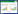扩展功能
文章信息
- 成莉凤, 戴小阳, 冯湘沅, 段盛文, 郑科, 刘正初
- CHENG Li-Feng, DAI Xiao-Yang, FENG Xiang-Yuan, DUAN Sheng-Wen, ZHENG Ke, LIU Zheng-Chu
- Bacillus subtilis BE-91生长及其胞外表达β-甘露聚糖酶的发酵条件优化
- Optimization of growth and shake flask fermentation conditions of Bacillus subtilis BE-91 producing extracellular β-mannanase
- 微生物学通报, 2015, 42(12): 2300-2307
- Microbiology China, 2015, 42(12): 2300-2307
- 10.13344/j.microbiol.china.150719
-
文章历史
- 收稿日期: 2015-09-23
- 接受日期: 2015-11-02
- 优先数字出版日期(www.cnki.net): 2015-11-03
β-甘露聚糖酶(β-Mannanase,EC3.2.1.78)是一类能够水解含有β-1,4-D-甘露糖苷键的甘露寡糖、甘露多糖,生成甘露二糖、三糖等小分子物质的水解酶[1]。结合甘露糖苷酶(β-Mannosidase,EC3.2.1.25)、半乳糖苷酶(α-Galactosidase,EC3.2.1.22)、葡萄糖苷酶(β-Glucosidase,EC3.2.1.21)、乙酰甘露聚糖脂酶(Acetylmannan esterase,EC3.2.1.6)等支链酶的协同作用,可彻底降解甘露聚糖[2]。目前,β-甘露聚糖酶已广泛应用于食品、医药、饲料、造纸、印染、纺织及石油开采等领域[3, 4, 5]。
β-甘露聚糖酶普遍存在于自然界中,但微生物生产的β-甘露聚糖酶是主要来源。迄今,已有许多关于微生物生产β-甘露聚糖酶的报道,例如:细菌中的芽孢杆菌(Paenibacillus thiaminolyticus、Bacillus nealsonii PN-11、Bacillus pumilus GBSW19)[6, 7, 8]等;真菌中的萨托菌(Neosartorya fischeri P1)[9]、篮状菌(Talaromyces leycettanus)[10]等,曲霉如构巢曲霉(Aspergillus nidulans XZ3)[11]、黑曲霉(Aspergillus niger BK01)[12]等;放线菌中的链霉菌(Streptomyces tendae)[13]等。微生物来源的β-甘露聚糖酶具有活性高,成本低,来源稳定,提取方便以及比动植物更广的作用pH、温度范围和底物适应性等显著特点,易被理论研究和工业化生产应用。随着β-甘露聚糖酶的工业化需求不断增长,如何利用微生物资源高效生产能适用于不同工业领域的β-甘露聚糖酶业已成为专家学者们关注的焦点,选育优良菌株并优化其产β-甘露聚糖酶的工艺条件是高效生产的基础。
本项目组选育到一个能同时高产耐热偏酸性β-甘露聚糖酶和木聚糖酶的半纤维降解高效菌株B. subtilis BE-91[14, 15],前期已对该菌木聚糖酶的发酵工艺、分离纯化和酶学性质等进行了系统研究[16, 17]。本研究拟在单因素试验基础上,采用正交试验法优化B.subtilis BE-91的生长条件和胞外表达β-甘露聚糖酶的摇瓶发酵条件,为开发B.subtilis BE-91在酶制剂产业的应用价值提供科学依据。
1 材料与方法 1.1 菌株半纤维降解高效菌株B.subtilis BE-91 (曾用编号:CXJZ11-01)由中国农业科学院麻类研究所酶工程项目组选育并保存。
1.2 培养基选择平板:在标准LB固体培养基(g/L,胰蛋白胨10,氯化钠10,酵母提取物5,琼脂15,加水至1 L,NaOH调整pH为7.4−7.6,1×105 Pa高压蒸汽灭菌20 min)配方的基础上,添加5 g/L魔芋胶、0.5 g/L曲利苯兰。
生长培养基、发酵培养基配方通过正交试验确定。
1.3 主要试剂魔芋胶(纯度>95%),成都路特实业有限公司;魔芋精粉(食品级),湖南龙山天食有限责任公司;曲利苯兰和甘露糖,美国Sigma公司;3,5-二硝基水杨酸,国药集团。
1.4 主要仪器酶标仪(MLtiskan GO),美国Thermo Scientific公司;分析天平(MS32000LE),瑞士梅特勒公司;pH计(HI9025),意大利HANNA公司;水浴摇床(THZ-82),中国荣华仪器公司。
1.5 菌种培养及β-甘露聚糖酶粗酶液制备取真空保存的B. subtilis BE-91菌种一环,接种于6 mL生长培养基,35 °C静置培养5.5 h;稀释涂于选择平板,35 °C培养18 h,挑取优良单菌落接种于6 mL生长培养基,35 °C静置培养6 h,即为一级培养菌悬液。将一级菌悬液接种于盛有100 mL培养液的小三角瓶,35 °C、180 r/min振荡培养至指数生长期,即为二级菌悬液。按接种量2%接种于300 mL发酵培养基,35 °C、180 r/min振荡培养。按相关实验步骤的时间要求取样(10 mL发酵液),4 °C、3 000 r/min离心10 min,上清液即为β-甘露聚糖酶粗酶液。
1.6 β-甘露聚糖酶活力测定参照Akino等方法[18],用魔芋胶和pH 6.5 0.025 mol/L柠檬酸-0.05 mol/L NaH2PO4缓冲液配制5 g/L (质量体积比)的底物,取1 mL适当稀释的酶液与2 mL预热的底物充分混匀,65 °C反应10 min,DNS法测还原糖生成量。用灭活的酶液做相同处理作对照。β-甘露聚糖酶活力定义:底物每分钟释放相当于1 μmol甘露糖的还原糖所需酶量为1个酶活力单位(以IU表示)。
1.7 生长培养条件优化 1.7.1 单因素试验:分别以培养温度(30、35、37和40 °C)和起始pH (4.5−8.0,间隔0.5)为变量,固定其他生长培养条件,转接摇瓶后,在适当的培养时间点取样,以测得的菌液OD600为衡量指标进行优化水平评价。每组试验设置3个重复。 1.7.2 正交试验:根据培养基的主要成分C源、N源和无机盐3因素设计正交实验L9(33)方案(表 1)[19, 20],以OD600值为衡量指标,结合极差和方差析评价生长培养基成分优化组合。每组试验设置分3个重复。| Test number | Factor A | Factor B | Factor C |
| 1 | 1 | 1 | 1 |
| 2 | 1 | 2 | 2 |
| 3 | 1 | 3 | 3 |
| 4 | 2 | 1 | 2 |
| 5 | 2 | 2 | 3 |
| 6 | 2 | 3 | 1 |
| 7 | 3 | 1 | 3 |
| 8 | 3 | 2 | 1 |
| 9 | 3 | 3 | 2 |
| Test number | Factor A | Factor B | Factor C | Factor D |
| 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 2 | 2 | 2 |
| 3 | 1 | 3 | 3 | 3 |
| 4 | 2 | 1 | 2 | 3 |
| 5 | 2 | 2 | 3 | 1 |
| 6 | 2 | 3 | 1 | 2 |
| 7 | 3 | 1 | 3 | 2 |
| 8 | 3 | 2 | 1 | 3 |
| 9 | 3 | 3 | 2 | 1 |
采用试验确定的优化生长和发酵条件,按照方法1.5菌种培养流程进行B. subtilis BE-91发酵,从发酵培养基培养第6 h开始取样,每小时取样一次,测定OD600和胞外β-甘露聚糖酶活力。
1.10 数据分析每个检测指标重复3次,取平均值。利用Excel 2007和统计分析软件SAS 9.0对3次重复试验结果进行分析并绘制图表。
2 结果与分析 2.1 生长培养条件优化 2.1.1 单因素对菌种生长的影响:测定在不同培养温度和起始pH条件下B. subtilis BE-91的生长趋势。(1) 培养温度:从图 1可以看出,不同温度明显影响了B. subtilis BE-91生长。35 °C条件下培养,B. subtilis BE-91的指数生长期呈现在接种后第4−8 h;40 °C条件下,B. subtilis BE-91接种4 h后,OD600不增长,甚至负增长;在30 °C和37 °C条件下,B. subtilis BE-91的对数生长曲线的斜率远不及35 °C。因此,可以肯定,35 °C是B. subtilis BE-91的适宜生长温度;若要进行扩大培养或发酵取6−8 h菌龄比较合适。(2) 起始pH:从表 3可以看出,当起始pH为4.5−8.0,培养第8 h的菌液OD600值都大于第6 h的菌液OD600值,说明B. subtilis BE-91的适宜生长pH范围比较宽;当起始pH 6.0时,培养第6 h和第8 h的菌液OD600值均较其余pH组大,说明起始 pH 6.0更适合于该菌生长。
|
| 图 1 温度对B.subtilis BE-91生长的影响 Figure 1 Effects of temperature on B.subtilis BE-91 growth |
| pH | OD 600 | |
| Culture in 6 h | Culture in 8 h | |
| 4.5 | 0.670 Bab | 0.722 Aa |
| 5.0 | 0.703 Bb | 1.046 CDb |
| 5.5 | 0.837 Dc | 1.214 Dc |
| 6.0 | 0.900 Ed | 1.356 Ecd |
| 6.5 | 0.816 Dc | 1.300 DEc |
| 7.0 | 0.782 Cc | 1.108 Db |
| 7.5 | 0.640 Aa | 0.890 Cb |
| 8.0 | 0.635 Aa | 0.788 Ba |
| Test number | N source | C source | Inorganic salt | OD 600 |
| 1 | 1 | 1 | 1 | 0.945 |
| 2 | 1 | 2 | 2 | 0.425 |
| 3 | 1 | 3 | 3 | 0.955 |
| 4 | 2 | 1 | 2 | 0.345 |
| 5 | 2 | 2 | 3 | 0.841 |
| 6 | 2 | 3 | 1 | 0.836 |
| 7 | 3 | 1 | 3 | 1.125 |
| 8 | 3 | 2 | 1 | 0.730 |
| 9 | 3 | 3 | 2 | 0.352 |
| K1 | 2.325 | 2.415 | 2.511 | |
| K2 | 2.022 | 1.996 | 1.122 | |
| K3 | 2.207 | 2.143 | 2.921 | |
| k1 | 0.775 | 0.805 | 0.837 | |
| k2 | 0.674 | 0.665 | 0.374 | |
| k3 | 0.736 | 0.714 | 0.974 | |
| R | 0.101 | 0.140 | 0.600 | |
| Primary and secondary factors: C>B>A | ||||
| Optimization levels: A 1B 1C 3 | ||||
| Source of variation | DF | SS | MS | F value | P |
| Model | 8 | 1.837 | 0.230 | 39.42 | <0.000 1 ** |
| A | 2 | 0.057 | 0.029 | 4.93 | 0.019 7 * |
| B | 2 | 0.077 | 0.039 | 6.64 | 0.006 9 ** |
| C | 2 | 1.702 | 0.851 | 146.12 | <0.000 1 ** |
| Error | 18 | 0.105 | 0.006 | ||
| Corrected total | 26 | 1.942 |
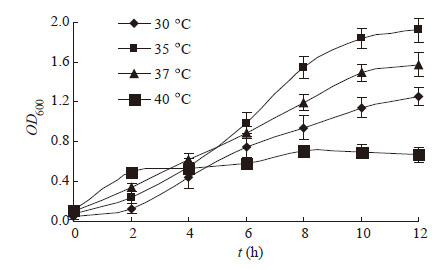
|
| 图 2 温度对β-甘露聚糖酶合成的影响 Figure 2 Effects of temperature on β-mannanase production |
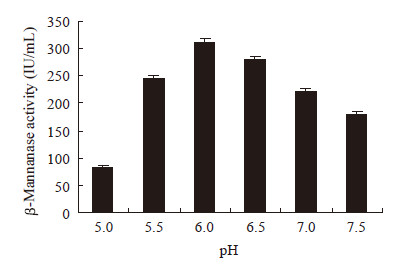
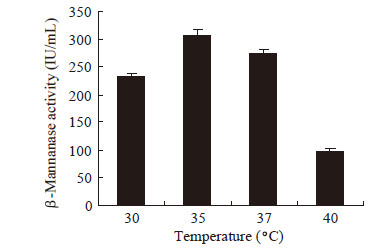
|
| 图 3 pH对β-甘露聚糖酶合成的影响 Figure 3 Effects of pH on β-mannanase production |

|
| 图 4 魔芋精粉浓度对β-甘露聚糖酶合成的影响 Figure 4 Effects of konjac powder concentration on β-mannanase production |
| Test number | C source | N source | pH | Temperature | β-Mannanase activity (IU/mL) |
| 1 | 1 | 1 | 1 | 1 | 203.4 |
| 2 | 1 | 2 | 2 | 2 | 244.5 |
| 3 | 1 | 3 | 3 | 3 | 203.4 |
| 4 | 2 | 1 | 2 | 3 | 213.7 |
| 5 | 2 | 2 | 3 | 1 | 215.9 |
| 6 | 2 | 3 | 1 | 2 | 208.0 |
| 7 | 3 | 1 | 3 | 2 | 324.3 |
| 8 | 3 | 2 | 1 | 3 | 233.1 |
| 9 | 3 | 3 | 2 | 1 | 296.9 |
| K1 | 651.3 | 741.4 | 644.5 | 716.2 | 586.3 |
| K2 | 637.6 | 693.5 | 755.1 | 776.8 | 662.7 |
| K3 | 854.3 | 708.3 | 743.6 | 650.2 | 651.2 |
| k1 | 217.1 | 247.1 | 214.8 | 238.7 | |
| k2 | 212.5 | 231.2 | 251.7 | 258.9 | |
| k3 | 284.8 | 236.1 | 247.9 | 216.7 | |
| R | 72.2 | 16.0 | 36.9 | 42.2 | |
| Primary and secondary factors: A>D>C>B | |||||
| Optimization levels: A 3B 1C 2D 2 | |||||
| Source of variation | DF | SS | MS | F value | P |
| Model | 10 | 46 239.28 | 4 623.93 | 59.89 | <0.000 1 ** |
| A | 2 | 29 470.52 | 14 735.26 | 190.84 | <0.000 1 ** |
| B | 2 | 1 204.41 | 602.21 | 7.80 | <0.000 1 ** |
| C | 2 | 7 405.65 | 3 702.82 | 47.96 | <0.000 1 ** |
| D | 2 | 8 023.29 | 4 011.641 | 51.96 | <0.000 1 ** |
| Error | 16 | 1 235.38 | 77.211 | ||
| Corrected total | 26 | 47 474.656 |
与菌体生长相比,B. subtilis BE-91菌株分泌β-甘露聚糖酶具有明显的同步效应(图 5),约6 h菌株进入指数生长期,10 h达到最高峰,OD600约为2.5。同时,β-甘露聚糖酶的产生在 10 h达到了最高值432.4 IU/mL,之后有小幅降低。据此,初步确定在该培养条件下,B.subtilis BE-91菌株分泌β-甘露聚糖酶的最佳时间为10 h。
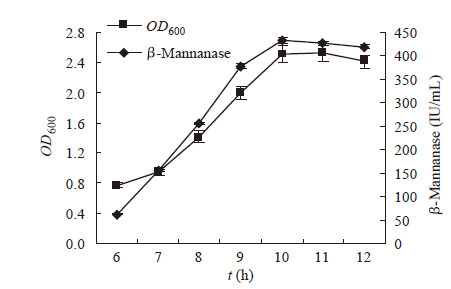
|
| 图 5 B. subtilis BE-91的产酶进程 Figure 5 β-Mannanase production course of B. subtilis BE-91 |
前期研究已表明,B. subtilis BE-91胞外表达β-甘露聚糖酶的最适pH为6.0,最适反应温度和热稳定性均大于60 °C,属于典型的耐热偏酸性β-甘露聚糖酶[14]。本研究获得的优化生长条件为:0.3%牛肉膏、0.2%酵母膏、0.1%葡萄糖、0.4%魔芋精粉、0.5% NaCl,初始pH 6.0、培养温度35 °C;胞外表达β-甘露聚糖酶活力的摇瓶发酵条件优化组合为:0.7%魔芋精粉、0.4%大豆蛋白、0.1% (NH4)2SO4、0.5% NaCl,发酵温度35 °C和起始pH 6.0。优化条件下发酵10 h,β-甘露聚糖酶活力最高达432.4 IU/mL。与国内外已有相关报道相比[21, 22, 23],发酵时间缩短了14-86 h、最高酶活力提高了5倍以上。由于本实验的发酵培养基采用价格低廉的魔芋精粉兼做碳源和产酶诱导物,大大节省了β-甘露聚糖酶的生产成本[24]。因此,B. subtilis BE-91培养与发酵周期短、胞外表达β-甘露聚糖酶的活性高,是酶制剂产业具有重大开发价值的菌种资源。本研究中的最适生长温度和发酵温度相同(均为35 °C),最适生长和发酵的初始pH值也相同(均为6.0)。由于二者的主要外界环境参数相同,不仅减轻了菌体生长和发酵产酶的同步最优化的技术难度,还可简化实际生产的操作流程。
本研究的目标菌株B. subtilis BE-91在发酵10 h产β-甘露聚糖酶达到432.4 IU/mL,较国内外其他报道具有产酶早、酶活力高的优势。综合考虑产量和生产成本,利用B.subtilis BE-91生产耐热偏酸性β-甘露聚糖酶,值得进一步中试[25]。
| [1] | Dhawan S, Kaur J. Microbial mannanases: an overview of production and applications[J]. Critical Reviews in Biotechnology, 2007, 27(4): 197-216 |
| [2] | Malgas S, van Dyk JS, Pletschke BI. A review of the enzymatic hydrolysis of mannans and synergistic interactions between β-mannanase, β-mannosidase and α-galactosidase[J]. World Journal of Microbiology and Biotechnology, 2015, 31(8): 1167-1175 |
| [3] | Wu G, Bryant MM, Voitle RA, et al. Effects of β-mannanase in corn-soy diets on commercial leghorns in second-cycle hens[J]. Poultry Science, 2005, 84(6): 894-897 |
| [4] | Ramachandran P, Zhao ZP, Singh R, et al. Characterization of a β-1, 4-mannanase from a newly isolated strain of Pholiota adiposa and its application for biomass pretreatment[J]. Bioprocess and Biosystems Engineering, 2014, 37(9): 1817-1824 |
| [5] | Adiguzel A, Nadaroglu H, Adiguzel G. Purification and characterization of β-mannanase from Bacillus pumilus (M27) and its applications in some fruit juices[J]. Journal of Food Science and Technology, 2015, 52(8): 5292-5298 |
| [6] | Dhawan S, Singh R, Kaur R, et al. A β-mannanase from Paenibacillus sp.: optimization of production and its possible prebiotic potential[J]. Biotechnology and Applied Biochemistry, 2015. DOI: 10.1002/bab.1419 |
| [7] | Chauhan PS, Tripathi SP, Sangamwar AT, et al. Cloning, molecular modeling, and docking analysis of alkali-thermostable β-mannanase from Bacillus nealsonii PN-11[J]. Applied Microbiology and Biotechnology, 2015, 99(21): 8917-8925 |
| [8] | Zang HY, Xie SS, Wu HJ, et al. A novel thermostable GH5_7 β-mannanase from Bacillus pumilus GBSW19 and its application in manno-oligosaccharides (MOS) production[J]. Enzyme and Microbial Technology, 2015, 78: 1-9 |
| [9] | Wang CH, Luo HY, Niu CF, et al. Biochemical characterization of a thermophilic β-mannanase from Talaromyces leycettanus JCM12802 with high specific activity[J]. Applied Microbiology and Biotechnology, 2015, 99(3): 1217-1228 |
| [10] | Yang H, Shi PJ, Lu HQ, et al. A thermophilic β-mannanase from Neosartorya fischeri P1 with broad pH stability and significant hydrolysis ability of various mannan polymers[J]. Food Chemistry, 2015, 173: 283-289 |
| [11] | Lu HQ, Luo HY, Shi PJ, et al. A novel thermophilic endo-β-1, 4-mannanase from Aspergillus nidulans XZ3: functional roles of carbohydrate-binding module and Thr/Ser-rich linker region[J]. Applied Microbiology and Biotechnology, 2014, 98(5): 2155-2163 |
| [12] | Huang JW, Chen CC, Huang CH, et al. Improving the specific activity of β-mannanase from Aspergillus niger BK01 by structure-based rational design[J]. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 2014, 1844(3): 663-669 |
| [13] | Yoo HY, Lee JH, Yang XG, et al. A novel bacterial mannanase from Streptomyces tendae: purification, characterization and application to hydrolysis of spent coffee ground[C]. Proceedings of the 37th Symposium on Biotechnology for Fuels and Chemicals. San Diego, CA: SBFC, 2015 |
| [14] | Cheng LF. Study on breeding of β-mannanase overproducing strain, purification and properties of β-mananase[D]. Beijing: Master’s Thesis of Chinese Academy of Agricultural Sciences, 2007 (in Chinese) 成莉凤. β-甘露聚糖酶高产菌株筛选及酶的纯化与性质研究 [D]. 北京: 中国农业科学院硕士学位论文, 2007 |
| [15] | Xu JF. Diversity, cloning and expression of xylanase gene from Bacillus subtilis BE-91[D]. Changsha: Doctoral Dissertation of Hunan Agricultural University, 2010 (in Chinese) 徐君飞. 枯草芽孢杆菌BE-91木聚糖酶基因多样性及其克隆 与表达研究[D]. 长沙: 湖南农业大学博士学位论文, 2010 |
| [16] | Liu ZC, Dai XY, Zhang JZ, et al. Screening of a xylanase high-producing strain and its rapid separation and purification[J]. Annals of Microbiology, 2011, 61(4): 901-906 |
| [17] | Liu ZC, Xu JF, Duan SW, et al. Expression of modified xynA gene fragments from Bacillus subtilis BE-91[J]. Annals of Microbiology, 2014, 64(1): 139-145 |
| [18] | Akino T, Nakamura N, Horikoshi K. Production of β-mannosidase and β-mannanase by an alkalophilic Bacillus sp.[J]. Applied Microbiology and Biotechnology, 1987, 26(4): 323-327 |
| [19] | Li YY, Hu CR. Experimental Design and Data Processing[M]. 2nd Edition. Beijing: Chemical Industry Press, 2008: 124-127 (in Chinese) 李云雁, 胡传荣. 试验设计与数据处理[M]. 第2版. 北京: 化 学工业出版社, 2008: 124-127 |
| [20] | Du LY, Wang H, Jin G, et al. Storage of Oenococcus oeni in liquid nitrogen[J]. Acta Microbiologica Sinica, 2011, 51(9): 1263-1269 (in Chinese) 杜立业, 王华, 金刚, 等. 酒酒球菌液氮超低温保存[J]. 微生 物学报, 2011, 51(9): 1263-1269 |
| [21] | Wang J, Li X, Zhu JJ, et al. Effects of medium components on β-mannanase production by Trichoderma reesei[J]. Journal of Nanjing Forestry University (Natural Science Edition), 2013, 37(1): 101-104 (in Chinese) 王静, 李鑫, 朱均均, 等. 培养基组成对里氏木霉合成β-甘 露聚糖酶的影响[J]. 南京林业大学学报: 自然科学版, 2013, 37(1): 101-104 |
| [22] | Yang XQ, Sun D, Yang WB, et al. Studies on the prodution of β-mannanase by Bacillus licheniformis NK-27[J]. Acta Scientiarum Naturalium Universitatis Nankaiensis, 2002, 35(2): 117-120 (in Chinese) 杨先芹, 孙丹, 杨文博, 等. 地衣芽孢杆菌NK-27菌株β-甘露 糖苷酶的产酶条件及粗酶性质[J]. 南开大学学报: 自然科学 版, 2002, 35(2): 117-120 |
| [23] | El-Sharounya EE, El-Toukhy NMK, El-Sersy NA, et al. Optimization and purification of mannanase produced by an alkaliphilic-thermotolerant Bacillus cereus N1 isolated from Bani Salama Lake in Wadi El-Natron[J]. Biotechnology & Biotechnological Equipment, 2015, 29(2): 315-323 |
| [24] | Blibech M, Ghorbel RE, Chaari F, et al. Improved mannanase production from Penicillium occitanis by fed-batch fermentation using acacia seeds[J]. ISRN Microbiology, 2011, 2011: 938347. DOI: 10.5402/2011/938347 |
| [25] | Yan Y, Yang SQ, Fan GS, et al. Optimization of xylanase production from Malbranchea cinnamomea by solid state fermentation[J]. Microbiology China, 2013, 40(8): 1339-1346 (in Chinese) 严烨, 杨绍青, 范光森, 等. 樟绒枝霉(Malbranchea cinnamomea)固体发酵产木聚糖酶的发酵条件优化[J]. 微生 物学通报, 2013, 40(8): 1339-1346 |
 2015, Vol. 42
2015, Vol. 42