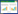扩展功能
文章信息
- 赵爱飞, 黄旭镇, 叶晓锋, 朱军莉, 励建荣
- ZHAO Ai-Fei, HUANG Xu-Zhen, YE Xiao-Feng, ZHU Jun-Li, LI Jian-Rong
- 气相色谱-质谱定量检测水产品腐败菌群体感应 DKPs信号分子
- Quantitative analysis of diketopiperazines of quorum sensing in spoilage bacteria of aquatic products by gas chromatography/mass spectrometry
- 微生物学通报, 2016, 43(2): 343-350
- Microbiology China, 2016, 43(2): 343-350
- 10.13344/j.microbiol.china.150385
-
文章历史
- 收稿日期: 2015-05-13
- 接受日期: 2015-07-16
- 优先数字出版日期(www.cnki.net): 2015-11-18
2. 辽宁省食品安全重点实验室 渤海大学 辽宁 锦州 121013
2. Food safety key laboratory of Liaoning Province,Bohai University,Jinzhou,Liaoning 121013,China
群体感应(Quorum sensing,QS)是细菌通过分泌可溶性自诱导物(Autoinducer,AI),作为信号分子来监测群体密度并协调细菌生物功能的信息交流机制,如生物发光、生物被膜形成、毒素产生、腐败调控、抗生素形成和群游现象等[1]。细菌QS首次发现于Vibrio fischeri的发光现象研究[2]。目前QS研究主要以细菌产生的自诱导物为着手点,主要包括大多数革兰氏阴性菌中的高丝氨酸内酯类(N-acyl-L-homoserine lactones,AHLs)、革兰氏阳性细菌的寡肽(Autoinducing peptides,AIP)及用于种间交流的呋喃酰硼酸二酯类化合物(Autoinducer-2,AI-2)。近年来,二酮哌嗪类化合物(Diketopiperazines,DKPs)也被证明是一种新的细菌群体感应信号分子[3],在铜绿假单胞菌(Pseudomonas aeruginosa)、奇异变形杆菌(Proteus mirabilis)、成团肠杆菌(Enterobacter agglomerans)、弗氏枸橼酸杆菌(Citrobacter freundii)等革兰氏阴性细菌的培养上清中都能检测到[4]。细菌分泌的DKPs能与特定的LuxR型蛋白结合,调节某些细菌的QS系统[3, 4]。DKPs还可参与QS系统的信息传递,根据细胞密度调节基因的表达,部分代替AHLs作用,与LuxR蛋白结合,促进或猝灭QS[5]。Gu等[3]发现DKPs作为波罗的海希瓦氏菌(Shewanella baltica)信号分子,促进腐败作用。
至今有多种方法应用于检测信号分子,主要包括微生物传感菌检测法(报告菌法,TLC等)和仪器检测法(HPLC,LC-MS,GC,GC-MS)。报告菌检测信号分子方便,但需要特定的报告菌株,稳定性较差,仅用于定性分析[6, 7]。TLC是检测信号分子常见的手段,方法灵敏有效,但也用于定性分析[8]。GC/GC-MS和HPLC/HPLC-MS常用来检测AHL信号分子,具有较高的准确度、灵敏性、抗干扰能力,在检测未知且复杂的信号分子方面有不可替代的作用[9, 10, 11]。目前,定量检测DKPs的研究仍较少。Axel等[12]采用HRGC/MS结合同位素稀释法检测发现,乳酸菌在MRS培养基中能够分泌cyclo-(L-Leu-L-Pro)和Cyclo-(L-Pro-L-Pro)。Brack等[13]采用GC-MS和LC-MS发现Bacillus pumilus能够分泌Cyclo-(L-Ala-L-Pro)、cyclo-(L-Gly-L-Pro)和cyclo-(L-Val-L-Pro)等9种DKPs。上述方法主要侧重于DKPs的定性分析,或者需要繁琐的前处理或需结合其他技术才能实现定量分析。鉴于此,本研究以GC-MS为分析平台,建立水产品特定腐败菌P. fluorescens和S. baltica中DKPs信号分子的定性和定量分析技术,以期为研究细菌群体感应的调控机制奠定良好的基础。
1 材料与方法 1.1 菌株和试剂P. fluorescens LP1和S. baltica XH2为实验室分离株;DKPs化合物Cyclo-(L-Pro-L-Gly)、cyclo-(L-Leu-L-Leu)、cyclo-(L-Pro-L-Leu)和cyclo-(L-Pro-L-Phe)标准品,上海生工生物工程股份有限公司合成,纯度为98%以上;甲醇(分析纯和色谱纯),国药集团化学试剂有限公司;乙酸乙酯和氯仿,上海凌峰化学试剂有限公司;LB和TSB培养基,青岛海博生物技术有限公司。
1.2 主要仪器GC-MS仪7890-5975N,美国安捷伦科技公司;台式高速冷冻离心机3-18K,美国Sigma公司;真空旋转蒸发仪EYELA N-1100,东京理化有限公司;循环水式多用真空泵,长城科工贸有限公司;低温冷却循环系统,杭州惠创仪器设备有限公司;LRH系列生化培养箱,上海一恒科技有限公司。
1.3 信号分子提取将甘油中保存的细菌培养物在LB培养基[3]28 °C活化后,接种于50 mL LB培养基,于28 °C、200 r/min培养12 h后,10 000×g离心10 min,取上清液用等体积氯仿萃取3次,收集有机相,再用同等体积超纯水清洗1次,所得有机相在37 °C旋转蒸发至干,用氯仿重新溶解上述提取物,定容至1 mL,经0.22 μm滤膜过滤后进行GC-MS分析。
1.4 色谱条件HP-5ms毛细柱(30 m×0.25 mm×0.25 nm,Agilent),载气高纯氦气(>99.999%),载气恒流流速l mL/min,分流比50:1,进样口温度为260 °C,进样量为l μL。升温程序设置为:起始温度50 °C,保持2 min后,以15 °C/min速率上升至260 °C,保持8 min,传输线温度220 °C。
1.5 质谱条件EI源温度230 °C,电子能70 eV,质色谱保留时间和质谱全扫描定性分析,质量扫描范围30−500 amu,扫描速率为2 s/scans,采用离子监控(SIM)模式定量。
1.6 培养基和提取溶剂对信号分子的影响将活化的P. fluorescens和S. baltica分别接种于LB和TSB培养基[3],在28 °C、200 r/min培养9 h,用氯仿提取信号分子,GC-MS-SIM检测DKPs含量。并且取两种细菌的LB培养物分别用氯仿和乙酸乙酯提取信号分子,分析DKPs含量。
1.7 细菌分泌DKPs的规律将P. fluorescens和S. baltica接种于LB培养基,在28 °C、200 r/min培养,每隔3 h取样,测定细菌OD600,并同时取样用氯仿提取信号分子,GC-MS-SIM检测DKPs活性。
1.8 数据处理实验重复3次,结果以均值±标准偏差表达。采用Origin 8.0进行处理和作图,单因素方差分析(Analysis of variance,ANOVA)用来比较各组间的差异,P<0.05表示差异性显著。
2 结果与分析 2.1 GC色谱条件优化研究分析了Cyclo-(L-Pro-L-Gly)、cyclo-(L-Pro-L-Leu)、cyclo-(L-Leu-L-Leu)和cyclo-(L-Pro-L-Phe) 4种标准品GC-MS的色谱条件。如图1所示,采用scan全扫描模式,优化升温程序,得到完全分离的标准品色谱图。
|
| 图1 DKPs 标准品GC-MS色谱图 Figure 1 The chromatogram of DKPs standards by GC-MS |
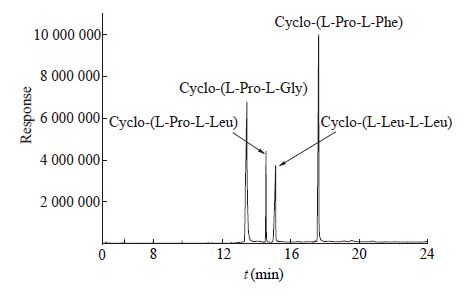
图2为4种标准品Cyclo-(L-Pro-L-Gly)、cyclo-(L-Pro-L-Leu)、cyclo-(L-Leu-L-Leu)和cyclo-(L-Pro-L-Phe)在GC-MS中所得的EI质谱图,特征离子的选择见表1,分别选择4种标准品中的基峰及其它高丰度的特征离子进行SIM扫描。高效率的SIM模式使DKPs检测和定量不需要繁琐的样品纯化,检测过程更方便快速。
|
|
图2
4种DKPs标准品EI质谱图
Figure 2
The EI mass spectrum of four kinds of DKPs standards by GC-MS
注:A:Cyclo-(L-Pro-L-Gly)质谱图;B:cyclo-(L-Pro-L-Leu)质谱图;C:cyclo-(L-Leu-L-Leu)质谱图;D:cyclo-(L-Pro-L-Phe)质谱图. Note: The EI mass spectrum of cyclo-(L-Pro-L-Gly); B: The EI mass spectrum of cyclo-(L-Pro-L-Leu); C: The EI mass spectrum of cyclo-(L-Leu-L-Leu); D: The EI mass spectrum of cyclo-(L-Pro-L-Phe). |
| DKPs | 特征离子 characteristic ion | 保留时间 retention time (min) | 扫描时间 scanning time (min) |
| cyclo-(L-Pro-L-Gly) | 83,111,154,70 | 13.443 | 12.576−13.609 |
| cyclo-(L-Pro-L-Leu) | 154,70,86,41 | 14.566 | 14.498−14.701 |
| cyclo-(L-Leu-L-Leu) | 170,86,140,113 | 15.071 | 14.968−15.141 |
| cyclo-(L-Pro-L-Phe) | 125,70,91,152 | 17.641 | 17.512−18.046 |
4种标准品利用GC-MS-SIM进行分析后得到的回归方程如表2所示。经GC-MS-SIM分析得出各回归方程的相关系数R2均大于0.998,相关性好。并且GC-MS经SIM定量后得到更低的检测限和定量限,其中检测限为0.04−0.10 mg/L,定量限为0.12−0.18 mg/L。
| DKPs | 保留时间 Retention time (min) | 线性方程 Linear range | 相关系数 Correlation coefficients | 检测限 LOD (mg/L) | 定量限 LOQ (mg/L) |
| cyclo-(L-Pro-L-Gly) | 13.443 | Y=0.000 20x+2.645 0 | 0.999 7 | 0.06 | 0.16 |
| cyclo-(L-Pro-L-Leu) | 14.566 | Y=0.000 05x+2.645 5 | 0.999 5 | 0.10 | 0.18 |
| cyclo-(L-Leu-L-Leu) | 15.071 | Y=0.000 10x−119.660 0 | 0.998 8 | 0.06 | 0.14 |
| cyclo-(L-Pro-L-Phe) | 17.641 | Y=0.000 08x−78.287 0 | 0.999 1 | 0.04 | 0.12 |
采用GC-MS-SIM分析4种DKPs信号分子2.5、50和150 mg/L混合标准溶液的精密度、准确度和回收率,如表3、4和5所示。得到日内精密度3.2%−9.1%,准确度96.4%−107.8%,日间精密度为2.2%−10.2%,准确度为97.1%−105.6%。加标量为2.5、50、150 mg/L时,平均回收率分别为51.8%−71.2%、66.2%−88.5%和64.1%−84.9%,标准偏差分别为2.7%−5.4%、1.4%−7.2%和1.7%−8.3%。该方法能满足实际检测要求。
| DKPs | 精密度 Precision (RSD%) | 准确度 Accuracy (%) | ||||
| L | M | H | L | M | H | |
| cyclo-(L-Pro-L-Gly) | 3.2 | 5.8 | 7.9 | 97.1 | 99.4 | 98.6 |
| cyclo-(L-Pro-L-Leu) | 7.3 | 5.4 | 4.8 | 106.4 | 96.4 | 102.3 |
| cyclo-(L-Leu-L-Leu) | 5.6 | 8.2 | 4.3 | 104.6 | 97.7 | 98.3 |
| cyclo-(L-Pro-L-Phe) | 8.3 | 6.5 | 9.1 | 96.2 | 102.6 | 107.8 |
| Note: L: 2.5 mg/L; M: 50 mg/L; H: 150 mg/L. | ||||||
| DKPs | 精密度 Precision (RSD%) | 准确度 Accuracy (%) | ||||
| L | M | H | L | M | H | |
| cyclo-(L-Pro-L-Gly) | 6.5 | 7.6 | 5.6 | 102.7 | 98.6 | 99.2 |
| cyclo-(L-Pro-L-Leu) | 2.2 | 3.1 | 5.5 | 104.2 | 97.9 | 103.8 |
| cyclo-(L-Leu-L-Leu) | 4.8 | 6.7 | 9.6 | 98.4 | 99.1 | 100.4 |
| cyclo-(L-Pro-L-Phe) | 4.2 | 10.2 | 3.6 | 97.1 | 101.3 | 105.6 |
| DKPs | 回收率 Recovery rate (SD%) | ||
| L | M | H | |
| cyclo-(L-Pro-L-Gly) | 65.7±5.4 | 67.4±7.2 | 77.2±8.3 |
| cyclo-(L-Pro-L-Leu) | 71.2±2.7 | 66.2±1.4 | 84.9±1.7 |
| cyclo-(L-Leu-L-Leu) | 68.3±4.2 | 88.5±1.8 | 74.3±5.2 |
| cyclo-(L-Pro-L-Phe) | 51.8±3.5 | 71.3±6.5 | 64.1±4.9 |
如表6所示,P. fluorescens在LB和TSB培养基中都形成两种DKPs信号分子Cyclo-(L-Pro-L-Leu)和Cyclo-(L-Pro-L-Phe),其中LB中含量为4.43 mg/L和3.91 mg/L,TSB中含量为3.95 mg/L和2.73 mg/L。S. baltica在两种培养基中也检测出相同的信号分子,LB中含量为5.43 mg/L和4.18 mg/L,TSB中含量为4.8 mg/L和1.12 mg/L。同时以未接种培养基为空白对照,检测结果未达到检测限,数据则未显示。结果表明,P. fluorescens和S. baltica在LB培养基中分泌的DKPs含量显著高于TSB培养基(p<0.05),其中Cyclo-(L-Pro-L-Phe)对培养基的变化更敏感。Ortori等[14]也报道铜绿假单胞菌在LB培养基中分泌的AHL信号分子浓度显著高于自制培养基(CDM)。
| DKPs | LB (mg/L) | TSB (mg/L) | ||
| P. fluorescens | S. baltica | P. fluorescens | S. baltica | |
| cyclo-(L-Pro-L-Leu) | 4.43±0.19b | 5.43±0.23a | 3.95±0.21b | 4.81±0.26b |
| cyclo-(L-Pro-L-Phe) | 3.91±0.17b | 4.18 ±0.25a | 2.73±0.15c | 1.12±0.08d |
| 注:表格中同行不同字母表示差异显著(P<0.05). Note: Different letters within the same line mean significant difference (P<0.05). | ||||
进一步比较提取溶剂(氯仿和乙酸乙酯)对DKPs活性的影响,如表7所示。氯仿和乙酸乙酯萃取溶剂显著影响细菌培养液中DKPs含量(P<0.05)。氯仿作为萃取溶剂时,P. fluorescens和S. baltica总DKPs含量分别为8.34 mg/L和9.61 mg/L,而乙酸乙酯萃取时,两种细菌中总DKPs含量分别为7.01 mg/L和9.12 mg/L,分别减少16.8%和5.8%。可见,氯仿作为细菌培养上清萃取溶剂获得的DKPs活性更高。
| DKPs | 氯仿 chloroform (mg/L) | 乙酸乙酯 ethyl acetate (mg/L) | ||
| P. fluorescens | S. baltica | P. fluorescens | S. baltica | |
| cyclo-(L-Pro-L-Leu) | 4.43±0.19b | 5.43±0.23a | 3.37±0.23c | 5.45±0.16a |
| cyclo-(L-Pro-L-Phe) | 3.91±0.17b | 4.18 ±0.25a | 3.64±0.15b | 3.67±0.21b |
| 注:表格中同行不同字母表示差异显著(P<0.05). Note: Different letters within the same line mean significant difference (P<0.05). | ||||
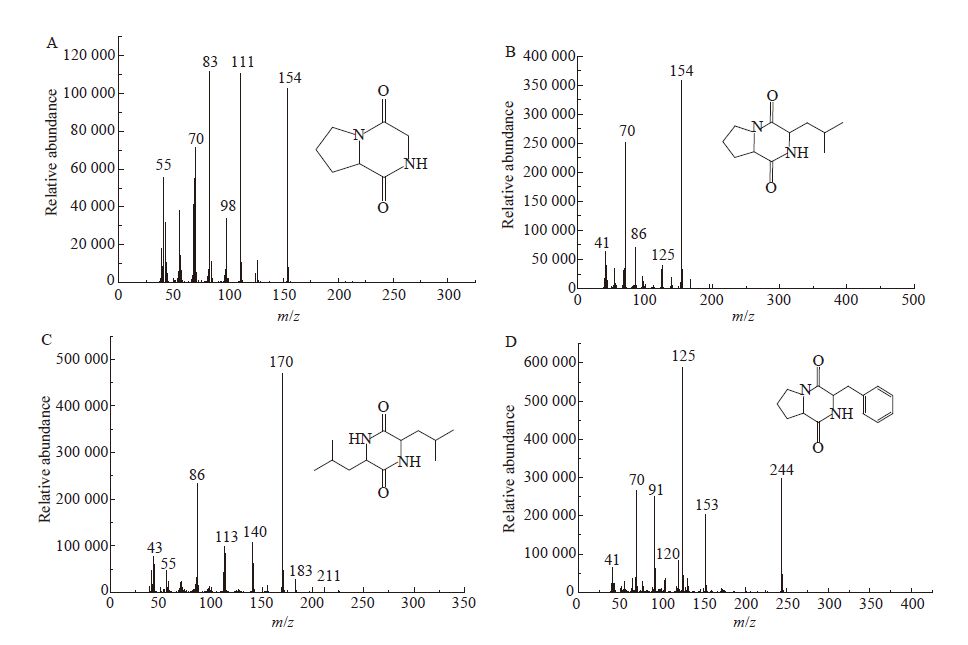
使用的P. fluorescens LP1和S. baltica XH2分别从冷藏大菱鲆和大黄鱼中分离鉴定的特定腐败菌,具有生长速度快,腐败活性高的特点[15]。如图3所示,利用上述优化的条件进行定量分析,两种细菌均产生两种相同的DKPs信号分子,出峰时间Cyclo-(L-Pro-L-Leu)为14.574 min,Cyclo-(L-Pro-L-Phe)为17.663 min。P. fluorescens形成的两种DKPs含量分别是4.43 mg/L和3.91 mg/L,S. baltica产生的两种DKPs含量分别是5.43 mg/L和4.18 mg/L,其中CycIo-(L-Pro-L-Leu)含量更高。进一步分析了培养时间对两种DKPs形成的影响,P. fluorescens和S. baltica在LB培养基中生长迅速,15 h达到稳定期。随着细菌的生长,培养基上清液中的DKPs逐步增加,在细菌对数后期12 h时DKPs的含量最高,分别达到8.34 mg/L和9.61 mg/L,之后逐步下降,其中18 h下降约20%,18 h后下降量较少。Gu等[3]发现S. baltica能够产生4种DKPs [Cyclo-(L-Pro-L-Gly)、Cyclo-(L-Pro-L-Leu)、Cyclo-(L-Leu-L-Leu)和Cyclo-(L-Pro-L-Phe)]参与腐败作用。綦国红等[16]发现鱼源P. fluorescens群体感应信号分子(N-3-oxo-C8-HSL)与腐败存在内在关联。然而,DKPs在腐败菌群体感应系统中的调控机制及在菌体内的代谢途径仍未明晰,有待于进一步的探究。
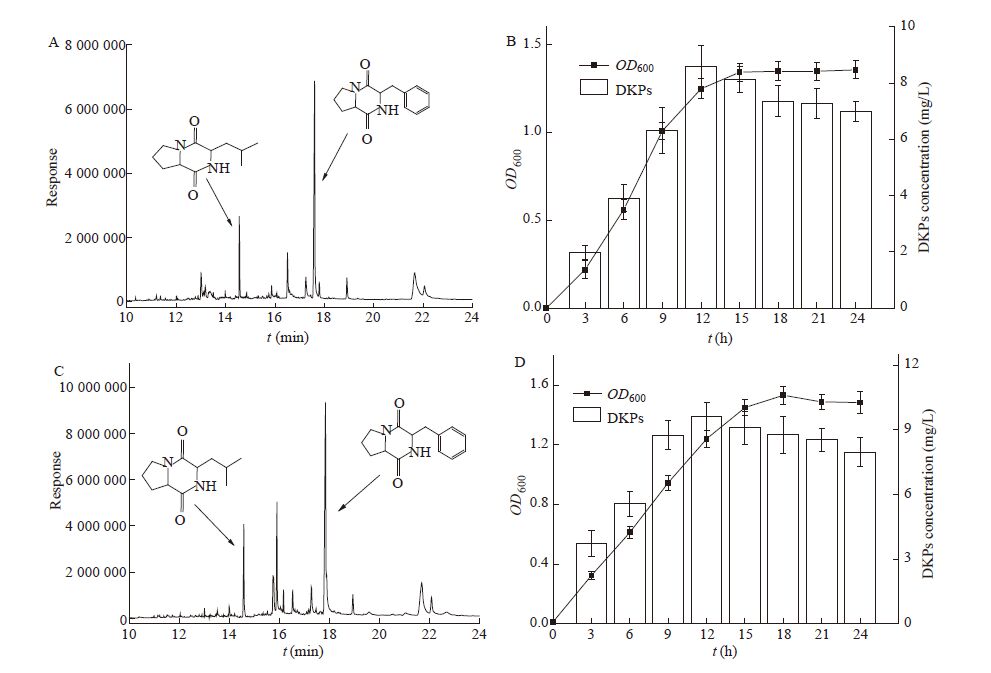
|
|
图3
P. fluorescens和S. baltica生长曲线及DKPs含量的变化
Figure 3
Changs in growth curve and DKPs content of P. fluorescens and S. baltica
注:A:P. fluorescens产生的DKPs GC-MS色谱图;B:P. fluorescens生长曲线及DKPs含量;C:S. baltica产生的DKPs GC-MS 色谱图;D:S. baltica生长曲线及DKPs含量. Note: A: Chromatogram of DKPs produced from P. fluorescens; B: Growth curve and DKPs content of P. fluorescens; C: Chromatogram of DKPs produced from S. baltica; D: Growth curve and DKPs content of S. baltica. |
本研究建立的GC-MS-SIM定量检测DKPs方法,具有精密度、灵敏度、准确度高的优点。同时发现采用LB培养基及提取溶剂氯仿检测细菌中的DKPs灵敏度更高。该方法应用于P. fluorescens和S. baltica两种腐败菌信号分子分析,发现都产生Cyclo-(L-Pro-L-Leu)和Cyclo-(L-Pro-L-Phe)两种DKPs,含量在细菌对数生长末期最高。因此,本方法能够适用于定量检测细菌的DKPs信号分子,为其功能研究奠定了技术支持。
| [1] | Waters CM,Bassler BL. Quorum sensing: cell-to-cell communication in bacteria[J]. Annual Review of Cell and Developmental Biology,2005,21(1): 319-346 |
| [2] | Nealson KH,Hastings JW. Bacterial bioluminescence: its control and ecological significance[J]. Microbiological Reviews,1979,43(4): 496-518 |
| [3] | Gu QQ,Fu LL,Wang YB,et al. Identification and characterization of extracellular cyclic dipeptides as quorum-sensing signal molecules from Shewanella baltica,the specific spoilage organism of Pseudosciaena crocea during 4 °C storage[J]. Journal of Agricultural and Food Chemistry,2013,61(47): 11645-11652 |
| [4] | Degrassi G,Aguilar C,Bosco M,et al. Plant growth-promoting Pseudomonas putida WCS358 produces and secretes four cyclic dipeptides: cross-talk with quorum sensing bacterial sensors[J]. Current Microbiology,2002,45(4): 250-254 |
| [5] | Holden MTG,Ram CS,de Nys R,et al. Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria[J]. Molecular Microbiology,1999,33(6): 1254-1266 |
| [6] | Steindler L,Venturi V. Detection of quorum-sensing N-acyl homoserine lactone signal molecules by bacterial biosensors[J]. FEMS Microbiology Letters,2007,266(1): 1-9 |
| [7] | Yang YH,Lee TH,Kim JH,et al. High-throughput detection method of quorum-sensing molecules by colorimetry and its applications[J]. Analytical Biochemistry,2006,356(2): 297-299 |
| [8] | Shaw PD,Ping G,Daly SL,et al. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography[J]. Proceedings of the National Academy of Sciences of the United States of America,1997,94(12): 6036-6041 |
| [9] | Fekete A,Frommberger M,Rothballer M,et al. Identification of bacterial N-acylhomoserine lactones (AHLs) with a combination of ultra-performance liquid chromatography (UPLC),ultra-high-resolution mass spectrometry,and in-situ biosensors[J]. Analytical and Bioanalytical Chemistry,2007,387(2): 455-467 |
| [10] | Cataldi TRI,Bianco G,Palazzo L,et al. Occurrence of N-acyl-L-homoserine lactones in extracts of some Gram-negative bacteria evaluated by gas chromatography-mass spectrometry[J]. Analytical Biochemistry,2007,361(2): 226-235 |
| [11] | LC-MS/MS检测水产品源致病菌和腐败菌群体感应AHLs信号分子[J]. 水产学报,2014,38(7): 1040-1046 Huang XZ,Zhu ZL,Zhao EK,et al. Detection of N-acylated-L-homoserine lactones of quorum sensing secreted by pathogen and spoilage bacteria from aquatic product using liquid chromatography-mass spectrometry/mass spectrometry[J]. Journal of Fisheries of China,2014,38(7): 1040-1046 (in Chinese) 黄旭镇,朱军莉,赵二科,等. |
| [12] | Axel C,Zannini E,Arendt EK,et al. Quantification of cyclic dipeptides from cultures of Lactobacillus brevis R2Δ by HRGC/MS using stable isotope dilution assay[J]. Analytical and Bioanalytical Chemistry,2014,406(9/10): 2433-2444 |
| [13] | Brack C,Mikolasch A,Schauer F. 2,5-Diketopiperazines produced by Bacillus pumilus during bacteriolysis of Arthrobacter citreus[J]. Marine Biotechnology,2014,16(4): 385-395 |
| [14] | Ortori CA,Dubern JF,Chhabra SR,et al. Simultaneous quantitative profiling of N-acyl-L-homoserine lactone and 2-alkyl-4(1H)-quinolone families of quorum-sensing signaling molecules using LC-MS/MS[J]. Analytical and Bioanalytical Chemistry,2011,399(2): 839-850 |
| [15] | Zhao EK,Zhu JL,Feng LF,et al. Preliminary mechanism of different spoilage potential of specific spoilage organism,Shewanella,in refrigerated Larimichthys crocea[J]. Journal of Fisheries of China,2015,39(2): 256-264 (in Chinese) 赵二科,朱军莉,冯立芳,等. 冷藏大黄鱼SSO希瓦氏菌致腐能力差异机制初探[J]. 水产学报,2015,39(2): 256-264 |
| [16] | GH,Dong MS,Chen XH,et al. Research on relationship between quorum sensing signal molecules and characteristics of spoilage in Pseudomonas spp. isolated from fish[J]. Scientia Agricultura Sinica,2007,40(7): 1486-1491 (in Chinese) Qi綦国红,董明盛,陈晓红,等. 鱼源假单胞菌群体感应信号分子与腐败特性相关关系的研究[J]. 中国农业科学,2007,40(7): 1486-1491 |
 2016, Vol. 43
2016, Vol. 43