扩展功能
文章信息
- 黄慧敏, 张小凤, 易根云, 吴生荣, 周建业, 李志强, 王静
- HUANG Hui-Min, ZHANG Xiao-Feng, YI Gen-Yun, WU Sheng-Rong, ZHOU Jian-Ye, LI Zhi-Qiang, WANG Jing
- 基于气相色谱-飞行时间质谱联用技术分析枯草芽孢杆菌对变异链球菌的抑制作用
- Measurement of inhibitory effect of Bacillus subtilis on Streptococcus mutans by gas-chromatography-time of flight-mass spectrometry
- 微生物学通报, 2018, 45(10): 2174-2182
- Microbiology China, 2018, 45(10): 2174-2182
- DOI: 10.13344/j.microbiol.china.170960
-
文章历史
- 收稿日期: 2017-11-17
- 接受日期: 2017-12-15
- 网络首发日期(www.cnki.net): 2018-01-17
2. 西北民族大学甘肃省口腔疾病研究重点实验室 甘肃 兰州 730000
2. Key Laboratory of Oral Diseases of Gansu Province, Northwest Minzu University, Lanzhou, Gansu 730000, China
龋病是一种严重危害人类健康的感染性疾病,变异链球菌(Streptococcus mutans)被认为是最主要的致龋菌,在龋病的发生发展中具有始动作用[1-3]。目前,从口腔中寻找能够抑制S. mutans生长的天然菌株并开发其相关抑菌代谢产物,是龋病防治研究的重要思路及方向之一。例如,有研究从健康人群的口腔牙菌斑中分离出多株具有益生作用的乳杆菌,能够抑制S. mutans的生长[4-6];同时另有报道显示,口腔中分离得到的血链球菌由于能产生H2O2,对S. mutans有一定的抑制作用[7-9]。但是,口腔中乳杆菌及血链球菌两者可能本身就有致龋作用[10],截至目前,二者仍不能作为理想的防龋益生菌株。
枯草芽孢杆菌(Bacillus subtilis)是一种G+兼性厌氧菌,能产生多种功能性产物,在植物学、食品、医疗等领域被广泛地研发并利用[11-13]。研究表明,B. subtilis属于非致病菌,其生长过程中能产生细菌素、枯草菌素、表面活性素等具有抑菌作用的物质,这些物质能抑制多种肠道菌群,具有较高的抑菌活性和较广的抑菌谱[12, 14],因此其已被用作肠道的益生菌使用,例如“妈咪爱”[15]。目前,B. subtilis虽在口腔中被检出[16-18],但其检出率较低,在口腔生态环境中的作用尚不明确[19],而且未见其对S. mutans抑制作用的研究。在我们的前期研究中,从健康人群口腔分离了一株B. subtilis (并将其命名为B. subtilis zh78),其细菌培养液经过滤除菌后能抑制S. mutans生长,但尚未探究其抑菌作用的代谢产物[19]。
非靶向代谢组学是对生物体小分子(Molecular weight,MW < 1 000)进行定性和半定量分析的组学技术,可以实现对目标样本小分子代谢物的初步筛选[20]。本研究拟利用该技术对前期发现的B. subtilis zh78进行检测,以分析B. subtilis zh78对S. mutans可能具有抑菌作用的代谢产物,为其在防龋益生方面的相关研究提供参考。
1 材料与方法 1.1 材料 1.1.1 菌种S. mutans (ATCC 25157)购自北京大学口腔医学院,B. subtilis zh78 (CGMCC No. 9952)保藏于中国微生物菌种保藏管理委员会,均由西北民族大学甘肃省口腔疾病研究重点实验室提供。
1.1.2 培养基B. subtilis zh78选择性培养基T2培养基(g/L):葡萄糖10.0,大豆蛋白胨10.0,牛肉膏5.0,酵母提取物2.0,无菌去离子水1 L,pH 7.0。S. mutans生长培养基BHI培养基(g/L):蛋白胨10.0,脱水小牛脑浸粉12.5,脱水牛心浸粉5.0,氯化钠5.0,葡萄糖2.0,磷酸氢二钠2.5,无菌去离子水1 L,pH 7.4±0.2。固体培养基每升加入琼脂20 g。
1.1.3 主要试剂和仪器衍生化试剂为双(三甲基硅烷基)三氟乙酰胺[Bis (trimethylsilyl) trifluoroacetamide,BSTFA],含有1% (体积比)三甲基氯硅烷(Trimethyl chlorosilane,TMCS)。衍生化试剂购自REGIS Technologies有限公司。
GC色谱仪,Agilent公司;质谱仪,LECO公司;气相色谱-飞行时间质谱联用仪配用的毛细管柱,J & W Scientific公司。
1.2 抑菌实验B. subtilis zh78在T2培养基活化后,挑取单克隆菌落转接至T2液体培养基培养,分别选取其在生长0 h [空白期,设为kb期(组)]、7 h [指数期,设为zs期(组)]、12 h [平台期,设为pt期(组)]、5 d [消亡期,设为sw期(组)]的生长菌液4 ℃、3 000 r/min离心5 min,弃沉淀,重复3次后0.22 μm滤器过滤。
向B. subtilis zh78生长各时期的上清液中按体积比(滤液/冷甲醇) 1:4加入冷甲醇,涡旋混匀后4 ℃、12 000 r/min离心10 min,取上清置于无菌离心管中,干燥得到萃取的代谢物。代谢物经无菌去离子水复溶后以牛津杯法进行S. mutans抑菌实验,以无菌去离子水作为阴性对照,每期产物设6个平行组,十字交叉法测抑菌圈直径,根据抑菌圈有无及大小判定其抑菌性。
1.3 代谢组学检测、分析 1.3.1 代谢物萃取及检测取B. subtilis zh78生长kb、zs、pt、sw期的菌液,4 ℃、3 000 r/min离心5 min,弃沉淀,重复3次后0.22 μm滤器过滤,得到B. subtilis zh78各生长期的上清液。取B. subtilis zh78各生长期的上清液100 μL (kb、zs、pt、sw每组各6例,共计24例),加入0.4 mL冷甲醇,漩涡混匀,4 ℃、12 000 r/min离心10 min,取0.4 mL上清经干燥、衍生化后,进行气相色谱-飞行时间质谱联用(GC-TOF-MS)检测。
1.3.2 数据分析利用LECO公司的Chroma TOF4.3X软件和LECO-Fiehn Rtx5数据库对采集得到的谱图进行过滤噪音、解卷积、峰强度校正、峰定性和定量等分析。数据预处理后,利用SIMCA-P+ (V13.0)统计软件、R语言包进行PCA (Principal component analysis)、OPLS-DA (Orthogonal partial least squares discriminant analysis)及Venn等分析,根据变量权重重要性排序(Variable importance in projection,VIP > 1),结合t检验(P < 0.05)寻找差异性表达代谢物;采用皮尔森相关分析,设r≥0.9,P < 0.05为相关性化合物。
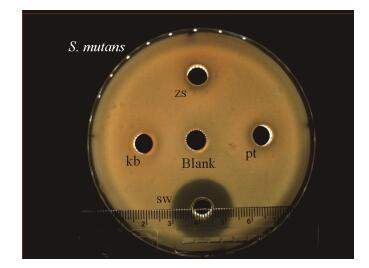
2 结果与分析 2.1 抑菌实验结果牛津杯法抑菌实验结果显示:在B. subtilis zh78的kb、zs、pt、sw各期冷甲醇萃取代谢产物抑菌实验(S. mutans)中,sw期代谢产物产生了明显的抑菌圈,而zs、pt期代谢产物产生的抑菌圈不明显,kb期和阴性对照组均未出现抑菌圈(图 1)。
|
| 图 1 B. subtilis zh78各生长时期代谢产物对S. mutans的抑制作用 Figure 1 Inhibitory effect of metabolites of B. subtilis zh78 on S. mutans at different growth stages 注:kb:空白期(组);zs:指数期(组);pt:平台期(组);sw:消亡期(组). sw期代谢物产生了明显的抑菌圈(平均直径为14.2 mm);zs、pt期代谢产物产生的抑菌圈不明显. Blank为阴性对照组,周围没有出现抑菌圈;kb组周围没有出现抑菌圈. Note: kb: Blank phase (group); zs: Exponential phase (group); pt: Plateau phase (group); sw: Extinction phase (group). The metabolite of sw phase produced an obvious inhibition zone (the average diameter of inhibition zone was 14.2 mm). The metabolite of zs and pt phases produced an unobvious inhibition zone. The negative control group (blank) and kb phase did not see an inhibition zone. |
|
|
如图 2所示,B. subtilis zh78在kb、zs、pt、sw各期代谢产物的主成分分别位于不同象限(累积解释率R2X=0.731,Q2=0.647),各组样本基本上可以区分。

|
| 图 2 24例B. subtilis zh78样本的PCA得分图 Figure 2 PCA scores plot of 24 samples of B. subtilis zh78 注:样本都处于95%置信区间内,各组样本基本上可以分开;kb为空白期(组),黑色;zs为指数期(组),红色;pt为平台期(组),蓝色;sw为消亡期(组),绿色,下同. Note: All samples are within 95% confidence intervals, samples of each group can be basically distinguished; kb refers to blank phase (group), black; zs refers to exponential phase (group), red; pt refers to plateau phase (group), blue; sw refers to extinction phase (group), green. The same below. |
|
|
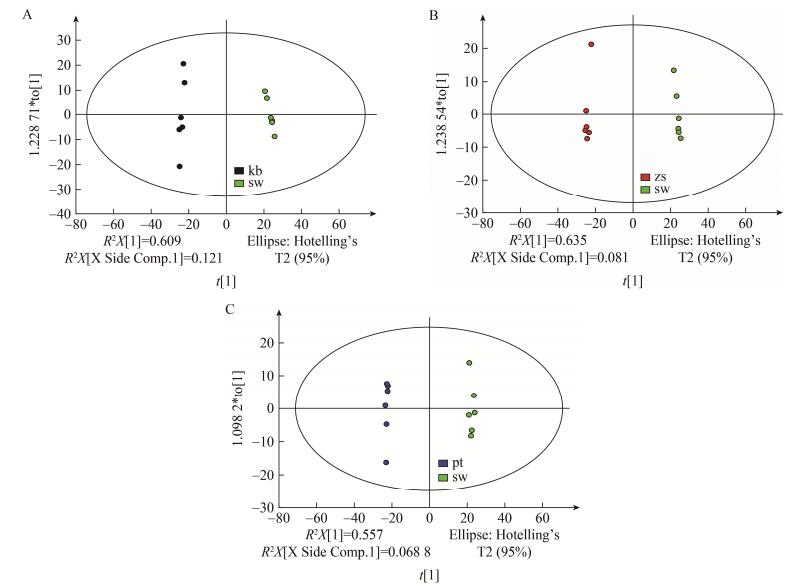
OPLS-DA组间差异分析结果显示,sw组代谢产物与kb、zs、pt各组代谢产物分别具有明显的差异(图 3),kb、zs、pt组分别与sw组对比分析,模型的R2X分别为0.73、0.64、0.644,R2Y分别为0.996、0.992、0.988,Q2分别为0.987、0.973、0.939。
|
| 图 3 B. subtilis zh78各对比组间的OPLS-DA得分图 Figure 3 OPLS-DA (Orthogonal partial least squares discriminant analysis) scores plot obtained from each comparative group of B. subtilis zh78 注:sw组代谢物与kb、zs、pt各组代谢物均有明显差异. A:sw组vs kb组;B:sw组vs zs组;C:sw组vs pt组. Note: The metabolites of sw group were significantly different from those of kb group, zs group, pt group. A: sw group vs kb group; B: sw group vs zs group; C: sw group vs pt group. |
|
|
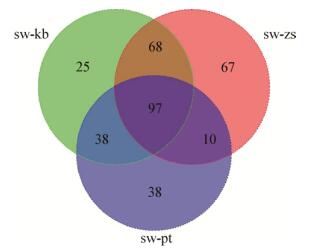
将B. subtilis zh78 sw期与生长各期的差异代谢物分别进行比对,寻找B. subtilis zh78 sw期特异性差异代谢物,结果显示其含有97种sw期的特异性代谢物,如Venn图(图 4)显示。将抑菌圈直径与筛选出的97种差异化合物进行皮尔森相关性分析,根据r≥0.9和P < 0.05筛选出36种与抑菌圈直径显著正相关的代谢物(表 1)。
|
| 图 4 差异代谢物Venn图 Figure 4 Venn map of differential metabolites 注:与kb、zs、pt组相比,sw组含有97种特异性代谢物. Note: The sw group contains 97 specific metabolites compared with kb group, zs group and pt phase. |
|
|
| No. | Name | r | P | Similarity | VIP value | FC | ||||
| sw/kb | sw/zs | sw/pt | sw/kb | sw/zs | sw/pt | |||||
| 1 | 木糖醇Xylitol | 0.900 | 0.031 | 812 | 1.27 | 1.18 | 1.27 | 4.61 | 3.75 | 2.50 |
| 2 | L-谷氨酸L-glutamic acid | 0.990 | 0.003 | 435 | 1.03 | 1.12 | 1.03 | 1.76 | 3.61 | 1.43 |
| 3 | L-酪氨酸L-tyrosine | 0.982 | 0.006 | 759 | 1.28 | 1.05 | 1.18 | 7.09 | 1.95 | 7.23 |
| 4 | 苯乙酸Phenylacetic acid | 0.996 | 0.001 | 868 | 1.27 | 1.24 | 1.33 | 25.76 | 26.32 | 21.94 |
| 5 | 羟基乙酸Glycolic acid | 0.990 | 0.003 | 805 | 1.27 | 1.14 | 1.33 | 3.43 | 2.17 | 2.03 |
| 6 | 丁醛Butyraldehyde | 0.990 | 0.003 | 469 | 1.26 | 1.24 | 1.32 | 24.78 | 24.68 | 24.22 |
| 7 | D-塔格糖D-tagatose 2 | 0.990 | 0.003 | 309 | 1.28 | 1.36 | 1.51 | 31.49 | 7.90 | 2.70 |
| 8 | L-苹果酸L-malic acid | 0.990 | 0.003 | 471 | 1.16 | 1.23 | 1.42 | 21.57 | 2.28 | 2.54 |
| 9 | 亚精胺Spermidine | 0.996 | 0.001 | 886 | 1.28 | 1.14 | 1.57 | 26.57 | 26.01 | 19.36 |
| 10 | 黄嘌呤Xanthine | 0.995 | 0.002 | 825 | 1.27 | 1.30 | 1.17 | 8.68 | 9.56 | 4.83 |
| 11 | 乳酰氨Lactamide 2 | 0.993 | 0.002 | 493 | 1.19 | 1.13 | 1.16 | 27.55 | 16.09 | 14.38 |
| 12 | 马来酰胺酸Maleamate 4 | 0.990 | 0.003 | 704 | 1.22 | 1.19 | 1.01 | 6.90 | 2.50 | 1.33 |
| 13 | 戊二酸Glutaric acid | 0.990 | 0.003 | 762 | 1.09 | 1.22 | 1.07 | 4.18 | 15.68 | 3.10 |
| 14 | 葡萄糖二酸Saccharic acid | 0.990 | 0.003 | 952 | 1.27 | 1.25 | 1.24 | 4.36 | 3.83 | 1.52 |
| 15 | 亚麻酸Linolenic acid | 0.990 | 0.003 | 468 | 1.28 | 1.25 | 1.33 | 24.33 | 24.90 | 24.80 |
| 16 | 蜡酸Cerotinic acid | 0.990 | 0.003 | 262 | 1.27 | 1.24 | 1.32 | 21.11 | 21.67 | 21.57 |
| 17 | 胸腺脱氧嘧啶核苷Thymidine 3 | 0.990 | 0.003 | 220 | 1.27 | 1.21 | 1.32 | 20.37 | 20.14 | 20.83 |
| 18 | 天冬酰胺Asparagine 3 | 0.990 | 0.002 | 944 | 1.24 | 1.21 | 1.15 | 28.51 | 29.08 | 22.90 |
| 19 | 谷氨酰胺Glutamine 2 | 0.990 | 0.003 | 296 | 1.35 | 1.04 | 1.29 | 7.17 | 9.46 | 3.26 |
| 20 | 胞苷Cytidine 2 | 0.987 | 0.003 | 543 | 1.28 | 1.25 | 1.34 | 22.35 | 22.92 | 22.81 |
| 21 | L-异亮氨酸L-isoleucine | 0.990 | 0.003 | 775 | 1.10 | 1.06 | 1.32 | 5.85 | 3.46 | 5.12 |
| 22 | L-正亮氨酸L-norleucine 1 | 0.989 | 0.003 | 442 | 1.25 | 1.22 | 1.30 | 22.41 | 22.98 | 22.88 |
| 23 | 乳糖醛酸Digalacturonic acid | 0.990 | 0.003 | 646 | 1.28 | 1.26 | 1.34 | 23.71 | 24.35 | 24.25 |
| 24 | 草酰乙酸Oxalacetic acid | 0.985 | 0.003 | 499 | 1.28 | 1.25 | 1.33 | 23.75 | 24.32 | 24.22 |
| 25 | 薄荷缩酮Menthone 1 | 0.990 | 0.003 | 305 | 1.53 | 1.18 | 1.46 | 21.12 | 21.29 | 21.58 |
| 26 | 甘油醚Diglycerol 1 | 0.990 | 0.003 | 520 | 1.06 | 1.10 | 1.32 | 26.38 | 23.24 | 22.27 |
| 27 | 肌酸Creatine degr | 0.990 | 0.003 | 227 | 1.26 | 1.24 | 1.32 | 21.67 | 22.24 | 22.14 |
| 28 | 松果体素Melatonin 3 | 0.989 | 0.003 | 237 | 1.33 | 1.25 | 1.28 | 25.66 | 26.22 | 26.12 |
| 29 | 甲基丙二酸Methylmalonic acid | 0.989 | 0.003 | 938 | 1.23 | 1.24 | 1.11 | 1.62 | 2.37 | 1.58 |
| 30 | D-赤酮酸内酯D-erythronolactone 2 | 0.988 | 0.004 | 587 | 1.24 | 1.26 | 1.23 | 1.42 | 2.08 | 1.48 |
| 31 | α-氨基己二酸Alpha-aminoadipic acid | 0.990 | 0.004 | 236 | 1.26 | 1.24 | 1.32 | 23.61 | 24.20 | 24.09 |
| 32 | N-乙酰-D-甘露糖胺N-acetyl-beta-D-mannosamine 3 | 0.990 | 0.003 | 789 | 1.27 | 1.25 | 1.33 | 39.05 | 45.35 | 46.82 |
| 33 | Nε-乙酰左旋赖氨酸N-epsilon-acetyl-L-lysine 2 | 0.990 | 0.003 | 702 | 1.25 | 1.22 | 1.31 | 29.38 | 29.94 | 29.83 |
| 34 | 氨基葡萄糖酸Glucosaminic acid | 0.990 | 0.003 | 331 | 1.27 | 1.23 | 1.33 | 23.40 | 26.42 | 22.75 |
| 35 | 苯甲酰甲酸Benzoylformic acid 2 | 0.990 | 0.003 | 264 | 1.25 | 1.22 | 1.31 | 26.10 | 26.67 | 26.56 |
| 36 | L-羟脯氨酸Trans-4-hydroxy-L-proline 2 | 0.990 | 0.003 | 839 | 1.17 | 1.15 | 1.22 | 26.27 | 26.84 | 26.73 |
| 注:r为相关系数,r越接近于1,代表相关性越高;P为相关系数的假设检验,P≤0.05,表明相关关系具有统计学意义;Similarity为代谢物与标准库中物质的匹配程度,满分为1 000,越接近1 000说明定性出的物质越准确;VIP值为代谢物引起两组之间差异所占的权重,VIP > 1是选择差异代谢物的标准;FC值为代谢物在两组中的峰面积比值,FC > 1,代表该物质含量sw组高于kb组(或sw组高于zs组,sw组高于pt).代谢物后面的数字表示代谢物经衍生化后所带的衍生化基团的个数,“代谢物+衍生化基团的数目”即表示该代谢物.
Notes: r refers to the correlation coefficient, the closer to 1, the higher the correlation is; P refers to the hypothesis test value of correlation coefficient, P≤0.05, showing the correlation is statistically significant; Similarity refers to the matching degree between metabolites and substance in standard library, the total value of which is 1 000, and the closer to 1 000, the more accurate the substance is; VIP refers to the variable importance in the projection value, VIP value greater than 1 is the criteria for selecting different metabolites; FC refers to the peak area ratio of metabolites between the two groups. FC > 1, representing the level of the tested component in sw phase is higher than that in kb phase (or that in sw phase higher than that in zs phase, or that in sw phase higher than pt phase). The number behind the metabolite represents the number of derivatization groups of the metabolite after derivatized. The form that metabolites+number of derivatization groupsrepresents the metabolite. | ||||||||||
我们前期研究在人体正常口腔中分离获得的B. subtilis zh78,其生长菌液能明显地抑制S. mutans生长[19]。本研究对其小分子代谢产物进行了非靶向性代谢组学初步筛查,以明确其可能的抑菌组分。
对B. subtilis zh78在各生长周期的代谢产物用冷甲醇萃取后,进行了S. mutans抑菌实验,发现sw期的代谢产物对S. mutans能够产生显著的抑制作用,其抑菌圈直径达到14.2 mm。该结果表明B. subtilis zh78的抑菌性代谢产物可由甲醇萃取得到,在后续利用代谢组学的方法,从其甲醇提取物中寻找抑菌物质是可行的。对B. subtilis zh78各期的代谢产物进行了PCA分析,结果显示其在kb、zs、pt、sw各期代谢产物具有明显差异。结合抑菌实验结果,推测B. subtilis zh78的抑菌物质主要在sw期产生。根据OPLS-DA组间差异分析,并结合差异化合物和抑菌圈的皮尔森相关性分析,结果显示,该菌在sw期能产生与抑制S. mutans生长显著相关的小分子代谢产物约36种,提示该36种物质均有可能在抑制S. mutans生长中发挥作用。对该36种物质进行了文献查证,结果显示,其中木糖醇、L-谷氨酸、L-酪氨酸是已报道的对S. mutans具有抑制作用的物质。苯乙酸、羟基乙酸、L-苹果酸、D-塔格糖等化合物的抑菌防龋性能在文献中有不同的报道,而其他物质是否参与到抑菌实验当中则有待于在后续的研究中进行进一步研究(表 1)。
木糖醇(Xylitol)是一种已明确具有防龋作用的物质,已被广泛应用于口香糖、糖果、糕点等食品中用以防龋[21]。文献报道,木糖醇对S. mutans具有抑制作用[22-23],而且抑制S. mutans生长的相关机理较为明确:木糖醇可经S. mutans的非特异性磷酸果糖转移酶系统进入S. mutans细胞内部,再通过磷酸化代谢产物的堆积抑制其生长[24];木糖醇可干扰S. mutans蛋白合成及热休克蛋白70、60的表达,从而影响S. mutans的生长[25]。结合本实验结果(不仅在sw组中明显升高,且与抑菌圈直径的相关系数r=0.990,P=0.031),提示木糖醇可能是B. subtilis zh78所产生小分子代谢产物中一种抑制S. mutans效果明确的物质。同时,B. subtilis zh78可能产生木糖醇这种具有典型防龋抑菌作用的物质,提示其在后续的益生菌研发中具有重要的价值。另外,有研究者将谷氨酸等20种游离氨基酸与变异链球菌共同培养后发现,D、L型谷氨酸可以抑制变异链球菌的生长和生物膜的形成,D、L型酪氨酸能延缓变异链球菌的生长[26]。结合本实验结果中它们与S. mutans抑菌作用的相关性,我们推测B. subtilis zh78可能产生L-谷氨酸、L-酪氨酸2种对S. mutans可能具有一定抑菌活性的小分子物质。
在上述与抑制S. mutans生长相关的化合物中,另有3种(苯乙酸、羟基乙酸、L-苹果酸)具有广谱抗菌能力的有机酸。有研究证实,有机酸由于能提高胞内渗透压、透化细菌外膜、抑制生物大分子合成、消耗营养等作用而具有杀菌作用[27]。苯乙酸在医药工业中常用于青霉素的产生,具有较强的杀菌作用[28];同样地,L-苹果酸[29]和羟基乙酸[30]也被证实具有抑菌作用;以上3种有机酸的r≥0.99且P < 0.01,因此,B. subtilis zh78的小分子代谢物中可能也含有该3种能广谱杀菌或抑菌的有机酸,从而能起到抑制S. mutans生长的作用。
此外,在与抑菌相关的物质中除了上述已报道具有抑菌作用的物质外,我们还发现了一种稀有单糖——D-塔格糖。D-塔格糖是半乳糖的酮糖形式,与木糖醇等多元醇类似,在口腔中产酸量低,也可以作为糖代用品在日常生活中使用,起到能防止牙釉质脱矿及防龋作用[31]。
非靶向代谢组学能全面、无偏倚地检测生物样本中的代谢物,可以对样本中代谢物进行初步盲选,寻找对比组别间的差异性代谢物。因此,该技术能研究目的对象在小分子代谢物方面的组成情况,全面筛查研究对象功能与代谢物之间的关系。然而,非靶向代谢组学仅为半定量分析,筛查到的代谢物尚不能通过其含量进行深入探讨,并且同其他组学研究一样,代谢组学存在一定的误差,因此需要同其他系统生物学研究方法相结合,才可以获得在含量和功能方面更确定的研究结果。
4 结论本研究对B. subtilis zh78进行了非靶向代谢组学检测及相关分析,结果显示,36种小分子物质与其对S. mutans抑制作用显著正相关(r≥0.95且P < 0.01)的物质被检测到,这些物质主要包括糖醇类、有机酸类和氨基酸类等。其对S. mutans生长的抑制作用在相关的研究中有不同的报道,结合本研究结果,初步推测B. subtilis zh78可能产生这36种代谢物,这些代谢物在B. subtilis zh78对S. mutans的抑菌作用中可能具有一定促进作用,这为将来深入研究B. subtilis zh78的抑菌作用机理,及深入分析其在龋病预防和口腔益生应用等方面的前景提供了一定的理论参考。
| [1] |
Bowen WH. Dental caries-not just holes in teeth! A perspective[J]. Molecular Oral Microbiology, 2016, 31(3): 228-233. DOI:10.1111/omi.2016.31.issue-3 |
| [2] |
Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms[J]. Caries Research, 2011, 45(1): 69-86. DOI:10.1159/000324598 |
| [3] |
Svensäter G, Borgström M, Bowden GHW, et al. The acid-tolerant microbiota associated with plaque from initial caries and healthy tooth surfaces[J]. Caries Research, 2003, 37(6): 395-403. DOI:10.1159/000073390 |
| [4] |
Yang J, Du GC, Chen J, et al. Characterization of a probiotic Lactobacillus strain isolated from oral cavity[J]. Acta Microbiologica Sinica, 2013, 53(4): 403-408. (in Chinese) 杨娟, 堵国成, 陈坚, 等. 口腔乳酸杆菌的分离及其益生特性[J]. 微生物学报, 2013, 53(4): 403-408. |
| [5] |
Samot J, Lebreton J, Badet C. Adherence capacities of oral Lactobacilli for potential probiotic purposes[J]. Anaerobe, 2011, 17(2): 69-72. DOI:10.1016/j.anaerobe.2011.04.001 |
| [6] |
Simark-Mattsson C, Emilson CG, Håkansson EG, et al. Lactobacillus-mediated interference of mutans streptococci in caries-free vs. caries-active subjects[J]. European Journal of Oral Sciences, 2007, 115(4): 308-314. DOI:10.1111/eos.2007.115.issue-4 |
| [7] |
Giacaman RA, Torres S, Gómez Y, et al. Correlation of Streptococcus mutans and Streptococcus sanguinis colonization and ex vivo hydrogen peroxide production in carious lesion-free and high caries adults[J]. Archives of Oral Biology, 2015, 60(1): 154-159. DOI:10.1016/j.archoralbio.2014.09.007 |
| [8] |
Fujishima K, Kawada-Matsuo M, Oogai Y, et al. dpr and sod in Streptococcus mutans are involved in coexistence with S. sanguinis, and PerR is associated with resistance to H2O2[J]. Applied and Environmental Microbiology, 2013, 79(5): 1436-1443. DOI:10.1128/AEM.03306-12 |
| [9] |
Zheng X, Zhang K, Zhou X, et al. Involvement of gshAB in the interspecies competition within oral biofilm[J]. Journal of Dental Research, 2013, 92(9): 819-824. DOI:10.1177/0022034513498598 |
| [10] |
Zhou XD. Cariology[M]. Beijing: People's Medical Publishing House, 2011: 41. (in Chinese) 周学东. 龋病学[M]. 北京: 人民卫生出版社, 2011: 41. |
| [11] |
Barbosa TM, Serra CR, La Ragione RM, et al. Screening for Bacillus isolates in the broiler gastrointestinal tract[J]. Applied and Environmental Microbiology, 2005, 71(2): 968-978. DOI:10.1128/AEM.71.2.968-978.2005 |
| [12] |
Huang HY, Wang JL, Ma R, et al. Antifungal activities of Bacillus subtilis STO-12 and analysis on its antifungal substances[J]. Acta Agriculturae Zhejiangensis, 2017, 29(1): 81-88. (in Chinese) 黄华毅, 王佳琳, 马荣, 等. 枯草芽孢杆菌STO-12抑菌活性及其抑菌物质分析[J]. 浙江农业学报, 2017, 29(1): 81-88. DOI:10.3969/j.issn.1004-1524.2017.01.12 |
| [13] |
Kumar KVK, Yellareddygari SK, Reddy MS, et al. Efficacy of Bacillus subtilis MBI 600 against sheath blight caused by Rhizoctonia solani and on growth and yield of rice[J]. Rice Science, 2012, 19(1): 55-63. DOI:10.1016/S1672-6308(12)60021-3 |
| [14] |
Poormontaseri M, Hosseinzadeh S, Shekarforoush SS, et al. The effects of probiotic Bacillus subtilis on the cytotoxicity of Clostridium perfringens type a in Caco-2 cell culture[J]. BMC Microbiology, 2017, 17: 150. DOI:10.1186/s12866-017-1051-1 |
| [15] |
Hu ZF, Xu L, Wang SW. The clinical efficacy of Xiaoerkang granule combined with MAMIAI to 98 children with autumn Ped-iatric diarrhea[J]. Chinese Journal of Biochemical Pharmaceutics, 2017, 37(5): 176-178. (in Chinese) 胡泽富, 徐玲, 王思为. 小儿康颗粒联合妈咪爱治疗小儿秋季腹泻98例临床观察[J]. 中国生化药物杂志, 2017, 37(5): 176-178. |
| [16] |
Naidorf IJ. Clinical microbiology in endodontics[J]. Dental Clinics of North America, 1974, 18(2): 329-344. |
| [17] |
Sunde PT, Olsen I, Lind PO, et al. Extraradicular infection: a methodological study[J]. Dental Traumatology, 2000, 16(2): 84-90. DOI:10.1034/j.1600-9657.2000.016002084.x |
| [18] |
Yamane K, Ogawa K, Yoshida M, et al. Identification and characterization of clinically isolated biofilm-forming gram-positive rods from teeth associated with persistent apical periodontitis[J]. Journal of Endodontics, 2009, 35(3): 347-352. DOI:10.1016/j.joen.2008.11.032 |
| [19] |
You XL, Wang SG, Zeng S, et al. In vitro inhibitive activity of metabolites Bacillus subtilis isolated from oral cavity[J]. Journal of Oral Science Research, 2015, 31(10): 991-994, 999. (in Chinese) 游祥磊, 王少果, 曾飒, 等. 口腔枯草芽孢杆菌代谢产物的抑菌活性研究[J]. 口腔医学研究, 2015, 31(10): 991-994, 999. |
| [20] |
Patti GJ, Yanes O, Siuzdak G. Metabolomics: the apogee of the omic triology[J]. Nature Reviews Molecular Cell Biology, 2013, 13(4): 263-269. |
| [21] |
Lynch H, Milgrom P. Xylitol and dental caries: an overview for clinicians[J]. Journal of the California Dental Association, 2003, 31(3): 205-209. |
| [22] |
Söderling EM. Xylitol, mutans streptococci, and dental plaque[J]. Advances in Dental Research, 2009, 21(1): 74-78. DOI:10.1177/0895937409335642 |
| [23] |
Söderling EM, Hietala-Lenkkeri AM. Xylitol and erythritol decrease adherence of polysaccharide-producing oral Streptococci[J]. Current Microbiology, 2010, 60(1): 25-29. DOI:10.1007/s00284-009-9496-6 |
| [24] |
Reiner AM. Xylitol and D-arabitol toxicities due to derepressed fructose, galactitol, and sorbitol phosphotransferases of Escherichia coli[J]. Journal of Bacteriology, 1977, 132(1): 166-173. |
| [25] |
Hrimech M, Mayrand D, Grenier D, et al. Xylitol disturbs protein synthesis, including the expression of HSP-70 and HSP-60, in Streptococcus mutans[J]. Molecular Oral Microbiology, 2000, 15(4): 249-257. |
| [26] |
Zhang LD, Ling JQ, Tong ZC. Effects of free amino acids on Streptococci mutans proliferation and bioflim formation[J]. Chinese Journal of Conservative Dentistry, 2014, 24(6): 313-316. (in Chinese) 张罗丹, 凌均棨, 童忠春. 游离氨基酸对变异链球菌生长及生物膜形成的影响[J]. 牙体牙髓牙周病学杂志, 2014, 24(6): 313-316. |
| [27] |
Zhang J, Tian ZG, Wang JH, et al. Advances in antimicrobial molecular mechanism of organic acids[J]. Acta Veterinaria et Zootechnica Sinica, 2011, 42(3): 323-328. (in Chinese) 张军, 田子罡, 王建华, 等. 有机酸抑菌分子机理研究进展[J]. 畜牧兽医学报, 2011, 42(3): 323-328. |
| [28] |
Madan SS, Wasewar KL. Removal of phenylacetic acid from aqueous streams[A]//Suresh S, Kumar A, Shukla A, et al. Biofuels and Bioenergy (BICE2016)[M]. Cham: Springer, 2017: 209-213
|
| [29] |
Eswaranandam S, Hettiarachchy NS, Johnson MG. Antimicrobial activity of citric, lactic, malic, or tartaric acids and Nisin-incorporated soy protein film against Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella gaminara[J]. Journal of Food Science, 2014, 69(3): FMS79-FMS84. |
| [30] |
Abels C, Kaszuba A, Michalak I, et al. A 10% glycolic acid containing oil-in-water emulsion improves mild acne: a randomized double-blind placebo-controlled trial[J]. Journal of Cosmetic Dermatology, 2011, 10(3): 202-209. DOI:10.1111/jcd.2011.10.issue-3 |
| [31] |
Wong D. Sweetener determined safe in drugs, mouthwashes, and toothpastes[J]. Dentistry Today, 2000, 19(5): 32, 34-35. |
 2018, Vol. 45
2018, Vol. 45




