扩展功能
文章信息
- 赵曦, 解云英, 白利平
- Zhao Xi, Xie Yun-ying, Bai Li-ping
- 微生物共培养在新活性化合物挖掘中的研究进展
- Progress of microbial co-culture in new compound mining
- 微生物学通报, 2017, 44(10): 2437-2442
- Microbiology China, 2017, 44(10): 2437-2442
- DOI: 10.13344/j.microbiol.china.160885
-
文章历史
- 收稿日期: 2016-12-02
- 接受日期: 2017-01-23
- 优先数字出版日期(www.cnki.net): 2017-02-27
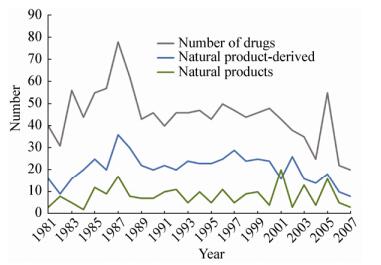
微生物次级代谢产物结构新颖、活性多样,一直以来都被作为药物及其先导化合物的重要来源。真菌次级代谢产物具有多种生理活性,如抗菌、抗肿瘤、抗氧化、免疫调节和细胞因子诱导等,具有巨大的药物开发价值[1-2]。但是,随着研究的不断深入,研究者们在微生物次级代谢产物中发现大量有价值的化合物的同时,筛选得到的微生物资源的重复率不断上升,具有新结构新活性的物质发现几率在逐年下降。据统计,从1990年后FDA批准的微生物天然产物及其衍生物数量逐年下降,在2001-2007这7年期间整体下降30% (图 1),基于上述原因,美国许多大公司甚至宣布放弃微生物来源的天然产物开发[3]。
针对这一情况,近20年来科学家们提出一系列解决办法:如开发特殊环境微生物,在其中寻找结构新颖、活性良好的物质[4];通过宏基因组方法,利用不可培养微生物中的基因资源,异源表达来获得新活性、新结构的化合物[5];利用已知化合物的生物合成基因簇,通过基因敲除、异源置换等手段,获得已知化合物的新衍生物[6];利用合成生物学手段对不同来源基因模块组装,绿色、高效合成药物前体等[7-8]。以上一系列研究发现了大量新化合物,为微生物天然产物的挖掘提供了前进动力。
然而,微生物在自然界是以混合的菌落形式存在的,与单一菌株相比,混合菌落能够完成更为复杂的任务,那些承袭了百年甚至千年的天然传统发酵工艺(天然混合培养),如食品和酒类,现今依然被广泛应用[9-10]。共培养最初是指在无菌条件下,不同微生物在厌氧或好氧条件下的混合培养[11]。今天的共培养同已往的天然混合培养有了本质的区别,它着重强调共培养是人们有目的、有意识地利用高通量技术和生物信息平台挖掘新的活性物质或提高产量[12]。真菌天然产物丰富,是天然药物的重要来源之一,然而随着重复发现越来越多,以及在常规的实验室培养条件下真菌中很多天然产物产量很低或根本不产生,因此通过真菌共培养挖掘新的天然产物正逐渐成为合成生物学的新前沿。
1 真菌vs真菌共培养历史上,人类很久以前就会利用真菌做面包和啤酒了,然而我们对真菌群落却并不了解。自1929年弗莱明从真菌中发现青霉素以来,真菌的研究大部分都基于真菌纯培养,从中相继发现了头孢霉素(抗生素)、环孢霉素(免疫抑制剂)、洛伐他汀(降胆固醇药物)、烟曲霉素(血管生长抑制剂)、苔色酸衍生物(抗骨质疏松药物)和麦角生物碱(抗偏头痛和降血压药物)等药物[13]。近5年来利用真菌与真菌共培养技术,结合高效、微量的分离纯化与多靶标(点)评价技术,已经发现了多种新型代谢产物(表 1),且数量呈逐年增加趋势,具有极高的药物开发潜力。
| Co-culture strains (fungi) | Product | Activity | Reference |
| Talaromyces siamensis & Phomopsis sp. | BE-31405 | Anti-fungal/bacteria | [14] |
| Penicillium citrinum & Beauveria felina | Citrifelins A & B | Anti-bacteria | [15] |
| Fusarium solani & Talaromyces sp. | Coculnol | Anti-virus | [16] |
| Phomopsis sp. K38 & Alternaria sp. E33 | Cyclopeptide | Anti-fungal | [17] |
| Alternaria tenuissima & Nigrospora sphaerica | Stemphyperylenol | Anti-fungal | [18] |
| Two unidentified fungi | Marinamide, methyl marinamide | Anti-tumor | [19] |
| Alternaria tenuissima & Fusarium culmorum/Fusarium graminearum | Deoxynivalenol, Zearalenone | Anti-bacteria | [20] |
| Two marine-derived mangrove epiphytic Aspergillus sp. | Aspergicin, Neoaspergillic acid | Anti-bacteria | [21] |
| Two unidentified fungi | Xanthone derivative | Anti-fungal | [22] |
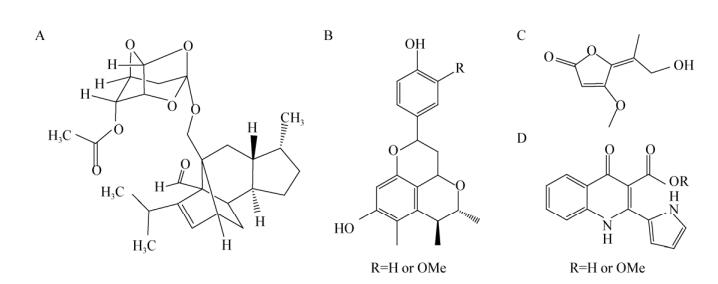
真菌共培养产物中发现的新化合物活性多样,如抗病毒、抗菌、抗肿瘤、免疫调节等,其中某些化合物还具有多种活性。如Nonaka等[14]将土壤来源的两株真菌Phomopsis sp. FKA-62和Talaromyces siamensis FKA-61共培养,发现Phomopsis sp. FKA-62能够刺激Talaromyces siamensis FKA-61产生新化合物BE-31405 (图 2A),10 μg该化合物能够显著抑制Phomopsis sp. FKA-62 (真菌)、Mucor racemosus IFO 4581 (真菌)、Bacillus subtilis ATCC 6633 (细菌)和Kocuria rhizophila ATCC 9341 (细菌)的生长,而对Talaromyces siamensis FKA-61无抑制(100 μg),显示出极好的开发前景;Meng等[15]从两株海洋来源真菌Penicillium citrinum MA-197和Beauveria felina EN-135共培养产物中分离得到2个新的橘霉素衍生物Citrifelins A和B (图 2B),对包括金黄色酿脓葡萄球菌、大肠杆菌在内的7种病原菌有抑制效果;利用真菌之间的共培养还发现了具有抗病毒活性的青霉酸衍生物Coculnol (图 2C)[16]、抗肿瘤活性的Marinamide和methyl marinamide (图 2D)[19]以及其他一系列具有抗菌活性的新化合物,极大地丰富了微生物天然产物的来源。
|
| 图 2 真菌共培养发现的新化合物结构 Figure 2 Structure of new compounds founded in fungi vs fungi co-culture products Note: A: BE-31405; B: Citrifelins A and B; C: Coculnol; D: Marinamide and methyl marinamide. |
|
|
我们课题组前期分离得到一小分子化合物2460A,它能显著抑制HT-29裸鼠移植瘤的生长[23]。通过进一步研究发现,其为两株真菌共培养产生,并且发酵产物中还有一系列红曲红胺类化合物[24]。2460A为其中一个化合物,通过一系列药理实验证明其可作用于结肠癌肿瘤细胞、结肠癌相关巨噬细胞IL-6/IL-6R信号通路及TNF信号通路,抑制细胞IL-6及TNFα的转录及分泌。
2 真菌vs细菌共培养细菌和真菌通过紧密接触,可以刺激真菌中某些沉默基因簇转录,进而诱导新的天然产物生成[25]。最近的研究证明,在土壤主要成分草酸盐的降解过程中,首先需在细菌与真菌的共同作用下使其pH发生改变,这一研究为土壤中细菌与真菌的共同存在提供了现实依据[26]。这些基础研究的结果极大地刺激了研究者们通过真菌与细菌共培养发现天然产物的热情。在2011-2015年间,大量新结构化合物被鉴定,除部分活性未定的新结构化合物外,表 2中罗列了5年期间通过真菌与细菌共培养发现的新化合物,这些化合物涵盖抗菌、抗肿瘤及抗寄生虫等各个方面,显示出通过真菌与细菌共培养挖掘结构新颖、活性良好的天然产物的巨大潜力。
| Co-culture strains (fungi & bacteria) | Product | Activity | Reference |
| Fusarium pallidoroseum & Saccharopolyspora erythraea | N-demethylophiosetin pallidorosetin A & B | Anti-tumor | [27] |
| Rhizopus microsporus & Burkholderia gladiol | iso-enacyloxin Ⅲa | Anti-fungal/bacteria | [28] |
| Aspergillus fumigatus & Streptomyces bullii | 1-10 | Anti-parasitic | [29] |
| Fusarium tricinctum & Bacillus subtilis | 1-3 (Figure 3)Enniatin A1 | Anti-bacteria | [30] |
| Aspergillus fumigatus & Sphingomonas sp. | Glionitrin B | Anti-tumor | [31] |
| Aspergillus fumigatus & Streptomyces peucetius | N, N′-((1Z, 3Z)-1, 4-bis(4-methoxyphenyl) buta-1, 3-diene-2, 3-diyl) diformamide | Anti-tumor | [32] |
Rateb等[29]通过烟曲霉菌(Aspergillus fumigatus)和链霉菌(Streptomyces bullii)共培养,发现产生了10个这两株菌单独无法产生的化合物,化合物分别为1:Ergosterol;2:Brevianamide F;3:Spirotryprostatin A;4:6-methoxy spirotryprostatin B;5和6:Fumitremorgin C及其衍生物;7:Fumitremorgin B;8:Verruculogen;9和10:11-O-methylpseurotin A及其异构体,其中化合物6、7、8具有显著的抗寄生虫活性及细胞毒作用;Ola等[30]通过三线镰孢(Fusarium tricinctum)和枯草芽孢杆菌(Bacillus subtilis)共培养,发现3个新结构化合物Macrocarpon C、N-(carboxymethyl)-anthranilic acid和Citreoisocoumarinol (图 3),而且具有抗菌活性的恩镰孢菌素A1 (Enniatin A1)产量提高78倍;Zuck等[32]通过烟曲霉菌(Aspergillus fumigatus)和链霉菌(Streptomyces peucetius)共培养,发现两个全新结构的化合物Fumiformamide和N, N'-((1Z, 3Z)-1, 4-bis(4-methoxyphenyl) buta-1, 3-diene-2, 3-diyl) diformamide,后一个化合物对多株肿瘤细胞的IC50都在nmol/L水平,显示出良好的应用前景。

|
| 图 3 三线镰孢和枯草芽孢杆菌共培养产生的新化合物 Figure 3 Structure of new compounds founded in Fusarium tricinctum vs Bacillus subtilis co-culture products Note: 1: Macrocarpon C; 2: N-(carboxymethyl)-anthranilic acid; 3: Citreoisocoumarinol. |
|
|
相较于纯培养微生物的基因突变或表观遗传修饰等手段,不同微生物共培养模拟自然生态驱动新天然产物生成,在新型天然产物挖掘方面发挥越来越重要的作用,但大部分通过共培养产生的新型天然产物生成机制并不明确。Nützmann等[33]研究发现,模式真菌构巢曲霉(Aspergillus nidulans)的组蛋白乙酰化可被Streptomyces rapamycinicus乙酰基转移酶调节,乙酰化水平增高,激活某些沉默基因簇,导致新型次级代谢产物的生成。但是在真菌与真菌之间是否也有类似机制存在未见报道,其他表观遗传修饰如甲基化修饰等是否在不同菌株共培养中发挥作用也不明确;此外,在菌株共培养中还存在一种交互饲喂现象,即两株菌之间利用各自代谢产物形成新化合物[34];这些不同菌株之间复杂的作用机制给共培养过程中新型天然产物生物合成机制的研究带来了新的挑战,而这些难题的攻克,对于研究者们利用已有的微生物基因组信息进行有目的的共培养来挖掘新型天然产物,将会起到极大的推动作用。
| [1] |
Moloney MG. Natural products as a source for novel antibiotics[J]. Trends in Pharmacological Sciences, 2016, 37(8): 689-701. DOI:10.1016/j.tips.2016.05.001 |
| [2] |
Whicher JR, Dutta S, Hansen DA, et al. Structural rearrangements of a polyketide synthase module during its catalytic cycle[J]. Nature, 2014, 510(7506): 560-564. DOI:10.1038/nature13409 |
| [3] |
Li JWH, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier?[J]. Science, 2009, 325(5937): 161-165. DOI:10.1126/science.1168243 |
| [4] |
Jin LM, Quan CS, Hou XY, et al. Potential pharmacological resources: natural bioactive compounds from marine-derived fungi[J]. Marine Drugs, 2016, 14(4): 76. DOI:10.3390/md14040076 |
| [5] |
Trindade M, van Zyl LJ, Navarro-Fernández J, et al. Targeted metagenomics as a tool to tap into marine natural product diversity for the discovery and production of drug candidates[J]. Frontiers in Microbiology, 2015, 6: 890. |
| [6] |
Sun H, Liu Z, Zhao H, et al. Recent advances in combinatorial biosynthesis for drug discovery[J]. Drug Design, Development and Therapy, 2015, 9: 823-833. |
| [7] |
Ro DK, Paradise EM, Ouellet M, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast[J]. Nature, 2006, 440(7086): 940-943. DOI:10.1038/nature04640 |
| [8] |
Ajikumar PK, Xiao WH, Tyo KEJ, et al. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli[J]. Science, 2010, 330(6000): 70-74. DOI:10.1126/science.1191652 |
| [9] |
Bader J, Mast-Gerlach E, Popović MK, et al. Relevance of microbial coculture fermentations in biotechnology[J]. Journal of Applied Microbiology, 2010, 109(2): 371-387. DOI:10.1111/jam.2010.109.issue-2 |
| [10] |
Bertrand S, Bohni N, Schnee S, et al. Metabolite induction via microorganism co-culture: a potential way to enhance chemical diversity for drug discovery[J]. Biotechnology Advances, 2014, 32(6): 1180-1204. DOI:10.1016/j.biotechadv.2014.03.001 |
| [11] |
Taniguchi M, Tanaka T. Clarification of interactions among microorganisms and development of co-culture system for production of useful substances[J]. Advances in Biochemical Engineering/Biotechnology, 2004, 90: 35-62. DOI:10.1007/b14089 |
| [12] |
Marmann A, Aly AH, Lin WH, et al. Co-cultivation — a powerful emerging tool for enhancing the chemical diversity of microorganisms[J]. Marine Drugs, 2014, 12(2): 1043-1065. DOI:10.3390/md12021043 |
| [13] |
Li W, Wu GW, Yang YP, et al. Discovery of fungal natural product in the post-genomic era[J]. Mycosystema, 2015, 34(5): 914-926. 李伟, 吴广畏, 杨玉萍, 等. 后基因组时代的真菌天然产物发现[J]. 菌物学报, 2015, 34(5): 914-926. |
| [14] |
Nonaka K, Iwatsuki M, Horiuchi S, et al. Induced production of BE-31405 by co-culturing of Talaromyces siamensis FKA-61 with a variety of fungal strains[J]. The Journal of Antibiotics (Tokyo), 2015, 68(9): 573-578. DOI:10.1038/ja.2015.28 |
| [15] |
Meng LH, Liu Y, Li XM, et al. Citrifelins A and B, citrinin adducts with a tetracyclic framework from cocultures of marine-derived isolates of Penicillium citrinum and Beauveria felina[J]. Journal of Natural Products, 2015, 78(9): 2301-2305. DOI:10.1021/acs.jnatprod.5b00450 |
| [16] |
Nonaka K, Chiba T, Suga T, et al. Coculnol, a new penicillic acid produced by a coculture of Fusarium solani FKI-6853 and Talaromyces sp. FKA-65[J]. The Journal of Antibiotics (Tokyo), 2015, 68(8): 530-532. DOI:10.1038/ja.2015.15 |
| [17] |
Li CY, Wang JH, Luo CP, et al. A new cyclopeptide with antifungal activity from the co-culture broth of two marine mangrove fungi[J]. Natural Product Research, 2014, 28(9): 616-621. DOI:10.1080/14786419.2014.887074 |
| [18] |
Chagas FO, Dias LG, Pupo MT. A mixed culture of endophytic fungi increases production of antifungal polyketides[J]. Journal of Chemical Ecology, 2013, 39(10): 1335-1342. DOI:10.1007/s10886-013-0351-7 |
| [19] |
Zhu F, Chen GY, Wu JS, et al. Structure revision and cytotoxic activity of marinamide and its methyl ester, novel alkaloids produced by co-cultures of two marine-derived mangrove endophytic fungi[J]. Natural Product Research, 2013, 27(21): 1960-1964. DOI:10.1080/14786419.2013.800980 |
| [20] |
Müller ME, Steier I, K ppen R, et al. Cocultivation of phytopathogenic Fusarium and Alternaria strains affects fungal growth and mycotoxin production[J]. Journal of Applied Microbiology, 2012, 113(4): 874-887. DOI:10.1111/jam.2012.113.issue-4 |
| [21] |
Zhu F, Chen GY, Chen X, et al. Aspergicin, a new antibacterial alkaloid produced by mixed fermentation of two marine-derived mangrove epiphytic fungi[J]. Chemistry of Natural Compounds, 2011, 47(5): 767-769. DOI:10.1007/s10600-011-0053-8 |
| [22] |
Li CY, Zhang J, Shao CL, et al. A new xanthone derivative from the co-culture broth of two marine fungi (strain No. E33 and K38)[J]. Chemistry of Natural Compound, 2011, 47: 382. DOI:10.1007/s10600-011-9939-8 |
| [23] |
Qi XQ, Zhu FC, Zhang Y, et al. Study of a novel compound 2460A with activities produced by fungus[J]. Acta Pharmaceutica Sinica, 2011, 46(2): 165-169. 齐小强, 朱凤昌, 张洋, 等. 真菌来源新活性化合物2460A的研究[J]. 药学学报, 2011, 46(2): 165-169. |
| [24] |
Ma M. Part Ⅰ: study on the mechanism of TNF-α production inhibitied by compound 1487B; Part Ⅱ: separation and activity study of the fungal co-culture metabolites[D]. Beijing: Master's Thesis of Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences & Peking Union Medical College, 2016(in Chinese). 马明. 第一部分: 化合物1487B抑制TNFα生成机制研究; 第二部分: 基于真菌共培养的代谢产物的分离及活性研究[D]. 北京: 中国医学科学院医药生物技术研究所硕士学位论文, 2016 |
| [25] |
Schroeckh V, Scherlach K, Nützmann HW, et al. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(34): 14558-14563. DOI:10.1073/pnas.0901870106 |
| [26] |
Martin G, Guggiari M, Bravo D, et al. Fungi, bacteria and soil pH: the oxalate-carbonate pathway as a model for metabolic interaction[J]. Environmental Microbiology, 2012, 14(11): 2960-2970. DOI:10.1111/j.1462-2920.2012.02862.x |
| [27] |
Whitt J, Shipley SM, Newman DJ, et al. Tetramic acid analogues produced by coculture of Saccharopolyspora erythraea with Fusarium pallidoroseum[J]. Journal of Natural Products, 2014, 77(1): 173-177. DOI:10.1021/np400761g |
| [28] |
Ross C, Opel V, Scherlach K, et al. Biosynthesis of antifungal and antibacterial polyketides by Burkholderia gladioli in coculture with Rhizopus microsporus[J]. Mycoses, 2014, 57(S3): 48-55. |
| [29] |
Rateb ME, Hallyburton I, Houssen WE, et al. Induction of diverse secondary metabolites in Aspergillus fumigatus by microbial co-culture[J]. RSC Advances, 2013, 3(34): 14444-14450. DOI:10.1039/c3ra42378f |
| [30] |
Ola ARB, Thomy D, Lai DW, et al. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through coculture with Bacillus subtilis[J]. Journal of Natural Products, 2013, 76(11): 2094-2099. DOI:10.1021/np400589h |
| [31] |
Park HB, Kim YJ, Park JS, et al. Glionitrin B, a cancer invasion inhibitory diketopiperazine produced by microbial coculture[J]. Journal of Natural Products, 2011, 74(10): 2309-2312. DOI:10.1021/np200563x |
| [32] |
Zuck KM, Shipley S, Newman DJ. Induced production of N-formyl alkaloids from Aspergillus fumigatus by co-culture with Streptomyces peucetius[J]. Journal of Natural Products, 2011, 74(7): 1653-1657. DOI:10.1021/np200255f |
| [33] |
Nützmann HW, Reyes-Dominguez Y, Scherlach K, et al. Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires saga/Ada-mediated histone acetylation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(34): 14282-14287. DOI:10.1073/pnas.1103523108 |
| [34] |
Seth EC, Taga ME. Nutrient cross-feeding in the microbial world[J]. Frontiers in Microbiology, 2014, 5: 350. |
 2017, Vol. 44
2017, Vol. 44