扩展功能
文章信息
- Xiao-zhen Gu, Wei-zhen Xue, Hui-Li Wang, Yi Xu
- 谷小珍, 薛玮祯, 汪惠丽, 徐毅
- Autolysis of Lactococcus lactis MG1363 induced by growth inhibitors
- 生长抑制剂所诱导的乳酸乳球菌MG1363的自溶表型及其生长阶段特异的性质
- Microbiology China, 2017, 44(6): 1322-1330
- 微生物学通报, 2017, 44(6): 1322-1330
- DOI: 10.13344/j.microbiol.china.160670
-
文章历史
- Received: September 20, 2016
- Accepted: December 26, 2016
- Published online(www.cnki.net): January 10, 2017
Lactic acid bacteria (LAB) is a group of gram positive bacteria utilizing sugar to generate lactic acid[1-2]. Due to its probiotic status, LAB held a crucial position in dairy industry. Autolysis is the bacterial physiological behavior to lyse themselves under stress conditions, through the action of peptidoglycan hydrolases (PGHs)[3-7]. Autolysis was generally induced by a range of rigid environmental conditions, including altered medium component, nutrition deficiency and growth inhibition[8-9]. The starter's autolysis during cheese manufacture could efficiently increase the free amino acid concentration in the curd, thus facilitating the development of flavor compounds and acceleration of the ripening process[9-11]. Therefore, it's of industrial and technological importance to screen the highly autolytic lactic acid bacteria strains and study their autolytic mechanisms under various environmental conditions.
The bacterial autolysis was generally involved in the cessation of peptidoglycan synthesis, such as elongated stationary phase or antibiotic treatment[12-14]. The autolytic profiles of Staphylococcus aureus strains were profoundly influenced by the antibiotic treatment[15-16]. Recently, it was evidenced that Bacillus subtilis and Enterococcus faecium could be used as the reporter strains to screen the autolysis-inducing agents[17-18]. The literature unraveled the importance to investigate the antibiotics' roles in the autolysis induction of the studied organism. However, despite the fact that the living environment of most LAB strains was frequently invaded by antibiotics, how antibiotics affected LAB's autolysis and survival still remains unknown.
It's aimed here to investigate the autolytic properties of various LAB strains primarily from Chinese traditional fermented milk, examine the enzymatic activities and transcription levels of various peptidoglycan hydrolases, and then investigate the autolytic profiles of L. lactis MG1363 elicited by a series of growth inhibitors.
1 Materials and Methods 1.1 Bacterial strains and growth conditionsThe strains involved in this study were listed in Table 1. L. lactis was grown in M17 broth[3], (Oxoid, Basingstoke, UK.) supplemented with 0.5% of glucose, and the culture condition was set as 30 ℃ without agitation. For the glucose-limited media used in the induction experiments, the glucose concentration was adjusted as 0.25% and 0.05% for 1/2 GM17 and 1/10 GM17, respectively. The same conditions were also used for the growth of enterococcal strains. The lactobacillus strains were grown at 37 ℃ without agitation in MRS broth (Oxoid, Basingstoke, UK.).
| Strains | Species | Origin and relative characteristics |
| MG1363 | Lactococcus lactis | Cheese starters |
| Wa | Lactococcus lactis | Chicken intestine, nisin-producing |
| W56 | Lactococcus lactis | Chicken intestine |
| F8 | Lactococcus lactis | Chicken intestine |
| KJ1002-4 | Lactococcus lactis | Fermented milk from Xinjiang |
| KJ2001-1 | Lactococcus lactis | Fermented milk from Xinjiang, nisin-producing |
| KJ1002-9 | Lactococcus lactis | Fermented milk from Xinjiang, nisin-producing |
| KJ2007-1 | Lactococcus lactis | Fermented milk from Xinjiang, nisin-producing |
| KJ2030-2 | Lactococcus lactis | Fermented milk from Xinjiang |
| KJ2024-2 | Lactococcus lactis | Fermented milk from Xinjiang |
| KJ9 | Enterococcus faecalis | Chicken intestine |
| N9 | Enterococcus faecium | Chicken intestine |
| KJ37 | Enterococcus faecium | Chicken intestine |
| KJ3 | Enterococcus faecium | Chicken intestine |
| S1 | Lactobacillus casei | Human feces, fast flocculating |
The autolytic phenotype of the LAB strains was evaluated as follows: the strains were cultured to the mid-exponential phase (OD600=1.0−1.5) or stationary phase (OD600=2.0) and then collected at 5 000×g for 3 minutes. The cells were washed once and resuspended in 50 mmol/L Tris-HCl (pH 7.0) or other buffer used, making the final OD600 reach 0.6−0.8. Subsequently the suspension was incubated at 30 ℃ for 72 h. The degree of autolysis was expressed as the reducing percentage of OD600 after 24 h or 72 h.
1.3 Total mRNA extraction and quantitative PCRTotal mRNA were extracted when MG1363 was grown for 5 h in GM17, in order to ensure the presence of autolysins. In addition, samples were also obtained from 1/10 GM17 culture at 2 h following the Amp's administration at 5 h of growth, in order to examine the alterations of autolysins upon the induced autolysis. The total RNAs were extracted using the RNA Extraction kit (RNA-Solv Reagent, Omega Biotech, USA), according to the manufacturer's instructions. Subsequently, the random primer was used to carry out the reverse transcription reaction according to the manufacturer's instructions, (PrimeScriptTMⅡ 1st strand cDNA Synthesis Kit, Takara Bio, Dalian, China.), resulting in the total cDNA.
The 25 µL reaction pool of fluorescent quantitative PCR was composed of: 12.5 µL of SYBR premix Extaq (TransStart Top Green qPCR Super Mix, TransGen Biotech, Beijing, China); 1 µL of 10 µmol/L forward and reverse primer each; 2 µL of 100 ng/µL cDNA template and 8.5 µL of deionized water. The real-time fluorescence PCR device was LC-96 (Light Cycler® 96 Real-Time PCR System, Roche Life Science; Beijing, China). The reaction procedure was set as one cycle of ℃ for 30 s; 40 cycles of 95 ℃ 5 s, 50 ℃ 30 s, 72 ℃ 1 min, followed by the dissociation stage of 95 ℃ 15 s, 60 ℃ 30 s and 95 ℃ 15 s. The transcription levels were calculated as the amounts relative to that of 16S rRNA gene under the same conditions. The primers used were listed as follows: AcmA: Forward primer: 5′-CTTATCAAGGAAAGAGCGTCGTA-3′, Reverse primer: 5′-CGCCATAACTTGGGTCGGTA-3′; AcmB: Forward primer: 5′-TATGGGAAATGGTGGAGAA-3′, Reverse primer: 5′-TGGCGTTCGCTGACA ATA-3′; AcmC: Forward primer: 5′-ATTCCGTTTCG GCTCATAC-3′, Reverse primer: 5′-TCAGTCGCATA GCCAGAGC-3′; AcmD: Forward primer: 5′-TTGGA ATCTAGTGGTGGTCA-3′, Reverse primer: 5′-TTGG GTCAGTAGCATAACG-3′; YjgB: Forward primer: 5′-AGGTGTTCCCTATGTTTGG-3′, Reverse primer: 5′-ATTCCCACATGGTCTCCAC-3′.
1.4 Protein extraction and renaturing SDS-PAGE4 mL of LAB culture were collected when grown to the mid-exponential phase. The cells were washed once and resuspended in 40 µL of deionized water. Subsequently, 10 µL 5×SDS loading buffer (250 mmol/L Tris-HCl (pH 6.8), 10% (W/V) SDS, 0.5% (W/V) BPB, 50% (V/V) Glycerol, 5% (W/V) β-mercaptoethanol) was added to the cell suspension and mixed thoroughly before boiling for 3 min. The cell debris was then removed by centrifugation at 12 000 r/min for 10 min, and 5 µL of the supernatant was subjected to the SDS-PAGE gel.
SDS-PAGE was performed with 12% (W/V) polyacrylamide separating gels. Renaturing SDS-PAGE was performed as previously described[19] with 0.2% autoclaved Micrococcus lysodeikticus cells included in the gel. The gel was then incubated in 25 mmol/L Tris-HCl (pH 7.0) buffer containing 1% Triton X-100 at 37 ℃ for 2−16 h or till the apparent degrading bands occurred.
1.5 Determination of glucose concentrationGlucose concentration of liquid medium was determined by dinitrosalicylic acid (DNS) method[8]. 3 mL of reagent (1% DNS, 0.2% phenol, 0.05% Na2SO3 and 1% NaOH) was added to 3 mL of glucose-containing solution. The mixture was boiled for 5 min and naturally cooled to room temperature. Optical density value was measured at 575 nm.
1.6 Autolysis induction assaysThe growth inhibitors used in this study were Cm (A protein synthesis inhibitor with MIC of 8 mg/L), Amp (A cell wall synthesis inhibitor with MIC of 4 mg/L), Km (A protein synthesis inhibitor with MIC of 4 mg/L) and SDS (A detergent and membrane disrupter). In order to examine autolysis of the L. lactis strains grown in 1/2 GM17 media, Cm or Amp was added to the culture at different growth points during the exponential phase (OD600 0.25−2.00). And then the bacterial growth curve continued to be depicted, till the diauxic growth ended. The same protocol was utilized in the induction assays pertaining to the strains in 1/10 GM17 media. The final concentration of Cm was 8 µg/mL, 50 µg/mL and 200 µg/mL, and the concentration of Amp was 4 µg/mL and 100 µg/mL, respectively.
To investigate the antibiotic effects on the buffered autolysis capability of L. lactis strains, Cm, Amp, Km and SDS with various concentrations were added to the GM17 culture with OD600 of 0.3. When the optical density reached 0.7, the cells was collected by centrifugation at 8 000×g for 3 min, washed once by sterilized water, and suspended in 25 mmol/L Tris-HCl (pH 7.0) containing 0.25% Triton X-100, making the final concentration reach 1.0. The bacterial suspension was incubated at 30 ℃, and the OD600 changes were determined after 5 h, which was used to represent the autolysis capability of this strain.
2 Results 2.1 Screening and species identification of LAB strainsTo extensively screen functional LAB strains, the selective media (GM17 and MRS) were utilized to isolate lactic acid bacteria from various natural habitats[20], such as chicken intestine, human faces, traditional yogurts from Xinjiang district. The isolates were subsequently subjected to 16S rRNA gene sequencing, leading to the identification of 14 new LAB strains, consisting of Lactococcus lactis (Wa, W56, F8, KJ1002-4, KJ2001-1, KJ1002-9, KJ2007-1, KJ2030-2, KJ2024-2), Enterococcus faecalis (KJ9), Enterococcus faecium (N9, KJ37, KJ3) and Lactobacillus casei (Table 1). Of note, all of the tested strains, as well as a model strain MG1363, were manifested as catalase-negative (Data not shown).
2.2 Autolysis of LAB strains and their peptidoglycan hydrolase activitySince it was previously shown that different growth phases were responsible for the distinct autolysis capabilities[3], all of the strains were tested for their autolysis rate at either or both growth phases (Exponential and stationary). As shown in Figure 1, the autolysis profiles among strains varied significantly, regardless of whether they belong to the same species or not (Lactococcus lactis or Enterococcus faecium). Thus lactococcal autolysis could be considered as strain-specific. Among the tested strains, strain KJ9, KJ3 and KJ1002-4 showed the highest degree of autolysis, rendering them candidates for any food industries warranted by the highly autolytic LAB strains. Besides, compared to stationary phase, the autolytic activities were readily released at exponential phase for almost all the strains (Figure 1).
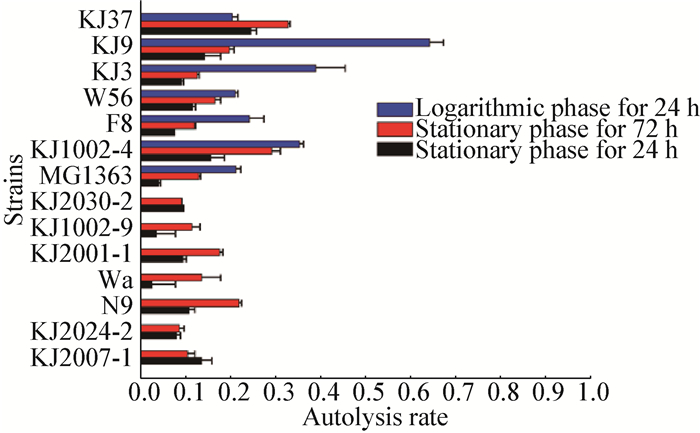
|
| Figure 1 The buffered autolysis of Lactococcus lactis and other LAB strains 图 1 乳酸乳球菌和其他乳酸菌株的自溶检测 Note: The strains cultured in GM17 medium were collected at exponential (blue bars) and stationary phases (red and black bars), and then resuspended in Tris-HCl buffer for 24 h (blue and black bars) or 72 h (red bars); the autolysis rate was calculated as the percentage of the decreased OD600 values during the incubation. 注:在GM17培养基中的菌株在指数生长期(蓝条)和稳定期(红条和黑条)分别进行收集, 并重悬在Tris-HCl缓冲液中24 h (蓝黑条)或72 h (红条); 自溶率计算为在孵育期间OD600的减少比率. |
|
|
To further detect the enzymes attributable to the varied autolytic profiles, renaturing SDS-PAGE was performed using the bacterial proteins collected from the mid-exponential phase. The electrophoresis (Figure 2) revealed that, L. lactis MG1363 showed a lysis band corresponding to the running position of AcmA, while strain KJ9 showed typical bands corresponding to the size of AtlA, either of which was thought to be the major peptidoglycan hydrolase for L. lactis and E. faecalis, respectively.
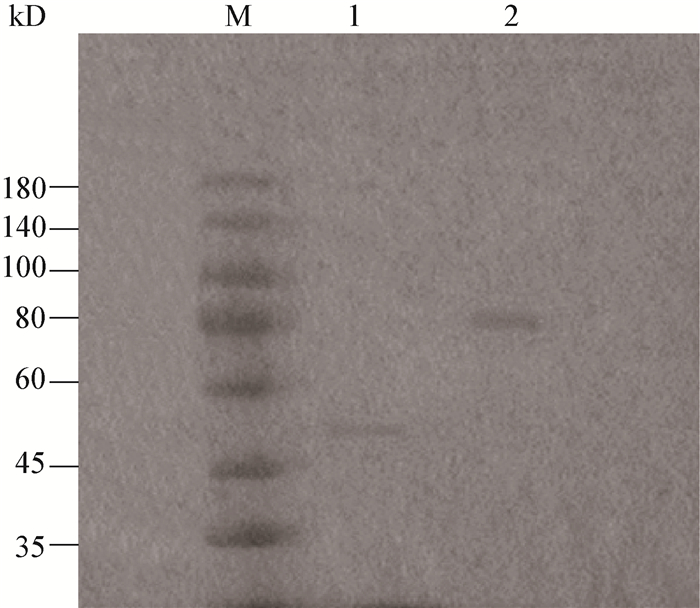
|
| Figure 2 Renaturing SDS-PAGE of L. lactis MG1363 and Enterococcus faecalis KJ9 图 2 乳酸乳球菌MG1363和粪肠球菌KJ9的复性SDS-PAGE电泳 Note: M: Marker; 1: L. lactis MG1363; 2: Enterococcus faecalis KJ9. Autoclaved Micrococcus lysodeikticus cells were incorporated into gels and served as the substrates of enzymatic degradation; the gels were incubated with the renaturing buffer for 24 h. 注:M:Marker; 1:乳酸乳球菌MG1363;2:粪肠球菌KJ9.灭活的溶壁微球菌细胞加入到凝胶中并作为酶解底物; 凝胶在复性缓冲液中孵育24 h完成复性反应. |
|
|
In order to further examine the expression profiles of lactococcal autolysins, the mRNA levels of all five autolysins, namely, AcmA, AcmB, AcmC, AcmD and YjgB[19-22], were determined at 5 h of growth in the culture of MG1363. It was shown (Figure 3) that, in contrast with control strain Lactobacillus casei BL23, all five autolysins were actively transcribed in this early and mid-exponential stage.
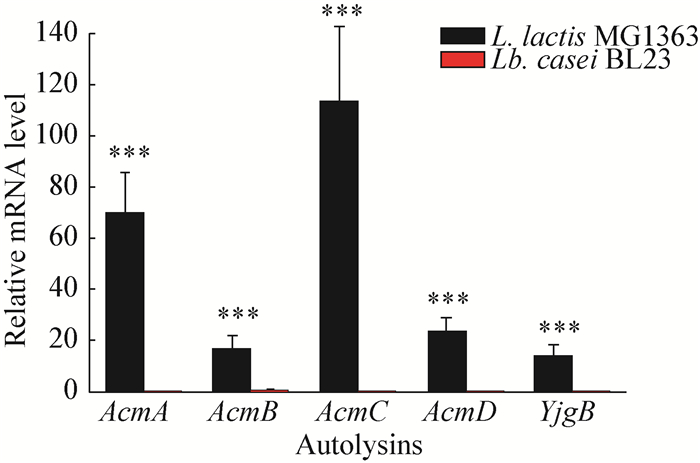
|
| Figure 3 Expression levels of autolysin genes for L. lactis and Lb. casei strains 图 3 乳酸乳球菌和干酪乳杆菌菌株自溶酶基因的表达水平 Note: Total RNAs were prepared from MG1363 cells grown in GM17 media for 5 h and analyzed by real-time fluorescence quantitative PCR with specific primers for each gene; The transcript amount was standardized by the amount of 16S rRNA gene in each sample; Lb. casei BL23 is a lactic acid bacteria strain besides L. lactis, thus not harboring the autolysins involved here; The same growth periods of this strain were also subjected to expressional analysis as control. *** stands for P < 0.001. 注:在GM17培养基中培养5 h的MG1363细胞的总RNA提取出来后进行荧光定量PCR的检测; 定量内参为16S rRNA基因; 干酪乳杆菌BL23是除去乳酸乳球菌之外的一种典型乳酸菌株, 因此并不含有本文所述的自溶酶; 处于同样生长时期的该菌株用作表达分析的对照使用. ***代表P值小于0.001. |
|
|
Bacillus subtilis was prone to autolysis induced by growth inhibitors when cultured in the carbon source-limited media[8]. Therefore, the glucose-limited (1/2 GM17) and strictly glucose-limited media (1/10 GM17) were used for the autolysis induction assays, respectively. All the antibiotic concentrations used in this study were in reference to their minimum inhibitory concentrations against L. lactis and minimum autolysis induction concentrations against B. subtilis[8]. Specifically, the growth inhibitors could be classified as protein synthesis inhibitor (Cm and Km), cell wall synthesis inhibitor (Amp) and membrane disrupter (SDS), respectively.
Given that growth conditions could considerably influence the lactococcal physiological behavior, the growth curve and glucose consumption were first determined for L. lactis MG1363 grown in 1/2 GM17 (Figure 4A). Glucose was exhausted when OD600 arrived at approximately 2.0 following 5 hours' incubation. However, no diauxic growth was observed in this glucose-limited medium. Subsequently, 50 µg/mL of Cm was added to the culture at a range of growth phases to determine the bacterial lysis profiles. Basically, Cm's adminstration triggered a profound bacteriacidal effect whenever the intervention occurred, with the bacterial growth ceased 1 h later in all cases (Figure 5). Following another 1 h's decline, the OD600 values remained unchanged, indicating that no sharp autolysis was induced under this condition, probably due to the fact the viable cell population had been reduced to a low number. Besides, the induction at glucose exhausting point (5 h) did not seem to generate any dramatically abnormal consequences.
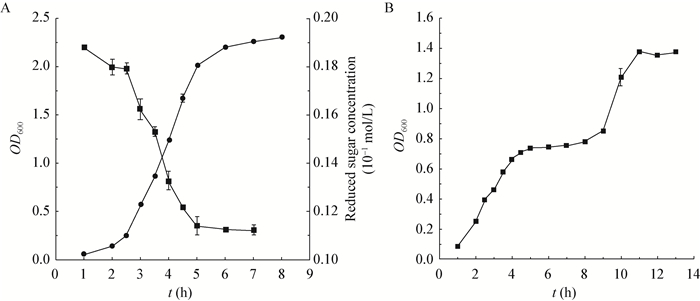
|
| Figure 4 The growth curve and glucose consumption of L. lactis MG1363 in 1/2 (A) and 1/10 (B) GM17 broth 图 4 乳酸乳球菌MG1363在1/2和1/10 GM17培养基中的生长曲线及葡萄糖消耗 Note: A: ●represent the OD600 values of the culture; ■represent the glucose concentration. B: ■indicated the OD600 values of the culture. 注:A:●代表培养液的OD600值; ■表示葡萄糖的含量. B:■代表培养液的OD600值. |
|
|
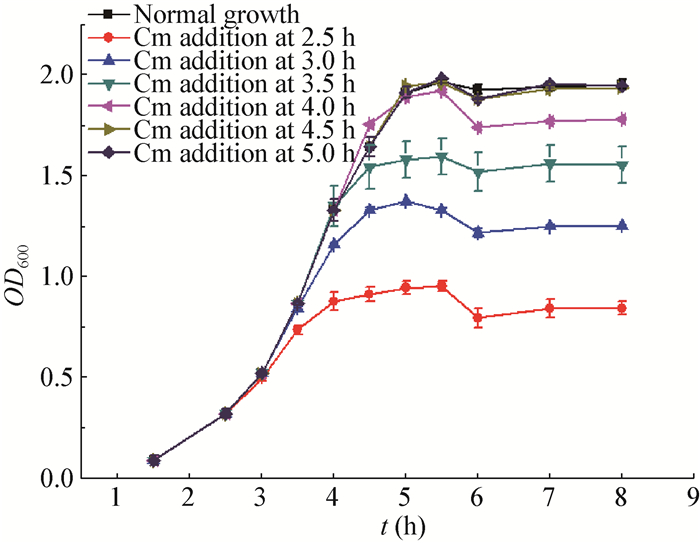
|
| Figure 5 OD600 changes of L. lactis MG1363 strains caused by the addition of Cm 图 5 氯霉素加入所导致的乳酸乳球菌MG1363的OD600值的改变 Note: Cm with the concentration of 50 mg/L was added to the culture at various time points; the altered growth curves under these conditions were then determined. 注:50 mg/L的氯霉素分别加入不同培养时间点的培养液中, 并对在上述条件下发生变化的生长曲线进行测定. |
|
|
Subsequently, Cm with changed concentration (8 µg/mL, 200 µg/mL) was subjected to the same assay. No obvious differences were observed (Data not shown). This suggests that Cm-induced autolysis in glucose-limited medium was concentration-independent.
Due to the above-mentioned data, a strictly glucose-limited medium (1/10 GM17) was utilized for the following experiments. Under this condition, L. lactis MG1363 exhibited a surprising diauxic growth (Figure 4B): the bacterial growth ceased with OD600 reaching 0.7, and then resumed 4 h later. The transition period between the two consecutive growth stages was supposed to be autolysis-susceptible, thus this period was chosen to assess the induced autolysis of MG1363 culture.
Figure 6 showed the various growing profiles following the addition of Cm or Amp at early-exponential phase (0.4), the turning point (0.7) and the diauxic point (7 h). It was revealed that the significant autolysis of MG1363 was induced by Amp, a cell wall synthesis inhibitor, only at the turning point of growth (when OD600 reached 0.7). In contrast to it, Amp with the same concentration could not even inhibit the bacterial growth when it was added at other stages (Figure 6). Besides, the concentration used here (4 mg/L) was less than its MIC in L. lactis. These data demonstrated that the autolysis caused by Amp was specifically dependent on the growth phases when the intervention occurred, and it could only be triggered when the strains transited from the first growth to the lag phase of the second growth. Additionally, the treatment of Cm did not seem to induce autolysis at any rates (Figure 6).
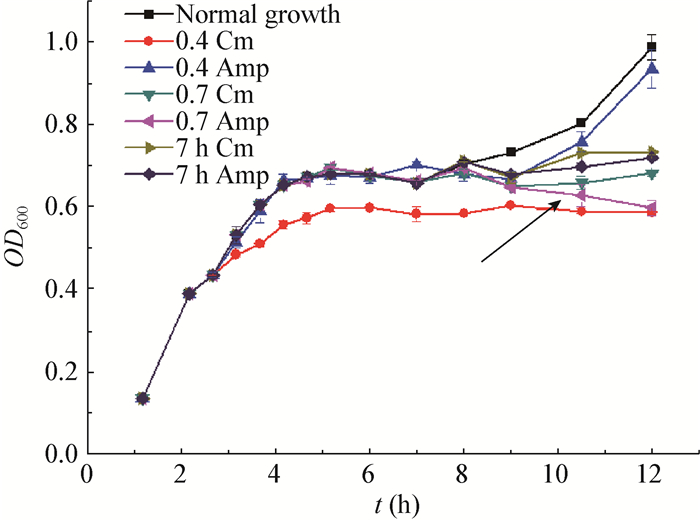
|
| Figure 6 Antibiotic-induced autolysis of L. lactis MG1363 propagated in 1/10 GM17 media 图 6 在1/10 GM17培养基中乳酸乳球菌MG1363受抗生素诱导的自溶 Note: Cm (8 mg/L) and Amp (4 mg/L) were added to the culture at various time points, respectively. 0.4 and 0.7 are adding antibiotics to the culture when the OD600 values of the strain reach up to 0.4 and 0.7 respectively; 7 h is adding antibiotics to the culture when the strain was grown for 7 hours; the black arrow indicated the growth curve under the condition of OD 0.7 Amp. 注:8 mg/L的氯霉素和4 mg/L的氨苄青霉素分别在不同时间点加入培养液中; 0.4和0.7表示抗生素加入的时间点是OD600分别到达0.4和0.7的时间; 7 h表示抗生素加入的时间点是菌株培养到7 h; 黑箭头表示在OD 0.7时刻加入氨苄青霉素时的生长曲线. |
|
|
In order to investigate whether autolysins were involved in this induced process, the transcription levels of AcmA, AcmB, AcmC, AcmD and YjgB, the five major autolysins in L. lactis, were determined in the presence or absence of Amp's administration. Surprisingly, it was shown from Figure 7 that, the mRNA levels of AcmA, AcmB, AcmD and YjgB exhibited significant decline with the influence of Amp, implying that autolysins probably did not play pivotal role in the induction of sharp autolysis.
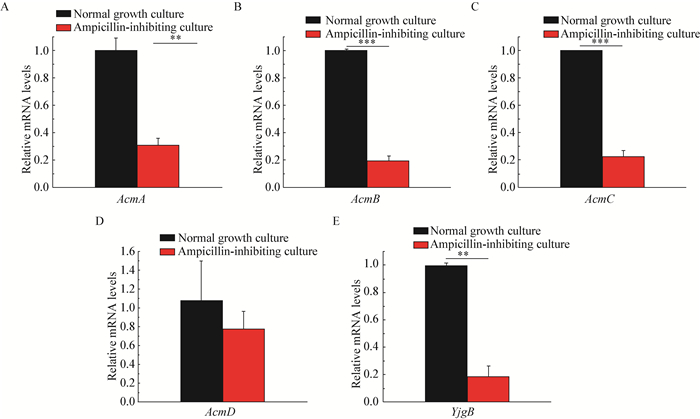
|
| Figure 7 Expression levels of autolysin genes for L. lactis MG1363 upon induction of autolysis 图 7 乳酸乳球菌MG1363在诱导下其自溶酶基因的表达水平 Note: Total RNAs were prepared from MG1363 cells grown in 1/10 GM17 media at 2 h following Amp's induction at 5 h of growth, and analyzed by real-time fluorescence quantitative PCR with specific primers for each gene (A, B, C, D, E for AcmA, AcmB, AcmC, AcmD and YjgB, respectively); the transcript amount was standardized by the amount of 16S rRNA gene in each sample. **: stands for P < 0.01; ***: stands for P < 0.001. 注:MG1363细胞在培养5 h后加入氨苄青霉素进行诱导, 之后继续培养2 h后收集细胞提取RNA, 并通过荧光定量PCR分别检测AcmA (A), AcmB (B), AcmC (C), AcmD (D)和YjgB (E)五个基因的表达水平; 转录体的定量以样本中16S rRNA基因的水平为内参. **:P值 < 0.01;***:P值 < 0.001. |
|
|
The buffer system was frequently utilized to determine the bacterial autolytic profiles[23], and Triton X-100 was added to the system to facilitate the release of the autolysins' activities. In the present investigation, the bacterial cells were collected and suspended in Tris-HCl buffer (pH 7.0) containing 0.25% Triton X-100, followed by the addition of Amp (4 µg/mL), Km (8 µg/mL), Cm (8 µg/mL) and SDS (20 µg/mL). The resulting autolysis profiles were presented in Figure 8.

|
| Figure 8 The buffered autolysis of MG1363 by the addition of different growth inhibitors 图 8 MG1363在不同生长抑制剂作用下的缓冲液自溶水平 Note: Cm, Amp, Km and SDS were added to the MG1363 culture with OD600 value of 0.3; subsequently the strains were collected when it reached 0.7 and then resuspended in 25 mmol/L Tris-HCl (pH 7.0) buffer with 0.25% Triton X-100, with its final concentration of 1.0; The suspension was incubated at 30 ℃, and the decreased percentage of OD600 values was used to represent the autolysis extent during 4 hours. 注:在生长到OD600值为0.3时的MG1363培养液中分别加入氯霉素、氨苄青霉素、卡那霉素和SDS; 随后菌株在生长至0.7时收集下来并重悬在25 mmol/L的Tris-HCl (pH 7.0) 并添加有0.25%的Triton X-100的缓冲液中, 使得菌体的吸光度值达到1.0;悬液在30 ℃条件下孵育4 h, 期间OD600的减少比值被用来表征自溶水平. |
|
|
Based on Figure 8, it was found that compared to the control group, the autolysis capabilities of L. lactis MG1363 was inhibited to a variable extent upon the addition of various growth inhibitors, and Cm showed the most prominent inhibitory effect. However, the tendencies seemed reversed as incubation time prolonged. This result might be a reflection of a diminished release of enzymatic activities of autolysins, when their cognate partners, cell wall synthetic enzymes, were strongly inhibited.
3 DISCUSSIONAutolysis was a strain-specific physiological phenomenon (As demonstrated in Figure 1), and its profiles were significantly influenced by the environmental conditions, such as salt concentration, pH, temperature, rennet's induction, etc[24]. Among them, medium compositions, as well as growth inhibitors, were generally regarded to play the predominant roles in autolysis induction[8]. The results here revealed that inhibitors' induction of autolysis could only occur when bacteria was grown in 1/10 GM17, instead of 1/2 GM17. Moreover, the induced autolysis was strictly growth phase-specific, that is, it could only occur at the turning point of its first growth. Based on these findings, it's tempting to speculate that, when bacteria was transited from glucose exhausting point to lag phase of the second growth, the rigid regulation of autolysin activities might be weakened, rendering it susceptible to the inhibitors' adverse effect. Therefore, it's the complex interplay of carbon deficiency and inhibitor's disruption that consequently contributed to the lysis of L. lactis strains.
Basically, this study is a proof that the LAB organisms are more prone to the induced autolysis when it's engaging in the remodeling process caused by energy conversion. This phenomenon could be interpreted by the disruption of proton motive forces, or alternatively the abnormal function of two-component regulatory system. Furthermore, it was also shown that autolysis could only be induced in the culture of 1/10 GM17, instead of 1/2 GM17. Therefore, it was reasonable to hypothesize that the timing that carbon source depletion occurred was also the key determinant for the autolysis induction. In other words, the growth inhibitors could trigger autolysis of L. lactis strains only in the case of glucose depletion, and only when the depletion was elicited in the early-exponential stage.
A strange observation manifested in this investigation is the down-regulation of lethal autolysins during the narrowly-scoped process of sharp lysis. So what is the leading cause of this bizarre lysis? Of note, the autolysins' expression was often co-regulated with cell wall synthetic enzymes[25], a molecular mechanism for bacteria to keep the balance of these antagonizing enzymes. Thus, the autolysins' decline might be attributed to either the deficiency of protein synthesis, or the concerted regulation with a diminished cell wall synthesis. In addition, it should not be ignored that the cell wall susceptibility might also be changed to some extent by the administration of growth inhibitors. Particularly, various peptidoglycan modifications, such as O-acetylation or de-N-acetylation, were able to lead to significant changes of autolysis phenotype, with its concrete mechanisms awaiting further investigation.
In summary, this study comprehensively investigated the autolysis profiles of L. lactis induced by a variety of growth inhibitors, and the growth phase-specific autolysis was first discovered in lactic acid bacteria. The findings here shed new light on the understanding of cellular mechanisms towards autolysis of lactococcal strains, paving way for the better elucidation and application of this bizarre physiological phenomenon.
ACKNOWLEDGEMENTS: The procedures of this study were approved by the lab of institute of Environment Toxicology and Food Safety, Hefei University of Technology.| [1] | Trias R, Ba eras L, Seguí EM, et al. Lactic acid bacteria from fresh fruit and vegetables as biocontrol agents of phytopathogenic bacteria and fungi[J]. International Microbiology: Official Journal of the Spanish Society for Microbiology, 2008, 11(4): 231–236. |
| [2] | Manzoor A, Ul-Haq I, Baig S, et al. Efficacy of locally isolated lactic acid bacteria against antibiotic-resistant uropathogens[J]. Jundishapur Journal of Microbiology, 2016, 9(1): e18952. |
| [3] | Ouzari H, Cherif A, Mora D. Autolytic phenotype of Lactococcus lactis strains isolated from traditional Tunisian dairy products[J]. Journal of Applied Microbiology, 2002, 92(5): 812–820. DOI:10.1046/j.1365-2672.2002.01585.x |
| [4] | Morya VK, Dong SJ, Kim EK. Production and characterization Te-peptide by induced autolysis of Saccharomyces cerevisiae[J]. Applied Biochemistry and Biotechnology, 2014, 172(7): 3390–3401. DOI:10.1007/s12010-014-0780-y |
| [5] | Gibert L, Didi J, Marlinghaus L, et al. The major autolysin of Staphylococcus lugdunensis, AtlL, is involved in cell separation, stress-induced autolysis and contributes to bacterial pathogenesis[J]. FEMS Microbiology Letters, 2014, 352(1): 78–86. DOI:10.1111/fml.2014.352.issue-1 |
| [6] | Szilágyi M, Kwon NJ, Bakti F, et al. Extracellular proteinase formation in carbon starving Aspergillus nidulans cultures–physiological function and regulation[J]. Journal of Basic Microbiology, 2011, 51(6): 625–634. DOI:10.1002/jobm.v51.6 |
| [7] | McIntyre M, Eade JK, Cox PW, et al. Quantification of autolysis in Penicillium chrysogenum by semiautomated image analysis[J]. Canadian Journal of Microbiology, 2001, 47(4): 315–321. DOI:10.1139/w01-016 |
| [8] | Chung JK, Yoon HE, Shin HC, et al. Induction of growth phase-specific autolysis in Bacillus subtilis 168 by growth inhibitors[J]. The Journal of Microbiology, 2009, 47(1): 50–59. DOI:10.1007/s12275-008-0256-2 |
| [9] | Lortal S, Di Blasi A, Madec MN, et al. Tina wooden vat biofilm: A safe and highly efficient lactic acid bacteria delivering system in PDO Ragusano cheese making[J]. International Journal of Food Microbiology, 2009, 132(1): 1–8. DOI:10.1016/j.ijfoodmicro.2009.02.026 |
| [10] | S ndergaard L, Ryssel M, Svendsen C, et al. Impact of NaCl reduction in Danish semi-hard Samsoe cheeses on proliferation and autolysis of DL-starter cultures[J]. International Journal of Food Microbiology, 2015, 213: 59–70. DOI:10.1016/j.ijfoodmicro.2015.06.031 |
| [11] | Gatti M, Fornasari ME, Mucchetti G, et al. Presence of peptidase activities in different varieties of cheese[J]. Letters in Applied Microbiology, 1999, 28(5): 368–372. DOI:10.1046/j.1365-2672.1999.00541.x |
| [12] | Meyrand M, Boughammoura A, Courtin P, et al. Peptidoglycan N-acetylglucosamine deacetylation decreases autolysis in Lactococcus lactis[J]. Microbiology, 2007, 153(10): 3275–3285. DOI:10.1099/mic.0.2007/005835-0 |
| [13] | Michelsen CF, Christensen AMJ, Bojer MS, et al. Staphylococcus aureus alters growth activity, autolysis, and antibiotic tolerance in a human host-adapted Pseudomonas aeruginosa lineage[J]. Journal of Bacteriology, 2014, 196(22): 3903–3911. DOI:10.1128/JB.02006-14 |
| [14] | Xue T, Zhao LP, Sun BL. LuxS/AI-2 system is involved in antibiotic susceptibility and autolysis in Staphylococcus aureus NCTC 8325[J]. International Journal of Antimicrobial Agents, 2013, 41(1): 85–89. DOI:10.1016/j.ijantimicag.2012.08.016 |
| [15] | Hsu CY, Lin MH, Chen CC, et al. Vancomycin promotes the bacterial autolysis, release of extracellular DNA, and biofilm formation in vancomycin-non-susceptible Staphylococcus aureus[J]. FEMS Immunology & Medical Microbiology, 2011, 63(2): 236–247. |
| [16] | Ledala N, Wilkinson BJ, Jayaswal RK. Effects of oxacillin and tetracycline on autolysis, autolysin processing and atl transcription in Staphylococcus aureus[J]. International Journal of Antimicrobial Agents, 2006, 27(6): 518–524. DOI:10.1016/j.ijantimicag.2006.03.008 |
| [17] | Lacriola CJ, Falk SP, Weisblum B. Screen for agents that induce autolysis in Bacillus subtilis[J]. Antimicrobial Agents and Chemotherapy, 2013, 57(1): 229–234. DOI:10.1128/AAC.00741-12 |
| [18] | Carvalho ME, Gon alves MH, Silva MT. Induction of autolysis in Streptococcus faecium[J]. Microbiology, 1987, 133(4): 985–993. DOI:10.1099/00221287-133-4-985 |
| [19] | Buist G, Kok J, Leenhouts KJ, et al. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation[J]. Journal of Bacteriology, 1995, 177(6): 1554–1563. DOI:10.1128/jb.177.6.1554-1563.1995 |
| [20] | Bourouni OC, El Bour M, Calo-Mata P, et al. Phylogenetic analysis of antimicrobial lactic acid bacteria from farmed seabass Dicentrarchus labrax[J]. Canadian Journal of Microbiology, 2012, 58(4): 463–474. DOI:10.1139/w2012-014 |
| [21] | Huard C, Miranda G, Redko Y, et al. Analysis of the peptidoglycan hydrolase complement of Lactococcus lactis: identification of a third N-acetylglucosaminidase, AcmC[J]. Applied and Environmental Microbiology, 2004, 70(6): 3493–3499. DOI:10.1128/AEM.70.6.3493-3499.2004 |
| [22] | Huard C, Miranda G, Wessner F, et al. Characterization of AcmB, an N-acetylglucosaminidase autolysin from Lactococcus lactis[J]. Microbiology, 2003, 149(3): 695–705. DOI:10.1099/mic.0.25875-0 |
| [23] | Riepe HR, Pillidge CJ, Gopal PK, et al. Characterization of the highly autolyticLactococcus lactis subsp. cremoris strains CO and 2250[J]. Applied and Environmental Microbiology, 1997, 63(10): 3757–3763. |
| [24] | Treimo J, Vegarud G, Langsrud T, et al. Use of DNA quantification to measure growth and autolysis of Lactococcus and Propionibacterium spp. in mixed populations[J]. Applied and Environmental Microbiology, 2006, 72(9): 6174–6182. DOI:10.1128/AEM.00515-06 |
| [25] | Antignac A, Sieradzki K, Tomasz A. Perturbation of cell wall synthesis suppresses autolysis in Staphylococcus aureus: Evidence for coregulation of cell wall synthetic and hydrolytic enzymes[J]. Journal of Bacteriology, 2007, 189(21): 7573–7580. DOI:10.1128/JB.01048-07 |
 2017, Vol. 44
2017, Vol. 44




