katagiri K, Maeda A, Shimonaka M, et al. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1[J]. Nature Immunology, 2003, 4(8): 741-748. DOI:10.1038/ni950 |
| [3] |
Jeong HW, Li ZG, Brown MD, et al. IQGAP1 binds Rap1 and modulates its activity[J]. The Journal of Biological Chemistry, 2007, 282(28): 20752-20762. DOI:10.1074/jbc.M700487200 |
| [4] |
Vigil D, Cherfils J, Rossman KL, et al. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy?[J]. Nature Reviews Cancer, 2010, 10(12): 842-857. DOI:10.1038/nrc2960 |
| [5] |
Raaijmakers JH, Bos JL. Specificity in Ras and Rap signaling[J]. The Journal of Biological Chemistry, 2009, 284(17): 10995-10999. DOI:10.1074/jbc.R800061200 |
| [6] |
Spaargaren M, Bischoff JR. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras, K-ras, and Rap[J]. Proceedings of the National Academy of Sciences of the United States of America, 1994, 91(26): 12609-12613. DOI:10.1073/pnas.91.26.12609 |
| [7] |
Parker WH, Qu ZC, May JM. Intracellular ascorbate prevents endothelial barrier permeabilization by thrombin[J]. The Journal of Biological Chemistry, 2015, 290(35): 21486-21497. DOI:10.1074/jbc.M115.662098 |
| [8] |
Xiong YW, Ye CJ, Yang NQ, et al. Ubc9 binds to ADAP and is required for Rap1 membrane recruitment, Rac1 activation, and integrin-mediated T cell adhesion[J]. Journal of Immunology, 2017, 199(12): 4142-4154. DOI:10.4049/jimmunol.1700572 |
| [9] |
Yang ZK, Kirton HM, Al-Owais M, et al. Epac2-Rap1 signaling regulates reactive oxygen species production and susceptibility to cardiac arrhythmias[J]. Antioxidants & Redox Signaling, 2017, 27(3): 117-132. |
| [10] |
Genova T, Grolez GP, Camillo C, et al. TRPM8 inhibits endothelial cell migration via a non-channel function by trapping the small GTPase Rap1[J]. The Journal of Cell Biology, 2017, 216(7): 2107-2130. DOI:10.1083/jcb.201506024 |
| [11] |
Zeng T, Cui ZM, Jiang W, et al. Optimization of the method for measurement of the activity of small GTP binding protein RhoA[J]. Journal of Biology, 2016, 33(4): 103-106. (in Chinese) 曾婷, 崔照盟, 蒋维, 等. 小G蛋白RhoA活性检测方法的优化[J]. 生物学杂志, 2016, 33(4): 103-106. DOI:10.3969/j.issn.2095-1736.2016.04.103 |
| [12] |
Caron E, Self AJ, Hall A. The GTPase Rap1 controls functional activation of macrophage integrin αMβ2 by LPS and other inflammatory mediators[J]. Current Biology, 2000, 10(16): 974-978. DOI:10.1016/S0960-9822(00)00641-2 |
| [13] |
Chung J, Serezani CH, Huang SK, et al. Rap1 activation is required for Fcγ receptor-dependent phagocytosis[J]. The Journal of Immunology, 2008, 181(8): 5501-5509. DOI:10.4049/jimmunol.181.8.5501 |
| [14] |
Wu AR, Chen H, Xu CF, et al. miR-203a is involved in HBx-induced inflammation by targeting Rap1a[J]. Experimental Cell Research, 2016, 349(1): 191-197. DOI:10.1016/j.yexcr.2016.10.016 |
| [15] |
Pizon V, Lerosey I, Chardin P, et al. Nucleotide sequence of a human cDNA encoding a ras-related protein (rap1B)[J]. Nucleic Acids Research, 1988, 16(15): 7719. DOI:10.1093/nar/16.15.7719 |
| [16] |
Minato N, Hattori M. Spa-1 (Sipa1) and Rap signaling in leukemia and cancer metastasis[J]. Cancer Science, 2009, 100(1): 17-23. DOI:10.1111/cas.2009.100.issue-1 |
| [17] |
Li SS, Guo XX, An S, et al. Biological function of the small G protein rap[J]. Progress in Physiological Sciences, 2016, 47(1): 14-20. (in Chinese) 李珊珊, 郭晓汐, 安输, 等. 小G蛋白Rap的信号通路与生物学功能[J]. 生理科学进展, 2016, 47(1): 14-20. |
| [18] |
Pizon V, Desjardins M, Bucci C, et al. Association of Rap1a and Rap1b proteins with late endocytic/phagocytic compartments and Rap2a with the Golgi complex[J]. Journal of Cell Science, 1994, 107: 1661-1670. |
| [19] |
Kawasaki H, Springett GM, Mochizuki N, et al. A family of cAMP-binding proteins that directly activate Rap1[J]. Science, 1998, 282(5397): 2275-2279. DOI:10.1126/science.282.5397.2275 |
 2018, Vol. 45
2018, Vol. 45