扩展功能
文章信息
- 王超, 李宏伟, 谢越盛, 郭坚华
- WANG Chao, LI Hong-Wei, XIE Yue-Sheng, GUO Jian-Hua
- 耐低温荧光假单胞菌筛选体系建立及其植物促生作用评价
- Construction of screening system for cold tolerant Pseudomonas fluorescens for plant growth-promotion
- 微生物学通报, 2016, 43(12): 2644-2656
- Microbiology China, 2016, 43(12): 2644-2656
- DOI: 10.13344/j.microbiol.china.160015
-
文章历史
- 收稿日期: 2016-01-07
- 接受日期: 2016-06-02
- 优先数字出版日期(www.cnki.net): 2016-06-08
2. 环境保护部南京环境科学研究所 江苏 南京 210042
2. Nanjing Institute of Environmental Sciences, Ministry of Environmental Protection of PR China, Nanjing, Jiangsu 210042, China
温带地区的农业生态系统常处于亚适温环境,植物和微生物会遭受低温胁迫,因此该类地区农作物生长季节短[1],耐低温微生物由此体现出重要的农业应用意义。尽管这类微生物的最适增殖温度在常温范围,它们在零上低温仍然能够增殖[2]。因此,有必要发掘和鉴定亚适温环境中能保持正常活力的微生物,这些微生物作为接种菌用于全球温带地区的农业生产将发挥重要作用。
地球上高山土壤环境的特点是物理、生化因子存在明显的季节变化[3],低温居多,耐低温微生物在高山及亚高山地区非常常见,以耐低温细菌为主[1]。Pandey等[4]在印度中部喜马拉雅亚高山山脉分离到了耐低温的具有溶磷、拮抗病原菌作用的恶臭假单胞菌。Negi等[5]在印度喜马拉雅山脉Garhwal地区鉴定到的假单胞菌能够分泌嗜铁素,具有植物促生作用。多数关于分离自高山及亚高山地区耐低温细菌的研究都有假单胞菌的报道[1],并且假单胞菌通常具有植物促生作用,同时能够增强植物对于病原物和非生物胁迫的抗性[6],称作植物促生细菌(plant growth-promoting bacteria,PGPB)[7]或植物根围促生细菌(plant growth-promoting rhizobacteria,PGPR)[8]。因此,分离和鉴定PGPR或PGPB类耐低温假单胞菌用于改善温带地区的农业生产力非常具有现实意义。
纵观假单胞菌PGPR或PGPB特性的报道,其植物促生作用机制主要包括以下几点:(1) 产生大量的植物激素吲哚-3-乙酸(indole-3-acetic acid,IAA)[9];(2) 增强土壤养分的矿化及溶解,尤其是不溶性磷酸盐类[10];(3) 分泌大量嗜铁素增进植物铁吸收[11];(4) 分泌1-氨基环丙烷羧酸(1-aminocyclopropane-1-carboxylate,ACC)脱氨酶降低植物根系乙烯含量[12]。荧光假单胞菌(Pseudomonas fluorescens)作为一种重要的生防细菌,可通过抑制病原菌的发生、诱导系统抗性、产生植物激素、提高植物生长量等促进植株的生长发育[13],是微生物肥料和生防制剂生产中最常见也是最重要的菌种之一[14]。Saravanakumar和Samiyappan[15]发现番茄(Lycopersicon esculentum)、黄瓜(Cucumis sativus)植株接种P. fluorescens 92rk、P190r后其根系总长度、总表面积及总体积显著上升。张鹏等[13]同样发现P. fluorescens能显著促进樱桃幼苗生长。前期研究中我们针对小麦全蚀病、赤霉病及茎基腐病的生物防治从小麦生境中分离了一批荧光假单胞菌[16-17],本研究进一步从中筛选耐低温菌株,评价其植物促生作用潜力,并选定潜力菌株进行温室和田间试验测定其植物促生效果。
1 材料与方法 1.1 主要试剂和仪器2, 4-二硝基苯肼、高氯酸、α-丁酮酸、牛血清蛋白、乙醇、次氯酸钠,购自南京寿德试验器材有限公司。
RDN-350-5C型人工气候箱,宁波东南仪器有限公司;ULT1786-4-V41超低温冰箱,北京隆盛科仪科技发展有限公司;普通天平,艾德姆衡器(武汉)有限公司;ZHWY-2102C恒温摇床,上海智城分析仪器制造有限公司。
1.2 供试菌株及培养条件853株细菌菌株系前期研究从我国江苏省和河南省小麦生境(根围土、根内、茎表、茎内、叶表、叶内)中分离保存[16-17],取保存于-70 ℃的荧光假单胞菌菌株划线培养于LB平板培养基,28 ℃活化24 h后转接于全营养琼脂平板培养基[18]于4 ℃培养,观察48 h内的生长情况。
耐低温荧光假单胞菌菌株划线培养于LB平板培养基,28 ℃活化24 h后转接于LB培养液中,28 ℃、200 r/min培养24 h成种子液。以1:100的比率在LB培养液中扩繁。28 ℃、200 r/min扩繁24 h后所得菌液在4 ℃、1 818×g离心10 min。 所得菌体用无菌0.85% NaCl溶液重悬成约 5×107 CFU/mL供温室试验使用,所得菌体用无菌LB培养液重悬成5×108 CFU/mL供田间试验使用。
蜡质芽胞杆菌(Bacillus cereus) AR156 (菌株保藏编号:CGMCC No. 1929) 从江苏省南京市下马坊菜园土壤中分离获得,枯草芽胞杆菌(B. subtilis) SM21 (菌株保藏编号:CGMCC No. 2058) 和沙雷氏菌(Serratia sp.) XY21 (菌株保藏编号:CGMCC No. 2059) 从江苏省镇江市森林土壤中分离获得,并鉴定保存。取保存于-70 ℃的生防菌菌株AR156、SM21和XY21划线培养于LB平板培养基。在28 ℃培养24 h后转接于LB培养液中,28 ℃、200 r/min培养24 h成种子液。以1:100的比率在LB培养液中扩繁。28 ℃、200 r/min扩繁24 h,所得菌液在4 ℃、1 818×g离心10 min,将浓度均调成5×108 CFU/mL后以1:1:1混合即得BBS菌剂。
1.3 耐低温菌株植物促生潜力评价分别在15 ℃和28 ℃培养温度下测定耐低温菌株产IAA、嗜铁素、ACC脱氨酶以及无机、有机磷溶解能力。
对于产IAA的定性测定,在菌株划线15 ℃和28 ℃培养的平板上滴加5 mL Salkowski试剂[19],37 ℃黑暗保温15 min,平板表层溶液变红说明能够产生IAA,再参照Patten和Glick的方法[20]进行定量测定。
对于产嗜铁素活性测定,菌株活化后分别点接于CAS平板培养基[21]上,15 ℃和28 ℃培养,分别观察菌落周围的透明圈。
对于产ACC脱氨酶活性的定性测定,菌株活化后接种于SMA固体培养基[22],15 ℃和28 ℃培养,选取传代5次后能够在ACC为唯一氮源的培养基上生长的菌株为产ACC脱氨酶阳性菌株,然后按下述方法进行定量测定。菌株在LB培养液中15 ℃和28 ℃、180 r/min培养24 h,4 ℃、 8 000 r/min离心10 min,菌体用SM培养液洗涤离心2次,然后菌体悬浮于SMA培养液,28 ℃、180 r/min培养24 h;4 ℃、8 000 r/min离心10 min收集菌体,用0.1 mol/L Tris-HCl缓冲液(pH 7.6) 洗涤离心2次,重新悬浮于600 μL 0.1 mol/L Tris-HCl缓冲液中(pH 8.5) ;加入30 μL甲苯并迅速振荡30 s以破碎细胞;取100 μL粗酶液4 ℃贮存用于测定蛋白浓度,其余粗酶液进行ACC脱氨酶活性测定。取粗酶液200 μL加入0.5 mol/L ACC 20 μL混匀,置于30 ℃水浴反应15 min,加入1 mL 0.56 mol/L HCl终止反应,12 000 r/min离心 5 min;取上清1 mL加入800 μL 0.56 mol/L HCl和300 μL 0.2% 2, 4-二硝基苯肼溶液,30 ℃保温 30 min;加入2 mL 2 mol/L NaOH溶液混匀,540 nm测吸光度值。对照α-丁酮酸标准曲线和蛋白测定标准曲线计算菌株的酶活性。ACC脱氨酶活性表示方法为:反应条件下,每毫克菌体蛋白每小时催化ACC脱氨形成α-丁酮酸的微摩尔数,单位为μmol α-ketobutyrate/(h·mg protein)。通过Bradford比色法测定蛋白质含量,以牛血清蛋白为标准物绘制标准曲线,计算获得线性方程。
对于不溶性磷溶解能力测定,菌株活化后分别点接于Pikovskaya平板培养基(无机磷测定)[23]和PSM平板培养基(有机磷测定)[24],15 ℃和28 ℃黑暗培养3-7 d后分别观察菌落周围的透明圈。
建立赋值系统,便于直观反映耐低温菌株的植物促生潜力,具体如下:以CAS平板、Pikovskaya平板和PSM平板上透明圈的大小判定菌株产嗜铁素以及无机磷、有机磷溶解能力的大小,没有透明圈或不能合成IAA及ACC脱氨酶,赋0分;透明圈直径1-5 mm或合成IAA浓度1-6 mg/L或合成ACC脱氨酶活性 0-0.5 μmol α-ketobutyrate/(h·mg protein),赋1分;透明圈直径5.1-10 mm或合成IAA浓度6-15 mg/L或合成ACC脱氨酶活性0.5-1 μmol α-ketobutyrate/ (h·mg protein),赋2分;透明圈直径10 mm以上或合成IAA浓度16 mg/L以上或合成ACC脱氨酶活性 1 μmol α-ketobutyrate/(h·mg protein)以上,赋3分;各项评估赋分加和即得菌株植物促生潜力总分。
1.4 耐低温菌株植物促生温室试验青菜[Brassica campestris L. ssp. chinensis (L.) Makino var. communis Tsen et Lee cv. Aijiaohuang]种子经表面消毒(70%乙醇浸泡3 min,然后用2%次氯酸钠溶液浸泡5 min,最后无菌水浸洗3次,每次1 min)后浸泡在菌悬液中,以0.85% NaCl溶液为对照,28 ℃静置5 min,然后用无菌水浸洗 2次,每次1 min,备用。试验1:将种子平铺在2.25%水琼脂平板上,每板10粒种子,每个处理 3个平板,独立重复3次;平板竖直放置于15 ℃黑暗培养,6 d后测量根长。试验2:将种子播种于塑料穴盘中(装有高温灭菌处理的营养土,组分为东北黑土和蛭石,二者以3:1 (体积比)均匀混合;东北黑土的理化性质:有机质含量34.87±1.56 g/kg,总氮含量84.33±10.19 mg/kg,总磷含量4.13±0.47 mg/kg,总钾含量529.67±39.11 mg/kg,pH 6.64±0.08,每个处理1盘(45株苗),独立重复3次;置于培养箱15±1 ℃培养,光照时间16 h,光照强度为 6 000 lx;培养20 d后每株青菜幼苗浇灌5 mL菌悬液,以0.85% NaCl溶液为对照;继续培养30 d后测定地上部生物量。
黄瓜(Cucumis sativus L. cv. Jinyou No. 1) 种子经表面消毒后浸泡在菌悬液中,以0.85% NaCl溶液为对照,28 ℃静置5 min,然后用无菌水浸洗 2次,每次1 min,然后将种子播种于容积 355.46 cm3的一次性塑料杯子中(装有无菌营养土),每个处理24株苗,独立重复3次;置于温室28±2 ℃培养,光照时间16 h,光照强度为 6 000 lx;培养15 d后每株青菜幼苗浇灌10 mL菌悬液,以0.85% NaCl溶液为对照;继续培养15 d后测定地上部生物量。
1.5 耐低温菌株1bYB22和3bJN2植物促生作用田间试验田间试验选取小麦(鲁麦23号)为供试植物,试验田位于山东省临沂市费县石井镇大安村(35.07°E,117.67°N),田块前茬种植作物为玉米,土壤养分含量:氮8.40 mg/kg,磷38.00 mg/kg,钾80.00 mg/kg,pH 7.11。
1bYB22和3bJN2菌悬液分别以清水稀释10倍作为浸种液,多菌合剂BBS稀释50倍作为浸种液,清水作对照,将小麦种子置于上述浸种液和清水中室温(约18 ℃)浸种5 min,滤出小麦种子后到田间播种。撒播播种,行间距25 cm,每处理(1bYB22、3bJN2、BBS和清水对照)播种4行,每处理播种10 m2 (播种行长10 m),每处理3个重复,随机区组排列。小区间间隔40 cm,并起垄作为隔离带。撒播前播种沟底浇适量水,覆土,踩实。返青后和扬花期分别进行菌剂浇灌处理(同浸种液),每株小麦平均10 mL,对照组以清水代替。除菌剂处理外,各处理组间田间管理措施相同。分别于出苗期和拔节期以五点取样法进行促生效果调查(地上部、地下部生物量及叶片数),收获前5点调查每m2有效穗数,并取样进行室内考种(穗长、穗粒数和千粒重)。
1.6 数据分析耐低温菌株植物促生性质以及青菜、黄瓜、小麦生物量等数据由Microsoft Excel 2007统计分析,显著水平由DPS v 7.05获得;菌株植物促生潜力总分与实际促生效果的相关性系数由Microsoft Excel 2007获得。
2 结果与分析 2.1 耐低温细菌的筛选以上述853株小麦生境分离细菌为菌株库,通过观察它们在4 ℃环境中全营养琼脂平板培养基上的生长情况,选取能够在48 h内正常形成菌落的菌株,共获得34株耐低温菌株(表 1)。
| 菌株 Strains | 来源 Source |
| Pseudomonas fluorescens 3bGN4 | 河南小麦根 Root of wheat |
| Pseudomonas fluorescens 3aYB7 | 江苏小麦叶表 Surface of leaves of wheat |
| Pseudomonas fluorescens 2bJN5 | 河南小麦茎内 Interior of stems of wheat |
| Pseudomonas fluorescens 4aYN9 | 江苏小麦叶内 Interior of leaves of wheat |
| Pseudomonas fluorescens 3bJN4 | 河南小麦茎内 Interior of stems of wheat |
| Pseudomonas fluorescens 2aYN7 | 江苏小麦叶内 Interior of leaves of wheat |
| Pseudomonas fluorescens 4aYN8 | 江苏小麦叶内 Interior of leaves of wheat |
| Pseudomonas fluorescens 1bYB14 | 河南小麦叶表 Surface of leaves of wheat |
| Pseudomonas fluorescens 4aYN6 | 江苏小麦叶内 Interior of leaves of wheat |
| Pseudomonas fluorescens 2bJN3 | 河南小麦茎内 Interior of stems of wheat |
| Pseudomonas fluorescens 1bYB22 | 河南小麦叶表 Surface of leaves of wheat |
| Pseudomonas fluorescens 4aYB2 | 江苏小麦叶表 Surface of leaves of wheat |
| Pseudomonas fluorescens 2aGN9 | 江苏小麦根 Root of wheat |
| Pseudomonas fluorescens 3aGN1 | 江苏小麦根 Root of wheat |
| Pseudomonas fluorescens 2bGN6 | 河南小麦根 Root of wheat |
| Pseudomonas fluorescens 4aGN7 | 江苏小麦根 Root of wheat |
| Pseudomonas fluorescens 3aYB8 | 江苏小麦叶表 Surface of leaves of wheat |
| Pseudomonas fluorescens 2aT19 | 江苏小麦根围土 Rhizosphere soil of wheat |
| Pseudomonas fluorescens 4aYN12 | 江苏小麦叶内 Interior of leaves of wheat |
| Pseudomonas fluorescens 2bJN7 | 河南小麦茎内 Interior of stems of wheat |
| Pseudomonas fluorescens 2bJN6 | 河南小麦茎内 Interior of stems of wheat |
| Pseudomonas fluorescens 3bT14 | 河南小麦根围土 Rhizosphere soil of wheat |
| Pseudomonas fluorescens 3bJN2 | 河南小麦茎内 Interior of stems of wheat |
| Pseudomonas fluorescens 1bYB44 | 河南小麦叶表 Surface of leaves of wheat |
| Pseudomonas fluorescens 3bT16 | 河南小麦根围土 Rhizosphere soil of wheat |
| Pseudomonas fluorescens 1bYB20 | 河南小麦叶表 Surface of leaves of wheat |
| Pseudomonas fluorescens 2aJN6 | 江苏小麦茎内 Interior of stems of wheat |
| Pseudomonas fluorescens 1bYB57 | 河南小麦叶表 Surface of leaves of wheat |
| Pseudomonas fluorescens 2bGN8 | 河南小麦根 Root of wheat |
| Pseudomonas fluorescens 2bJN1 | 河南小麦茎内 Interior of stems of wheat |
| Pseudomonas fluorescens 2aJB15 | 江苏小麦茎表 Surface of stem of wheat |
| Pseudomonas fluorescens 4aT6 | 江苏小麦根围土 Rhizosphere soil of wheat |
| Pseudomonas fluorescens 2aYB8 | 江苏小麦叶表 Surface of leaves of wheat |
| Pseudomonas fluorescens 1bYB11 | 河南小麦叶表 Surface of leaves of wheat |
以产IAA、ACC脱氨酶、嗜铁素,溶解无机、有机磷能力为评价指标,分别在15 ℃和28 ℃环境中测定了34株耐低温菌株的产IAA、ACC脱氨酶、嗜铁素活性以及溶解无机、有机磷能力。结果发现,除不能分泌嗜铁素外,菌株1bYB22在两种温度环境中具有上述所有特性,其在15 ℃和28 ℃环境中合成的IAA分别为16.18、36.30 mg/L;菌株3bJN2在28 ℃环境中合成的IAA达29.64 mg/L,此外,10个菌株在两种温度环境中都能合成IAA,4个菌株不能合成IAA;菌株4aYN9在28 ℃环境中合成ACC脱氨酶的活性高达1.89 μmol α-ketobutyrate/(h·mg protein),1bYB22等5个菌株在两种温度环境中都能合成ACC脱氨酶;1bYB22等8个菌株在两种温度环境中同时具有溶解无机、有机磷能力,5个菌株没有溶磷能力;4aYB2等10个菌株在两种温度环境中都能合成嗜铁素。根据耐低温菌株在培养平板上形成的透明圈大小以及合成ACC脱氨酶活性、IAA的浓度,对菌株植物促生潜力评估赋分,结果如表 2所示。
| 菌株 Strains | 产ACC脱氨酶 ACC deaminase production | 产吲哚乙酸 IAA production | 无机磷溶解 Inorganic phosphate solubilisation | 有机磷溶解 Organic phosphate solubilisation | 产嗜铁素 Siderophores production | 植物促生潜力 Assessed growth promoting potential | |||||||||
| 28 ℃ | 15 ℃ | 28 ℃ | 15 ℃ | 28 ℃ | 15 ℃ | 28 ℃ | 15 ℃ | 28 ℃ | 15 ℃ | ||||||
| 1bYB22 | 1 | 1 | 3 | 3 | 1 | 1 | 2 | 2 | 0 | 0 | 14 | ||||
| 4aYB2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 1 | 7 | ||||
| 2aGN9 | 0 | 0 | 1 | 0 | 2 | 1 | 2 | 3 | 1 | 0 | 10 | ||||
| 3aGN1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 4 | ||||
| 2bGN6 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 5 | ||||
| 2bJN7 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 3 | 1 | 7 | ||||
| 2bJN6 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ||||
| 3bT14 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | ||||
| 3bJN2 | 0 | 0 | 3 | 0 | 1 | 3 | 1 | 3 | 0 | 0 | 11 | ||||
| 1bYB44 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 7 | ||||
| 3bT16 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 3 | 2 | 0 | 12 | ||||
| 1bYB20 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 6 | ||||
| 2aJN6 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 3 | 0 | 5 | ||||
| 1bYB57 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 4 | ||||
| 2bGN8 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 5 | ||||
| 2aJB15 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 0 | 11 | ||||
| 4aT6 | 0 | 0 | 1 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 9 | ||||
| 2aYB8 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 3 | 0 | 8 | ||||
| 1bYB11 | 1 | 0 | 1 | 0 | 1 | 2 | 1 | 3 | 2 | 0 | 11 | ||||
| 4aGN7 | 2 | 0 | 1 | 0 | 2 | 2 | 1 | 0 | 0 | 0 | 8 | ||||
| 3aYB8 | 2 | 0 | 1 | 0 | 2 | 2 | 1 | 1 | 0 | 0 | 9 | ||||
| 2aT19 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 3 | 1 | 8 | ||||
| 3bGN4 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 5 | ||||
| 3aYB7 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 7 | ||||
| 2bJN5 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 4 | ||||
| 4aYN9 | 3 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 8 | ||||
| 3bJN4 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 8 | ||||
| 2aYN7 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 2 | 1 | 6 | ||||
| 4aYN8 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 2 | 6 | ||||
| 1bYB14 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 6 | ||||
| 4aYN6 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 0 | 7 | ||||
| 2bJN3 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 4 | ||||
| 2bJN1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 4 | ||||
| 4aYN12 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 7 | ||||
| 注:没有透明圈或不能合成IAA及ACC脱氨酶,赋0分;透明圈直径1-5 mm或合成IAA浓度1-6 mg/L或合成ACC脱氨酶活性0-0.5 μmol α-ketobutyrate/(h·mg protein),赋1分;透明圈直径5.1-10.0 mm或合成IAA浓度6-15 mg/L或合成ACC脱氨酶活性 0.5-1.0 μmol α-ketobutyrate/(h·mg protein),赋2分;透明圈直径10 mm以上或合成IAA浓度16 mg/L以上或合成ACC脱氨酶活性 1 μmol α-ketobutyrate/(h·mg protein)以上,赋3分. Note: The in vitro biochemistry activity was graded with 0, 1, 2, or 3 based on the diameter (in mm) of the semicircular hyaline zones: grade 0, no antagonism; grade 1, 1-5 mm; grade 2, 5.1-10 mm; grade 3, >10 mm. The ability of synthesizing IAA was graded with 0, 1, 2, or 3 based on the concentration of IAA: grade 0, no IAA; grade 1, 1-6 mg/L; grade 2, 6-15 mg/L; grade 3, >16 mg/L. The ACC deaminase activity was expressed as μmol α-ketobutyrate/(h·mg protein) and graded with 0, 1, 2, or 3: grade 0, 0 μmol α-ketobutyrate/(h·mg protein); grade 1, 0-0.5 μmol α-ketobutyrate/(h·mg protein); grade 2, 0.5-1.0 μmol α-ketobutyrate/(h·mg protein); grade 3, >1 μmol α-ketobutyrate/(h·mg protein). | |||||||||||||||
根据耐低温菌株植物促生潜力评估得分情况,选取了总分9分(含)以上的菌株(1bYB22、3bJN2、2aGN9、2aJB15、1bYB11、3bT16、3aYB8和4aT6) 进行温室试验。青菜种子播种50 d后进行结果统计,发现菌株1bYB22对青菜生长具有显著促生作用,促生效果达25.11%,菌株3bT16、3aYB8和4aT6没有促生作用(图 1A Ⅱ,表 3,图 2A);青菜种子在水琼脂平板上黑暗生长了6 d,统计发现菌株1bYB22和3bJN2处理组的根长显著高于对照组,前者促生效果达116.76%,菌株1bYB11、3aYB8和4aT6没有促生作用 (图 1B,表 3,图 2B);黄瓜种子播种25 d后进行结果统计,发现菌株1bYB22、3bJN2、1bYB11和3bT16对黄瓜生长具有显著促生作用,其中菌株3bJN2促生效果达28.87%,仅菌株4aT6没有促生作用(图 1A Ⅰ,表 3,图 2C)。

|
| 图 1 耐低温菌株P. fluorescens 1bYB22对青菜和黄瓜的促生作用 Figure 1 Growth promoting effect of cold tolerant P. fluorescens 1bYB22 on no-heading Chinese cabbage and cucumber under greenhouse condition 注:A:温室培养的幼苗;Ⅰ:黄瓜幼苗;Ⅱ:青菜幼苗. B:发芽的青菜种子. 1bYB22:菌株1bYB22处理组;Control:对照组. Note: A: Seedlings in greenhouse condition; Ⅰ: seedlings of cucumber; Ⅱ: seedlings of cabbage. B: germinating seeds. Seedlings of 1bYB22-treated (1bYB22) and control (Control). |
|
|

|
| 图 2 耐低温假单胞菌菌株植物促生作用与促生潜力之间的相关性 Figure 2 The coincident relationship between the assessed growth promoting potential and the plant growth promoting effect on non-heading Chinese cabbage and cucumber 注:A:土壤中15 ℃温室培养的青菜,相关系数为0.85;B:平板上15 ℃萌发的青菜种子,相关系数为0.83;C:土壤中28 ℃温室培养的黄瓜,相关系数为0.62;数据标签标示促生效果(%). Note: A: the biomass increase of non-heading Chinese cabbage in greenhouse condition at 15 ℃, and the correlation coefficiency was 0.85; B: The root length increase of non-heading Chinese cabbage on Agar plates at 15 ℃, and the correlation coefficiency was 0.83; C: The biomass increase of cucumber under greenhouse condition at 28 ℃, and the correlation coefficiency was 0.62; Data labels indicate the plant growth promoting effect (%). |
|
|
| 处理 Treatments | 青菜地上部鲜重 Shoot weight of non-heading Chinese cabbage (g) | 青菜种子根长 Root length of non-heading Chinese cabbage (cm) | 黄瓜地上部鲜重 Shoot weight of cucumber (g) | |
| 1bYB22 | 6.17±0.99a | 3.75±0.24a | 4.31±0.76a | |
| 3bJN2 | 5.22±0.93b | 2.54±0.30b | 4.33±1.16a | |
| 2aGN9 | 5.14±0.64b | 2.08±0.28bcd | 3.64±0.62bcde | |
| 2aJB15 | 5.13±0.52b | 2.42±0.28bc | 3.60±0.42cde | |
| 1bYB11 | 5.08±0.58b | 1.55±0.27def | 4.22±1.06ab | |
| 3bT16 | 4.88±1.41bc | 1.84±0.21bcde | 3.99±0.51abc | |
| 4aT6 | 4.84±0.87bc | 1.33±0.22ef | 3.28±0.66e | |
| 3aYB8 | 4.41±0.62c | 1.42±0.28def | 3.96±0.63abcd | |
| Control | 4.93±0.85bc | 1.73±0.24cdef | 3.36±0.74de | |
| 注:数值为平均值±标准差,不同字母表示处理间在P<0.05的显著水平差异性显著(LSD test). Note: The data are the averages±SD of three replicates. Values with the same English letter within the same column don't differ from each other significantly according to Duncan' s test at P<0.05. | ||||
基于耐低温菌株的产IAA、ACC脱氨酶、嗜铁素以及无机、有机磷溶解能力,构建了评估系统用于温室试验前评价菌株的植物促生潜力,为了验证该系统的有效性,我们分析了8个菌株在温室试验中的实际促生作用与植物促生潜力之间的相关性。结果发现,对于15 ℃温室土壤培养的青菜,该相关性系数为0.85 (图 2A);对于15 ℃水琼脂平板培养的青菜,该相关性系数为0.83 (图 2B);对于28 ℃温室土壤培养的黄瓜,该相关性系数为0.62 (图 2C)。
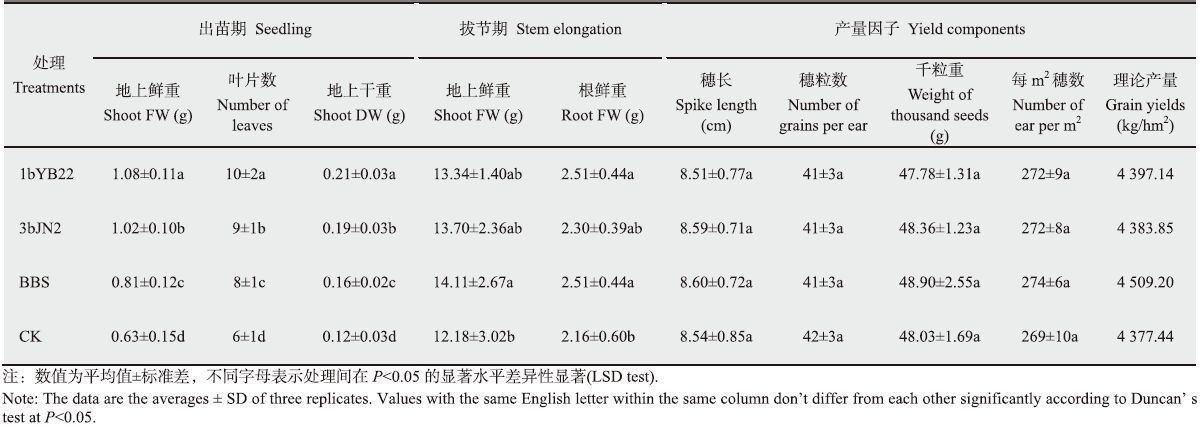
2.4 耐低温菌株1bYB22和3bJN2对小麦的田间促生作用分别于小麦出苗期、拔节期及收获时统计了耐低温菌株的田间促生效果,出苗期菌株1bYB22和3bJN2对小麦表现出了显著的促生作用,菌株处理组小麦植株地上部、地下部鲜重和叶片数均显著高于对照组,且显著高于多菌合剂BBS (图 3A,表 4);拔节期两个菌株都有促生作用,但效果不显著,然而BBS却表现出了显著的促生效果(图 3B,表 4);测定产量因子发现,耐低温菌株及BBS处理组植株穗长、穗粒数、千粒重,每m2有效穗数及理论产量均高于对照组,但效果都不显著,菌株1bYB22和3bJN2分别增产0.58%、0.19%,低于BBS的3.90% (图 3C,表 4)。

|
| 图 3 耐低温菌株对小麦的田间促生作用 Figure 3 Growth promoting effect of cold tolerant Pseudomonads on wheat in field 注:A、B:不同生长时期的小麦幼苗;C:小麦穗. 1bYB22:菌株1bYB22处理组;3bJN2:菌株3bJN2处理组;BBS:BBS菌剂处理组;CK:清水对照组. Note: A, B: wheat seedlings at different stages; C: wheat ears. Seedlings of 1bYB22-treated (1bYB22) , 3bJN2-treated (3bJN2) , BBS-treated (BBS) and water-treated (CK). |
|
|
 |
本研究从小麦生境分离细菌中成功筛选到 34株耐低温菌株,通过菌株植物促生性质生物测定、温室及田间促生试验,最终获得了具有农业生产实际应用价值的耐低温PGPB P. fluorescens 1bYB22;首次报道了从小麦生境筛选和评价耐低温PGPR/PGPB,其他关于耐低温PGPR/PGPB的报道大多来源于印度喜马拉雅山区[5, 22, 25-26]。
近年来,有益微生物大量用于农业生产,可有效减少化学肥料的使用。对于温带地区而言,筛选具有植物促生作用的耐低温微生物是必不可少的。为了实现此目标,首先是获得耐低温微生物。小麦是我国重要的粮食作物,其生长周期跨冬季、春季,甚至是初夏,因此,小麦生境中的微生物能适应很广的温度范围,小麦生境中的假单胞菌是获得耐低温菌株的良好来源。另外,在获得耐低温微生物的基础上,建立恰当的微生物植物促生作用潜力评价系统对于高效率得到耐低温PGPR/PGPB也是至关重要的。本研究建立的评价系统从34株耐低温菌株中筛选出8株植物促生潜力菌株(表 2),其中部分菌株在温室试验中表现出了较好的促生效果(图 1,表 3),并且8株耐低温菌株的温室促生效果与植物促生潜力得分之间存在较好的相关性,相关性系数均在0.62以上(图 2),表明该评价系统在筛选耐低温PGPR/PGPB中是有效的,对筛选用于温带地区农业生产的微生物类投入品具有指导意义。当然,PGPR/PGPB能否成功定殖同样决定其促生作用的发挥,后期研究中将把耐低温菌株定殖能力考虑到评价系统中。
作为植物体内活跃的生长激素之一,IAA能激发植株快速(比如细胞伸长)而长久(比如细胞分裂、分化)的生长反应[27]。据统计,80%左右的植物根围分离菌能够产生IAA,特别是当培养基中加入了色氨酸[28]。我们测定了耐低温菌株的产IAA能力,发现30株细菌能够在15 ℃和(或) 28 ℃环境中产生IAA (表 2) ,这些菌株产生的IAA在一定程度上会促进植物生长,比如菌株1bYB22,它在15 ℃培养温度下能产生16.18 mg/L的IAA,该浓度的IAA相比空白处理可能足以显著促进15 ℃环境中青菜种子根的伸长(图 1B)。在植物和微生物生长发育不可或缺的营养元素中,磷是仅次于氮的矿质营养元素,参与细胞代谢所需能量的积累和释放[29]。通过接种溶磷微生物增加土壤中植物可利用磷元素含量在温室试验[30]和田间应用中[31]都有报道。本研究中29株耐低温菌株在15 ℃和(或) 28 ℃环境中具有溶解无机和(或)有机磷的能力(表 2),这些菌株在土壤定殖后能增加可溶性磷元素含量,一定程度上解释了菌株接种的青菜和黄瓜植株相比对照组有更高的地上部生物量(图 1A,表 3)。Çakmakçı等[32]同样发现9株产IAA且具有溶磷能力的假单胞菌和芽胞杆菌菌株能够增加小麦及菠菜的生物量,促进氮磷钾吸收,增强酶的活力。PGPR/PGPB的植物促生作用同样体现在根的形态上,比如延长主根、增生侧根和不定根[33],进而促进植物对营养元素的吸收[34]。研究同样发现耐低温菌株在水琼脂平板上增长了青菜主根(6.36%-116.76%,图 2B),也增加了土壤培养青菜的地上部生物量(3.10%-25.11%,图 2A)。Mishra等[35]在耐低温P. putida PGRs4定殖的小麦幼苗上发现了相似结果,并且PGRs4促进了小麦幼苗对氮磷的吸收。
耐低温P. fluorescens 1bYB22在温室中表现出了广谱、显著的促生作用(图 1,图 2,表 3),对其促生性质测定发现,1bYB22在15、28 ℃下均能产ACC脱氨酶、IAA,并能溶解不溶性磷,但不会产生嗜铁素(表 2),说明1bYB22可能通过降低植物根围乙烯含量、增加吲哚乙酸含量促进植物生长,同时增加土壤中植物可以利用的磷元素含量。已有很多关于耐低温假单胞菌类PGPR/PGPB的相关报道[5, 22, 25, 36],但均来自印度喜马拉雅山区,1bYB22是首个分离自小麦生境的耐低温PGPB。
田间试验中,耐低温菌株1bYB22和3bJN2在出苗期表现出了显著的促生作用,且效果均优于多菌合剂BBS (图 3A,表 4);拔节期,两个耐低温菌株的促生作用均不显著,且效果均差于BBS (图 3B,表 4);表明耐低温菌株在温度较低的情况下仍能够正常生长和发挥促生作用,而在常温下效果较好的BBS在出苗期时的低温环境中作用不佳,可能是因为芽胞杆菌在低温下不能正常代谢。
| [1] | Maheshwari DK. Plant Growth and Health Promoting Bacteria[M]. Berlin Heidelberg: Springer, 2011: 18 . |
| [2] | Morita RY. Psychrophilic bacteria[J]. Bacteriological Reviews 1975, 39(2) : 147–167. |
| [3] | Bowman WD, Seastedt TR. Structure and Function of An Alpine Ecosystem: Niwot Ridge, Colorado[M]. New York: Oxford University Press, 2001: 15 . |
| [4] | Pandey A, Trivedi P, Kumar B, et al. Characterization of phosphate solubilizing and antagonistic strain of Pseudomonas putida (Bo) Isolated from a sub-alpine location in the Indian Central Himalaya[J]. Current Microbiology 2006, 53(2) : 102–107. DOI:10.1007/s00284-006-4590-5 |
| [5] | Negi YK, Garg SK, Kumar J. Cold-tolerant fluorescent Pseudomonas isolates from Garhwal Himalayas as potential plant growth promoting and biocontrol agents in pea[J]. Current Science 2005, 89(12) : 2151–2156. |
| [6] | Wu X, Monchy S, Taghavi S, et al. Comparative genomics and functional analysis of niche-specific adaptation in Pseudomonas putida[J]. FEMS Microbiology Reviews 2011, 35(2) : 299–323. DOI:10.1111/j.1574-6976.2010.00249.x |
| [7] | Bashan Y, Holguin G. Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant growth-promoting bacteria) and PGPB[J]. Soil Biology and Biochemistry 1998, 30(8/9) : 1225–1228. |
| [8] | Kloepper JW, Schroth MN. Plant growth promoting rhizobacteria on radishes[A]//Angers J. Proceedings of the 4th International Conference on Plant Pathogenic Bacteria, Station de Pathologie Végétale et Phytobactériologie[C]. Tours, France: INRA, 1978: 879-882 |
| [9] | Viruel E, Lucca ME, SiñerizF. Plant growth promotion traits of phosphobacteria isolated from Puna, Argentina[J]. Archives of Microbiology 2011, 193(7) : 489–496. DOI:10.1007/s00203-011-0692-y |
| [10] | Miller SH, Browne P, Prigent-Combaret C, et al. Biochemical and genomic comparison of inorganic phosphate solubilization in Pseudomonas species[J]. Environmental Microbiology Reports 2010, 2(3) : 403–411. |
| [11] | Fernández-Piñar R, Cámara M, Dubern JF, et al. The Pseudomonas aeruginosa quinolone quorum sensing signal alters the multicellular behaviour of Pseudomonas putida KT2440[J]. Research in Microbiology 2011, 162(8) : 773–781. DOI:10.1016/j.resmic.2011.06.013 |
| [12] | Penrose DM, Glick BR. Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase-containing plant growth-promoting bacteria[J]. Canadian Journal of Microbiology 2001, 47(4) : 368–372. DOI:10.1139/w01-014 |
| [13] | Zhang P, Qin SJ, Zhou WJ, et al. Effects of Pseudomonas fluorescens inoculation on root respiration and seedling growth of Cherry[J]. Scientia Agricultura Sinica 2014, 47(19) : 3857–3865. (in Chinese) 张鹏, 秦嗣军, 周文杰, 等. 荧光假单胞菌对樱桃根系呼吸和幼苗生长的影响[J]. 中国农业科学 2014, 47(19) : 3857–3865. |
| [14] | Liu H, Wu XQ, Ren JH, et al. Phosphate-dissolving characteristics and growth promoting effect of Pseudomonads fluorescent JW-JSI on poplar seedlings[J]. Scientia Silvae Sinicae 2013, 49(9) : 112–118. (in Chinese) 刘辉, 吴小芹, 任嘉红, 等. 一株荧光假单胞菌的溶磷特性及其对杨树的促生效果[J]. 林业科学 2013, 49(9) : 112–118. |
| [15] | Saravanakumar D, Samiyappan R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants[J]. Journal of Applied Microbiology 2007, 102(5) : 1283–1292. DOI:10.1111/jam.2007.102.issue-5 |
| [16] | Yang MM, Mavrodi DV, Mavrodi OV, et al. Biological control of take-all by fluorescent Pseudomonas spp. from Chinese wheat fields[J]. Phytopathology 2011, 101(12) : 1484–1491. |
| [17] | Wang LY, Wang C, Shen CM, et al. Biocontrol of wheat Fusarium head blight and Fusarium crown rot caused by Fusarium graminearum[J]. Journal of Triticeae Crops 2014, 34(5) : 703–708. (in Chinese) 王路遥, 王超, 申成美, 等. 引发小麦赤霉病和茎基腐病禾谷镰孢菌的生物防治初探[J]. 麦类作物学报 2014, 34(5) : 703–708. |
| [18] | Atlas RM. The Handbook of Microbiological Media for the Examination of Food[M]. Boca Raton: CRC Press, 1995: 197 . |
| [19] | Roca A, Pizarro-Tobías P, Udaondo Z, et al. Analysis of the plant growth-promoting properties encoded by the genome of the rhizobacterium Pseudomonas putida BIRD-1[J]. Environmental Microbiology 2013, 15(3) : 780–794. DOI:10.1111/1462-2920.12037 |
| [20] | Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system[J]. Applied and Environmental Microbiology 2002, 68(8) : 3795–3801. DOI:10.1128/AEM.68.8.3795-3801.2002 |
| [21] | Alexander DB, Zuberer DA. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria[J]. Biology and Fertility of Soils 1991, 12(1) : 39–45. DOI:10.1007/BF00369386 |
| [22] | Glick BR, Karaturovíc DM, Newell PC. A novel procedure for rapid isolation of plant growth promoting pseudomonads[J]. Canadian Journal of Microbiology 1995, 41(6) : 533–536. DOI:10.1139/m95-070 |
| [23] | Naik PR, Sahoo N, Goswani D, et al. Genetic and functional diversity among fluorescent Pseudomonads isolated from the rhizosphere of Banana[J]. Microbial Ecology 2008, 56(3) : 492–504. DOI:10.1007/s00248-008-9368-9 |
| [24] | Jorquera MA, Hernández MT, Rengel Z, et al. Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil[J]. Biology and Fertility of Soils 2008, 44(8) : 1025–1034. DOI:10.1007/s00374-008-0288-0 |
| [25] | Mishra PK, Mishra S, Selvakumar G, et al. Characterisation of a psychrotolerant plant growth promoting Pseudomonas sp. strain PGERs17 (MTCC 9000) isolated from North Western Indian Himalayas[J]. Annals of Microbiology 2008, 58(4) : 561–568. DOI:10.1007/BF03175558 |
| [26] | Selvakumar G, Joshi P, Nazim S, et al. Phosphate solubilization and growth promotion by Pseudomonas fragi CS11RH1 (MTCC 8984) , a psychrotolerant bacterium isolated from a high altitude Himalayan rhizosphere[J]. Biologia 2009, 64(2) : 239–245. |
| [27] | Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling[J]. FEMS Microbiology Reviews 2007, 31(4) : 425–448. DOI:10.1111/j.1574-6976.2007.00072.x |
| [28] | Patten CL, Glick BR. Bacterial biosynthesis of indole-3-acetic acid[J]. Canadian Journal of Microbiology 1996, 42(3) : 207–220. DOI:10.1139/m96-032 |
| [29] | Johri JK, Surange S, Nautiyal CS. Occurrence of salt, pH, and temperature-tolerant, phosphate-solubilizing bacteria in alkaline soils[J]. Current Microbiology 1999, 39(2) : 89–93. DOI:10.1007/s002849900424 |
| [30] | Kumar V, Behl RK, Narula N. Establishment of phosphate-solubilizing strains of Azotobacter chroococcum in the rhizosphere and their effect on wheat cultivars under green house conditions[J]. Microbiological Research 2001, 156(1) : 87–93. DOI:10.1078/0944-5013-00081 |
| [31] | Chabot R, Antoun H, Cescas MP. Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarum biovar. phaseoli[J]. Plant and Soil, 1996, 184(2) : 311-321 |
| [32] | Çakmakçı R, Erat M, Erdoğan Ü, et al. The influence of plant growth-promoting rhizobacteria on growth and enzyme activities in wheat and spinach plants[J]. Journal of Plant Nutrition and Soil Science 2007, 170(2) : 288–295. |
| [33] | Mantelin S, Desbrosses G, Larcher M, et al. Nitrate-dependent control of root architecture and N nutrition are altered by a plant growth-promoting Phyllobacterium sp[J]. Planta 2006, 223(3) : 591–603. DOI:10.1007/s00425-005-0106-y |
| [34] | Canbolat MY, Bilen S, Çakmakçı R, et al. Effect of plant growth-promoting bacteria and soil compaction on barley seedling growth, nutrient uptake, soil properties and rhizosphere microflora[J]. Biology and Fertility of Soils 2006, 42(4) : 350–357. DOI:10.1007/s00374-005-0034-9 |
| [35] | Mishra PK, Bisht SC, Ruwari P, et al. Alleviation of cold stress in inoculated wheat (Triticum aestivum L.) seedlings with psychrotolerant Pseudomonads from NW Himalayas[J]. Archives of Microbiology 2011, 193(7) : 497–513. DOI:10.1007/s00203-011-0693-x |
| [36] | Selvakumar G, Kundu S, Joshi P, et al. Growth promotion of wheat seedlings by Exiguobacterium acetylicum 1P (MTCC 8707) a cold tolerant bacterial strain from the Uttarakhand Himalayas[J]. Indian Journal of Microbiology 2009, 50(1) : 50–56. |
 2016, Vol. 43
2016, Vol. 43