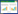扩展功能
文章信息
- 白滨, 何苏琴, 荆卓琼, 申培增, 张广荣, 文朝慧
- BAI Bin, HE Su-Qin, JING Zhuo-Qiong, SHEN Pei-Zeng, ZHANG Guang-Rong, WEN Zhao-Hui
- 分离自黄瓜的多主棒孢霉不同表型菌株对杀菌剂的敏感性
- Fungicide sensitivity of Corynespora cassiicola strains with different phenotypes isolated from cucumber in Baiyin of Gansu
- 微生物学通报, 2016, 43(2): 322-329
- Microbiology China, 2016, 43(2): 322-329
- 10.13344/j.microbiol.china.150316
-
文章历史
- 收稿日期: 2015-04-14
- 接受日期: 2015-07-14
- 优先数字出版日期(www.cnki.net): 2015-09-09
2. 甘肃省农业科学院 植物保护研究所 甘肃 兰州 730070;
3. 甘肃省白银市农业技术服务中心 甘肃 白银 730900;
4. 甘肃省白银市植保植检站 甘肃 白银 730900;
5. 甘肃出入境检验检疫局 甘肃 兰州 730020
2. Institute of Plant Protection, Gansu Academy of Agricultural Sciences, Lanzhou, Gansu 730070, China;
3. Baiyin Agricultural Technology Service Center, Baiyin, Gansu 730900, China;
4. Baiyin Station of Plant Protection and Quarantine, Baiyin, Gansu 730900, China;
5. Gansu Entry-Exit Inspection and Quarantine Bureau, Lanzhou, Gansu 730020, China
多主棒孢霉[Corynespora cassiicola (Berk. & Curt.) Wei]是一种寄主范围广泛、世界性分布的重要植物病原菌,不同寄主和不同地域来源的菌株呈现出丰富的表型特征和遗传多样性[1, 2, 3]。由于多主棒孢霉菌株间对杀菌剂敏感性的差异及抗性菌株的出现,给病害的防治带来了更多的困难[4, 5, 6, 7, 8, 9, 10, 11, 12]。
黄瓜褐斑病是近年来发生在甘肃省白银地区日光温室的新病害,除造成黄瓜叶片产生褐斑和叶枯外,还侵染嫁接端口处的黄瓜接穗茎,造成植株枯萎死亡[13]。从该地区日光温室黄瓜病叶和病茎上分离到的多主棒孢霉菌株呈现出多种表型特征,本研究旨在通过对分离自黄瓜的多主棒孢霉不同表型菌株的适宜生长温度、产孢量等生物学特性和对杀菌剂的敏感性研究,为该地区多主棒孢霉侵染引起的黄瓜叶斑病和茎疫病的化学防治提供技术支持。
1 材料与方法 1.1 试验材料 1.1.1 试验用菌株:试验菌株于2014年6−11月采用孢子稀释法从甘肃省白银地区日光温室黄瓜褐斑病病叶和病茎上分离得到,经科赫氏法则验证,确认为引起该地区黄瓜叶片褐斑病和茎疫病的病原菌;经形态学和分子生物学鉴定,确定试验菌株为多主棒孢霉Corynespora cassiicola。菌株来源及菌落形态见表1、图1。| 菌株编号 Strain No. | GenBank accession No. | 获得时间 Obtained time | 分离部位 Host tissue | 菌落形态(在PDA平板上25 °C培养7 d) Colony morphology (7 d on PDA at 25 °C) |
| cu-1 | KT002182 | 2014.06 | 茎 | 气生菌丝灰白色至灰褐色,丰茂;菌落圆形,边缘整齐;无色素泌出 |
| cu-2 | - | 2014.06 | 茎 | 气生菌丝灰白色至灰褐色,丰茂;菌落圆形,边缘整齐;无色素泌出 |
| cu-3 | KT002180 | 2014.06 | 茎 | 气生菌丝白色至灰白色,丰茂;菌落圆形,边缘整齐;有酒红色素泌出 |
| cu-4 | KT002181 | 2014.11 | 叶 | 几无气生菌丝;菌落近黑色,表面粉状;菌落边缘不整齐;无色素泌出 |
| cu-5 | - | 2014.11 | 叶 | 几无气生菌丝;菌落近黑色,表面粉状;菌落边缘不整齐;无色素泌出 |

|
| 图1 分离自黄瓜的多主棒孢霉不同表型菌株菌落形态 Figure 1 The colony morphology of Corynespora cassiicola strains with different phenotypes isolated from cucumber 注:A−C:菌株cu-1 (A:7 d,正面;B:7 d,背面;C:30 d,正面);D−F:菌株cu-2 (D:7 d,正面;E:7 d,背面;F:30 d,正面);G−I:菌株cu-3 (G:7 d,正面;H:7 d,背面;I:30 d,正面);J−L:菌株cu-4 (J:7 d,正面;K:7 d,背面;L:30 d,正面);M−O:菌株cu-5 (M:7 d,正面;N:7 d,背面;O:30 d,正面). 培养条件:PDA平板25 °C培养,培养皿内径72 mm. Note: A−C: Strain cu-1 (A: 7 d,front; B: 7 d,back; C: 30 d,front); D−F: Strain cu-2 (D: 7 d,front; E: 7 d,back; F: 30 d,front); G−I: Strain cu-3 (G: 7 d,front; H: 7 d,back; I: 30 d,front); J−L: Strain cu-4 (J: 7 d,front; K: 7 d,back; L: 30 d,front); M−O: Strain cu-5 (M: 7 d,front; N: 7 d,back; O: 30 d,front). Culture conditions: Cultured on PDA at 25 °C,Petri dish inner diameter 72 mm. |
| 杀菌剂种类 Variety of fungicide | 生产厂商 Manufacturer | 推荐使用浓度(稀释倍数) Recommended dose (dilution ratios) |
100 mL培养基加药量(商品量)
fungicide added/100 mL medium (commodity dose) |
| 75%百菌清可湿性粉剂 Chlorothalonil 75% WP | 江阴苏利化学股份有限公司 | 500× | 200.0 mg |
| 50%多菌灵可湿性粉剂 Carbendazim 50% WP | 河北冠龙农化有限公司 | 500× | 200.0 mg |
| 70%代森锰锌可湿性粉剂 Mancozeb 70% WP | 天津市东方农药有限公司 | 500× | 200.0 mg |
| 50%速克灵可湿性粉剂 Procymidone 50% WP | 宜宾川安高科农药有限责任公司 | 1 000× | 100.0 mg |
| 10%苯醚甲环唑水分散粒剂 Difenoconazole 10% WDG | 永农生物科学有限公司 | 1 000× | 100.0 mg |
| 430 g/L戊唑醇悬浮剂 Tebuconazole 430 g/L SC | 上海禾本药业有限公司 | 5 000× | 20.0 mg |
| 400 g/L氟硅唑乳油 Flusilazole 400 g/L EC | 兴农药业(中国)公司 | 5 000× | 20.0 µL |
| 250 g/L嘧菌酯悬浮剂 Azoxystrobin 250 g/L SC | 先正达(苏州)作物保护有限公司 | 1 000× | 100.0 µL |
菌落生长抑制率(%)=[(对照菌落直径-处理菌落直径)/对照菌落直径]×100。
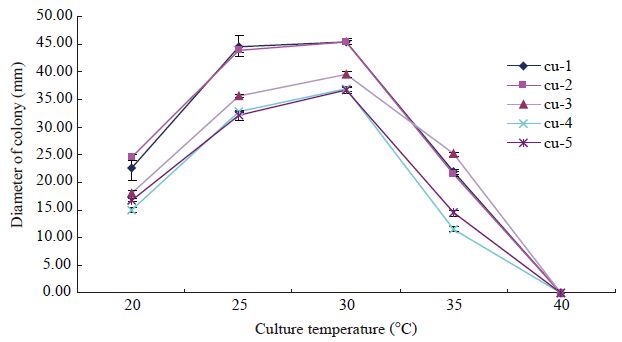
2 结果与分析 2.1 试验菌株适宜生长温度菌株cu-1和cu-2的适宜生长温度为25−30 °C,菌株cu-3、cu-4和cu-5的适宜生长温度为30 °C (图2)。40 °C培养4 d未生长的菌株转放至25 °C 7 d后,菌株cu-1有1/3的菌丝块恢复生长,菌株cu-2有2/3的菌丝块恢复生长,菌株cu-3所有菌丝块都未能恢复生长,菌株cu-4和cu-5的菌丝块全部恢复生长(图3A)。说明菌株cu-4和cu-5对40 °C亚高温的耐受性最强,其次为菌株cu-2和cu-1,菌株cu-3的耐受性最弱。
|
| 图2 培养温度对5个菌株菌落生长的影响 Figure 2 Effects of culture temperature on colonial growth of five strains of Corynespora cassiicola Note: Cultured 4 d on PDA. |
|
| 图3 亚高温对分离自黄瓜的多主棒孢霉的影响及杀菌剂对气生菌丝稀少型菌株的诱变作用 Figure 3 The effect of sub-high temperature on Corynespora cassiicola isolates collected from cucumber and fungicides mutagenesis to the strain with scanty aerial hyphae 注:A:供试菌株在40 °C培养4 d未生长,转放至25 °C培养7 d,菌株cu-1有1/3的菌丝块恢复生长,菌株cu-2有2/3的菌丝块恢复生长,菌株cu-3所有菌丝块都未能恢复生长,菌株cu-4和cu-5的菌丝块全部恢复生长;B−C:菌株cu-4的氟硅唑诱变菌株,在PDA平板上25 °C培养7 d和30 d的菌落形态,示气生菌丝明显增多(B:7 d;C:30 d);D:菌株cu-4及其氟硅唑诱变菌株,在氟硅唑含药平板上25 °C培养7 d,示诱变菌株对诱变源杀菌剂的敏感性低于野生菌株;E−I:菌株cu-4在添加不同杀菌剂的含药平板上25 °C培养30 d的菌落形态,示速克灵、戊唑醇、苯醚甲环唑和氟硅唑对菌株cu-4的诱变作用,菌落形态与原始菌株明显不同. 培养皿内径72 mm. Note: A: Tested strains cultured 4 d at 40 °C,did not grow,then conversion to 25 °C and cultured 7 d,one-third of hypha block to restore growth in strain cu-1,two-thirds of hypha block to restore growth in strain cu-2,all hypha blocks failed to restore growth in strain cu-3,all hypha blocks restore growth in strain cu-4 and cu-5; B,C: Colony morphology of mutant strain induced by flusilazole,cultured on PDA at 25 °C,show that aerial hyphae obviously increased (B: 7 d; C: 30 d); D: Strain cu-4 and its flusilazole induced strain,cultured 7 d at 25 °C on flusilazole amendment medium,show that the sensitivity of mutant strain is lower than wild strain to mutagenic source fungicide; E−I: Colony morphology of cu-4 cultured 30 d at 25 °C on media medicated by different fungicides,show that fungicides mutagenesis of procymidone,tebuconazole,difenoconazole and flusilazole,colonial morphotypes were differ with original strain.Petri dish inner diameter 72 mm. |
供试菌株在PDA平板上25 °C黑暗培养5 d和21 d,不同表型菌株分生孢子产生数量差异显著,无气生菌丝的菌株(cu-4和cu-5)培养5 d的产孢量即可达1.08×105−1.49×105个孢子/cm2,显著多于气生菌丝丰茂的菌株(表3)。
| 菌株编号 Strain No. | 5 d Conidia amount (number/cm2) | 21 d Conidia amount (number/cm2) |
| cu-1 | 1.70×102±1.47×102 C | 9.68×103±1.78×103 B |
| cu-2 | 5.94×102±1.47×102 C | 9.17×103±6.74×102 B |
| cu-3 | 8.49×101±1.47×102 C | 5.10×103±6.74×102 B |
| cu-4 | 1.49×105±1.20×103 A | 1.28×106±1.84×105 A |
| cu-5 | 1.08×105±7.10×103 B | 1.46×106±2.75×105 A |
| 极值相差倍数 Times between the maximum and the minimum | 1.75×103 | 2.87×102 |
|
注:同列数据后的不同字母表示差异极显著,下同.
Note: Different letters in the same column indicate highly significantly different according to the LSD test (P≤0.01). The same below. |
||
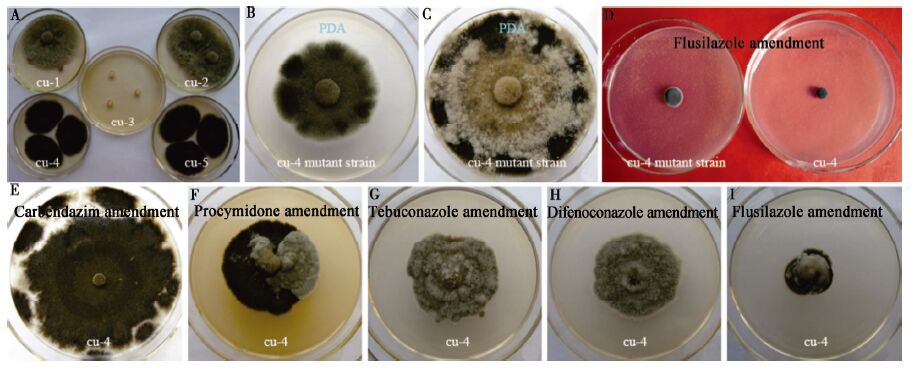
分离自黄瓜的多主棒孢霉不同表型菌株对杀菌剂的敏感性存在差异,菌株cu-3对8种杀菌剂的平均敏感性最低。在试验浓度下,5个试验菌株对8种杀菌剂的敏感性(以5个菌株的平均值计)依次为:代森锰锌>氟硅唑>戊唑醇>苯醚甲环唑>速克灵>百菌清>嘧菌酯>多菌灵(表4)。在试验中还观察到,速克灵、苯醚甲环唑、戊唑醇和氟硅唑对气生菌丝稀少型菌株cu-4和cu-5具有诱变作用,菌落的气生菌丝明显增多(图3E−I)。
| 处理 Treatment | 抑制率 Inhibition rate (%) | |||||
| cu-1 | cu-2 | cu-3 | cu-4 | cu-5 | Average | |
| 75%百菌清可湿性粉剂(500×) Chlorothalonil 75% WP (500×) | 49.68 E | 51.06 E | 15.41 E | 65.53 D | 64.18 C | 49.17 |
| 50%多菌灵可湿性粉剂(500×) Carbendazim 50% WP (500×) | 2.72 G | 4.43 G | 3.84 E | 19.90 E | 24.30 D | 11.04 |
| 70%代森锰锌可湿性粉剂(500×) Mancozeb 70% WP (500×) | 100.00 A | 100.00 A | 80.60 AB | 93.73 A | 87.26 A | 92.32 |
| 50%速克灵可湿性粉剂(1 000×) Procymidone 50% WP (1 000×) | 79.04 D | 77.38 D | 67.10 C | 74.10 C | 75.52 B | 74.63 |
| 10%苯醚甲环唑水分散粒剂(1 000×) Difenoconazole 10% WDG (1 000×) | 78.20 D | 78.52 D | 74.83 BC | 84.61 B | 86.29 A | 80.49 |
| 430 g/L戊唑醇悬浮剂(5 000×) Tebuconazole 430 g/L SC (5 000×) | 84.49 C | 83.94 C | 81.64 AB | 85.85 B | 85.67 A | 84.32 |
| 400 g/L氟硅唑乳油(5 000×) Flusilazole 400 g/L EC (5 000×) | 88.68 B | 88.16 B | 87.53 A | 87.05 B | 86.92 A | 87.67 |
| 250 g/L嘧菌酯悬浮剂(1 000×) Azoxystrobin 250 g/L SC (1 000×) | 37.53 F | 37.00 F | 32.86 D | 23.31 E | 26.18 D | 31.37 |
| Average | 65.04 | 65.06 | 55.48 | 66.76 | 67.04 | 63.88 |
|
注:以PDA为基础培养基,25 °C培养7 d.
Note: PDA as basal medium, cultured 7 d at 25 °C. |
||||||
自从化学农药,特别是内吸性杀菌剂开始应用于农业生产,病原菌对杀菌剂的抗性问题就一直备受关注。化学农药对病原菌的影响是多方面的,除了病菌对化学农药敏感性的改变外,还包括菌落形态、生长速率、产孢量、致病性等特征特性的变化[14, 15, 16]。
本研究中,分离自黄瓜的多主棒孢霉不同表型菌株在适宜生长温度、菌丝生长速度、产孢量及对杀菌剂的敏感性等方面均存在差异:分泌酒红色素且气生菌丝丰茂的菌株cu-3对8种杀菌剂的平均敏感性最低,但对40 °C的亚高温敏感(40 °C培养4 d,病菌即死亡);气生菌丝灰色、丰茂,无色素分泌的cu-1和cu-2菌株菌丝生长速度最快,且对代森锰锌的敏感性最高(被完全抑制);气生菌丝稀少型菌株对40 °C亚高温的耐受性和产孢量明显高于气生菌丝丰茂的菌株,对多菌灵的敏感性稍高于气生菌丝丰茂型菌株。从试验结果来看,供试菌株对多菌灵和嘧菌酯的敏感性极低(抑制率<40%),这2种杀菌剂已失去对该地区黄瓜褐斑病的防控作用,而代森锰锌表现出较高的杀菌和抑菌活性,可在病害防治中与氟硅唑、戊唑醇、苯醚甲环唑、速克灵等内吸性杀菌剂交替使用,以延缓病菌抗药性的产生、延长农药使用寿命,提高防治效果。
在延伸试验中发现,菌株cu-4的氟硅唑诱变菌株对多菌灵、速克灵、苯醚甲环唑、戊唑醇和氟硅唑的敏感性较野生菌株降低,但对百菌清和嘧菌酯的敏感性较野生菌株增高,变异菌株在PDA平板上的菌丝生长速率与野生菌株无明显差异(图3B−D)。变异菌株在致病性等方面与野生菌株有无差异,还有待于进一步试验研究。
化学杀菌剂的诱变作用是否是造成多主棒孢霉拥有丰富的表型特征和遗传多样性的原因之一,值得进行更深入的研究。
| [1] | Dixon LJ,Schlub RL,Pernezny K,et al. Host specialization and phylogenetic diversity of Corynespora cassiicola[J]. Phytopathology,2009,99(9): 1015-1027 |
| [2] | Qi YX,Zhang X,Pu JJ,et al. Morphological and molecular analysis of genetic variability within isolates of Corynespora cassiicola from different hosts[J]. European Journal of Plant Pathology,2011,130(1): 83-95 |
| [3] | Shimomoto Y,Sato T,Hojo H,et al. Pathogenic and genetic variation among isolates of Corynespora cassiicola in Japan[J]. Plant Pathology,2011,60(2): 253-260 |
| [4] | Liu MT,Xu RF,Jiang XJ,et al. Preliminary study on leaf spot caused by Corynespora cassiicola on cucumber[J]. Journal of Henan Agricultural Sciences,2003(8): 33-35 (in Chinese) 刘鸣韬,徐瑞富,蒋学杰,等. 黄瓜靶斑病研究初报[J]. 河南农业科学,2003(8): 33-35 |
| [5] | Kan LN,Li BJ,Ji MS,et al. In vivo studies on screening effective fungicides against Corynespora cassiicola[J]. China Vegetables,2007(4): 22-24 (in Chinese) 阚琳娜,李宝聚,纪明山,等. 黄瓜褐斑病防治药剂的活体筛选[J]. 中国蔬菜,2007(4): 22-24 |
| [6] | Tang P,Li ZC,Peng DY,et al. Field efficiency of 25% azoxystrobin suspension concentrate against cucumber target leaf spot[J]. Journal of Liaoning Agricultural Sciences,2008(3): 69-70 (in Chinese) 唐鹏,李中朝,彭大勇,等. 25%嘧菌酯悬浮剂对黄瓜褐斑病的防效[J]. 辽宁农业科学,2008(3): 69-70 |
| [7] | Vawdrey LL,Grice KRE,Westerhuis D. Field and laboratory evaluations of fungicides for the control of brown spot (Corynespora cassiicola) and black spot (Asperisporium caricae) of papaya in far north Queensland,Australia[J]. Australasian Plant Pathology,2008,37(6): 552-558 |
| [8] | Qi ZQ,Ji MS,Lu T,et al. In vitro screening of effective fungicides against Corynespora cassiicola[J]. Plant Protection,2009,35(2): 140-143 (in Chinese) 祁之秋,纪明山,陆田,等. 黄瓜褐斑病防治药剂的离体活性筛选[J]. 植物保护,2009,35(2): 140-143 |
| [9] | Yang Y,Wang LY,Zhang Y,et al. Sensitivity of two strains of Corynespora cassiicola on rubber trees to twelve fungicides[J]. Plant Protection,2010,36(1): 118-121 (in Chinese) 杨叶,王兰英,张宇,等. 两株橡胶多主棒孢霉对12种杀菌剂原药的敏感性[J]. 植物保护,2010,36(1): 118-121 |
| [10] | Teramoto A,Martins MC,Ferreira LC,et al. Reaction of hybrids,inhibition in vitro and target spot control in cucumber[J]. Horticultura Brasileira,2011,29(3): 342-348 |
| [11] | Li JT,Mo SX,Xin XL,et al. Research on effective duration of fungicides against cucumber Corynespora cassiicola in vitro[J]. Journal of Changjiang Vegetables,2012(8): 67-68 (in Chinese) 李金堂,默书霞,辛相龙,等. 黄瓜靶斑病药剂防治持效期的离体研究[J]. 长江蔬菜: 学术版,2012(8): 67-68 |
| [12] | Xavier SA,Canteri MG,Barros DCM,et al. Sensitivity of Corynespora cassiicola from soybean to carbendazim and prothioconazole[J]. Tropical Plant Pathology,2013,38(5): 431-435 |
| [13] | Zhang GR,Bai B,He SQ,et al. The occurring features and control suggestions of cucumber brown spot at sunlight greenhouse in Baiyin area in Gansu province[A]//Chen WQ. Ecological civilization construction and green plant protection (Proceedings of China society of plant protection 2014 academic annual meeting)[C]. Beijing: China Agricultural Science and Technology Press,2014: 383 (in Chinese) 张广荣,白滨,何苏琴,等. 甘肃省白银地区日光温室黄瓜棒孢褐斑病发生特点及防治建议[A]//陈万权. 生态文明建设与绿色植保(2014年中国植保年会论文集)[C]. 北京: 中国农业科学技术出版社,2014: 383 |
| [14] | Slifkin MK. Apparent induction of mutants and enhancement of conidial formation by fungicides in Alternaria mali[J]. Mycopathologia et Mycologia Applicata,1973,50(3): 233-240 |
| [15] | Dekker J. The fungicide-resistance problem[J]. Netherlands Journal of Plant Pathology,1977,83(Suppl 1): 159-167 |
| [16] | Possiede YM,Gabardo J,Kava-Cordeiro V,et al. Fungicide resistance and genetic variability in plant pathogenic strains of Guignardia citricarpa[J]. Brazilian Journal of Microbiology,2009,40(2): 308-313 |
 2016, Vol. 43
2016, Vol. 43