扩展功能
文章信息
- 陶怡乐, 温东辉
- TAO Yi-le, WEN Dong-hui
- 细菌硝酸盐异化还原成铵过程及其在河口生态系统中的潜在地位与影响
- Dissimilatory nitrate reduction to ammonium: thepotential and impacts in estuarine regions
- 微生物学通报, 2016, 43(1): 172-181
- Microbiology China, 2016, 43(1): 172-181
- 10.13344/j.microbiol.china.150288
-
文章历史
- 收稿日期: 2015-04-05
- 接受日期: 2015-06-05
- 优先数字出版日期(www.cnki.net): 2015-07-03
氮是一种在自然界广泛存在且所有生命活动不可缺少的营养元素,氮循环更是地球生物化学循环中不可缺少的重要组成部分之一。但过量氮素滞留水体易导致水体富营养化、引发赤潮、消耗大量溶解氧,同时水体脱氮过程还涉及温室气体排放,因此会直接威胁水生态安全、饮用水安全以及全球气候等重大环境问题[1, 2, 3]。
固氮作用、硝化作用和反硝化作用是氮循环中较为重要、受主要关注的过程,然而越来越多的研究表明,一些被忽视的氮转化过程可能在一些特定的环境中起着举足轻重的作用。
1 硝酸盐异化还原成铵作用的过程与分类尽管早在1938年硝酸盐异化还原成铵(Dissimilatory nitrate reduction to ammonium,DNRA)就在纯培养条件下被发现了[4],但由于常规分析方法难以鉴别DNRA的产物和活性,且另一种硝酸盐异化还原过程——反硝化往往更易发生[5],DNRA长时间内未受到足够重视。
DNRA过程以NO3−/NO2−为电子受体,氧化NADH产生NH4+,存在于细菌和真菌中[6]。与植物和少数细菌消耗能量进行的硝酸盐同化还原成铵不同,DNRA过程的主要目的不是进一步将产生的铵转化为供自身利用的有机氮,而是产能或减少对细胞有毒害作用的NO3−/NO2−,因此多数DNRA过程属于异养发酵产能过程,以Klebsiella为例:无NO3−/NO2−时,NADH氧化形成乙醇[7]如公式(1);存在NO3−/NO2−时,几乎不产生乙醇,反应式如公式(2)。
| $ {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}}\to \text{C}{{\text{H}}_{\text{3}}}\text{COOH}+{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{OH}+\text{HCOOH}$ | (1) |
| $\text{3}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}}+\text{2N}{{\text{O}}_{\text{2}}}^{\text{-}}+\text{2}{{\text{H}}_{\text{2}}}\text{O}\to \text{6C}{{\text{H}}_{\text{3}}}\text{COOH}+\text{2N}{{\text{H}}_{\text{4}}}^{\text{+}}+\text{6HCOOH}$ | (2) |
该类DNRA过程可以分为两个阶段:
第一阶段类似反硝化,异化硝酸还原酶(NaR)将NO3−还原成NO2−[8]。电子供体不足时,DNRA过程会停留在这一步,造成NO2−的积累[9]。研究显示一些河流中NO2−的积累主要就是由河底沉积物中厌氧DNRA细菌引起的[10]。
目前已知的NaR酶有两种:一种在质膜上,与含细胞色素的电子传递有关,为反硝化细菌和多数DNRA细菌所共有。另一种是溶解性的,目前仅发现于专性厌氧的Closridium perfringens中,只含一个亚单位非血红素铁硫蛋白,其功能与含细胞色素的电子传递无关,其电子供体为铁氧还原蛋白[11]。
第二个阶段,亚硝酸还原酶(NiR)将NO2−还原成NH4+。DNRA的NiR酶活性比反硝化的NiR更强,可以进行6个而非3个电子的传递。
目前已知的DNRA的NiR酶是一种胞浆周围酶,存在于Escherichiacoli中,由nrfA基因编码,其辅基为四细胞色素C复合体[12],电子供体可能是甲酸,但电子传递链中的具体氢载体还有待确定。
上述DNRA过程会产生N2O,产量一般约为NO3−或NO2−总量的1%。但是Citrobacter C48的N2O产量高达23.5%[11],而Clostridium KDHS2是已报道的唯一不能产生N2O的菌株。N2O的产生机理尚未确定,但可能不和NH4+来自同一酶系统,例如Escherichiacoli的NaR酶是DNRA过程中产生N2O必需的,而NiR酶则可以缺失[13]。对于DNRA过程是否产生NO还鲜有报道[14]。
除上述DNRA过程外,还有一种较为特殊的自养DNRA方式:自养硫细菌利用硝酸盐氧化硫化物(H2S、S2−)以及单质S[4, 15],其反应式如公式(3)[16]。
| $\text{4}{{\text{H}}_{\text{2}}}\text{S+4NO}{{\text{3}}^{\text{-}}}\text{+4}{{\text{H}}_{\text{2}}}\text{O}\to \text{4N}{{\text{H}}_{\text{4}}}^{\text{+}}\text{4S}{{\text{O}}_{\text{4}}}{{\text{2}}^{\text{-}}}$ | (3) |
一些硫细菌既能进行DNRA产生NH4+,又能进行反硝化产生N2[17],但只有硫化物能产生NH4+和N2O,其对反硝化作用则可能存在抑制。一些硫细菌,如Desulfovibrio desulfuricans,不能代谢NO3−,需要依靠外源NO2−进行代谢[18]。
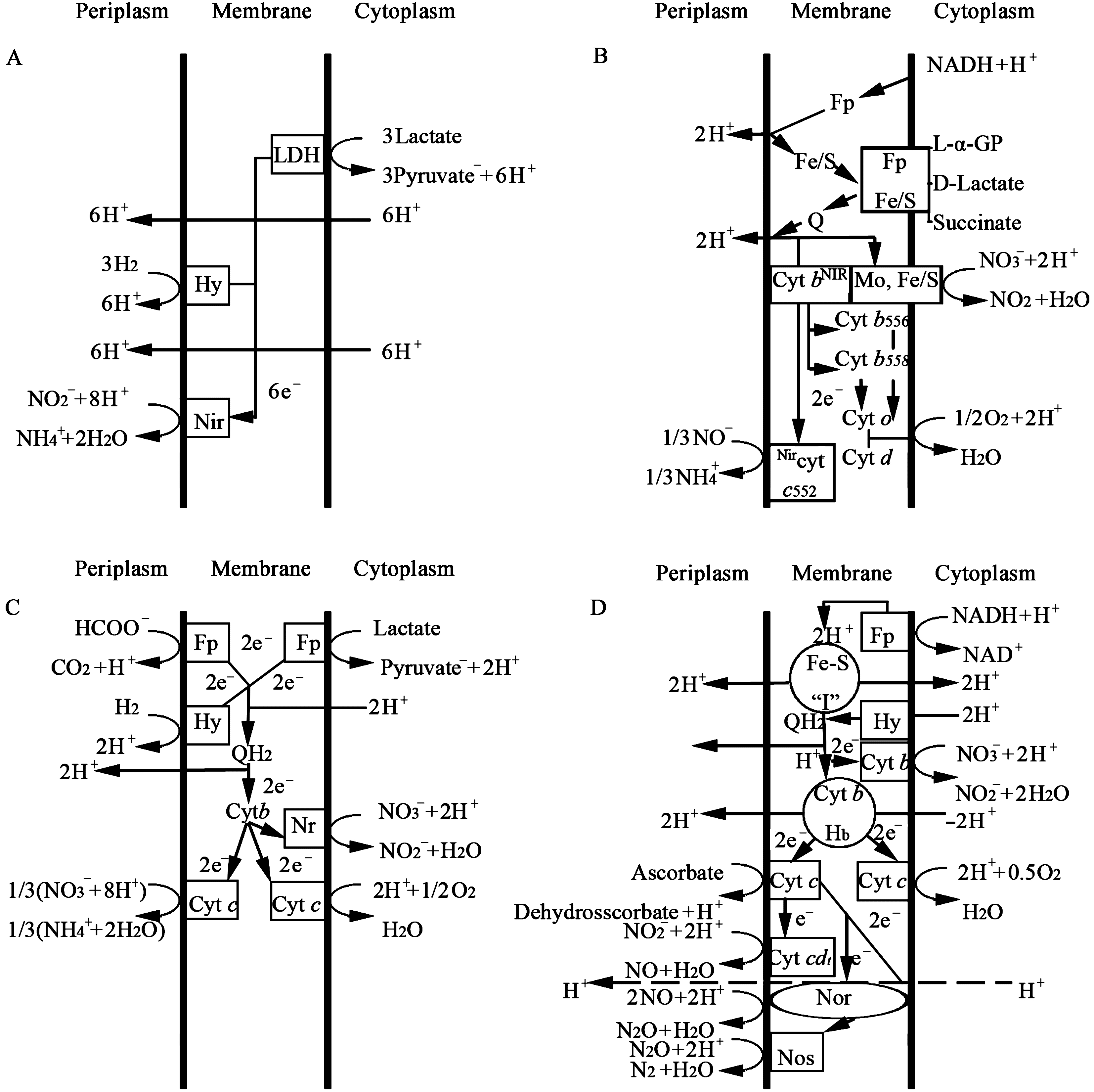
NO3−是比O2产能效率低的电子受体[19],因此DNRA细菌多为专性厌氧菌和兼性厌氧菌,如Escherichia、Klebsiella、Citrobacter、Proteus、Desulfovibrio、Wolinella、Haemophilus、Achromobacter、Clostridium、Streptococcus、Neisseriasubflava等11属[18]。在常见的存在DNRA的生境中,一般主导区系是Aeromonas、Enterobacteria 等兼性厌氧发酵细菌[18],但也有具备DNRA功能的微嗜氧菌和严格好氧菌(表 1)[20]。Campylobacter sputorum是目前报道的唯一具备DNRA功能的微嗜氧细菌,其电子传递链与专性厌氧DNRA细菌、兼性厌氧DNRA细菌的电子传递链差异都较大,与反硝化细菌的电子传递链较为相似(图 1)[9]。而好氧细菌种类相对较多,如Bacillus属、Pscudomonas属中的多种细菌都具备DNRA功能,但其具体机制尚不明确。
| 严格厌氧细菌 Strict anaerobic bacteria | 兼性厌氧细菌 Facultative anaerobic bacteria | 好氧细菌 Aerobic bacteria |
| Veillonella alcaleseens | Salmonella typH imurium | Bacillus licheniformis[31] |
| Closridium spp. | Citrobacter sp. | Bacillus macerans[32] |
| Wolinelle succimogenes | Escherichia coli | Bacillus subtilis[33, 34] |
| Desulfovibrio desulfuricans | Enterobacter aerogenes | Bacillus spp. |
| Desulfovibrio sp. | Klebsiella sp. | Bacillus sp.[35] |
| Desulfovibrio gigas | Serratia marcescens | Neisseria subflava |
| Kuenenia stuttgartiensis | Erwinia carotovora | Pseudomonas spp. |
| Selenomonas ruminantium | Photobacterium fischeri | Pseudomonas aeruginosa |
| Closridium perfringens[13] | Vibrio sp. | Campylobacter sputoum |
| Vibrio fischeri |
DNRA广泛存在于土壤[21, 22, 23, 24]、稻田[25]、湿地、消化污泥[26],反刍动物的瘤胃[27]以及淡水[10]、河口、海洋沉积物[28, 29]等生境中。由于DNRA过程有利于土壤中氮素的保存[30],我国对其的研究主要集中在稻田水淹土壤中[13],缺乏对其他生境的研究。
2 河口生态系统氮循环概述河口是连接河流与海洋的过渡地带,流场受到径流动力和海洋动力相互作用的影响。河口区域受到潮汐循环、咸淡水交替、出露淹没交替、泥沙冲淤交替等海陆互作的影响,各种环境因子复杂多变,多种氮循环过程在此并存,其中河口沉积物既是氮素营养盐的源和汇,也是以氮生化反应为主的微生物富集的场所,在氮循环的建立和平衡中起着主导作用。
河口又往往经济发达、人口密集,人为干扰向河口输入大量外源氮素,使河口区域的环境因子更加复杂,影响微生物群落及其相互作用[36, 37, 38, 39],引发水生态安全、饮用水安全和全球变暖等重大环境问题,故研究河口的氮循环过程对可持续发展有重要意义。
目前关于河口地区的氮循环形成的研究主要得出了以下两个结论[40, 41]:首先,反硝化是减少入海氮通量及富营养化水环境中氮素去除的最好手段。全球范围内河口地区总氮负荷中约50%由反硝化去除。其次,河口地区通常可去除径流中10%−60%的氮负荷。
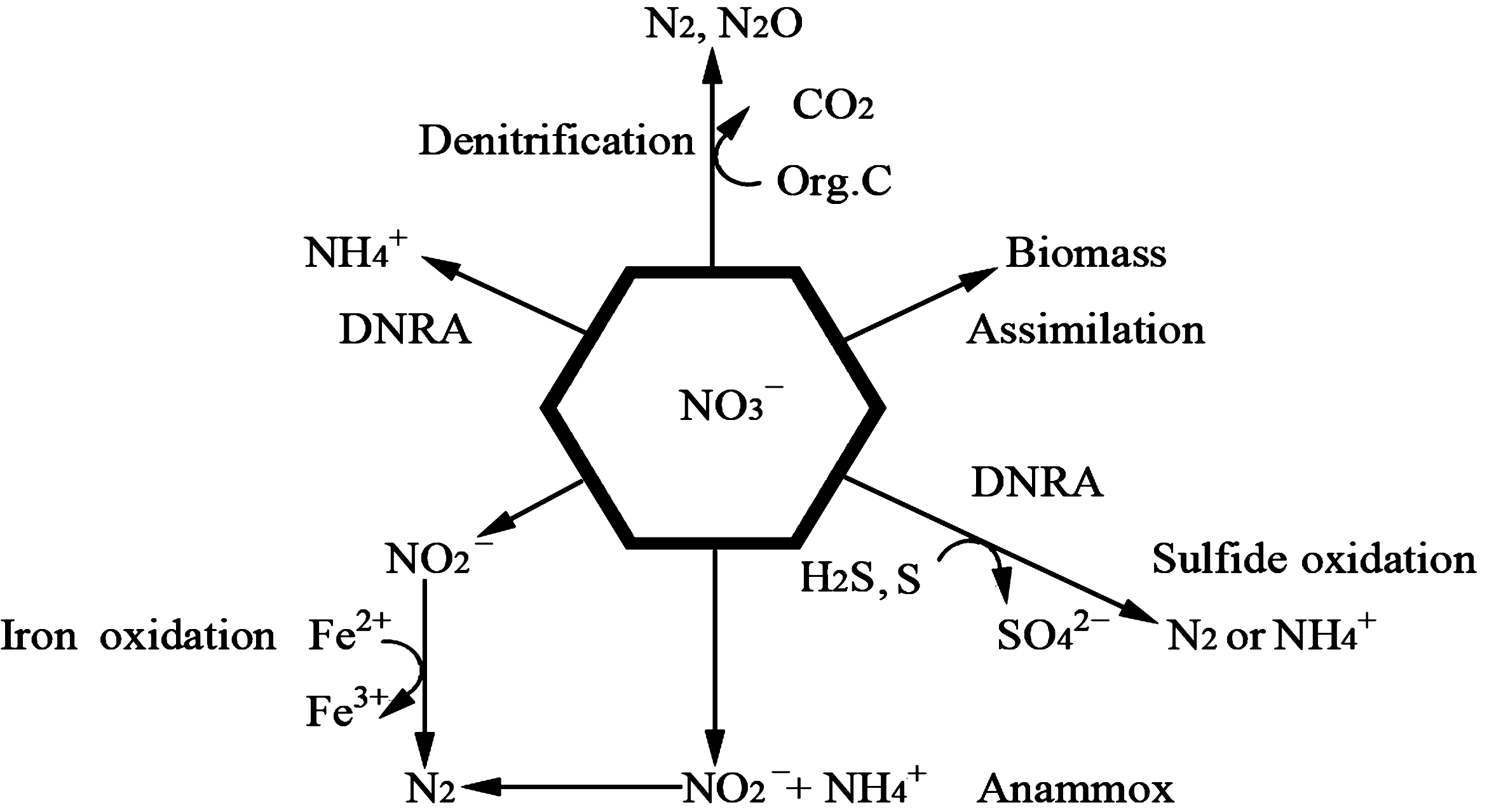
然而,由于氮转化途径复杂多样,现有海洋氮循环通量模型还无法计算达到氮平衡的范围[42]。可见,可能还存在一些被忽视的重要氮循环途径。针对新发现的氮循环途径和河口区的特殊环境,Santoro[43]在2010年提出了河口区氮循环的主要途径(图 2)[43],较为全面地推测了此类区域的氮素的来龙去脉。
3 河口生态系统氮循环中DNRA的地位与影响尽管自20世纪80年代末,DNRA过程存在于自然生态系统的氮循环过程之中的事实就已经被确认了[18],但是对DNRA过程的重要性的认识还是在近十年之内的事情[44]:不同生态微环境下广泛存在DNRA过程,占初始NO3span>−的10%−90%[45],DNRA在近海河口更是一种氮素内循环的重要过程[45, 46, 47],比如丹麦的Norsmide Fjord,其表层沉积物只发生DNRA过程[48]。
一般DNRA过程在亚热带河口水体较为剧烈,例如Gardener等[49, 50]的同位素实验表明:德克萨斯州海湾中15%−75%的NO3−通过DNRA转化为NH4+,仅5%−29%的NO3−转化为N2。而他们对佛罗里达州海湾的研究表明:在该相对高温高盐的河口滨海系统中,夏季富营养化严重,DNRA过程剧烈,河口富营养化加剧[51]。澳大利亚的塔斯马尼亚州的河口也出现相同的现象:在水体富营养化过程中氮素内循环生成的NH4+占NH4+总量的10%−50%,并发生积累[52]。
我国对于河口DNRA途径的研究较少,主要集中在珠江口。韦宗敏等[53]在研究微生物好氧反硝化机理时,在利用珠江口沉积物筛选好氧反硝化菌种过程中发现了能在碳源充足的情况下进行DNRA的微生物群落,其转化效率可达30%;蒋然等[52]测试了不同碳源对珠江河口沉积物中DNRA过程的影响;徐继荣等[54]研究发现珠江口入海河段水样和沉积物NO3−的还原速率远大于反硝化速率,都说明NO3−生化转化途径中硝化-反硝化耦合反应不是唯一的主要途径。在长江口的氮循环方面,林啸[55]研究发现:N2O排放与沉积物水界面NH4+-N通量而非NOx-N通量呈正相关,因此长江口滨岸沉积物-水气界面N2O排放的主要氮转化过程更为可能是DNRA。
但即使在相近的河口区域,DNRA过程也会有很大差异,例如林啸[55]对密西西比河口氮循环研究表明DNRA过程对NO3−的去除和NH4+的生成的贡献都很小,与Childs等[56]在墨西哥湾相近区域的研究结果较不同。可见,进一步认识、了解调控该过程的环境因子的重要性。
4 河口生态系统环境因子对DNRA过程的影响 4.1 盐度与硫酸盐盐度是河口生态系统中变化最为剧烈的环境因子。Laverman等[57]在咸-淡水交汇区的沉积物水土体系氮循环模拟实验中,观察到随盐度升高,DNRA过程的反应速率比淡水环境中提高35 mmol/(m3·h),可见,咸潮入侵对DNRA过程可能有促进作用。而在长时间尺度上,Giblin等[58]对Parker河口进行了长达13年的监测研究后,发现沉积物-水界面营养通量以铵为主,铵通量与盐度有较高相关性,DNRA过程的季节变化模式与盐度时间尺度变化一致。
对于盐度对DNRA过程的影响有两种解释:第一种认为这是由于与DNRA过程竞争的反硝化过程受到盐度的抑制作用,但也有研究表明反硝化对盐度并不敏感,故这一观点还存在争议[43, 48, 49, 50, 51];另一个较受认可的解释认为,随着盐度升高,SO42−含量下降,硫还原细菌的DNRA过程的电子供体(即低价态硫化物)就相应增多了,这既能抑制反硝化过程又能促进DNRA过程[13, 49, 50, 59]。
4.2 氧化还原电位(Eh)一般认为,只有在强烈还原条件下 (Eh<−200 mV) DNRA过程才会在NO3−异化还原中起到重要作用[21],例如殷士学[60]对淹水土壤的培养实验显示:Eh在−100 mV之上时对DNRA影响不大,但从−100 mV降至−340 mV时DNRA过程明显增强。但也有对土壤的研究表明,DNRA过程对Eh的敏感性并不及反硝化过程[61],该结论是否适用于河口沉积物还有待研究。
4.3 溶解氧(DO)虽然存在对氧浓度不敏感的DNRA细菌[13],但一般认为,低DO让DNRA菌群比反硝化菌群更具竞争力[15, 18],如宋国栋[62]对长江口和东海沉积物监测显示:随DO减少至严重缺氧,厌氧氨氧化(Anammox)和反硝化速率分别降低了38%和43%,DNRA速率则增加了3倍。
这种现象可以归因于以下3点:(1)严重缺氧条件下沉积物有机氮矿化速率降低,硝化速率降低,从而抑制了反硝化过程等硝酸盐代谢途径[60]。(2) 缺氧意味着氧化还原电位较低,对DNRA过程,尤其是硫还原菌的DNRA过程更为有利。(3) DNRA菌群一般以兼性厌氧发酵细菌为主。缺氧会迫使这些细菌更多地进行DNRA过程产能。
4.4 碳源、氮源及碳氮比(C/N)一般认为,DNRA过程在氮源缺乏、碳源丰富而不稳定的环境中更容易发生,并成为硝酸盐异化还原的主要途径[13, 57, 63]。
氮源方面,一些细菌不适合利用NO2−作为氮源,这可能与NO2−对生物体有较大的毒害作用有关;但是也有少数细菌,如Desulfovibrio desulfuricans只能以NO2−为氮源进行代谢。因此,NO3−可能是更有利于DNRA细菌生长的氮源[60]。但高浓度NO3−对DNRA也存在抑制作用,例如在盐沼沉积物中,可利用的NO3−增加会使DNRA过程去除NO3−的效率由52%降至4%[64]。另外,虽然低浓度氮源对N2O的产出无关,但高浓度氮源有利于N2O产出[59]。DNRA过程的产物氨氮浓度则对DNRA过程没有影响,DNRA过程产生的氨氮可以成为其他微生物的氮源[10, 65]。
碳源方面,在有机质富集的沉积物中DNRA过程去除NO3−可达50%,与反硝化相当[41, 66]。Tiedje[20]认为易分解的碳源可被高效利用电子受体的DNRA细菌充分利用,因为DNRA过程中1 mol NO3−接受8 mol电子,而反硝化过程中仅接受5 mol电子。该观点在之后的研究中得到了证明[10],即DNRA更趋向于在氧化程度较弱的碳源环境中发生。韦宗敏[13]对珠江口沉积物培养实验表明:在C/N为2−10的条件下,DNRA过程都会发生,但碳源氧化状态越强,DNRA过程所需要的适宜C/N越高。
4.5 温度DNRA过程在亚热带河口、海洋区域较为剧烈,可见其对温度的要求较高。韦宗敏[13]对珠江口沉积物培养实验显示:该区域混合菌群的产铵率在30 °C达到最高,为47.59%,随后产铵率随温度上升下降,高于Anammox过程的最佳温度12 °C和反硝化过程的最佳温度24 °C。
4.6 pH韦宗敏[13]对珠江口沉积物进行培养实验后发现:初始pH值为7.0时,产铵率最高达25.71%,N2O的产出量也达最高,pH 6.0−10.0之间时,DNRA过程都可以发生;殷士学[59]通过淹水土壤的培养实验得出:DNRA过程发生于pH 5.0−8.0之间。一般认为DNRA过程多发生于偏碱性的环境中,而且DNRA过程本身也是产碱过程,因此环境pH和DNRA过程可能存在相互影响。
4.7 反硝化过程与厌氧氨氧化过程硝酸盐有较多的生化转化途径(图 3)[18]。其中DNRA过程、反硝化过程和Anammox过程是沉积物厌氧环境中氮循环的3种关键过程[62]。
反硝化过程将NO3−还原为N2O或N2,而Anammox过程以NO2−为电子受体,NH4+为电子供体产生N2O[67]和N2。两个过程会与DNRA过程竞争相同的底物,但生化过程一般是复杂的,每种过程都适应于特定的外界条件(图 4)[68]。
一般认为反硝化过程是DNRA过程的主要竞争者,但两类细菌的共培养实验显示:无论哪类细菌数量占优,都共同利用环境中的氮源和碳源,不抑制对方生长[53, 69]。
而Anammox过程既参与对氮源的竞争,也可以与DNRA过程耦合达到脱氮的效果,因为DNRA过程可以为Anammox过程提供底物——NO2−和NH4+。DNRA-Anammox耦合过程中执行DNRA过程的不仅可以是DNRA细菌,也可能是Anammox细菌,如Kueneniastuttgartiensis[47]。
虽然DNRA-Anammox耦合过程与反硝化过程不同,但最终结果都是将NO3−转化为N2并伴随少量N2O产生,因此难以用传统的同位素示踪法区分,需要辅助其他示踪技术和基因标记方法来研究[67]。因此虽然3种过程在沉积物中的发生频率和效率的比例决定了沉积物中氮的收支,但目前的研究一般只考虑3种过程中的一种或两种[70, 71, 72, 73]。尽管目前亦有能在三者共存条件下区分各自贡献的方法[74],但需要建立开放的稳态体系,尚未见研究报道。
5 总结与展望DNRA过程将在水体中易转移的NO3−转换为不易转移的NH4+,与两种主要脱氮过程——反硝化和Anammox过程竞争相同底物,可能加剧水体富营养化;但同时DNRA-Anammox耦合过程意味着DNRA过程可能有利于水体的脱氮。因此,探究DNRA过程对其它氮循环过程的影响和相互关系是非常有意义的。
国内目前对DNRA过程的研究较少,尤其是对于河口这样一个环境复杂、连接咸淡水两种生态系统的重要脱氮场所,DNRA过程所扮演的具体角色、受到哪些环境因子的调控以及具体调控机理还有待具体研究,同时新的实验技术和研究方法有待开发。此外,进一步研究DNRA细菌的生理生化特征和代谢途径,也将深入揭示DNRA过程的微生物基础。
综上,研究河口生态系统中的DNRA过程有助于加深我们对氮循环的认识,为我国河口地区甚至近海的水资源保护和生态治理提供科学指导。
| [1] | Chen SY, Wu ZM, Yu WB, et al. Formation, harmfulness, prevention, control and treatment of waters eutrophication[J]. Environmental Science and Technology, 1999(2): 11-15 (in Chinese) 陈水勇, 吴振明, 俞伟波, 等. 水体富营养化的形成、危害和防治[J]. 环境科学与技术, 1999(2): 11-15 |
| [2] | Fan B, Qu JH, Liu SX, et al. Removal of nitrate in drinking water[J]. Techniques and Equipments for Environmental Pollution Control, 2000, 1(3): 44-50 (in Chinese) 范彬, 曲久辉, 刘锁祥, 等. 饮用水中硝酸盐的脱除[J]. 环境污染治理技术与设备, 2000, 1(3): 44-50 |
| [3] | Luo ZJ, Jin MG. Research progress of ammonia, nitrite and nitrate pollution in grountwater[J]. Hydrogeology and Engineering Geology, 2002, 29(4): 65-69 (in Chinese) 罗泽娇, 靳孟贵. 地下水三氮污染的研究进展[J]. 水文地质工程地质, 2002, 29(4): 65-69 |
| [4] | Woods DD. The reduction of nitrate to ammonia by Clostridium welchii[J]. Biochemical Journal, 1938, 32(11): 2000-2012 |
| [5] | He JZ, Zhang LM. Key processes and microbial mechanisms of soil nitrogen transformation[J]. Microbiology China, 2013, 40(1): 98-108 (in Chinese) 贺纪正, 张丽梅. 土壤氮素转化的关键微生物过程及机制[J]. 微生物学通报, 2013, 40(1): 98-108 |
| [6] | Huang C, He QM, Wu HD, et al. Study progress on the mechanism of dissimilatory Nitrate Reduction in Fungi[J]. Microbiology China, 2009, 36(7): 1052-1057 (in Chinese) 黄灿, 何清明, 邬红东, 等. 真菌异化硝酸盐还原机理的研究进展[J]. 微生物学通报, 2009, 36(7): 1052-1057 |
| [7] | Chèneby D, Hartmann A, Hénault C, et al. Diversity of denitrifying microflora and ability to reduce N2O in two soils[J]. Biology and Fertility of Soils, 1998, 28(1): 19-26 |
| [8] | Li XP, Fang T, Ao HY, et al. Vertical variation of dissimilatory nitrate reductase activity and nitrate reducer in the sediments of Lake Donghu[J]. China Environmental Science, 2010, 30(2): 228-232 (in Chinese) 李小平, 方涛, 敖鸿毅, 等. 东湖沉积物中dNaR 活性和硝酸盐还原菌的垂向分布[J]. 中国环境科学, 2010, 30(2): 228-232 |
| [9] | Yin SX, Shen QR. Physiological and biochemical characteristics of nitrate reducers in anaerobic soils[J]. Acta Pedologica Sinica, 2003, 40(4): 624-630 (in Chinese) 殷士学, 沈其荣. 缺氧土壤中硝态氮还原菌的生理生化特征 [J]. 土壤学报, 2003, 40(4): 624-630 |
| [10] | Kelso BHL, Smith RV, Laughlin RJ, et al. Dissimilatory nitrate reduction in anaerobic sediments leading to river nitrite accumulation[J]. FEMS Microbiology Ecology, 1997, 63(12): 4679-4685 |
| [11] | Yin SX, Lu JF. The microorganisms’ mediated processes of dissmilatory reduction of nitrate to ammonium[J]. Microbiology China, 1997, 24(3): 170-173 (in Chinese) 殷士学, 陆驹飞. 硝酸异化还原成铵的微生物学过程[J]. 微生物学通报, 1997, 24(3): 170-173 |
| [12] | Darwin A, Hussain H, Griffiths L, et al. Regulation and sequence of the structural gene for cytochrome C552 from Escherichia coli —— not a hexahaem but a 50 kD tetrahaem nitrite reductase[J]. Molecular Microbiology, 1993, 9(6): 1255-1265 |
| [13] | Wei ZM. Preliminary research on dissimilatory nitrate reduction to ammonium in microaerobic condition[D]. Guangzhou: Master’s Thesis of South China University of Technology, 2012 (in Chinese) 韦宗敏. 微好氧环境中硝酸盐异化还原成铵的影响研究[D]. 广州: 华南理工大学硕士学位论文, 2012 |
| [14] | Cai YJ, Ding WX, Xiang J. Mechanisms of nitrous oxide and nitric oxide production in soils: a review[J]. Soil, 2012, 44(5): 712-718 (in Chinese) 蔡延江, 丁维新, 项剑. 土壤N2O 和NO 产生机制研究进展 [J]. 土壤, 2012, 44(5): 712-718 |
| [15] | Tiedje JM, Sexstone AJ, Myrold DD, et al. Denitrification: ecological niches, competition and survival[J]. Antonie Van Leeuwenhoek, 1982, 48(6): 569-583 |
| [16] | Sayama M, Risgaard-Petersen N, Nielsen LP, et al. Impact of bacterial NO3 - transport on sediment biogeochemistry[J]. Applied and Environmental Microbiology, 2005, 71(11): 7575-7577 |
| [17] | Zopfi J, Kjær T, Nielsen LP, et al. Ecology of Thioploca spp.: Nitrate and sulfur storage in relation to chemical microgradients and influence of Thioploca spp. on the sedimentary nitrogen cycle[J]. Applied and Environmental Microbiology, 2001, 67(12): 5530-5537 |
| [18] | Steenkamp DJ, Peck HD Jr. Proton translocation associated with nitrite respiration in Desulfovibrio desulfuricans[J]. The Journal of Biological Chemistry, 1981, 256(11): 5450-5458 |
| [19] | Alexander RB, Smith RA, Schwarz GE. Effect of stream channel size on the delivery of nitrogen to the Gulf of Mexico[J]. Nature, 2000, 403(6771): 758-761 |
| [20] | Tiedje JM. Ecology of denitrification and dissimilatory nitrate reduction to ammonium[A]//Biology of Anaerobic Microorganisms[M]. New York: John Wiley & Son, 1988: 179-244 |
| [21] | Stanford G, Legg JO, Dzienia S, et al. Denitrification and associated nitrogen transformation in soils[J]. Soil Science, 1975, 120(2): 147-152 |
| [22] | Buresh RJ, Patrick WH. Nitrate reduction to ammonium in anaerobic soil[J]. Soil Science Society of America Journal, 1978, 42(6): 913-918 |
| [23] | Caskey WH, Tiedje JM. Evidence for Clostridia as agents of dissimilatory reduction of nitrate to ammonium in soils[J]. Soil Science Society of America Journal, 1979, 43(5): 931-936 |
| [24] | Fazzolari E, Matiotti A, Germon JC. Nitrate reduction to ammonia: a dissimilatory process in Enterobacter amnigenus[J]. Canadian Journal of Microbiology, 1990, 36(11): 779-785 |
| [25] | Yin SX, Chen D, Chen LM, et al. Dissimilatory nitrate reduction to ammonium and responsible microorganisms in two Chinese and Australian paddy soils[J]. Soil Biology and Biochemistry, 2002, 34(8): 1131-1137 |
| [26] | Kasper HF, Tiedje JM, Firestone RB. Denitrification and dissimilatory nitrate reduction to ammonium in digested sludge[J]. Canadian Journal of Microbiology, 1981, 27(9): 878-885 |
| [27] | Kaspar HF, Tiedje JM. Dissimilatory reduction of nitrate and nitrite in the bovine rumen: nitrous oxide production and effect of acetylene[J]. Applied and Environmental Microbiology, 1981, 41(3): 705-709 |
| [28] | Bonin P, Omnes P, Chalamet A. Simultaneous occurrence of denitrification and nitrate ammonification in sediments of the French Mediterranean Coast[J]. Hydrobiologia, 1998, 389(1/3): 169-182 |
| [29] | Koike I, Hattori A. Denitrification and ammonia formation in anaerobic coastal sediments[J]. Applied and Environmental Microbiology, 1978, 35(2): 278-282 |
| [30] | Dalagaard T, Bak F. Nitrate reduction in a sulfate-reducing bacterium, Desulfovibrio desulfuricans, isolated from rice paddy soil: sulfide inhibition, kinetics and regulation[J]. Applied and Environmental Microbiology, 1994, 60(1): 291-297 |
| [31] | Shariat P, Mitchell WJ, Boyd A, et al. Anaerobic metabolism in Bacillus licheniformis NCIB 6346[J]. Microbiology, 1995, 141(5): 1117-1124 |
| [32] | Schirawski J, Uden G. Anaerobic respiration of Bacillus macerans with fumarate, TMAO, nitrate and nitrite and regulation of the pathways by oxygen and nitrate[J]. Archives of Microbiology, 1995, 163(2): 148-154 |
| [33] | Nakano MM, Zuber P. Anaerobic growth of a “strict aerobe” (Bacillus subtilis)[J]. Annual Review of Microbiology, 1998, 52(1): 165-190 |
| [34] | Hoffmann T, Frankenberg N, Marino M, et al. Ammonification in Bacillus subtilis utilizing dissimilatory nitrite reductase is dependent on resDE[J]. Journal of Bacteriology, 1998, 180(1): 186-189 |
| [35] | Smith MS, Zimmerman K. Nitrous oxide production by nondenitrifying soil nitrate reducers[J]. Soil Science Society of America Journal, 1981, 45(5): 865-871 |
| [36] | Sun RH, Wen DH. Responses to external ammonium source by the ammonia oxidation microorganisms from Hangzhou bay[J]. Acta Scientiarum Naturalium Universitatis Pekinensis, 2013, 49(5): 908-914 (in Chinese) 孙仁华, 温东辉. 杭州湾水环境中氨氧化微生物对外源氨氮的响应[J]. 北京大学学报: 自然科学版, 2013, 49(5): 908-914 |
| [37] | Zhang Y, Chen LJ, Dai TJ, et al. Ammonia manipulates the ammonia-oxidizing Archaea and bacteria in the coastal sediment-water microcosms[J]. Applied Microbiology and Biotechnology, 2015, 99(15): 6481-6491 |
| [38] | Zhang Y, Chen LJ, Sun RH, et al. Effect of wastewater disposal on the bacterial and archaeal community of sea sediment in an industrial area in China[J]. FEMS Microbiology Ecology, 2014, 88(2): 320-332 |
| [39] | Zhang Y, Chen LJ, Sun RH, et al. Ammonia-oxidizing bacteria and archaea in wastewater treatment plant sludge and nearby coastal sediment in an industrial area in China[J]. Applied Microbiology and Biotechnology, 2015, 99(10): 4495-4507 |
| [40] | Jetten MSM. The microbial nitrogen cycle[J]. Environmental Microbiology, 2008, 10(11): 2903-2909 |
| [41] | Herbert RA. Nitrogen cycling in coastal marine ecosystems[J]. FEMS Microbiology Reviews, 1999, 23(5): 563-590 |
| [42] | Codispoti LA, Brandes JA, Christensen JP, et al. The oceanic fixed nitrogen and nitrous oxide budgets: moving targets as we enter the Anthropocene?[J]. Scientia Marina, 2001, 65(S2): 85-105 |
| [43] | Santoro AE. Microbial nitrogen cycling at the saltwater-freshwater interface[J]. Hydrogeology Journal, 2010, 18(1): 187-202 |
| [44] | Burgin AJ, Hamilton SK. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways[J]. Frontiers in Ecology and the Environment, 2007, 5(2): 89-96 |
| [45] | Jørgensen KS. Annual pattern of denitrification and nitrate ammonification in estuarine sediment[J]. Applied and Environmental Microbiology, 1989, 55(7): 1841-1847 |
| [46] | Enoksson V, Samuelsson MO. Nitrification and dissimilatory ammonium Production and their effects on nitrogen flux over the sediment-water interface in bioturbated coastal sediments[J]. Marine Ecology Progress Series, 1987, 36: 181-189 |
| [47] | Kartal B, Kuypers MMM, Lavik G, et al. Anammox bacteria disguised as denitrifiers: nitrate reduction to dinitrogen gas via nitrite and ammonium[J]. Environmental Microbiology, 2007, 9(3): 635-642 |
| [48] | Hansen JI, Henriksen K, Blackburn TH. Seasonal distribution of nitrifying bacteria and rates of nitrification in coastal marine sediments[J]. Microbial Ecology, 1981, 7(4): 297-304 |
| [49] | An S, Gardner WS. Dissimilatory nitrate reduction to ammonium(DNRA) as a nitrogen link versus denitrification as a sink in a shallow estuary(Laguna Madre/Baffin Bay, Texas)[J]. Marine Ecology Progress Series, 2002, 237: 41-50 |
| [50] | Gardner WS, McCarthy MJ, An S, et al. Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries[J]. Limnology and Oceanography, 2006, 51(2): 558-568 |
| [51] | Gardner WS, McCarthy MJ. Nitrogen dynamics at the sediment-water interface in shallow, sub-tropical Florida Bay: why denitrification efficiency may decrease with increased eutrophication[J]. Biogeochemistry, 2009, 95(2/3): 185-198 |
| [52] | Jiang R, Li ZX. Active nitrogen accumulation mechanism in typical estuarine regions based on dissimilatory nitrate reduction to ammonium[J]. Water Resources Protection, 2014(4): 10-13 (in Chinese) 蒋然, 李召旭. 典型河口区硝态氮短程还原成铵的活性氮累积途径研究进展[J]. 水资源保护, 2014(4): 10-13 |
| [53] | Wei ZM, Huang SB, Jiang R. Effect of carbon on dissimilatory Nitrate reduction to ammonium process[J]. Industrial Safety and Environmental Protection, 2012, 38(9): 4-7, 14 (in Chinese) 韦宗敏, 黄少斌, 蒋然. 碳源对微生物硝酸盐异化还原成铵过程的影响[J]. 工业安全与环保, 2012, 38(9): 4-7, 14 |
| [54] | Xu JR, Wang YS, Yin JP, et al. Transformation of dissolved inorganic nitrogen species and nitrigication and denitrification processes in the near sea section of Zhujiang River[J]. Acta Scientiae Circumstantiae, 2005, 25(5): 686-692 (in Chinese) 徐继荣, 王友绍, 殷建平, 等. 珠江口入海河段DIN 形态转化与硝化和反硝化作用[J]. 环境科学学报, 2005, 25(5): 686-692 |
| [55] | Lin X. Nitrogen cycle processes and influence mechanism research from the typical estuarine area[D]. Shanghai: Doctoral Dissertation of East China Normal University, 2011 (in Chinese) 林啸. 典型河口区氮循环过程和影响机制研究[D]. 上海: 华东师范大学博士学位论文, 2011 |
| [56] | Childs CR, Rabalais NN, Turner RE, et al. Sediment denitrification in the Gulf of Mexico zone of hypoxia[J]. Marine Ecology Progress Series, 2002, 240(1): 285-290 |
| [57] | Laverman AM, Canavan RW, Slomp CP, et al. Potential nitrate removal in a coastal freshwater sediment (Haringvliet Lake, The Netherlands) and response to salinization[J]. Water Research, 2007, 41(14): 3061-3068 |
| [58] | Giblin AE, Weston NB, Banta GT, et al. The effects of salinity on nitrogen losses from an Oligohaline Estuarine sediment[J]. Estuaries and Coasts, 2010, 33(5): 1054-1068 |
| [59] | Seitzinger S. Nitrogen cycle: out of reach[J]. Nature, 2008, 452(7184): 162-163 |
| [60] | Yin SX. Dissimilatory nitrate reduction to ammonium in submerged soils[D]. Nanjing: Doctoral Dissertation of Nanjing Agricultural University, 2000 (in Chinese) 殷士学. 淹水土壤中硝态氮异化还原成铵过程的研究[D]. 南京: 南京农业大学博士学位论文, 2000 |
| [61] | Pett-Ridge J, Silver WL, Firestone MK. Redox fluctuations frame microbial community impacts on N-cycling rates in a humid tropical forest soil[J]. Biogeochemistry, 2006, 81(1): 95-110 |
| [62] | Song GD. Benthic nitrogen loss and transformations in the East China Sea[D]. Qingdao: Doctoral Dissertation of Ocean University of China, 2013 (in Chinese) 宋国栋. 东海沉积物中氮循环的关键过程[D]. 青岛: 中国海洋大学博士学位论文, 2013 |
| [63] | Seitzinger SP. Denitrification in freshwater and coastal marine ecosystems: Ecological and geochemical significance[J]. Limnology and Oceanography, 1988, 33(4): 702-724 |
| [64] | Dong LF, Thornton DCO, Nedwell DB, et al. Denitrification in sediments of the River Colne Estuary, England[J]. Marine Ecology Progress Series, 2000, 203(1): 109-122 |
| [65] | Guo Y. The study of nitrate as a sole nitrogen source for the growth of heterotrophic denitrifying bacteria in activated sludge[D]. Xi’an: Master’s Thesis of Xi’an University of Architecture and Technology, 2013 (in Chinese) 郭瑜. 硝酸盐氮为唯一氮源时异养微生物增长特性的研究 [D]. 西安: 西安建筑科技大学硕士学位论文, 2013 |
| [66] | Abril G, Riou SA, Etcheber H, et al. Transient, tidal time-scale, nitrogen transformations in an estuarine turbidity maximum-fluid mud system (The Gironde, South-west France)[J]. Estuarine, Coastal and Shelf Science, 2000, 50(5): 703-715 |
| [67] | Guo JH, Peng YZ. Heterotrophic nitrification, anaerobic ammonia oxidation and archaeal ammonia oxidation in a new nitrogen cycle[J]. Acta Scientiae Circumstantiae, 2008, 28(8): 1489-1498 (in Chinese) 郭建华, 彭永臻. 异养硝化、厌氧氨氧化及氨氧化古菌与新的氮循环[J]. 环境科学学报, 2008, 28(8): 1489-1498 |
| [68] | Weber KA, Picardal FW, Roden EE. Microbially catalyzed nitrate-dependent oxidation of biogenic solid-phase Fe(Ⅱ) compounds[J]. Environmental Science & Technology, 2001, 35(8): 1644-1650 |
| [69] | Chen LM. The amount, the physiological groups and the reducing characteristics of denitrifiers and DNRA bacteria in submerged soils[D]. Yangzhou: Master’s Thesis of Yangzhou University, 2001 (in Chinese) 陈丽敏. 淹水土壤中反硝化菌和硝酸还原菌数量、生理类群及其还原特点的研究[D]. 扬州: 扬州大学硕士学位论文, 2001 |
| [70] | Nielsen LP. Denitrification in sediment determined from nitrogen isotope pairing[J]. FEMS Microbiology Letters, 1992, 86(4): 357-362 |
| [71] | Thamdrup B, Dalsgaard T. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments[J]. Applied and Environmental Microbiology, 2002, 68(3): 1312-1318 |
| [72] | Risgaard-Petersen N, Nielsen LP, Rysgaard S, et al. Application of the isotope pairing technique in sediments where anammox and denitrification co-exist[J]. Limnology and Oceanography: Methods, 2003, 1(1): 63-73 |
| [73] | Jensen MM, Lam P, Revsbech NP, et al. Intensive nitrogen loss over the Omani Shelf due to anammox coupled with dissimilatory nitrite reduction to ammonium[J]. The ISME Journal, 2011, 5(10): 1660-1670 |
| [74] | Spott O, Stange CF. A new mathematical approach for calculating the contribution of anammox, denitrification and atmosphere to an N2 mixture based on a 15N tracer technique[J]. Rapid Communications in Mass Spectrometry, 2007, 21(14): 2398-2406 |
 2016, Vol. 43
2016, Vol. 43