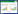扩展功能
文章信息
- 陈玉洁, 敖日格乐, 王纯洁, 畅旺东, 徐东贺, 白丹丹
- CHEN Yu-Jie, CHEN Aorigele, WANG Chun-Jie, CHANG Wang-Dong, XU Dong-He, BAI Dan-Dan
- 酸马奶提取Kluyveromyces marxianus代谢抗菌复合物对感染致病性Escherichia coli O8小鼠的免疫机能及其盲肠菌群的影响
- Effect of antimicrobial compounds of Kluyveromyces marxianus in Koumiss on immune function and caecal microflora in mice challenged with pathogenic Escherichia coli O8
- 微生物学通报, 2015, 42(12): 2386-2385
- Microbiology China, 2015, 42(12): 2386-2385
- 10.13344/j.microbiol.china.150340
-
文章历史
- 收稿日期: 2015-04-22
- 接受日期: 2015-05-11
- 优先数字出版日期(www.cnki.net): 2015-06-02
2. 内蒙古农业大学 兽医学院 内蒙古 呼和浩特 010018
2. College of Veterinary Medicine, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia 010018, China
酸马奶是经酵母菌等微生物以马奶为原料发酵而成的卫生保健乳饮料,可防治心血管病、消化系统病、肺结核、糖尿病和腹泻等疾病[1]。在内蒙古和新疆地区的酸马奶样品中,马克斯克鲁维酵母Kluyveromyces marxianus为酵母菌中的优势菌[2, 3],有报道称其对单核细胞增生李斯特菌Listeria monocytogenes有抑菌作用[4, 5],源于该菌代谢产生毒素蛋白、抗生素因子、有机酸、过氧化氢等抗菌复合物[6],其中毒素蛋白是一种有抑菌活性的糖蛋白,能结合于敏感菌的细胞壁,降低敏感菌细胞内pH,抑制细胞代谢,导致细胞死亡[7]。近些年,以酿酒酵母Saccharomyces属酵母菌为主的益生菌或微生态制剂如活酵母菌、酵母菌细胞壁、酵母菌培养物逐渐被应用到动物生产中[8, 9, 10],但针对酸马奶提取K.marxianus代谢抗菌复合物在动物上的应用未见报道。
养牛业中致病性大肠杆菌Escherichia coli会引起犊牛腹泻——一种急性肠道疾病,常用抗生素来治疗。但抗生素的长期使用会引起严重的耐药性,且在牛体中的残留会直接威胁到人体健康和安全[11]。酸马奶提取K. marxianus代谢抗菌复合物在体外实验中能有效抑制致病性E. coli O8的生长,有望成为新的抗生素替代品[12]。为此,本文给小鼠连续灌胃K. marxianus代谢抗菌复合物K. marxianus pH 2.0和K. marxianus pH 8.0,并用致病性E.coli O8感染小鼠进行对比实验,论证K. marxianus代谢抗菌复合物对致病性E. coli O8感染小鼠的免疫机能及其盲肠菌群的影响,为进一步的应用研究提供理论依据。
1 材料与方法 1.1 实验材料 1.1.1 供试材料: 1号液体培养基(g/L,麦芽提取物39,酵母浸膏3,细菌学蛋白胨5,乳糖10,pH 6.0),广东环凯生物技术有限公司。免疫荧光单克隆抗体:PE anti-mouse CD4+(12-0041),PE anti-mouse CD8+(12-0081),美国Affymetrix公司。E. coli O8:奶牛粪样中分离的内蒙古优势血清型致病性E.coli。K. marxianus:内蒙古锡林郭勒盟地区牧民家庭的酸马奶样品中分离的酵母菌,由常规生化鉴定方法和26S rDNA扩增共同鉴定。K. marxianus代谢抗菌复合物:将K. marxianus接种于1号液体培养基中25 °C下培养72 h,过滤培养液后参照文献[13]采用乙酸乙酯萃取法制得抗菌复合物K. marxianus pH 2.0 (K2)和K. marxianus pH 8.0 (K8),主要成分为丙酸(分别为2 711.3 mg/100 g和1 874.7 mg/100 g)、乳酸(680.8 mg/100 g和672.0 mg/100 g)、苹果酸(673.1 mg/100 g和13.2 mg/100 g)、抗坏血酸 (743.9 mg/100g和405.3 mg/100 g)和毒素蛋白(75.0 mg/100 g和74.1 mg/100 g)。 1.1.2 实验动物与实验设计:选用128只昆明系小白鼠,体重20±2 g,雌雄各半,随机分为4组,空白组、致病对照组、K2组和K8组,每组32只,实验方案见表 1。50%致死浓度(Minimum lethal dose,MLD) E. coli O8为4.7×109 CFU/mL。小鼠饲喂颗粒饲料,不含任何抗生素,笼养,自由饮水。| 组别 Group |
实验方案 Testing program |
| 空白组 The control group |
连续灌胃无菌磷酸盐缓冲液PBS 7 d,每天一次,每次0.2 mL/只 |
| 致病对照组 The challenged control group |
连续灌胃无菌PBS 7 d,第4天腹腔注射50% MLD E. coli O 8 菌悬液0.3 mL/只 |
| K2组 K2 group |
连续灌胃溶于无菌PBS中的K2 [剂量为2 500 mg/(kg·bw)] 7 d,第4天腹腔注射50% MLD E. coli O 8 菌悬液0.3 mL/只 |
| K8组 K8 group |
同K2组类似,灌胃溶于无菌PBS中的K8 [剂量为2 500 mg/(kg·bw)],并注菌 |
数据以 x±s 表示,采用SAS 8.0进行单因素方差分析,组间差异进行Duncan多重比较,P<0.05为差异有统计学意义。
2 结果与分析 2.1 K2和K8对感染致病性E. coli O8小鼠临床症状和小肠病理变化的影响 2.1.1 K2和K8对感染致病性E. coli O8小鼠临床症状的影响:空白组小鼠7 d内无明显变化。致病对照组小鼠在实验第4天注菌后8−12 h,部分小鼠精神不振,开始聚堆。注菌后24−36 h出现明显临床症状,主要为眯眼、腹泻、厌食、精神萎靡、行动迟缓、聚堆,部分小鼠死亡。注菌后48−72 h (实验第7天)死亡小鼠数量增加,但未死亡小鼠精神状态出现好转,行动比先前敏捷。K2组和K8组小鼠整体精神状态好于致病对照组,死亡小鼠数量较少。 2.1.2 K2和K8对感染致病性E. coli O8小鼠小肠病理变化的影响:由图 1可知,空白组小鼠小肠绒毛排列整齐,且细长紧密。致病对照组小鼠在第7天小肠组织出现病理改变,小肠绒毛矮短,松散,小肠绒毛间间隙变大,有些绒毛甚至断裂脱落。K2组和K8组小鼠在第4天小肠绒毛比空白组更长且紧密;在第7天小肠绒毛形态也有病理改变,但相对于致病对照组有所好转。表明小鼠注射致病性E. coli O8后小肠组织出现病理变化,灌胃K2和K8可以一定程度上缓解和改善感染致病性E. coli O8小鼠的小肠组织病理变化。

|
|
图 1
小鼠小肠组织病理形态学变化(HE染色,200×)
Fig.1
Pathomorphology of small intestinal in mice (HE staining, 200×)
注:A:空白组;B:7 d致病对照组;C:4 d K2组;D:7 d K2组;E:4 d K8组;F:7 d K8组.
Note: A: The control group at 0 d; B: The challenged control group at 7 d; C: K2 group at 4 d; D: K2 group p at 7 d; E: K8 group at 4 d; F: K8 group at 7 d. |
由表 2可知,各组小鼠在第0天脏器指数无差别(P>0.05)。致病对照组在第4天脏器指数与空白组无差别(P>0.05)。致病对照组在第7天脾脏指数与空白组无差别(P>0.05),胸腺指数显著低于空白组(P<0.05)。K2组和K8组在第4天和第7天脾脏指数显著高于空白组(P<0.05),胸腺指数与空白组无差别(P>0.05)。表明小鼠注射致病性E. coli O8后会降低胸腺指数,灌胃K2和K8可以提高感染致病性E. coli O8小鼠的脏器指数。
| 项目 Item |
时间 Time (d) |
组别 Group |
|||
| 空白组 The control group |
致病对照组 The challenged control group |
K2组 K2 group |
K8组 K8 group |
||
| 脾脏指数 Spleen index |
0 | 5.63±0.52 | 5.59±0.89 | 5.56±0.77 | 5.60±0.80 |
| 4 | 5.71±1.48 b | 5.75±1.70 b | 8.19±2.54 a | 8.22±2.34 a | |
| 7 | 5.83±1.72 b | 5.40±0.35 b | 9.35±1.54 a | 8.64±1.98 a | |
| 胸腺指数 Thymus index |
0 | 2.64±0.49 | 2.60±0.31 | 2.65±0.90 | 2.61±0.27 |
| 4 | 2.78±0.67 | 2.70±0.52 | 3.22±2.04 | 2.99±1.60 | |
| 7 | 3.32±0.86 a | 1.72±0.54 b | 3.00±0.17 a | 2.93±0.23 a | |
Note: In the same row, values with different small letter superscripts mean significant difference (P<0.05), while with the same or no letter superscripts mean no significant difference (P>0.05).
由表 3可知,各组在第0天各免疫球蛋白无差别(P>0.05)。致病对照组在第4天各免疫球蛋白与空白组无差别(P>0.05)。致病对照组在第7天IgA显著低于空白组(P<0.05),IgG和IgM与空白组无差别(P>0.05)。K2组在第4天IgA、IgG高于空白组(P<0.05),IgM与空白组无差别(P>0.05)。K2组在第7天IgA与空白组无差别(P>0.05),IgG和IgM显著高于空白组(P<0.05)。K8组在第4天各免疫球蛋白与空白组无差别(P>0.05)。K8组在第7天IgA和IgG与空白组无差别(P>0.05),IgM显著高于空白组(P<0.05)。表明小鼠注射致病性E. coli O8后体内IgA降低,灌胃K2和K8可以提高感染致病性E. coli O8小鼠免疫球蛋白,其中K2组效果更优。
| 项目 Item |
时间 Time (d) |
组别 Group |
|||
| 空白组 The control group |
致病对照组 The challenged control group |
K2组 K2 group |
K8组 K8 group |
||
| IgA | 0 | 83.08±0.64 | 82.87±1.70 | 83.50±1.48 | 82.44±0.85 |
| 4 | 83.29±0.42 b | 83.71±1.27 b | 94.10±3.18 a | 90.50±1.27 ab | |
| 7 | 84.77±0.64 ab | 74.17±1.48 c | 88.59±1.91 a | 81.59±0.85 b | |
| IgG | 0 | 116.59±0.90 | 116.29±1.79 | 115.39±2.09 | 116.89±2.99 |
| 4 | 116.89±1.19 b | 117.19±3.29 b | 132.12±3.88 a | 127.04±2.39 ab | |
| 7 | 117.19±0.30 bc | 112.41±2.69 c | 130.33±3.88 a | 124.65±1.19 ab | |
| IgM | 0 | 309.17±3.01 | 310.37±1.81 | 306.16±8.42 | 308.56±14.44 |
| 4 | 305.55±4.21 | 309.17±13.84 | 351.89±13.24 | 328.42±16.25 | |
| 7 | 306.76±5.42 b | 270.05±2.41 b | 421.68±15.64 a | 392.20±15.04 a | |
Note: In the same row, values with different small letter superscripts mean significant difference (P<0.05), while with the same or no letter superscripts mean no significant difference (P>0.05).
由表 4可知,各组CD4+无差别(P>0.05)。各组在第0天CD8+和CD4+/CD8+无差别(P>0.05)。致 病对照组在第4天和第7天CD8+和CD4+/CD8+与空白组无差别(P>0.05)。K2组在第4天和第7天CD8+显著低于空白组(P<0.05),CD4+/CD8+显著高于空白组(P<0.05)。K8组在第4天CD8+显著低于空白组(P<0.05),CD4+/CD8+与空白组无差别(P>0.05)。K8组在第7天CD8+显著低于空白组(P<0.05),CD4+/CD8+显著高于空白组(P<0.05)。表明小鼠注射致病性E. coli O8后T细胞亚群变化不大,灌胃K2和K8会降低感染致病性E.coli O8小鼠CD8+,提高CD4+/CD8+,且K2组效果更优。
| (%) | |||||
| 项目 Item |
时间 Time (d) |
组别 Group |
|||
| 空白组 The control group |
致病对照组 The challenged control group |
K2组 K2 group |
K8组 K8 group |
||
| CD4+ | 0 | 36.25±0.85 | 36.43±0.49 | 36.16±0.37 | 36.63±0.47 |
| 4 | 36.35±0.94 | 36.41±0.80 | 36.59±1.04 | 36.46±2.79 | |
| 7 | 36.28±2.06 | 36.17±5.87 | 36.85±4.35 | 36.52±2.61 | |
| CD8+ | 0 | 25.57±3.43 | 25.31±2.89 | 25.32±2.93 | 25.56±1.43 |
| 4 | 25.60±4.86 a | 25.50±1.86 a | 19.17±2.25 b | 19.94±2.16 b | |
| 7 | 25.70±1.32 a | 26.69±0.59 a | 17.86±2.30 b | 17.97±1.89 b | |
| CD4+/CD8+ | 0 | 1.45±0.21 | 1.46±0.16 | 1.45±0.15 | 1.44±0.09 |
| 4 | 1.47±0.29 b | 1.43±0.10 b | 1.94±0.23 a | 1.85±0.24 ab | |
| 7 | 1.42±0.13 b | 1.36±0.23 b | 2.12±0.46 a | 2.04±0.13 a | |
Note: In the same row, values with different small letter superscripts mean significant difference (P<0.05), while with the same or no letter superscripts mean no significant difference (P>0.05).
由表 5可知,各组在第0天和第4天E. coli、肠球菌、乳酸杆菌、双歧杆菌数量无差别(P>0.05)。致病对照组在第7天E. coli数量显著高于空白组(P<0.05),肠球菌数量与空白组无差别(P>0.05),乳酸杆菌数量与空白组无差别(P>0.05),双歧杆菌数量显著低于空白组(P<0.05)。K2组在第7天E. coli数量显著低于空白组(P<0.05),肠球菌数量显著低于空白组(P<0.05),乳酸杆菌数量显著高于空白组(P<0.05),双歧杆菌数量与空白组无差别(P>0.05)。K8组在第7天E. coli数量与空白组无差别(P>0.05),肠球菌数量显著低于空白组(P<0.05),乳酸杆菌数量显著高于空白组(P<0.05),双歧杆菌数量与空白组无差别(P>0.05)。表明小鼠注射致病性E. coli O8后会增加肠道中E. coli数量,减少双歧杆菌数量,灌胃K2和K8会降低感染致病性E. coli O8小鼠肠道中E. coli的数量,增加乳酸杆菌和双歧杆菌的数量,且K2组效果更优。
| (LgCFU/g of cecum wet weight) | |||||
| 项目 Item |
时间 Time (d) |
组别 Group |
|||
| 空白组 The control group |
致病对照组 The challenged control group |
K2组 K2 group |
K8组 K8 group |
||
| 大肠杆菌
E. coli |
0 | 6.85±0.88 | 6.82±0.01 | 6.83±0.16 | 6.80±0.14 |
| 4 | 6.87±0.85 | 6.86±0.79 | 5.95±0.31 | 6.13±0.62 | |
| 7 | 6.83±0.84 b | 10.05±0.14 a | 5.29±0.23 c | 5.56±0.77 bc | |
| 肠球菌
Enterococcus |
0 | 6.60±0.32 | 6.55±0.14 | 6.57±0.20 | 6.62±0.09 |
| 4 | 6.53±0.24 | 6.55±0.74 | 5.93±0.35 | 5.96±0.06 | |
| 7 | 6.64±0.26 a | 6.56±0.74 a | 4.59±0.38 b | 5.22±0.32 b | |
| 乳酸杆菌
Lactobacillus |
0 | 6.11±0.06 | 6.09±0.15 | 6.08±0.07 | 6.11±0.09 |
| 4 | 6.10±0.23 | 6.06±0.14 | 6.86±0.58 | 6.64±0.13 | |
| 7 | 6.17±0.04 b | 5.58±0.55 b | 7.34±0.27 a | 7.27±0.69 a | |
| 双歧杆菌
Bifidobacterium |
0 | 10.02±0.05 | 10.01±0.03 | 9.91±0.07 | 10.01±0.13 |
| 4 | 10.03±0.30 | 10.00±0.57 | 10.53±0.38 | 10.41±0.36 | |
| 7 | 10.17±0.21 a | 6.62±0.23 b | 10.03±0.15 a | 10.01±0.59 a | |
Note: In the same row, values with different small letter superscripts mean significant difference (P<0.05), while with the same or no letter superscripts mean no significant difference (P>0.05).
致病对照组小鼠腹腔注射致病性E.coli O8出现一系列的病理变化,如腹泻、厌食、精神萎靡等,与斯木吉德研究的致病性E.coli病理变化相似[18],观察小肠病理切片发现小肠绒毛形态结构遭到破坏,与Girard等[19]的报道相一致。灌胃K2和K8可以一定程度上缓解和改善感染致病性E.coli O8小鼠的病症和小肠组织病理变化。
胸腺是机体淋巴细胞分化和成熟的主要中枢免疫器官,而脾脏是机体最重要的外周淋巴器官。在胸腺中淋巴细胞发育成熟后,经循环系统转移至脾脏及各级淋巴组织和淋巴器官中储存,并发挥免疫作用。脾脏和胸腺增重是免疫器官生长发育快的表现,而其指数提高意味着免疫系统成熟较快[20]。实验结果表明:致病对照组小鼠注射致病性E. coli O8后,导致胸腺指数降低。但实验鼠灌胃K2和K8可以提高感染致病性E. coli O8小鼠脾脏和胸腺指数,认为K2和K8促进了感染致病性E. coli O8小鼠脾脏和胸腺的生长发育,进而提高机体免疫机能。本实验结果与郭剑英等研究的中药复方对小鼠脏器指数的影响相一致[21]。
免疫球蛋白是介导体液免疫的主要抗体,血清抗体的高低在一定程度上反映机体对疾病的抵抗能力。IgA、IgG和IgM可以代表血清免疫球蛋白的水平[22]。实验结果表明:致病对照组小鼠注射致病性E. coli O8后,血清IgA显著降低,而且IgG和IgM也有降低的趋势。实验在第4天K2组小鼠 血清IgA、IgG显著高于空白组,该结果与菊糖酸奶对小鼠免疫球蛋白的影响结果相一致[23]。在第7天K2组小鼠血清IgG和IgM显著高于空白组。K8组小鼠血清IgM显著高于空白组,认为K2组和K8组由于K2和K8的干预在第7天时缓解了小鼠感染致病性E.coli O8的症状,使部分指标恢复到正常水平。另外K2和K8有益于增强感染致病性E. coli O8小鼠机体的体液免疫能力,从而提高其免疫机能,起到抗炎免疫作用。
免疫细胞的数量和活性的变化也能反映机体细胞免疫机能状态。参与机体细胞免疫功能的T淋巴细胞主要有CD4+细胞和CD8+细胞。在一定范围之内,CD4+细胞百分含量较高或CD4+/CD8+值较大,说明机体免疫功能状态良好[24, 25]。实验结果表明:小鼠灌胃K2和K8可以显著提高感染致病性E. coli O8小鼠血液中CD4+/CD8+,认为K2和K8有益于提高机体免疫功能状态。
肠道菌群指人体或动物肠道内存在的大量细菌,随宿主自身饮食和环境因素的改变而变化,肠道菌群平衡稳定是保障机体健康的必要条件,盲肠菌群可以直接或间接反映肠道菌群变化。盲肠菌群主要由厌氧菌构成,其优势菌包括双歧杆菌和乳酸杆菌等[26]。E. coli、肠球菌是盲肠菌群中的条件致病菌。这几种细菌数量的变化是肠道健康的重要指标[27, 28]。实验结果表明:致病对照组小鼠注射致病性E. coli O8后,盲肠中E. coli数量增多,双歧杆菌数量降低。认为致病性E.coli O8可使小鼠正常菌群失调,促进E.coli快速增殖,减少双歧杆菌的数量。小鼠灌胃K2和K8可以抑制感染致病性E. coli O8小鼠盲肠中E.coli和肠球菌的数量,提高乳酸杆菌和双歧杆菌的数量,由此可见K2和K8会促进有益菌在肠道内的生长和定殖。另外乳酸杆菌代谢产生的乳酸,双歧杆菌发酵产生的醋酸,K2和K8中的有机酸,调节肠道内pH,抑制有害细菌繁殖和生长,保持动物正常盲肠菌群比例,防治疾病[29, 30]。本实验结果与魏刚研究的中药复方对小鼠肠道菌群的影响一致[31]。
4 结论(1) 小鼠注射致病性E. coli O8,出现患病临床症状,其小肠组织出现病理改变。盲肠中E. coli数量增多,胸腺指数、血清IgA、双歧杆菌数量降低。
(2) 酸马奶提取K. marxianus代谢抗菌复合物K2影响感染致病性E. coli O8小鼠小肠病理变化。提高小鼠的脾脏指数和血清免疫球蛋白,增高CD4+/CD8+比值,减少CD8+。同时抑制盲肠中E. coli数量和肠球菌,促进乳酸杆菌繁衍。
(3) 酸马奶提取K. marxianus代谢抗菌复合物K8影响感染致病性E. coli O8小鼠小肠病理变化。提高感染致病性E. coli O8小鼠的脾脏指数和IgM,增高CD4+/CD8+比值,减少CD8+。同时抑制盲肠中肠球菌,促进乳酸杆菌繁衍。
(4) 酸马奶提取K. marxianus代谢抗菌复合物K2和K8对感染致病性E. coli O8小鼠的影响K2更优。
| [1] | Liu SN,Han Y,Zhou ZJ. Lactic acid bacteria in traditional fermented Chinese foods[J]. Food Research International,2011, 44(3): 643-651 |
| [2] | Ishii S,Kikuchi M,Takao S. Isolation and identification of lactic acid bacteria and yeasts from “Chigo” in Inner Mongolia, China[J]. Nihon Chikusan Gakkaiho,1997,68(3): 325-329 |
| [3] | Ni HJ,Bao QH,Sun TS,et al. Identification and biodiversity of yeasts isolated from Koumiss in Xinjiang of China[J]. Acta Microbiologica Sinica,2007,47(4): 578-582 (in Chinese) 倪慧娟,包秋华,孙天松,等. 新疆地区酸马奶中酵母菌的鉴定及其生物多样性分析[J]. 微生物学报,2007,47(4): 578-582 |
| [4] | Goerges S,Aigner U,Silakowski B,et al. Inhibition of Listeria monocytogenes by food-borne yeasts[J]. Applied and Environmental Microbiology,2006,72(1): 313-318 |
| [5] | Goerges S,Koslowsky M,Velagic S,et al. Anti-listerial potential of food-borne yeasts in red smear cheese[J]. International Dairy Journal,2011,21(2): 83-89 |
| [6] | Viljoen BC. Yeast ecological interactions. Yeast’yeast, yeast’bacteria,yeast’fungi interactions and yeasts as biocontrol agents[A]//Querol A,Fleet G. Yeasts in Food and Beverages[M]. Berlin: Springer,2006: 83-110 |
| [7] | Wen WR,Chen H,Dai GS,et al. The killer activity of the yeast killer system[J]. Chinese Journal of Microecology,1999,11(4): 217-219 (in Chinese) 温旺荣,陈红,戴庚孙,等. 酵母菌杀菌系统的杀菌活性[J]. 中国微生态学杂志,1999,11(4): 217-219 |
| [8] | Swanson KS,Grieshop CM,Flickinger EA,et al. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function,ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs[J]. Journal of Nutrition,2002,132(5): 980-989 |
| [9] | Swanson KS,Grieshop CM,Flickinger EA,et al. Effects of supplemental fructooligosaccharides plus mannanoligosaccharides on immune function and ileal and fecal microbial populations in adult dogs[J]. Archiv für Tierernaehrung,2002,56(4): 309-318 |
| [10] | Chiquette J. Evaluation of the protective effect of probiotics fed to dairy cows during a subacute ruminal acidosis challenge[J]. Animal Feed Science and Technology,2009,153(3/4): 278-291 |
| [11] | Piao FZ,Xia C. The occurrence characteristics of cow disease and their popular trend[J]. Veterinary Orientation,2011(2): 33-35 (in Chinese) 朴范泽,夏成. 当前牛病的发生特点和流行趋势[J]. 兽医导刊,2011(2): 33-35 |
| [12] | Chen YJ,Chen A,Wang CJ,et al. Effects of antimicrobial compounds of Kluyveromyces marxianus in Koumiss on pathogenic Escherichia coli and its cell surface characteristics[J]. Microbiology China,2015,42(4): 683-689 (in Chinese) 陈玉洁,敖日格乐,王纯洁,等. 酸马奶提取 Kluyveromyces marxianus 代谢抗菌复合物对致病性大肠杆菌的抑菌和细胞 表面特性的影响[J]. 微生物学通报,2015,42(4): 683-689 |
| [13] | He YF. Studies on isolation,identification of microorganisms and their antibacterial factors from koumiss[D]. Hohhot: Doctoral Dissertation of Inner Mongolia Agricultural University, 2008 (in Chinese) 贺银凤. 酸马奶酒中微生物的分离鉴定及抗菌因子的研究 [D]. 呼和浩特: 内蒙古农业大学博士学位论文,2008 |
| [14] | Zhan YB. Screening of compound Mongolian medicine treating bovine mastitis and research of its mechanism on anti-inflammation and immunoenhancement[D]. Hohhot: Doctoral Dissertation of Inner Mongolia Agricultural University, 2010 (in Chinese) 战永波. 治疗奶牛乳房炎蒙药复方的筛选及其抗炎免疫机理 的研究[D]. 呼和浩特: 内蒙古农业大学博士学位论文,2010 |
| [15] | Shen GX,Zhou RL. Modern Immunology Experiment Technology[M]. 2nd Edition. Wuhan: Hubei Science & Technology Press,2002: 228-253 (in Chinese) 沈关心,周汝麟. 现代免疫学实验技术[M]. 第2版. 武汉: 湖 北科学技术出版社,2002: 228-253 |
| [16] | Tuo JJ. Research on effect of the caulerpa racemosa var peltata polysaccharides and selenizing product on T lymphocytes and NK cells in mice[D]. Guangzhou: Master’s Thesis of Ji’nan University,2007 (in Chinese) 庹菁菁. 总状蕨藻盾叶变种多糖及其硒化物对小鼠 T 细胞和 NK 细胞的调节[D]. 广州: 暨南大学硕士学位论文,2007 |
| [17] | Wu SG,Gao CQ,Zhang HJ,et al. Effect of dietary isomalto-oligosaccharide on performance,caecal microflora and immune function of laying hens[J]. Chinese Journal of Animal Nutrition,2011,23(9): 1560-1568 (in Chinese) 武书庚,高春起,张海军,等. 低聚异麦芽糖对产蛋鸡生产性能、盲肠微生物和免疫机能的影响[J]. 动物营养学报,2011, 23(9): 1560-1568 |
| [18] | Huasai S,Chen A,Wang CJ,et al. Isolation and identification of pathogenic Escherichia coli in fresh cow faeces[J]. Chinese Journal of Veterinary Medicine,2009,45(8): 42-43 (in Chinese) 斯木吉德,敖日格乐,王纯洁,等. 奶牛新鲜粪便致病性大肠杆菌分离鉴定[J]. 中国兽医杂志,2009,45(8): 42-43 |
| [19] | Girard F,Frankel G,Phillips AD,et al. Interaction of enterohemorrhagic Escherichia coli O157: H7 with mouse intestinal mucosa[J]. FEMS Microbiology Letters,2008,283(2): 196-202 |
| [20] | Chen FX,Chen WY,Zhong XH. Effect of Taraxacum polysaccharides on immune function in mice[J]. Chinese Journal of Veterinary Medicine,2009,45(3): 19-20 (in Chinese) 陈福星,陈文英,钟秀会. 蒲公英多糖对小鼠免疫机能的影响[J]. 中国兽医杂志,2009,45(3): 19-20 |
| [21] | Guo JY,Yan C,Wang JH,et al. Effect on blood metabolites and immunologic organ index in response to Chinese herbal ultra-fine powder treatment of mouse[J]. China Animal Husbandry & Veterinary Medicine,2012,39(5): 240-243 (in Chinese) 郭剑英,颜诚,王杰豪,等.饲料中添加复方中药对小鼠血 液生理生化指标和免疫器官指数的影响[J]. 中国畜牧兽医, 2012,39(5): 240-243 |
| [22] | Shangguan MJ,Wang F,Zhang HG,et al. Effects of inulin on growth performance,immune organs indices and serum immunoglobulin of laying chicklings[J]. Chinese Journal of Animal Nutrition,2009,21(1): 118-122 (in Chinese) 上官明军,王芳,张红岗,等. 菊粉对蛋雏鸡生长性能、免疫器官指数和血清免疫球蛋白的影响[J]. 动物营养学报,2009, 21(1): 118-122 |
| [23] | Xiong ZW,Zheng Y,Lan Y,et al. Effect of inulin-supplemented yogurt on immune organ indexes and serum immunoglobulin in mice[J]. Food Science,2014,35(21): 216-218 (in Chinese) 熊政委,郑韵,兰洋,等. 菊糖酸奶对小鼠免疫器官指数和血清免疫球蛋白的影响[J]. 食品科学,2014,35(21): 216-218 |
| [24] | Hasisurong,Du XY,Jiang JS,et al. Study on immune stimulating properties of compound butafosfan solution in mice[J]. Chinese Journal of Veterinary Science and Technology, 2005,35(7): 574-578 (in Chinese) 哈斯苏荣,杜小燕,蒋金书,等. 复方丁氨丙磷溶液对小鼠免疫机能的影响[J]. 中国兽医科技,2005,35(7): 574-578 |
| [25] | Hu YJ,Lin YC,Zheng L,et al. The effect of dietary active yeast supplement on performance and immunity of early weaned piglets[J]. Acta Zoonutrimenta Sinica,2003,15(4): 49-53 (in Chinese) 胡友军,林映才,郑黎,等. 活性酵母对早期断奶仔猪生产性能和免疫机能的影响[J]. 动物营养学报,2003,15(4): 49-53 |
| [26] | Wu SL,Liu F,Luo XQ,et al. Effect of Yacon powder on intestinal flora in mice[J]. Journal of Southwest China Normal University (Natural Science Edition),2012,37(2): 42-45 (in chinese) 吴三林,刘芳,罗小琴,等. 灌服雪莲果粉对小鼠肠道菌群的影响[J]. 西南师范大学学报: 自然科学版,2012,37(2): 42-45 |
| [27] | Hooper LV,Gordon JI. Commensal host-bacterial relationships in the gut[J]. Science,2001,292(5519): 1115-1118 |
| [28] | Holzapfel WH,Haberer P,Snel J,et al. Overview of gut flora and probiotics[J]. International Journal of Food Microbiology, 1998,41(2): 85-101 |
| [29] | Qu MR,Ling BM,Lu DX,et al. Effect of fructooligosaccharides infusion on rumen fermentation characteristic in sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2006,37(8): 779-784 (in Chinese) 瞿明仁,凌宝明,卢德勋,等. 灌注果寡糖对生长绵羊瘤胃发酵功能的影响[J]. 畜牧兽医学报,2006,37(8): 779-784 |
| [30] | Ling BM,Qu MR,Lu DX,et al. Effects of functional oligosaccharides on the rumen fermentation of the growing sheep in vitro[J]. Chinese Journal of Animal Nutrition,2007, 19(2): 129-134 (in Chinese) 凌宝明,瞿明仁,卢德勋,等. 利用体外法研究功能性寡糖对生长绵羊瘤胃发酵特性的影响[J]. 动物营养学报,2007, 19(2): 129-134 |
| [31] | Wei G,Ba CF. Effects of traditional Chinese medicine compound on intestinal flora and activity of digestive enzymes in mice[J]. Feed Research,2014(21): 86-88 (in Chinese) 魏刚,巴彩凤. 中药复方对小鼠肠道菌群和消化酶活性的影响[J]. 饲料研究,2014(21): 86-88 |
 2015, Vol. 42
2015, Vol. 42