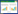扩展功能
文章信息
- 谢华蓉, 徐在超, 刘军, 史亚楠, 袁保红, 邓祖军
- XIE Hua-Rong, XU Zai-Chao, LIU Jun, SHI Ya-Nan, YUAN Bao-Hong, DENG Zu-Jun
- 广藿香内生真菌多样性及其对青枯菌的拮抗活性
- Diversity and the antagonistic activities of endophytic fungi from patchouli against Ralstonia solanacearum
- 微生物学通报, 2017, 44(5): 1171-1181
- Microbiology China, 2017, 44(5): 1171-1181
- DOI: 10.13344/j.microbiol.china.160781
-
文章历史
- 收稿日期: 2016-10-26
- 接受日期: 2017-03-21
- 优先数字出版日期(www.cnki.net): 2017-04-05
2. 广东药科大学基础学院 广东省生物活性药物研究重点实验室 广东 广州 510006
2. School of Basic Courses, Guangdong Provincial Key Laboratory of Pharmaceutical Bioactive Substances, Guangdong Pharmaceutical University, Guangzhou, Guangdong 510006, China
广藿香[Pogostemon cablin (Blanco) Benth]是唇形科(Lamiaceae)刺蕊草属(Pogostemon)植物,以叶、梗入药,具芳香化湿、和胃止呕、祛暑解表的功效,是广东道地药材,著名的“十大南药”之一[1]。广藿香为多年生草本,以广藿香为主药组成的成方制剂达30多种,如著名的国家中药保护品种“藿香正气丸(胶囊、水)”和“抗病毒口服液”,从广藿香中提取的精油可用于配制各种剂型的化妆品、定香剂和杀虫剂等,其已成为医药工业和轻化工业的重要原料[2-3],目前国内外对广藿香的需求量极大[4-5]。由青枯菌[Ralstonia solanacearum (Smith) Yabuuchi]侵染引起的青枯病是广藿香种植过程中危害最为严重的系统性病害,严重威胁广藿香产业的生存与发展[6-7]。对广藿香青枯病的防治目前主要以化学农药为主,但效果有限,难以控制病害蔓延,而且易产生耐药,制约了高产、优质、低残留药材的生产[4]。利用转基因、辐射诱变等现代生物技术培育广藿香抗病新品种的研究虽有开展[8-9],但仍未获得具有应用前景的稳定的广藿香抗病品种[4]。因此,寻找新的安全有效的青枯病防治方法对于推广广藿香的种植、解决广藿香资源紧缺、发展广藿香产业具有重要的意义。
植物内生真菌一般是指在全部或部分生活周期内生活在植物体内,但对宿主植物组织不引起明显病害症状的一类真菌[10]。现有研究表明内生真菌与宿主植物在漫长的进化过程中已建立密切的联系,并能通过多种机制,如产生抗菌物质、铁载体、发生营养竞争、诱导植物的系统抗性等,提高植物的抗病害能力[11-12]。有报道显示,内生真菌Colletotrichum gloeosporioides和C. rosea接种到可可树幼苗后,均能显著降低其黑荚果腐病和冻荚果腐病的发病几率,并减轻其发病症状[13];内生真菌Cladorrhinum foecundissimum S8的接种可使棉花根腐病的发生率降低45.5%-70.0%[14],显然内生真菌在植物病害生物防治中具有良好的应用前景。再者,由于其生长微环境的特殊性以及与宿主长期的协同进化过程,内生真菌的次生代谢产物十分丰富,其中有许多不仅具有特殊结构或新颖的生物活性,而且往往还存在与其宿主植物相同或相似生理活性的次生代谢产物[15],因此植物内生真菌已成为筛选新化合物和新药的重要资源库[16-17]。
广藿香提取物已被发现具有调节胃肠道功能、抗虫、抗菌、抗病毒、抗肿瘤等多种生物活性[18-20],有研究者从一些广藿香内生真菌菌株,如Diaporthe longicolla A616、Myrothecium roridum A553和Bipolaris sorokiniana A606的代谢产物中也分离到一些具有抗肿瘤活性的化合物[21-23],然而目前对广藿香内生真菌的生物多样性缺乏全面的认识,对于其在广藿香青枯病防治中的潜在作用也尚未涉及。本研究拟采用组织块培养法从广藿香植株分离内生真菌,结合形态学及分子生物学方法对内生真菌进行鉴定,系统分析广藿香各部位内生真菌的类群分布及多样性,并从中筛选对青枯菌具拮抗活性的菌株,为全面挖掘广藿香内生真菌的药用资源以及构建“内生真菌-广藿香”青枯病生防系统提供前期基础。
1 材料与方法 1.1 材料 1.1.1 广藿香植株: 供试植株为处于营养生长期阶段(没有开花)、株高50 cm左右的健康广藿香植株,于2015年11月30日(20 ℃,相对湿度55%-80%)采集自广州市广东药科大学大学城校区中药园(N23°03′31.9″,E113°24′31.8″)。采样地区处于南亚热带,属南亚热带典型的季风海洋性气候,全年平均气温22-23 ℃,平均相对湿度约77%,年均降水量介于1 623 mm-1 900 mm之间。采样时将整株植物拔出,用无菌塑料袋装好后放入冰盒中送至实验室,再置于4℃保存,2 h内进行内生真菌的分离。 1.1.2 青枯病病原菌: 青枯菌(Ralstonia solanacearum)菌株GIM1.70由广东省微生物研究所提供。 1.1.3 主要试剂和仪器: 引物ITS1F、ITS4、NL1、NL1,生工生物工程(上海)股份有限公司;2×EasyTaq PCR SuperMix,北京全式金生物技术有限公司。真菌DNA提取试剂盒E.Z.N.A.® Fungal DNA Midi Kit,Omega公司。ETC811基因扩增仪,北京东胜创新生物科技有限公司;JY-C电泳仪,北京君意东方电泳设备有限公司;ZF-258凝胶成像分析仪,上海嘉鹏科技有限公司;LRH-150生化培养箱,上海一恒科技有限公司。 1.1.4 培养基: PDA培养基(g/L):马铃薯200.0,葡萄糖20.0,琼脂15.0,氯霉素0.1,pH 6.0;LB培养基(g/L):蛋白胨10.0,牛肉膏粉3.0,氯化钠5.0,pH 7.3;半固体营养琼脂(g/L):蛋白胨10.0,牛肉膏粉3.0,氯化钠5.0,琼脂粉7.0,pH 7.3;促孢培养基(g/L):磷酸二氢钾1.0,硝酸钾1.0,硫酸镁0.5,氯化钾0.5,淀粉0.2,葡萄糖0.2,蔗糖0.2,琼脂15,pH 6.0。 1.2 方法 1.2.1 内生真菌的分离与纯化: 采用文献[24]中的组织块方法分离广藿香内生真菌:将采集到的健康广藿香的根、茎、叶样品,用自来水冲洗掉表面残留的泥土等杂质,将其切成2 cm左右的小段,在超净台将其依次浸泡在75%酒精1 min,3%-5%有效氯的NaClO溶液3 min,75%的酒精0.5 min,然后再用无菌水冲洗3遍,用无菌滤纸吸干多余的水分,晾干,将其剪成约0.5 cm×0.5 cm的小片。将处理好后剪成的小片铺于PDA平板上,在26 ℃恒温培养箱中培养2-3周,待菌丝从组织材料切口处长出,及时将其转接到另一新的PDA平板上,纯化2-3次后接种于PDA固体斜面培养基上保存。同时收集表面消毒后最后一次冲洗样品的水样按照文献[25]进行表面消毒效果的检查。 1.2.2 内生真菌的形态学特征: 采用载玻片法培养真菌[24],然后在显微镜下观察真菌的菌丝、孢子及子实体结构,同时结合其菌落特征,根据《真菌鉴定手册》[26]进行分类鉴定。该培养条件下不产孢子的菌株可使用促孢培养基培养或紫外线照射来诱导孢子的产生,仍不产孢者将其归为不产孢类,有待后续的分子鉴定来确认其具体的种属类别。 1.2.3 内生真菌的分子鉴定: 通过形态学鉴定获得内生真菌的类群信息后,从每个类群中随机挑取3-5株菌通过分子鉴定进一步确认内生真菌的种属类别(不产孢类的菌株则全部都要进行分子鉴定)。采用真菌DNA提取试剂盒提取内生真菌总DNA,然后利用引物ITS1F (5′-CTTGGTCATTTAGAGAA GTAA-3′)和ITS4 (5′-TCCTCCGCTTAGATATGC-3′)扩增其内转录间隔区序列(Internal transcribed spacer,ITS),利用NL1 (5′-GCATATCAATAAGCGGAGGAA AAG-3′)和NL4 (5′-GGTCCGTGTTTCAAGACGG-3′)扩增核糖体大亚基基因序列(Large subunit,LSU)。ITS及LSU扩增的PCR反应体系(50μL)为:2×EasyTaq PCR SuperMix 25 μL,10 μmol/L正反引物各1 μL,模板DNA 1 μL,用ddH2O定容至50 μL。PCR反应条件:94 ℃ 5 min;94 ℃ 30 s,55 ℃ 30 min,72 ℃ 90 s,30个循环;72 ℃ 10 min。获得的PCR产物送生工生物工程(上海)股份有限公司测序。将ITS (或LSU)序列的测序结果在NCBI数据库中进行BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi)比对,从而获得分子鉴定结果。对青枯菌有拮抗活性的内生真菌,利用BLAST获得高相似性的菌种序列信息(可初步判断待测菌株所在的属水平),同时参考文献[27-31]选取与高相似性序列同属不同种的模式菌株序列作为参考序列,并选取与待测菌株亲缘关系适中的同科不同属的序列作为外类群,然后使用MEGA 7.0软件,采用邻接法构建系统发育树。 1.2.4 青枯菌拮抗菌株的筛选: 采用双层平板拮抗法筛选具有青枯菌拮抗活性的内生真菌[32]:将受试菌接种在PDA平板上,28 ℃培养5-7 d后往长菌的PDA平板上倾注10 mL半固体营养琼脂培养基(温度40 ℃),待其凝固后即制成上层培养基。最后将100 μL青枯菌悬液(1×106 CFU/mL)均匀涂布于上层培养基上,37 ℃培养48 h后观察并测量受试菌对青枯菌的抑菌圈。 1.2.5 数据分析: 采用定殖率(Colonization rate,CR)分析广藿香不同组织受到内生真菌侵染的程度,用分离率(Isolation frequency,IF)分析在一个特定的植物组织样品中内生真菌的丰度以及植物组织样品中多重侵染的频率,用相对分离频率(Relative frequency,RF)分析及确定各部位内生真菌的优势类群,用Shannon-Wiener多样性指数(H′)分析广藿香各部位内生真菌的多样性,用均匀度指数(E)分析内生真菌类群在各部位分布的均匀程度,用相似性指数(Cs)分析不同部位内生真菌多样性的差异。相关计算公式[33]如下:CR (%)=出现内生真菌的组织块数/总分离组织块数×100;
IF (%)=分离到内生真菌的总株数/总分离组织块数×100;
RF (%)=样本中分到的某种内生真菌的菌株数/分离到的总菌株数×100;
H′=-ΣPi×lnPi;
E=H′/ln(S);
Cs=2j/(a+b)
其中,Pi是指某特定种类内生真菌的菌株数占分离到内生真菌菌株总数的比值;S为物种数,N为个体总数;j为两个部位共有的内生真菌属数;a、b是两个部位各自有的内生真菌属数。根据Jaccard相似性系数原理,当Cs介于0.00-0.25时为极不相似;Cs介于0.25-0.50时为中等不相似;Cs介于0.50-0.75时为中等程度相似;Cs介于0.75-1.00时为极为相似。
采用针对两个百分数样本差异比较的t检验方法[34]分析广藿香不同部位的定殖率、分离率、多样性指数等是否存在显著性差异。
2 结果与分析 2.1 广藿香内生真菌的定殖率与分离率将表面消毒后样品最后一遍冲洗液涂布PDA平板,26℃培养14 d后均无菌落长出。同时取15株已分离获得的代表性真菌菌株的菌丝体(根、茎、叶分离物各5株),分别按相同的表面消毒程序处理后再接种到PDA平板上,26 ℃培养14 d后,所有平板均未见真菌生长蔓延。以上结果表明,经过表面消毒过程后样品表面附生菌已被消除,分离到的菌株来自样品内部,属于广藿香内生真菌。
从402个广藿香组织块中分离到313株内生真菌,其中来自根98株、茎104株和叶111株(表 1)。样品内生真菌总的定殖率和分离率分别为76.37%和77.86%,内生真菌在广藿香叶中的定殖率最高为94.74%,其次为茎部(91.07%),而根的定殖率最低,为55.11%。各部位内生真菌的分离率与定殖率呈现相同的变化规律,从高到低依次为叶(97.37%)、茎(92.85%)、根(55.68%)。
| 项目 Item | 根 Root | 茎 Stem | 叶 Leaf | 总计 Total |
| 组织块数Numder of tissue segments | 176 | 112 | 114 | 402 |
| 长菌组织块数Numder of tissue segments with fungi | 97 | 102 | 108 | 307 |
| 菌株数Number of fungal strains | 98 | 104 | 111 | 313 |
| 定殖率Colonization rate (%) | 55.11b | 91.07a | 94.74a | 76.37 |
| 分离率Isolation frequency (%) | 55.68b | 92.85a | 97.37a | 77.86 |
| 注:不同小写字母表示不同部位的定殖率或分离率具有显著性差异(P < 0.05). Note: Different small letters indicate that the comparison differences in the colonization rate or isolation rate are significant (P < 0.05) under the different parts of patchouli. | ||||
从广藿香共分离到的313株内生真菌,经形态学特征观察和rDNA-ITS序列分析,鉴定归为30个属,其主要类群为链格孢属(Alternaria)和拟茎点霉属(Phomopsis),分别占分离菌株总数的28.75%和23.00% (表 2)。不同部位内生真菌的组成及其优势类群具有一定的差异:根部内生真菌分属于19个属,其中链格孢属(Alternaria)和棒孢霉属(Corynespora)为优势类群,分别占根部内生真菌总数的32.65%和12.24%;茎一共分离到10个属的内生真菌,其优势类群为链格孢属(49.04%)、炭疽菌属(Colletotrichum,占17.31%)和腐皮壳属(Diaporthe,占15.38%);叶中共分离到12个属,以拟茎点霉属(50.45%)和炭疽菌属(17.12%)为优势类群(表 2)。
| 真菌种类 Species of fungal | 根(98株) Root (98 strains) | 茎(104株) Stem (104 strains) | 叶(111株) Leaf (111 strains) | 合计(313株) Total (313 strains) |
| 犁头霉属Absidia | 1.02 | 0.00 | 0.00 | 0.32 |
| 链格孢属Alternaria | 32.65 | 49.04 | 6.30 | 28.57 |
| 截顶炭团菌属Annulohypoxylon | 0.00 | 0.00 | 0.90 | 0.32 |
| 曲霉属Aspergillus | 2.04 | 0.00 | 0.00 | 0.64 |
| 炭皮菌属Biscogniauxia | 0.00 | 0.00 | 1.80 | 0.32 |
| 葡萄座腔菌属Botryosphaeria | 0.00 | 0.96 | 0.00 | 0.32 |
| 毛壳属Chaetomium | 0.00 | 0.96 | 0.00 | 0.32 |
| 枝孢属Cladosporium | 0.00 | 0.00 | 0.90 | 0.32 |
| 炭疽菌属Colletotrichum | 1.02 | 17.31 | 17.12 | 12.14 |
| 棒孢霉属Corynespora | 12.24 | 0.00 | 0.00 | 3.83 |
| 腐皮壳属Diaporthe | 1.02 | 15.38 | 2.70 | 6.39 |
| 弯孢聚壳属Eutypella | 0.00 | 0.96 | 0.00 | 0.32 |
| 镰刀菌属Fusarium | 5.10 | 0.00 | 0.00 | 1.60 |
| 小丛壳属Glomerella | 1.02 | 0.96 | 1.80 | 1.28 |
| Gibellulopsis | 1.02 | 0.00 | 0.00 | 0.32 |
| 球座菌属Guignardia | 0.00 | 0.00 | 13.51 | 4.79 |
| 壳球孢属Macrophomina | 1.02 | 0.00 | 0.00 | 0.32 |
| 巨座壳科Magnaporthaceae | 0.00 | 0.00 | 1.80 | 0.64 |
| 单链孢属Monocillium | 1.02 | 0.00 | 0.00 | 0.32 |
| 漆斑菌属Myrothecium | 4.08 | 0.00 | 0.00 | 1.28 |
| 新萨托菌属Neosartorya | 1.02 | 0.00 | 0.00 | 0.32 |
| 新壳梭孢属Neofusicoccum | 0.00 | 0.96 | 0.00 | 0.32 |
| 黑孢霉属Nigrospora | 0.00 | 3.85 | 1.80 | 1.92 |
| 拟青霉属Paecilomyces | 9.18 | 0.00 | 0.00 | 2.88 |
| 青霉属Penicillium | 7.14 | 0.00 | 0.00 | 2.24 |
| 拟茎点霉属Phomopsis | 6.12 | 9.61 | 50.45 | 23.00 |
| 炭角菌科Xylariaceae | 0.00 | 0.00 | 0.90 | 0.32 |
| 篮状菌属Talaromyces | 9.18 | 0.00 | 0.00 | 2.88 |
| 木霉属Trichoderma | 2.04 | 0.00 | 0.00 | 0.64 |
| 丝核菌属Rhizoctonia | 2.04 | 0.00 | 0.00 | 0.64 |
从Shannon-Wiener多样性指数(H′)来看,广藿香总体的H′为2.32,根、茎和叶的H′值分别为2.64、2.00和1.97,其中根部内生真菌的多样性最高;而3个部位的均匀度指数(E)则与多样性指数呈现不同的变化规律,叶(0.72) 和茎(0.74) 的E值明显高于根部(0.66),这表明根部内生真菌虽然种类较为丰富,但各类群的分布却不均匀(表 3)。
| 多样性指标 Divesity index | 根 Root | 茎 Stem | 叶 Leaf |
| 总菌株数Number of fungal strains (N) | 98 | 104 | 110 |
| 物种数Number of fungal species (S) | 27 | 15 | 16 |
| Shannon-Wiener多样性指数Shannon-Wiener diversity index (H′) | 2.64a | 2.00a | 1.97a |
| 均匀度指数Evenness index (E) | 0.66b | 0.74a | 0.72a |
| 相似性指数-根Similarity index-root (Cs root) | 1.00 | 0.20 | 0.19 |
| 相似性指数-茎Similarity index-stem (Cs stem) | - | 1.00 | 0.35 |
| 相似性指数-叶Similarity index-leaf (Cs leaf) | - | - | 1.00 |
| 注:不同小写字母表示不同部位某一项指数之间具有显著性差异(P < 0.05). Note: Different small letters indicate that the comparison differences under the different parts of patchouli are significant (P < 0.05). | |||
比较根、茎和叶3个部位之间的真菌种类的相似性指数(Cs)发现,茎与叶的相似性指数(Cs stem-leaf=0.35) 最高,其次是根与茎(Csroot-stem=0.20),根与叶(Cs root-leaf=0.19) 的最低(表 3)。根据Jaccard相似性系数原理,茎与叶两个部位的内生真菌类群组成为中等不相似,而根与茎、根与叶之间的群落组成均为极不相似。广藿香共分离到30种不同类群的内生真菌,其中除了链格孢属、拟茎点霉属、炭疽菌属、腐皮壳属、小丛壳属(Glomerella)等5个属在3种组织都存在,黑孢霉属(Nigrospora)存在于茎和叶中外,其余24个类群只分布于其中某一特定组织,例如葡萄座腔菌属(Botryosphaeria)、毛壳属(Chaetomium)和弯孢聚壳属(Eutypella) 3个属只在茎部分离到,而截顶炭团菌属(Annulohypoxylon)、炭皮菌属(Biscogniauxia)、枝孢属(Cladosporium)、球座菌属(Guignardia)、巨座壳科(Magnaporthaceae)和炭角菌科(Xylariaceae) 6个类群只在叶中分离到,根部则存在15个特有的内生真菌类群(表 2)。
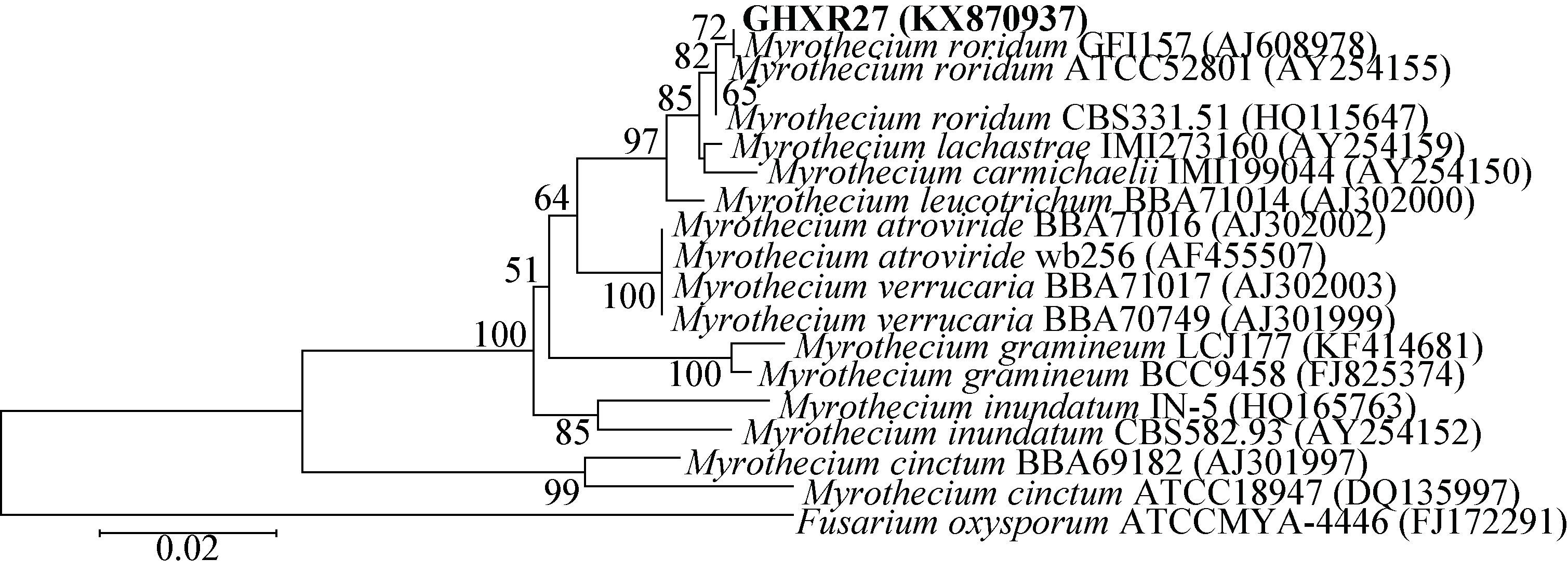
2.4 具青枯菌拮抗活性广藿香内生真菌菌株的筛选与鉴定发现16株内生真菌对青枯菌有抑制活性(表 4),其中菌株GHXR07、GHXR27和GHXR29的抑菌效果较为显著,其抑菌圈相对半径分别达到2.8、1.1和3.0 cm。将菌株GHXR07、GHXR27和GHXR29的ITS-rDNA序列提交GenBank数据库,获得的登录号分别为KX870936、KX870937和KX870938。然后在GenBank中进行一致性搜索,选择一致性较高且具代表性的菌株ITS序列进行多序列比对,构建系统发育树获得其分子鉴定信息,并结合形态学特征对高活性菌株进行鉴定。根据Blast结果可知,菌株GHXR07的ITS和LSU序列均与GenBank中Talaromyces属的序列最为相似,其最高相似性分别为97%和99%。在基于ITS基因分析的系统发育树中(图 1),GHXR07与Talaromyces radicus亲缘关系相对较近,而在基于LSU基因分析的系统发育树(图 2)中,其与Talaromyces rugulosus关系最为密切,但它在这两个发育树中均能与Talaromyces属的参考序列很好地聚类在一起,结合GHXR07菌落形态及孢子特征(图 3A)可将其鉴定为Talaromyces sp.。菌株GHXR27的ITS序列与漆斑菌属中的Myrothecium roridum (HQ115647.1) 的一致性高达99%,其在系统发育树上与4个Myrothecium roridum序列形成一个支持度为82%的支(图 4),同时结合其菌落与孢子形态特征(图 3B),可以将其鉴定为Myrothecium roridum Tode。GHXR29的ITS序列与篮状菌属中的Talaromyces wortmannii (KF984827) 的一致性高达99%,在系统发育树中与3个Talaromyces wortmannii序列形成一个支持度为98%的支(图 5),同时结合其形态学特征(图 3C),可将其鉴定为Talaromyces wortmannii (Kl cker) Benjamin。
| 菌株编号 Strain no. | 抑菌圈相对半径* The relative radius of inhibition zone* (cm) |
| GHXL17 | 0.2 |
| GHXL12 | 0.2 |
| GHXL26 | 0.2 |
| GHXS16 | 0.2 |
| GHXR06 | 0.4 |
| GHXR07 | 2.8 |
| GHXR16 | 0.6 |
| GHXR17 | 0.2 |
| GHXR19 | 0.6 |
| GHXR26 | 0.3 |
| GHXR27 | 1.1 |
| GHXR29 | 3.0 |
| GHXR33 | 0.5 |
| GHXR35 | 0.9 |
| GHXR36 | 0.5 |
| GHXR37 | 0.9 |
| 注:*:抑菌圈相对半径=抑菌圈半径-真菌菌落半径. Note: *: The relative radius of inhibition zone = the inhibition zone radius -fungal colony radius. | |

|
| 图 1 基于ITS rDNA序列构建的GHXR07系统发育树 Figure 1 Phylogenetic tree based on the ITS rDNA gene sequence of GHXR07 注:建树方法为邻接法;外类群为Trichocoma paradoxa CBS788.83 (JN899398);系统树分支点处的数字为系统树的自展值(1 000次重复);标尺长度代表遗传距离;括号中编号为所用菌株在GenBank中的登录号. Note: The phylogenetic tree was constructed by neighbour-joining method; Trichocoma paradoxa CBS788.83 (JN899398) was used as the out-group; The number in each branch point indicates the number of trees from 1 000 bootstrap replications in which the branch occurs; Scale length represented genetic distance. GenBank accession numbers were shown in the parentheses. |
|
|

|
| 图 2 基于LSU rDNA序列构建的GHXR07系统发育树 Figure 2 Phylogenetic tree based on the LSU rDNA gene sequence of GHXR07 注:建树方法为邻接法;外类群为Trichocoma paradoxa IAM14601(AB047220);系统树分支点处的数字为系统树的自展值(1 000次重复);标尺长度代表遗传距离;括号中编号为所用菌株在GenBank中的登录号. Note: The phylogenetic tree was constructed by neighbour-joining method; Trichocoma paradoxa IAM14601 (AB047220) was used as the out-group; The number in each branch point indicates the number of trees from 1 000 bootstrap replications in which the branch occurs; Scale length represented genetic distance. GenBank accession numbers were shown in the parentheses. |
|
|

|
| 图 3 内生真菌GHXR07 (A)、GHXR27 (B)、GHXR29 (C)的菌落及孢子形态 Figure 3 The colony and spores morphology of endophytic fungi GHXR07 (A), GHXR27 (B), and GHXR29 (C) 注:a:菌落的正面;b:菌落的背面;c:孢子及菌丝. Note: a: The front of the colony; b: The back of the colony; c: Spore and hyphae. |
|
|
|
| 图 4 基于ITS rDNA序列构建的GHXR27系统发育树 Figure 4 Phylogenetic tree based on the ITS rDNA gene sequence of GHXR27 注:建树方法为邻接法;外类群为Fusarium oxysporum strain ATCC MYA-444618S (FJ172291);系统树分支点处的数字为系统树的自展值(1 000次重复);标尺长度代表遗传距离;括号中编号为所用菌株在GenBank中的登录号. Note: The phylogenetic tree was constructed by neighbour-joining method; Fusarium oxysporum strain ATCC MYA-444618S (FJ172291) was used as the out-group; The number in each branch point indicates the number of trees from 1 000 bootstrap replications in which the branch occurs; Scale length represented genetic distance. GenBank accession numbers are shown in the parentheses. |
|
|
|
| 图 5 基于ITS rDNA序列构建的GHXR29系统发育树 Figure 5 Phylogenetic tree based on the ITS rDNA gene sequence of GHXR29 注:建树方法为邻接法;外类群为Trichocoma paradoxa CBS788.83 (JN899398);系统树分支点处的数字为系统树的自展值(1 000次重复);标尺长度代表遗传距离;括号中编号为所用菌株在GenBank中的登录号. Note: The phylogenetic tree was constructed by neighbour-joining method; Trichocoma paradoxa CBS 788.83 (JN899398) was used as the outgroup; The number in each branch point indicates the number of trees from 1 000 bootstrap replications in which the branch occurs; Scale length represented genetic distance. GenBank accession numbers are shown in the parentheses. |
|
|
从采集自广东药科大学大学城校区中药园的广藿香共分离到30个属的内生真菌,内生真菌总的定殖率和分离率分别为76.37%和77.86%,其总多样性指数为2.32,表明该区域生长的广藿香蕴含着丰富的内生真菌资源。本研究中广藿香内生真菌定殖率和多样性指数与其他同处亚热带或热带区域的植物较接近[35-36],但明显高于北方温带地区生长的药用植物[37-38],这可能与宿主植物所处地区的环境和气候有关,热带和亚热带地区比温带地区具有更加丰富的物种多样性,并且降水较多,湿度较大,更有利于内生真菌在植物体内的侵染和定殖[35, 39-40]。同时本研究还在广藿香中分离到一些在其他药用植物中未曾分离到的较为新颖和少见的内生真菌类群[41],如炭皮菌属(Biscogniauxia)、弯孢聚壳属(Eutypella)、Gibellulopsis、新萨托菌属(Neosartorya)、新壳梭孢属(Neofusicoccum)和篮状菌属(Talaromyces),这些真菌在广藿香特异性地分布或许可能与广藿香体内所具有的独特微环境有关[41]。大量研究表明植物内生真菌的次生代谢产物存在许多具有新结构或新活性的物质,目前已成为新药开发的重要资源库[16-17, 42],因此本研究对广藿香内生真菌的分离及其类群的系统分析将为广藿香内生真菌代谢产物的后续开发和挖掘奠定基础。
广藿香根部内生真菌的种类数量及生物多样性指数均明显高于茎和叶,在一些其他植物的内生菌研究中也得到了相似的结果[38, 43-45]。土壤微生物生物量大且种类丰富[46],它们也是植物内生菌的主要来源[47-48]。植物根系的能量和物质代谢异常活跃且与土壤微生物存在直接和紧密的相互作用,其受到土壤真菌侵染的机会较多[49],因此根部内生真菌的多样性相对较高是极易理解的。虽然茎和叶部内生真菌的种类少于根部,但其定殖率及分离率却高于根部,这可能与广藿香叶和茎部的空间和养分相对于根部较为充足,更有利于内生真菌定殖和繁衍有关[38],而广藿香多数由扦插繁殖而成,其根系并不发达,提供的营养和空间有限。
内生真菌在广藿香不同部位的类群结构与分布具有一定的差异性和专一性:根部的优势类群为链格孢属(32.65%)和棒孢霉属(12.24%),茎为链格群相似性指数均小于0.5,这表明它们的类群不相似程度较高。广藿香3个部位共分离到30个不同类群的内生真菌,其中24个类群只分布于某一特定组织内,例如棒孢霉属只在根中分离到,球座菌属只在叶中分离到,而葡萄座腔菌属只在茎中分离到,这一结果可能与广藿香不同部位的组织结构及营养条件不同所导致的体内微环境差异有关[50-51]。链格孢属、拟茎点霉属和炭疽菌属的内生真菌在广藿香体内有较高的分离率,已有研究报道它们在其他药用草本植物中也有广泛的分布[41],这可能与链孢属(49.04%)、炭疽菌属(17.31%)和腐皮壳属(15.38%),而叶为拟茎点霉属(50.45%)和炭疽菌属(17.12%);根、茎、叶3个部位之间的内生真菌类格孢属、拟茎点霉属及炭疽菌属真菌的侵染和定殖对宿主及生长微环境具有较强的适应性有关[52]。链格孢属真菌主要分布于植物的茎和叶中,根部较为少见[53-54],而本研究从广藿香根部分离到较高比例的链格孢属真菌,其他研究者也在沙棘、芦荟、绒毛草等植物的根部分离到较高比例的链格孢属内生真菌[55-57],这可能与这些植物根部存在适合链格孢属真菌生长和繁殖的营养成分、组织结构及其他微环境有关。
青枯菌侵染可导致多种植物比如番茄、烟草、辣椒、香蕉、广藿香出现青枯病。研究者已从植物根际土壤中分离到多种对番茄、辣椒、烟草青枯菌具有较好抗性的细菌,如芽胞杆菌、越南伯克氏菌等,并在盆栽试验中取得了一定的防治效果[58-59]。但根际细菌在应用过程中较易受到土壤土著微生物竞争、原生动物的吞噬以及其他环境因子的影响,造成效果不稳定[60-61]。相对于细菌,真菌呈菌丝生长,接触面积广,生物量大,在环境中具有极强的适应能力[62],因而内生真菌可能更容易与宿主植物形成紧密的共生关系,对其产生持续、稳定的作用。已有研究发现内生真菌可通过多种机制提高植物抗病害能力[11-12]。本研究从广藿香内生真菌分离到16株对青枯菌具有拮抗活性的菌株,其中3株内生真菌的拮抗活性尤为显著,作为广藿香青枯病潜在的生防菌,值得进一步研究。我们的前期研究还发现内生真菌可在逆境环境下促进宿主植物生长并降低其地上部分重金属含量[63]。因此,广藿香-内生真菌防治系统有望在预防青枯病的同时,在缩短广藿香生产周期、降低重金属残留、改善药材质量等方面发挥作用,这对于广藿香种植产业具有重要的应用价值,有必要开展进一步的深入研究。
| [1] | Lin XH, He H, Wu LR, et al. Studies on callus induction and plantlets regeneration of Pogostemon cablin (Blanco) Benth[J]. Journal of Guangzhou University of Traditional Chinese Medicine, 2009, 26(2) : 171–175. (in Chinese) 林小桦, 贺红, 吴立蓉, 等. 广藿香愈伤组织诱导和分化再生植株的研究[J]. 广州中医药大学学报, 2009, 26(2) : 171–175. |
| [2] | Wu YG, Hu XW. Research and Example on the Standardized Cultivation of Medicinal Plan from Southern China[M]. Beijing: China Forestry Publishing House, 2014: 78 -102. (in Chinese) 吴友根, 胡新文. 南药植物规范化栽培研究与实例[M]. 北京: 中国林业出版社, 2014: 78 -102. |
| [3] | Chen YS. Progresses in the research on the rare Pogostemon cablin[J]. Guangdong Agricultural Sciences, 2007(1) : 109–112. (in Chinese) 陈元生. 珍稀南药广藿香的研究进展[J]. 广东农业科学, 2007(1) : 109–112. |
| [4] | He GZ, Li JK, Gao W, et al. The strategy to promote the development of planting industry of Pogostemon cablin in in Guangdong Province[J]. Chinese Agricultural Science Bulletin, 2012, 28(31) : 288–292. (in Chinese) 何国振, 李锦坤, 高伟, 等. 广东省广藿香种植业发展策略[J]. 中国农学通报, 2012, 28(31) : 288–292. DOI:10.3969/j.issn.1000-6850.2012.31.055 |
| [5] | Chen WW, Xu HH. Study on the protection and sustainable utilization of Southern medicinal resources[J]. Journal of Guangzhou University of Traditional Chinese Medicine, 2009, 26(3) : 201–203. (in Chinese) 陈蔚文, 徐鸿华. 南药资源的保护与可持续利用研究[J]. 广州中医药大学学报, 2009, 26(3) : 201–203. |
| [6] | Chen Y, Jin CZ. Research advance on biological control of plant bacterial wilt[J]. Hunan Argicultural Sciences, 2009(7) : 82–85. (in Chinese) 陈勇, 金晨钟. 植物青枯病的生物防治研究进展[J]. 湖南农业科学, 2009(7) : 82–85. |
| [7] | Yang CY, Zhang Z, Wei JH, et al. The investigation and analysis of the distribution of Ralstonia solanacearum in Hainan Pogostemon cablin[J]. Traditional Medicine, 2010, 19(10) : 78–79. (in Chinese) 杨春雨, 张争, 魏建和, 等. 海南广藿香青枯病原菌分布的调查与分析[J]. 传统医药, 2010, 19(10) : 78–79. |
| [8] | Zhang JM, Sun XP, Zheng XQ. Construction of cecropin B and D double gene expression vector and transformation of patchouli (Pogostemon cablin Benth.)[J]. Chinese Journal of Tropical Crops, 1997, 18(1) : 50–57. (in Chinese) 张家明, 孙雪飘, 郑学勤. 抗菌肽B、D双基因表达载体的构建及转化广藿香的研究[J]. 热带作物学报, 1997, 18(1) : 50–57. |
| [9] | He H, Xu SY, Wu LR, et al. Selection of bacterial-wilt-resistance mutants of Pogostemon cablin (Blanco) Benth. by radiation mutation[J]. Journal of Guangzhou University of Traditional Chinese Medicine, 2012, 29(2) : 185–189. (in Chinese) 贺红, 许仕仰, 吴立蓉, 等. 广藿香辐射诱变筛选抗病突变体的研究[J]. 广州中医药大学学报, 2012, 29(2) : 185–189. |
| [10] | Stone JK, Bacon CW, White Jr JF. An overview of endophytic microbes: endophytism defined[A]//Bacon CW, White Jr JF. Microbial Endophytes[M]. New York: Marcel Dekker, 2000: 3-29 |
| [11] | Backman PA, Sikora RA. Endophytes: an emerging tool for biological control[J]. Biological Control, 2008, 46(1) : 1–3. |
| [12] | Berg G, Krechel A, Ditz M, et al. Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi[J]. FEMS Microbiology Ecology, 2005, 51(2) : 215–229. DOI:10.1016/j.femsec.2004.08.006 |
| [13] | Mejía LC, Enith I, Maynard Z, et al. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens[J]. Biological Control, 2008, 46(1) : 4–14. DOI:10.1016/j.biocontrol.2008.01.012 |
| [14] | Gasoni L, de Gurfinkel S. Biocontrol of Rhizoctonia solani by the endophytic fungus Cladorrhinum foecundissimum in cotton plants[J]. Australasian Plant Pathology, 2009, 38(4) : 389–391. DOI:10.1071/AP09013 |
| [15] | Kusari S, Pandey SP, Spiteller M. Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites[J]. Phytochemistry, 2013, 91 : 81–87. DOI:10.1016/j.phytochem.2012.07.021 |
| [16] | Kaul S, Gupta S, Ahmed M, et al. Endophytic fungi from medicinal plants: a treasure hunt for bioactive metabolites[J]. Phytochemistry Reviews, 2012, 11(4) : 487–505. DOI:10.1007/s11101-012-9260-6 |
| [17] | Shukla ST, Habbu PV, Kulkarni VH, et al. Endophytic microbes: a novel source for biologically/pharmacologically active secondary metabolites[J]. Asian Journal of Pharmacology and Toxicology, 2014, 2(3) : 1–16. |
| [18] | Zhang Y, Zhang JC, Chen Y, et al. Current progresses in pharmacognosy, Chemistry, and pharmacology of Pogostemon cablin[J]. Chinese Traditional and Herbal Drugs, 2006, 37(5) : 786–790. (in Chinese) 张英, 张金超, 陈瑶, 等. 广藿香生药、化学及药理学的研究进展[J]. 中草药, 2006, 37(5) : 786–790. |
| [19] | Gao XL, Xiong S, Wang YF, et al. The preliminary study on the resistance of three effective parts of patchouli to coxsackie virus B3 in vitro[J]. Journal of Chinese Medicinal Materials, 2009, 32(5) : 761–764. (in Chinese) 高相雷, 熊盛, 王一飞, 等. 广藿香三种有效部位体外抗柯萨奇病毒B3作用的初步研究[J]. 中药材, 2009, 32(5) : 761–764. |
| [20] | Jeong JB, Choi J, Lou ZY, et al. Patchouli alcohol, an essential oil of Pogostemon cablin, exhibits anti-tumorigenic activity in human colorectal cancer cells[J]. International Immunopharmacology, 2013, 6(2) : 184–190. |
| [21] | Wang M, Chen YC, Sun ZH, et al. Study on cytotoxic secondary metabolites of endophytic fungus Diaporthe longicolla A616 from Pogostemon cablin[J]. China Journal of Chinese Materia Medica, 2016, 41(11) : 2112–2117. (in Chinese) 王沫, 陈玉婵, 孙章华, 等. 广藿香内生真菌Diaporthe longicolla A616细胞毒活性次级代谢产物研究[J]. 中国中药杂志, 2016, 41(11) : 2112–2117. |
| [22] | Liu WZ, Tan GH, Pan QL, et al. Chemical constituents of the mycelia of an endophytic Myrothecium roridum from Pogostemon cablin (Blanco) Benth[J]. Journal of Guangdong Pharmaceutical University, 2014, 30(4) : 427–429. (in Chinese) 刘威振, 谭国慧, 潘清灵, 等. 广藿香内生真菌露湿漆斑菌菌丝体化学成分研究[J]. 广东药学院学报, 2014, 30(4) : 427–429. |
| [23] | Wang M, Chen YC, Li HH, et al. Study on secondary metabolites of endophytic fungus Bipolaris sorokiniana from Pogostemon cablin and their antitumor activities[J]. Journal of Guangdong Pharmaceutical University, 2015, 31(5) : 576–580. (in Chinese) 王沫, 陈玉婵, 李浩华, 等. 广藿香内生真菌Bipolaris sorokiniana的次级代谢产物及其抗肿瘤活性研究[J]. 广东药学院学报, 2015, 31(5) : 576–580. |
| [24] | Deng ZJ, Cao LX, Zhou SN. Isolation and metabolite bioactivities of mangrove fungal endophytes[J]. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2010, 49(2) : 100–104. (in Chinese) 邓祖军, 曹理想, 周世宁. 红树林内生真菌的分离及代谢产物生物活性的初步研究[J]. 中山大学学报:自然科学版, 2010, 49(2) : 100–104. |
| [25] | Cao LX, You JL, Zhou SN. Endophytic fungi from Musa acuminata leaves and roots in South China[J]. World Journal of Microbiology and Biotechnology, 2002, 18(2) : 169–171. DOI:10.1023/A:1014491528811 |
| [26] | Wei JC. Manual of Determinative Fungi[M]. Shanghai: Shanghai Scientific and Technical Publishers, 1979 . (in Chinese) 魏景超. 真菌鉴定手册[M]. 上海: 上海科学技术出版社, 1979 . |
| [27] | Liu JK, Hyde KD, Jones EBG, et al. Fungal diversity notes 1-110: taxonomic and phylogenetic contributions to fungal species[J]. Fungal Diversity, 2015, 72(1) : 1–197. DOI:10.1007/s13225-015-0324-y |
| [28] | Kwon HW, Kim JY, Choi MA, et al. Characterization of Myrothecium roridum isolated from imported anthurium plant culture medium[J]. Mycobiology, 2014, 42(1) : 82–85. DOI:10.5941/MYCO.2014.42.1.82 |
| [29] | Samson RA, Yilmaz N, Houbraken J, et al. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium[J]. Studies in Mycology, 2011, 70 : 159–183. DOI:10.3114/sim.2011.70.04 |
| [30] | Fujii T, Hoshino T, Inoue H, et al. Taxonomic revision of the cellulose-degrading fungus Acremonium cellulolyticus nomen nudum to Talaromyces based on phylogenetic analysis[J]. FEMS Microbiology Letters, 2014, 351(1) : 32–41. DOI:10.1111/fml.2014.351.issue-1 |
| [31] | Sang H, An TJ, Kim CS, et al. Two novel Talaromyces species isolated from medicinal crops in Korea[J]. Journal of Microbiology, 2013, 51(5) : 704–708. DOI:10.1007/s12275-013-3361-9 |
| [32] | Chen DH, Zhu Y, Gai TX, et al. Inhibition effect of Lactic acid bacteria to mold isolated from yogurt[J]. China Dairy Industry, 2014, 42(1) : 21–23. (in Chinese) 陈大欢, 朱忆, 盖天欣, 等. 乳酸菌对酸乳中霉菌的抑制作用研究[J]. 中国乳品工业, 2014, 42(1) : 21–23. |
| [33] | Wang YN, Wang LK, Miao ZB, et al. Endophytic fungi diversity in root of Dendrobium fimbiratum (Orchidaceae)[J]. Ecological Science, 2014, 33(4) : 672–679. (in Chinese) 王亚妮, 王丽琨, 苗宗保, 等. 流苏石斛根部内生真菌多样性研究[J]. 生态科学, 2014, 33(4) : 672–679. |
| [34] | Li SG. Practical Biostatistics[M]. 2nd Edition. Beijing: Peking University Press, . (in Chinese) 李松岗. 实用生物统计[M]. 第2版.北京: 北京大学出版社, . |
| [35] | Zang W, Sun X, Sun JQ, et al. Diversity and community structure of endophytic fungi from Taxus chinensis var. mairei[J]. Chinese Journal of Applied Ecology, 2014, 25(7) : 2071–2078. (in Chinese) 臧威, 孙翔, 孙剑秋, 等. 南方红豆杉内生真菌的多样性与群落结构[J]. 应用生态学报, 2014, 25(7) : 2071–2078. |
| [36] | Fróhlich J, Hyde KD, Petrini O. Endophytic fungi associated with palms[J]. Mycological Research, 2000, 104(10) : 1202–1212. DOI:10.1017/S095375620000263X |
| [37] | Sun JQ, Guo LD, Zang W, et al. Diversity and ecological distribution of endophytic fungi associated with medicinal plants[J]. Science in China Series C: Life Sciences, 2008, 38(5) : 475–484. (in Chinese) 孙剑秋, 郭良栋, 臧威, 等. 药用植物内生真菌多样性及生态分布[J]. 中国科学C辑:生命科学, 2008, 38(5) : 475–484. |
| [38] | Zhu J, Li XJ, Sun L, et al. Ecological distribution and diversity of medical Ferula species produced in Xinjiang[J]. China Journal of Chinese Materia Medica, 2015, 40(2) : 356–361. (in Chinese) 朱军, 李晓瑾, 孙丽, 等. 新疆药用阿魏属植物内生真菌的生态分布与多样性[J]. 中国中药杂志, 2015, 40(2) : 356–361. |
| [39] | Pan TY, Yin ZQ, Li XJ, et al. Diversity of foliar endophytic fungi of invasive plant purpurstem eupatorium (Eupatorium adenophorum)[J]. Journal of Microbiology, 2015, 35(5) : 73–78. (in Chinese) 潘田扬, 殷照青, 李仙杰, 等. 入侵植物紫茎泽兰叶内生真菌多样性研究[J]. 微生物学杂志, 2015, 35(5) : 73–78. |
| [40] | Porras-Alfaro A, Bayman P. Hidden fungi, emergent properties: endophytes and microbiomes[J]. Annual Review of Phytopathology, 2011, 49(1) : 291–315. DOI:10.1146/annurev-phyto-080508-081831 |
| [41] | Tan XM, Zhou YQ, Chen J, et al. Advances in research on diversity of endophytic fungi from medicinal plants[J]. Chinese Pharmaceutical Journal, 2015, 50(18) : 1563–1580. (in Chinese) 谭小明, 周雅琴, 陈娟, 等. 药用植物内生真菌多样性研究进展[J]. 中国药学杂志, 2015, 50(18) : 1563–1580. |
| [42] | Wani K, Saboo S, Solanke P, et al. Production of novel secondary metabolites from endophytic fungi by using fermentation process[J]. Indo American Journal of Pharmaceutical Research, 2016, 6(3) : 4957–4961. |
| [43] | Du XN, Xu HJ, Huang PP, et al. Diversity and antimicrobial activity of endophytic bacteria isolated from Lycium barbarum of Ningxia[J]. Microbiology China, 2015, 42(9) : 1779–1787. (in Chinese) 杜晓宁, 徐惠娟, 黄盼盼, 等. 宁夏枸杞内生细菌的多样性及其抑菌活性研究[J]. 微生物学通报, 2015, 42(9) : 1779–1787. |
| [44] | Angelini P, Rubini A, Gigante D, et al. The endophytic fungal communities associated with the leaves and roots of the common reed (Phragmites australis) in lake Trasimeno (Perugia, Italy) in declining and healthy stands[J]. Fungal Ecology, 2012, 5(6) : 683–693. DOI:10.1016/j.funeco.2012.03.001 |
| [45] | Jin H, Yan ZQ, Liu Q, et al. Diversity and dynamics of fungal endophytes in leaves, stems and roots of Stellera chamaejasme L. in northwestern China[J]. Antonie van Leeuwenhoek, 2013, 104(6) : 949–963. DOI:10.1007/s10482-013-0014-2 |
| [46] | Torsvik V, vre s L. Microbial diversity and function in soil: from genes to ecosystems[J]. Current Opinion in Microbiology, 2002, 5(3) : 240–245. DOI:10.1016/S1369-5274(02)00324-7 |
| [47] | Compant S, Saikkonen K, Mitter B, et al. Editorial special issue: soil, plants and endophytes[J]. Plant and Soil, 2016, 405(1/2) : 1–11. |
| [48] | Compant S, Sessitsch A, Mathieu F. The 125th anniversary of the first postulation of the soil origin of endophytic bacteria-a tribute to M.L.V. Galippe[J]. Plant and Soil, 2012, 356(1/2) : 299–301. |
| [49] | Schulz B, Boyle C. The endophytic continuum[J]. Mycological Research, 2005, 109(6) : 661–686. DOI:10.1017/S095375620500273X |
| [50] | Wu JB, Zhan RL, Liu F, et al. Isolation, identification and diversity analysis of endophytic fungi in Mangifera indica[J]. Journal of Fruit Science, 2013, 30(6) : 1030–1039. (in Chinese) 吴婧波, 詹儒林, 柳凤, 等. 杧果内生真菌分离鉴定及多样性分析[J]. 果树学报, 2013, 30(6) : 1030–1039. |
| [51] | Thongsandee W, Matsyda Y, Ito S. Temporal variations in endophytic fungal assemblages of Ginkgo biloba L[J]. Journal of Forest Research, 2012, 17(2) : 213–218. DOI:10.1007/s10310-011-0292-3 |
| [52] | Fernandes EG, Pereira OL, da Silva CC, et al. Diversity of endophytic fungi in Glycine max[J]. Microbiological Research, 2015, 181 : 84–92. DOI:10.1016/j.micres.2015.05.010 |
| [53] | Gu W. Bioactive metabolites from Alternaria brassicicola ML-P08, an endophytic fungus residing in Malus halliana[J]. World Journal of Microbiology and Biotechnology, 2009, 25(9) : 1677–1683. DOI:10.1007/s11274-009-0062-y |
| [54] | Guo LD, Xu L, Zheng WH, et al. Genetic variation of Alternaria alternata, an endophytic fungus isolated from Pinus tabulaeformis as determined by random amplified microsatellites (RAMS)[J]. Fungal Diversity, 2004, 16 : 53–65. |
| [55] | Li Q, Sun GY. Isolation and identification of endophytes from Hippophae[J]. Chinese Agricultural Science Bulletin, 2006, 22(10) : 300–302. (in Chinese) 李琦, 孙广宇. 沙棘内生菌的分离与初步鉴定[J]. 中国农学通报, 2006, 22(10) : 300–302. DOI:10.3969/j.issn.1000-6850.2006.10.072 |
| [56] | Ye X, Lu L, Cong S, et al. Diversity analysis of endophytic fungi in reed[J]. Liaoning Agricultural Sciences, 2016(1) : 5–8. (in Chinese) 叶馨, 陆蕾, 丛珊, 等. 芦苇内生真菌的多样性分析[J]. 辽宁农业科学, 2016(1) : 5–8. |
| [57] | Márquez SS, Bills GF, Acu a LD, et al. Endophytic mycobiota of leaves and roots of the grass Holcus lanatus[J]. Fungal Diversity, 2010, 41(1) : 115–123. DOI:10.1007/s13225-009-0015-7 |
| [58] | Liu W, Shen XY, Duan JN, et al. Screening, identification of antagonistic rhizospheric Bacillus sp. LW-6 against tobacco bacterial wilt[J]. Acta Phytophylacica Sinica, 2013, 40(1) : 95–96. (in Chinese) 刘伟, 沈小英, 段军娜, 等. 抗烟草青枯病菌的芽胞杆菌筛选和鉴定[J]. 植物保护学报, 2013, 40(1) : 95–96. |
| [59] | Qiao JQ, Chen ZY, Liang XJ, et al. Biocontrol efficacy on tomato bacterial wilt by Bacillus subtilis Bs916[J]. Chinese Journal of Biological Control, 2016, 32(2) : 229–234. (in Chinese) 乔俊卿, 陈志谊, 梁雪杰, 等. 枯草芽孢杆菌Bs916防治番茄青枯病[J]. 中国生物防治学报, 2016, 32(2) : 229–234. |
| [60] | Qiu JP, Huang YX, Wang C, et al. Effects of bacterial consortium EG03 on control of pepper bacterial wilt and rhizosphere microbial community characteristics in fields[J]. Chinese Journal of Applied Ecology, 2014, 25(5) : 1468–1474. (in Chinese) 邱敬萍, 黄艳霞, 王超, 等. EG03菌剂对辣椒青枯病的防治效果及对根围土壤微生物群落的影响[J]. 应用生态学报, 2014, 25(5) : 1468–1474. |
| [61] | Jiang M, Cao LX, Zhang RD. The relationship of heavy metal resistant endophyte and the heavy metal resistance ability of their host plants[J]. Journal of Agro-Environment Science, 2007, 26(6) : 2038–2042. (in Chinese) 姜敏, 曹理想, 张仁铎. 重金属抗性内生真菌与其宿主植物重金属抗性关系初探[J]. 农业环境科学学报, 2007, 26(6) : 2038–2042. |
| [62] | Harms H, Schlosser D, Wick LY. Untapped potential: exploiting fungi in bioremediation of hazardous chemicals[J]. Nature Reviews Microbiology, 2011, 9(3) : 177–192. DOI:10.1038/nrmicro2519 |
| [63] | Deng ZJ, Cao LX, Zhang RD, et al. Enhanced phytoremediation of multi-metal contaminated soils by interspecific fusion between the protoplasts of endophytic Mucor sp. CBRF59 and Fusarium sp. CBRF14[J]. Soil Biology and Biochemistry, 2014, 77 : 31–40. DOI:10.1016/j.soilbio.2014.06.005 |
 2017, Vol. 44
2017, Vol. 44