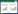扩展功能
文章信息
- 李玲霞, 吴锦艳, 曹小安, 冯倩, 杜国玉, 尹双辉, 李江伟, 尚佑军
- LI Ling-Xia, WU Jin-Yan, CAO Xiao-An, FENG Qian, DU Guo-Yu, YIN Shuang-Hui, LI Jiang-Wei, SHANG You-Jun
- 卵泡刺激素受体FSHR234纳米抗体的分离纯化及多克隆抗体的制备
- Isolation and identification of follicle-stimulating hormone receptor (FSHR234) nanobody and polyclonal antibody
- 微生物学通报, 2018, 45(11): 2463-2469
- Microbiology China, 2018, 45(11): 2463-2469
- DOI: 10.13344/j.microbiol.china.180311
-
文章历史
- 收稿日期: 2018-04-17
- 接受日期: 2018-08-06
- 网络首发日期(www.cnki.net): 2018-08-10
2. 新疆大学 生物资源基因工程重点实验室 新疆 乌鲁木齐 830046
2. Xinjiang Key Laboratory of Biological Resources and Genetic Engineering, Xinjiang University, Urumqi, Xinjiang 830046, China
卵泡刺激素受体(Follicle-stimulating hormone receptor,FSHR)是垂体前叶细胞分泌的,属于G蛋白偶联受体超家族中的糖蛋白亚家族成员,是一个7次跨膜糖蛋白,在睾丸和卵巢血管内皮细胞低表达。相关研究证明,FSHR与成年女性绝经后雌激素降低有密切联系,因此对于骨质疏松症的治疗也有一定效果,抗FSHR纳米抗体在血管形成中具有一定作用。FSH是由垂体分泌的一种糖蛋白激素,主要作用是促进和维持正常的性腺发育和生殖功能。FSH与其受体FSHR特异性结合,在女性卵泡发生和男性精子发生的启动和维持中分别起着至关重要的作用[1-2]。在女性绝经前后骨代谢调节方面也发挥着重要作用。FSH的生理作用是通过其靶细胞——颗粒细胞上特异性受体FSHR介导而完成的。因此,FSH水平作为雌激素变化引起骨质疏松的重要因子,在动物实验中用于骨质疏松症治疗效果的研究[3-7]。
FSHR的激素结合位点局限在细胞外域,存在多个参与激素受体相互作用的区域[8-9],通过X-ray可以从FSH和FSHR结合的晶体结构研究中推测出许多重要的结合位点,包括胞外区、胞内区和富含亮氨酸重复(Leucine-rich repeats,LRR)区等。FSHR特异性表达于男性睾丸支持细胞(Sertoli cell)表面,在男性青春期生精的启动和成年期生精过程的维持中发挥重要作用[10]。由于FSHR含有较多二硫键,使其空间结构较为复杂,因而FSHR蛋白较多以包涵体的形式存在,其变性-复性过程复杂耗时,不便于快速表达和纯化。因此,本试验通过构建重组质粒以获得表达较高的原核蛋白,为后期制备高纯度的FSHR纳米抗体奠定基础。
纳米抗体是骆驼及骆驼科动物体内自然产生的具有完整功能的最小抗原结合片段[11],软骨鱼如鲨鱼等动物体内也产生纳米抗体[12],与传统抗体相比,具有分子量小、特异性强、亲和力高、溶解性及热稳定性高等优点,适合于原核表达和各种真核表达系统,正是这些特征使得该抗体成为新一代治疗性抗体,具有很大的开发应用价值[13]。FSHR是卵巢癌靶向T细胞治疗癌症患者的潜在靶点,在免疫缺陷小鼠中,FSHR定向T细胞过继转移能够显著抑制人类卵巢癌异种移植瘤的生长,表明FSHR是一种很有前景的卵巢癌免疫治疗靶点,因而可以进一步探索FSHR针对癌症患者的免疫治疗方法[14]。也有研究表明,FSHR异位表达于许多性腺外肿瘤(尤其是内分泌肿瘤)的瘤内血管内皮细胞,在肿瘤细胞中也有发现,包括垂体腺瘤、神经内分泌瘤等,通过人T细胞转导表达抗FSHR免疫受体已在卵巢癌细胞上得到证实[15]。目前研究领域还缺少针对FSHR靶点的检测抗体,本试验采用噬菌体展示技术从骆驼免疫文库中筛选制备靶向FSHR纳米抗体,以期为今后卵巢癌及肿瘤诊断和骨质疏松症等的治疗提供新的研究方法。
1 材料与方法 1.1 材料大肠杆菌DH5α、BL21(DE3)感受态细胞,北京全式金生物技术有限公司;pGEX-4T-1载体为新疆大学生物资源基因工程重点实验室保存;限制性内切酶、还原型谷胱甘肽等,宝生物工程(大连)有限公司;抗His标签鼠单克隆抗体、Anti-FSHR IgG单克隆抗体、山羊抗鼠IgG(H & L)-HRP抗体、山羊抗骆驼IgG(H & L)-HRP抗体,北京义翘神州生物技术有限公司。凝胶成像仪、核酸电泳仪、SDS-PAGE电泳仪,Bio-Rad公司;隔水式电热恒温培养箱,上海跃进医疗器械有限公司。
LB培养基(g/L):胰蛋白胨10.0,酵母提取物5.0,氯化钠10.0,pH 7.2。
1.2 方法 1.2.1 重组质粒pGEX-4T-1-FSHR234的构建选择限制性内切酶XhoⅠ、BamHⅠ将pET30a- FSHR于37 ℃进行双酶切8 h,得到FSHR胞外区片段,切胶回收目的基因片段。利用相同的限制性内切酶对pGEX-4T-1进行双酶切,16 ℃连接pGEX- 4T-1与FSHR过夜,并将重组质粒pGEX-4T-1-FSHR转化DH5α,挑取单克隆进行PCR和酶切鉴定,将鉴定的阳性克隆进行测序以确定是否构建成功。
1.2.2 谷胱苷肽S转移酶标签FSHR234蛋白的表达及条件优化将pGEX-4T-1-FSHR表达菌按1%接种到氨苄抗性(100 mg/L)的LB培养基,在37 ℃、220 r/min培养4 h至OD600达0.6−0.8,加入终浓度为0.1 mmol/L的IPTG于37 ℃诱导4 h,于4 ℃、12 000 r/min离心5 min分离上清和沉淀,用12% SDS-PAGE分别检测上清和沉淀蛋白表达。进一步改变温度为18、20、25、30 ℃,IPTG终浓度为0.05、0.1、0.2、0.5及1 mmol/L,每个温度分别对应以上5个不同IPTG浓度以确定最佳诱导条件。转速120−220 r/min诱导4−6 h。离心分离上清和沉淀,沉淀用含有6 mol/L尿素的Tris-HCl溶液溶解变性,经过透析液Ⅰ、Ⅱ、Ⅲ分别复性24 h。经GST柱料分别纯化上清与包涵体FSHR234蛋白,Bradford法检测蛋白浓度。
1.2.3 ELISA及Western blot试验FSHR234蛋白定量浓度为300 μg/mL,ELISA检测FSHR234蛋白表达试验中FSHR蛋白包被浓度为2、5、10 μg/mL,阴性对照为3%牛血清蛋白(Bovine serum albumin,BSA),一抗为抗FSHR鼠单克隆抗体(1:500),二抗为羊抗鼠IgG-HRP (1:5 000)。Western blot实验中蛋白上样量12 μg,采用辣根过氧化氢酶DAB显色试剂盒显色。ELISA检测纳米抗体的结合能力时FSHR234包被浓度为2 μg/mL,一抗为VHH-3F9,稀释浓度分别为0.25、0.5、2.0以及10.0 μg/mL,阳性对照为抗FSHR单克隆抗体(1:1 000),二抗为羊抗驼IgG-HRP (1:5 000)。
1.2.4 FSHR234抗原筛选获得的VHH-X基因序列分析FSHR免疫骆驼制得VHH噬菌体展示文库,抗原FSHR234包被浓度分别为5、2.5、1.25 μg/μL,每孔100 μL,经过3轮淘洗筛选,获得了63个阳性克隆。其中选择丰度最高的2个序列VHH-3F9与VHH-3F19,丰度较低的1个序列VHH-3F20以及单独序列中随机2个序列VHH-3F8与VHH-3F10共5个VHH基因序列,将它们分别构建至pET22b载体,于0.1 mmol/L IPTG条件下37 ℃诱导4 h,共表达5株抗FSHR纳米抗体,Ni柱纯化抗体蛋白。
1.2.5 细胞免疫化学检测抗FSHR抗体在coav-3表面的结合将对数期生长的coav-3细胞按2×105个/mL铺于24孔板,37 ℃培养过夜。次日4%多聚甲醛固定细胞,加入3%脱脂奶粉于37 ℃封闭2 h,然后加入一抗即抗FSHR骆驼多抗IgG (1:500)在37 ℃放置2 h,再加入二抗即抗FSHR纳米抗体VHH-3F9 (1:50),37 ℃放置1.5 h,期间用PBS洗3次,每次5 min,对照组为未免骆驼纯化血清(1:500),二抗为羊抗驼-HRP (1:2 000),室温避光DAB显色。
2 结果与分析 2.1 重组质粒pGEX-4T-1-FSHR234的构建FSHR234胞外区全长为702 bp,提取质粒pGEX-4T-1与pET30a-FSHR并分别进行双酶切,从图 1可以看出,重组质粒酶切后目的基因大小正确。进一步双酶切鉴定重组质粒pGEX-4T-1-FSHR234,核酸电泳结果表明目的片段大小与预计值一致,说明构建成功。

|
| 图 1 pGEX-4T-1与pET30a-FSHR双酶切以及重组质粒pGEX-4T-1-FSHR的酶切鉴定 Figure 1 Double enzyme digestion of pGEX-4T-1 and pET30a-FSHR and enzyme identification of pGEX-4T-1-FSHR 注:M:DL5000 marker;1:质粒pGEX-4T-1;2:双酶切pGEX-4T-1;3:质粒pET30a-FSHR;4:双酶切pET30a-FSHR;5:质粒pET30a;6:双酶切pGEX-4T-1-FSHR;7:质粒pGEX-4T-1;8:双酶切pGEX-4T-1-FSHR. Note: M: DL5000 marker; 1: pGEX-4T-1 plasmid; 2: Double enzyme digestion of pGEX-4T-1; 3: pET30a-FSHR plasmid; 4: Double enzyme digestion of pET30a-FSHR; 5: pET30a plasmid; 6: Double enzyme digestion of pGEX-4T-1-FSHR; 7: pGEX-4T-1 plasmid; 8: Double digestion of pGEX-4T-1-FSHR. |
|
|
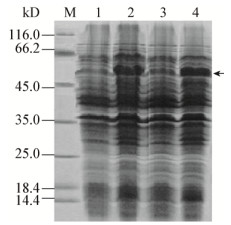
FSHR基因胞外区全长为702 bp,蛋白大小约为52 kD,IPTG诱导之后有明显的蛋白表达且大小正确(图 2)。由图 3可以看出,在不同温度及IPTG条件下,蛋白表达量不同,最终在18 ℃、0.1 mmol/L IPTG、转速120 r/min条件下诱导16 h得到可溶性表达较高的FSHR234,浓度测定为0.3 mg/mL。
|
| 图 2 FSHR234蛋白的诱导表达 Figure 2 Protein expression of FSHR234 注:M:蛋白Marker;1、3:未诱导蛋白;2、4:诱导后蛋白. Note: M: Protein marker; 1, 3: Uninduced proteins; 2, 4: Induced proteins. |
|
|
|
| 图 3 12% SDS-PAGE检测条件优化后FSHR蛋白表达 Figure 3 Expression of FSHR protein under optimum condition detected by 12% SDS-PAGE 注:A:1、2、3、4和5、6、7、8分别为37 ℃和30 ℃条件下未诱导、诱导、上清、沉淀所表达的蛋白;B:1、2、3、4和5、6、7、8分别为25 ℃和18 ℃条件下的未诱导、诱导、上清、沉淀所表达的蛋白;M:蛋白Marker. Note: A: 1, 2, 3, 4 and 5, 6, 7, 8 are uninduced, induced, supernatant and inclusion-body protein under 37 ℃ and 30 ℃; B: 1, 2, 3, 4 and 5, 6, 7, 8 are uninduced, induced, supernatant and inclusion body protein under 25 ℃ and 18 ℃; M: Protein marker. |
|
|
ELISA及Western blot结果表明,FSHR234蛋白能与抗FSHR单克隆抗体特异性结合。FSHR234按300 μg免疫骆驼,每2周一次,共免疫5次,得到抗FSHR多克隆抗体,经ELISA检测抗体效价达1:128 000。Planeix等通过RT-PCR已发现在coav-3细胞表面具有FSHR表达[10],本试验利用细胞免疫化学法进一步直观地发现抗FSHR抗体在coav-3细胞表面的表达。DAB显色结果表明,与阴性血清相比,抗FSHR234骆驼多克隆抗体IgG在coav-3细胞表面具有大量表达(图 4D)。

|
| 图 4 抗FSHR多克隆抗体结合能力的鉴定 Figure 4 Identification of the binding ability of anti-FSHR polyclonal antibody 注:M:蛋白Marker. A:FSHR234纯化结果,1、2、3、4分别为粗蛋白、流穿液、洗脱1、洗脱2;B:Western blot结果;C:ELISA结果,***:P < 0.001;D:细胞免疫组化结果,a为阴性对照,b为抗FSHR骆驼多抗,c为VHH-3F9纳米抗体(200×). Note: M: Protein marker. A: Purification of FSHR234, 1, 2, 3, 4 are crude protein, impure liquid, elution 1 and elution 2; B: Results of Western blot; C: Results of ELISA, ***: P < 0.001; D: Cell immunohistochemistry, a is control, b is FSHR polyclonal antibody, c is VHH-3F9 nanobody (200×). |
|
|
作者前期于新疆大学生物资源基因工程重点实验室建立了骆驼免疫抗体文库,库容为8.3×106 PFU。本试验采用亲和淘洗法进行了3轮筛选,获得5个VHH基因序列构建于pET22b载体,并利用IPTG诱导表达原核蛋白即为纳米抗体。诱导蛋白分离上清和沉淀,发现VHH-3F9可以在上清和胞间质均表达,且浓度及纯度较高(图 5A、B),其余纳米抗体上清表达量较低。经ELISA验证纳米抗体结合性表明,5个表达的纳米抗体中只有VHH-3F9和VHH-3F19能与FSHR234结合,且VHH-3F9结合能力较高(图 5C),经测定其温度稳定性得知VHH-3F9在37 ℃处理12 h仍保留70%的活性。细胞免疫化学实验结果表明VHH-3F9纳米抗体和抗FSHR骆驼多克隆抗体IgG与coav-3细胞表面的FSHR结合,且多抗IgG结合能力高于VHH-3F9。

|
| 图 5 VHH-3F8、VHH-3F10、VHH-3F19、VHH-3F20以及VHH-3F9蛋白表达及抗原结合力检测 Figure 5 The expression of VHH-3F8, VHH-3F10, VHH-3F19, VHH-3F20 and VHH-3F9 proteins and identification of binding capacity 注:M:蛋白Marker. A:1:VHH-3F8上清蛋白;2:VHH-3F8胞间质蛋白;3:VHH-3F10上清蛋白;4:VHH-3F10胞间质蛋白;5:VHH-3F19上清蛋白;6:VHH-3F19胞间质蛋白;7:VHH-3F20上清蛋白;8:VHH-3F20胞间质蛋白. B:1、4为未诱导VHH-3F9蛋白;2、5为VHH-3F9上清蛋白;3、6为VHH-3F9胞间质蛋白. C:ELISA鉴定VHH-3F9与FSHR234结合能力,***:P < 0.001. Note: M: Protein marker. A: 1: Supernatant of VHH-3F8; 2: Intercellular protein of VHH-3F8; 3: Supernatant of VHH-3F10; 4: Intercellular protein of VHH-3F10; 5: Supernatant of VHH-3F19; 6: Intercellular protein of VHH-3F19; 7: Supernatant of VHH-3F20; 8: Intercellular protein of VHH-3F20. B: 1, 4 are uninduced VHH-3F9; 2, 5 are supernatant of VHH-3F9; 3, 6 are intercellular protein of VHH-3F9. C: The combining capacity of VHH-3F9 with FSHR234 by ELISA, ***: P < 0.001. |
|
|
噬菌体展示技术(Phage display technology)可以靶向亲和筛选特异性抗体,通过抗体的富集可以获得cDNA或开放阅读框精确的序列,在生物医学领域占据重要地位,是一种经典的制备特异性抗体的方法。通过高度多样化的噬菌体肽库可以获得具有阻断功能及高亲和力的多肽,目前该技术已用于抗原表位的分析、应用疫苗的制备、新型生物催化剂的选择和研究、酶抑制剂的筛选以及蛋白之间相互作用的研究。大肠杆菌同样也应用于噬菌体展示技术产生抗体,通过对噬菌体展示文库的筛选,可以获得大量与抗原决定簇结合的抗体,是一种很有效的选择靶抗体的方法。
本试验通过将目的基因构建于不同载体进行蛋白表达,再经过条件优化获得了可溶性表达的FSHR234蛋白。pET22b载体N端携带有pel-B信号肽序列,能够将表达的目的蛋白定位于外周质腔,也称为胞间质;同时载体含有C端His标签,以便于蛋白的制备和纯化,避免了大量包涵体的形成,因此我们将FSHR纳米抗体基因构建于pET22b载体以提高可溶性表达率。本文经过亚克隆诱导表达了5个抗FSHR纳米抗体VHH-3F8、VHH-3F9、VHH-3F10、VHH-3F19以及VHH-3F20,它们均可在上清和胞间质得到表达,其中VHH-3F9和VHH-3F19表达量较高。本文获得的纳米抗体结合能力不同,可能原因是真核表达的骆驼VHH基因在原核系统中存在机制差异,导致蛋白表达情况各异且在淘洗筛选过程中不免有非特异结合抗体产生。VHH-3F9结合能力较高,主要是因其序列为重复次数最多的克隆序列,很有可能是高度富集的特异结合FSHR的纳米抗体。然而,产生亲和力更高的抗FSHR单抗有待进一步探究。
免疫骆驼产生的多克隆抗体靶点多、效价强,本试验制备了效价较高的抗FSHR多克隆抗体,通过噬菌体展示技术筛选获得了结合能力较高的抗FSHR纳米抗体VHH-3F9,并且在coav-3细胞表面高表达。已有动物实验证实,FSH对骨的吸收产生直接影响,亮丙瑞林(Leuprorelin,LE)是一种常用的激素类抗恶性肿瘤药物,作用于垂体前叶后也具有促使FSH、LH释放的功能,LE对OVX大鼠实验性根尖周损伤后牙槽骨丢失有保护作用,FSHR阳性细胞数与牙槽骨丢失面积具有显著相关性[16]。阻断FSH与其受体FSHR的结合能够抑制骨质流失,不管是临床还是科研领域,力求寻找靶向针对FSHR的单克隆抗体势在必得。本文制备的纳米抗体属于一种单抗,来自骆驼血清的纳米抗体是完全没有轻链的最小功能抗原结合片段,它可以呈现独特的表位并结合到靶抗体的空腔[17],为今后基于该靶点的临床研究提供有价值的技术参考[18]。
| [1] |
Zhu LL, Blair H, Cao J, et al. Blocking antibody to the β-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(36): 14574-14579. DOI:10.1073/pnas.1212806109 |
| [2] |
Sun L, Peng YZ, Sharrow AC, et al. FSH directly regulates bone mass[J]. Cell, 2006, 125(2): 247-260. DOI:10.1016/j.cell.2006.01.051 |
| [3] |
Sun L, Zhang ZY, Zhu LL, et al. Further evidence for direct pro-resorptive actions of FSH[J]. Biochemical and Biophysical Research Communications, 2010, 394(1): 6-11. DOI:10.1016/j.bbrc.2010.02.113 |
| [4] |
Qin RP, Li LX, Li JW, et al. Inhibition of osteoporosis in ovariectomized rats using follicle-stimulating hormone receptor specific polyclonal antibody[J]. China Biotechnology, 2017, 37(6): 9-16. (in Chinese) 秦瑞坪, 李玲霞, 李江伟, 等. 抗人卵泡刺激素受体多克隆抗体的制备及其对实验大鼠骨质疏松的抑制作用[J]. 中国生物工程杂志, 2017, 37(6): 9-16. |
| [5] |
Gracia CR, Sammel MD, Freeman EW, et al. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition[J]. Menopause, 2005, 12(2): 128-135. DOI:10.1097/00042192-200512020-00005 |
| [6] |
Sowers MR, Jannausch M, McConnell D, et al. Hormone predictors of bone mineral density changes during the menopausal transition[J]. The Journal of Clinical Endocrinology & Metabolism, 2006, 91(4): 1261-1267. |
| [7] |
Sowers MR, Zheng HY, Jannausch ML, et al. Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause[J]. The Journal of Clinical Endocrinology & Metabolism, 2010, 95(5): 2155-2162. |
| [8] |
Zhang XY, Hong SS, Kang Y, et al. Expression and purification of the extracellular domain of the human follicle-stimulating hormone receptor using Escherichia coli[J]. Journal of Obstetrics and Gynaecology Research, 2014, 40(2): 501-508. DOI:10.1111/jog.12203 |
| [9] |
Wang J, Zhang WW, Yu CX, et al. Follicle-stimulating hormone increases the risk of postmenopausal osteoporosis by stimulating osteoclast differentiation[J]. PLoS One, 2015, 10(8). |
| [10] |
Planeix F, Siraj MA, Bidard FC, et al. Endothelial follicle-stimulating hormone receptor expression in invasive breast cancer and vascular remodeling at tumor periphery[J]. Journal of Experimental & Clinical Cancer Research, 2015, 34: 12. |
| [11] |
Muyldermans S. Nanobodies: natural single-domain antibodies[J]. Annual Review of Biochemistry, 2013, 82(1): 775-797. DOI:10.1146/annurev-biochem-063011-092449 |
| [12] |
Zhu L, Zhang DP. Advances in the study of natural small molecular antibody[J]. Acta Pharmaceutica Sinica, 2012, 47(10): 1281-1286. |
| [13] |
Ghannam A, Kumari S, Muyldermans S, et al. Camelid nanobodies with high affinity for broad bean mottle virus: a possible promising tool to immunomodulate plant resistance against viruses[J]. Plant Molecular Biology, 2015, 87(4/5): 355-369. |
| [14] |
Urbanska K, Stashwick C, Poussin M, et al. Follicle-stimulating hormone receptor as a target in the redirected T-cell therapy for cancer[J]. Cancer Immunology Research, 2015, 3(10): 1130-1137. DOI:10.1158/2326-6066.CIR-15-0047 |
| [15] |
Pawlikowski M. Expression of follicle stimulating hormone receptors in intra-tumoral vasculature and in tumoral cells—the involvement in tumour progression and the perspectives of application in cancer diagnosis and therapy[J]. Endokrynologia Polska, 2018, 69(2): 192-198. DOI:10.5603/EP.2018.0022 |
| [16] |
Liu SB, Yong C, Xu WM, et al. Protective effects of follicle-stimulating hormone inhibitor on alveolar bone loss resulting from experimental periapical lesions in ovariectomized rats[J]. Journal of Endodontics, 2010, 36(4): 658-663. DOI:10.1016/j.joen.2010.01.011 |
| [17] |
Qiu YL, Li P, Dong S, et al. Phage-mediated competitive chemiluminescent immunoassay for detecting Cry1Ab toxin by using an anti-idiotypic camel nanobody[J]. Journal of Agricultural and Food Chemistry, 2018, 66(4): 950-956. DOI:10.1021/acs.jafc.7b04923 |
| [18] |
Chung HH, Lee JC, Minn I. Follicle-stimulating hormone receptor in gynecological cancers[J]. Molecular & Cellular Toxicology, 2018, 14(1): 1-7. |
 2018, Vol. 45
2018, Vol. 45