扩展功能
文章信息
- 张艳, 杜海, 吴群, 徐岩
- ZHANG Yan, DU Hai, WU Qun, XU Yan
- 酱香型白酒发酵中两株主要乳酸菌对酿造微生物群体的影响
- Impacts of two main lactic acid bacteria on microbial communities during Chinese Maotai-flavor liquor fermentation
- 微生物学通报, 2015, 42(11): 2087-2097
- Microbiology China, 2015, 42(11): 2087-2097
- 10.13344/j.microbiol.china.150120
-
文章历史
- 收稿日期: 2015-02-03
- 接受日期: 2015-04-14
- 优先数字出版日期(www.cnki.net): 2015-04-24
乳酸菌广泛存在于多种混合菌发酵食品中,例如乳制品、面制品、泡菜等。这些传统发酵食品在多种微生物类群相互作用下形成独特的风味特征[1, 2, 3]。针对传统发酵体系中的乳酸菌,国内外学者进行了广泛深入的研究。研究发现乳酸菌在发酵食品中的作用是双重的[4, 5, 6, 7]:一方面,乳酸菌代谢物与其他微生物发酵产生的酸、醇、酮等物质相互作用,使产品具有独特的风味;另一方面,乳酸菌的代谢产物过量积累也会影响发酵食品的品质,使发酵过程异常,最终影响产品的品质与口感。
在中国白酒中,乳酸是代表白酒特征的一种有机酸类,对酒精有掩盖作用,在酒中起到调和酒味的缓冲功能;乳酸乙酯在酒体的呈味和呈香中发挥重要作用,其在中国白酒中较高的含量是区别于国外蒸馏酒的显著特征[8]。乳酸菌的主要代谢产物乳酸是形成乳酸乙酯及其他香味成分的重要基础物质。酱香型白酒具有二次投料、多轮次循环发酵的工艺特点,上一轮次的发酵产物,尤其乳酸等不易挥发物质的不断积累,必然影响到下一轮次的微生物结构以及产酒品质[9]。随着基于分子生物学的未培养技术的应用,越来越多研究者在中国白酒发酵中的细菌结构组成中发现了乳酸菌的准确种属,并确定其在发酵过程中的变化趋势。如吴莉莉等[10]发现清香型和酱香型白酒发酵中优势乳酸菌的种类和含量均有不同,并通过可培养和未培养的方法确定了酱香型白酒中的主要乳酸菌是Lactobacillus homohiochii和Lactobacillus buchneri。邵明凯[11]在酱香型白酒发酵中的细菌结构中发现,随着发酵轮次的增加乳酸菌的含量不断增加,发酵到最后一轮次时乳酸菌成为绝对优势细菌。因此,深入研究乳酸菌在酱香型白酒发酵中的地位与作用,对全面了解影响酱香白酒品质的因素,并以此指导生产提高优质酱香出酒率有重要意义。
目前,对中国白酒酿造微生物的研究集中在主要酵母和芽孢杆菌等酿造功能方面。例如,酿酒酵母作为最主要的酿造微生物群体,对乙醇的产生及其他风味物质的产生有重要作用[11, 12];霉菌在白酒发酵过程中能够分泌多种酶降解淀粉、蛋白质等大分子物质,为酵母和细菌的生长提供营养基质[13, 14]。传统观点认为,乳酸菌在中国白酒发酵过程中主要产生负面影响,对乳酸菌的关注主要体现在生产上控制乳酸菌的数量以控制乳酸乙酯的含量,从而保证白酒的品质[15]。针对乳酸菌在中国白酒发酵中的地位及作用并未有相关深入研究报道。
针对以上研究现状及科学问题,本研究以从酱香型白酒发酵酒醅中分离筛选到的两株主要乳酸菌——L. homohiochii XJ-L1和L. buchneri XJ-L2[10]为主要研究对象,明确其抑菌效果。并从环境耐受性以及风味贡献等多角度探究与酵母的组合发酵,分析阐述乳酸菌对酱香型白酒酿造微生物群体的作用,探究乳酸菌在酱香型白酒生产过程中的作用及其对最终酱香品质的影响。为后期全面深入研究乳酸菌在酱香型白酒发酵中的功能奠定基础,并以此指导实际生产,提高优质酱香白酒出酒率。
1 材料与方法 1.1 材料 1.1.1 菌种:乳酸菌:Lactobacillus homohiochii XJ-L1、Lactobacillus buchneri XJ-L2;酵母:Saccharomyces cerevisiae XJ-Y1、Zygosaccharomyces bailii XJ-Y2、Pichia galeiformis XJ-Y3、Schizosaccharomyces pombe XJ-Y4、Geotrichum candidum XJ-Y5、Pichia membranifaciens XJ-Y6、Issatchenkia orientalis XJ-Y7;芽孢杆菌:Bacillus amyloliquefaciens XJ-B1、Bacillus subtilis XJ-B2、Bacillus licheniformis XJ-B3;霉菌:Aspergillus oryzae XJ-M1、Aspergillus niger XJ-M2、Aspergillus flavus XJ-M3、Aspergillus albicans XJ-M4、Rhizopus oryzae XJ-M5;所有菌种均分离自同一贵州地区典型酱香型白酒的发酵环境和生产过程中。 1.1.2 药品及培养基:MRS培养基、酵母浸膏、蛋白胨购自Oxoid公司;PDA培养基、氯化钠、无水葡萄糖、乳酸、乙醇、琼脂粉购自国药集团化学试剂(北京)有限公司;用于HPLC和GC-MS的乳酸、乙醇、薄荷醇、磷酸二氢钠、甲醇、硫酸均为色谱级,购自Sigma公司。高粱汁培养基:高粱:水=1:4 (质量体积比),加淀粉酶蒸煮液化,加糖化酶糖化,过滤离心,调节糖度至10 °Bx。
1.1.3 主要仪器和设备:厌氧培养箱(BUG BOX)购自英国Ruskinn公司;5 mm单孔琼扩打孔器购自北京普博斯生物科技有限公司;Corning Transwell 12孔细胞培养板购自Corning公司;酶标仪购自Thermo公司;pH计购自Mettler Toledo公司;超声波清洗仪购自天津Autoscience公司;高效液相色谱仪Agilent 1200和气相色谱质谱联用仪GC 6890N-MSD 5975均购自美国Agilent公司。 1.2 实验方法 1.2.1 抑菌实验:(1) 打孔法检测乳酸菌对细菌和酵母的抑制特性:参照Schillinger等[16]的方法。无菌发酵液制备:L. homohiochii XJ-L1和L. buchneri XJ-L2种子液分别按1%接种量接种于4 mL无菌MRS培养液中,37 °C厌氧箱中静置培养4 d,12 000 r/min离心10 min取上清,0.22 μm水系针头式滤器过滤,收集滤液即为乳酸菌无菌发酵液。待检测细菌和酵母的种子液用无菌生理盐水(0.9% NaCl)稀释适当倍数,分别涂布于LB和YPD平板,固定1 h后打孔,将无菌发酵液加入孔中,正置培养(细菌12 h,酵母48 h),十字交叉法测量抑菌圈半径。(2) 双层平板法检测乳酸菌对霉菌的抑制特性:参照Magnusson等[17]的方法。底层平板为MRS平板,将L. homohiochii XJ-L1和L. buchneri XJ-L2在平板的两侧分别密集划线,37 °C厌氧箱中培养4 d。上层平板:配制0.7%的素琼脂半固体试管,1×105 Pa灭菌30 min,冷却至50 °C,将混合均匀的孢子液加入试管中并振荡混匀后倾倒在底层平板上,轻轻晃动以使孢子液均匀分布,静置30 min至上层平板凝固,30 °C正置培养3 d,观察霉菌生长情况。
1.2.2 混菌发酵实验:选取2株优势乳酸菌L. homohiochii XJ-L1和L. buchneri XJ-L2,与3株主要酵母S. cerevisiae XJ-Y1、Z. bailii XJ-Y2和P. galeiformis XJ-Y3,分别组合发酵。具体操作步骤如下:采用Stadie等[18]所使用的Coning Transwell培养系统。将灭菌后的高粱汁培养液分装到孔内,静置平衡2 h,将乳酸菌与酵母种子液按1:1的比例分别接种于Transwell嵌套内和孔内,每个组合设置3组平行,以纯培养组为对照,30 °C静置培养3 d,间隔24 h取样,测定乳酸菌和酵母OD600,比较其生长情况。 1.2.3 乳酸菌的乙醇耐受性和酵母的乳酸耐受性实验:(1) 乳酸菌的乙醇耐受性:调节MRS培养液乙醇浓度分别为0、2%、4%、6%、8%、10%、12%,接种L. homohiochii XJ-L1和L. buchneri XJ-L2 (1%),37 °C厌氧箱静置培养48 h,测定OD600比较其生长情况。(2) 酵母的乳酸耐受性:乳酸调节YPD液体培养基pH为2.0、3.0、4.0、5.0、6.0,接种S. cerevisiae XJ-Y1、Z. bailii XJ-Y2和P. galeiformis XJ-Y3 (1%),30 °C、200 r/min摇床培养24 h,测定OD600比较其生长情况。
1.2.4 乳酸和乙醇的检测:使用HPLC的方法检测纯培养和混合发酵体系中乳酸和乙醇的含量,实验方法参照文献[11, 19]。乳酸标准曲线为:y=0.002 853x+0.005 389,R²=0.999,x为峰面积,y为乳酸浓度(g/L)。乙醇标准曲线为:y=0.000 003x+0.002,R2=0.998,x为峰面积,y为乙醇浓度(g/L)。 1.2.5 挥发性风味物质的检测:使用SPME GC-MS检测纯培养和混合发酵体系中挥发性代谢物的含量,方法参照文献[20];薄荷醇(100.00 mg/L)作为内标。 2 结果与分析 2.1 优势乳酸菌对主要酿造微生物的影响情况分析了从酱香型白酒发酵酒醅中分离筛选到的2株优势乳酸菌L. homohiochii XJ-L1和L. buchneri XJ-L2,对从同一发酵环境中分离筛选到的酿造微生物[11, 21] (包括7株酵母、3株芽孢杆菌和5株霉菌)生长的抑制情况,以探究乳酸菌在酿造微生物区系中的作用。
由表 1、2可知,L. homohiochii XJ-L1对所选7株酵母、3株细菌和5株霉菌的生长均无抑制作用;而L. buchneri XJ-L2对7株酵母中的2株(Sc. pombe XJ-Y4、G. candidum XJ-Y5)以及3株芽孢杆菌和5株霉菌的生长均有抑制作用(图 1)。根据吴莉莉等[10]的研究,L. homohiochii存在于整个发酵过程中,而L. buchneri仅存在于发酵中后期,且在上层发酵酒醅中的含量高于中下层。二者与其他微生物的生态关系与其在微生物发酵过程中的动态变化具有相关性。
| 类别 Classes | 实验菌种 Experimental strains | L. buchneri XJ-L2 | L. homohiochii XJ-L1 | 空白对照 Blank control |
| 酵母 Yeast | S. cerevisiae XJ-Y1 | − | − | − |
| Z. bailii XJ-Y2 | − | − | − | |
| P. galeiformis XJ-Y3 | − | − | − | |
| Sc. pombe XJ-Y4 | ++ | − | − | |
| G. candidum XJ-Y5 | + | − | − | |
| P. membranifaciens XJ-Y6 | − | − | − | |
| I. orientalis XJ-Y7 | − | − | − | |
| 细菌 Bacteria | B. amyloliquefaciens XJ-B1 | + | − | − |
| B. subtilis XJ-B2 | + | − | − | |
| B. licheniformis XJ-B3 | + | − | − |
Note: −: No inhibition zone; +: The radius of inhibition zone was from 0.2 to 0.3 cm; ++: The radius of inhibition zone was greater than 0.3 cm.
| 类别 Classes | 实验菌种 Experimental strains | L. buchneri XJ-L2 | L. homohiochii XJ-L1 |
| 霉菌 Mold | A. oryzae XJ-M1 | + | − |
| A. niger XJ-M2 | + | − | |
| A. flavus XJ-M3 | + | − | |
| A. albicans XJ-M4 | + | − | |
| R. oryzae XJ-M5 | + | − |
Note: −: no inhibition; +: inhibition.

|
|
图 1
buchneri XJ-L2对芽孢杆菌、酵母和霉菌的抑制情况
Figure 1
Inhibition of L. buchneri XJ-L2 to Bacillus, yeasts and mold
注:A:B. licheniformis XJ-B3;B:B. subtilis XJ-B2;C:B. amyloliquefaciens XJ-B1;D:Sc. pombe XJ-Y4;E:G. candidum XJ-Y5; F:R. oryzae XJ-M5;G:A. albicans XJ-M4;H:A. niger XJ-M2;I:A. flavus XJ-M3;J:A. oryzae XJ-M1. A−E中无抑菌圈的孔中所加为无菌MRS培养液作为空白对照,另外3个孔中均为L. buchneri XJ-L2的无菌发酵液. F−J的底层平板右侧为L. homohiochii XJ-L1,左侧为L. buchneri XJ-L2.
Note: A: B. licheniformis XJ-B3; B: B. subtilis XJ-B2; C: B. amyloliquefaciens XJ-B1; D: Sc. pombe XJ-Y4; E: G. candidum XJ-Y5; F: R. oryzae XJ-M5; G: A. albicans XJ-M4; H: A. niger XJ-M2; I: A. flavus XJ-M3; J: A. oryzae XJ-M1. A−E: MRS was in one of four holes and L. buchneri XJ-L2 was in three others. Left of F−J was L. buchneri XJ-L2 and right of F−J was L. homohiochii XJ-L1. |
L. buchneri XJ-L2的抑制作用可防止微生物过量生长,有助于维持酿造微生物区系平衡。文献已报道多种发酵环境中,L. buchneri能够抑制多种革兰氏阳性菌、革兰氏阴性菌和真菌的生长,维持发酵体系的稳定[22, 23]。此外,研究发现L. homohiochii XJ-L1和L. buchneri XJ-L2对主要酿酒酵母S. cerevisiae XJ-Y1均无抑制作用。据报道,以S. cerevisiae为主的酱香型白酒发酵酵母菌群产酯丰富,对优质酱香白酒风味的形成有重要的作用[11]。同时乳酸菌与酵母共培养时,乳酸菌能够通过酸化培养环境促进酵母的生长[24]。因此,进一步研究L. homohiochii XJ-L1和L. buchneri XJ-L2对酱香型白酒发酵中主要酵母的影响,对进一步了解乳酸菌对酿造微生物群体的影响具有重要意义。
2.2 优势乳酸菌与主要功能酵母的相互作用2株乳酸菌与3株酵母纯培养和混合培养方式及对应编号如表 3所示。纯培养和共培养条件下酵母和乳酸菌的生长情况如图 2和图 3所示。
| 对应编号 Number | L. homohiochii XJ-L1 | L. buchneri XJ-L2 | |
| X | N | ||
| P. galeiformis XJ-Y3 | Y1 | X1 | N1 |
| Z. bailii XJ-Y2 | Y2 | X2 | N2 |
| S. cerevisiae XJ-Y1 | Y9 | X9 | N9 |

|
|
图 2
纯培养和共培养体系中酵母菌的生长变化情况
Figure 2
The growth change of yeasts in pure culture and co-culture systems
注:Y1:P. galeiformis XJ-Y3的纯培养;X1:P. galeiformis XJ-Y3和L. homohiochii XJ-L1的共培养;N1:P. galeiformis XJ-Y3和L. buchneri XJ-L2的共培养;Y2:Z. bailii XJ-Y2的纯培养;X2:Z. bailii XJ-Y2和L. homohiochii XJ-L1的共培养;N2:Z. bailii XJ-Y2和L. buchneri XJ-L2的共培养;Y9:S. cerevisiae XJ-Y1的纯培养;X9:S. cerevisiae XJ-Y1和L. homohiochii XJ-L1的共培养;N9:S. cerevisiae XJ-Y1和L. buchneri XJ-L2的共培养.
Note: Y1: pure culture of P. galeiformis XJ-Y3; X1: co-culture of P. galeiformis XJ-Y3 and L. homohiochii XJ-L1; N1: co-culture of P. galeiformis XJ-Y3 and L. buchneri XJ-L2; Y2: pure culture of Z. bailii XJ-Y2; X2: co-culture of Z. bailii XJ-Y2 and L. homohiochii XJ-L1; N2: co-culture of Z. bailii XJ-Y2 and L. buchneri XJ-L2; Y9: pure culture of S. cerevisiae XJ-Y1; X9: Co-culture of S. cerevisiae XJ-Y1 and L. homohiochii XJ-L1; N9: co-culture of S. cerevisiae XJ-Y1 and L. buchneri XJ-L2. |

|
|
图 3
纯培养和共培养体系中乳酸菌的生长变化情况
Figure 3
The growth change of lactic acid bacteria in pure culture and co-culture systems
注:X:L. homohiochii XJ-L1的纯培养;X1:P. galeiformis XJ-Y3和L. homohiochii XJ-L1的共培养;X2:Z. bailii XJ-Y2和L. homohiochii XJ-L1的共培养;X9:S. cerevisiae XJ-Y1和L. homohiochii XJ-L1的共培养;N:L. buchneri XJ-L2的纯培养;N1:P. galeiformis XJ-Y3和L. buchneri XJ-L2的共培养;N2:Z. bailii XJ-Y2和L. buchneri XJ-L2的共培养;N9:S. cerevisiae XJ-Y1和L. buchneri XJ-L2的共培养.
Note: X: Pure culture of L. homohiochii XJ-L1; X1: Co-culture of P. galeiformis XJ-Y3 and L. homohiochii XJ-L1; X2: Co-culture of Z. bailii XJ-Y2 and L. homohiochii XJ-L1; X9: Co-culture of S. cerevisiae XJ-Y1 and L. homohiochii XJ-L1; N: Pure culture of L. buchneri XJ-L2; N1: Co-culture of P. galeiformis XJ-Y3 and L. buchneri XJ-L2; N2: Co-culture of Z. bailii XJ-Y2 and L. buchneri XJ-L2; N9: Co-culture of S. cerevisiae XJ-Y1 and L. buchneri XJ-L2. |
与纯培养时相比,2株乳酸菌对3株酵母的生长均有促进作用,且L. buchneri XJ-L2的促进作用较L. homohiochi XJ-L1明显(图 2)。P. galeiformis XJ-Y3促进了2株乳酸菌的生长,而S. cerevisiae XJ-Y1则抑制了2株乳酸菌的生长,Z. bailii XJ-Y2对2株乳酸菌的生长无明显作用(图 3)。这种相互作用关系的差异,与酵母的产乙醇能力及乳酸菌的乙 醇耐受能力相关。因此研究乳酸菌的乙醇耐受性和酵母菌的乳酸耐受性,可以从环境耐受性方面进一步了解乳酸菌和酵母菌混合培养时影响彼此生长的原因。
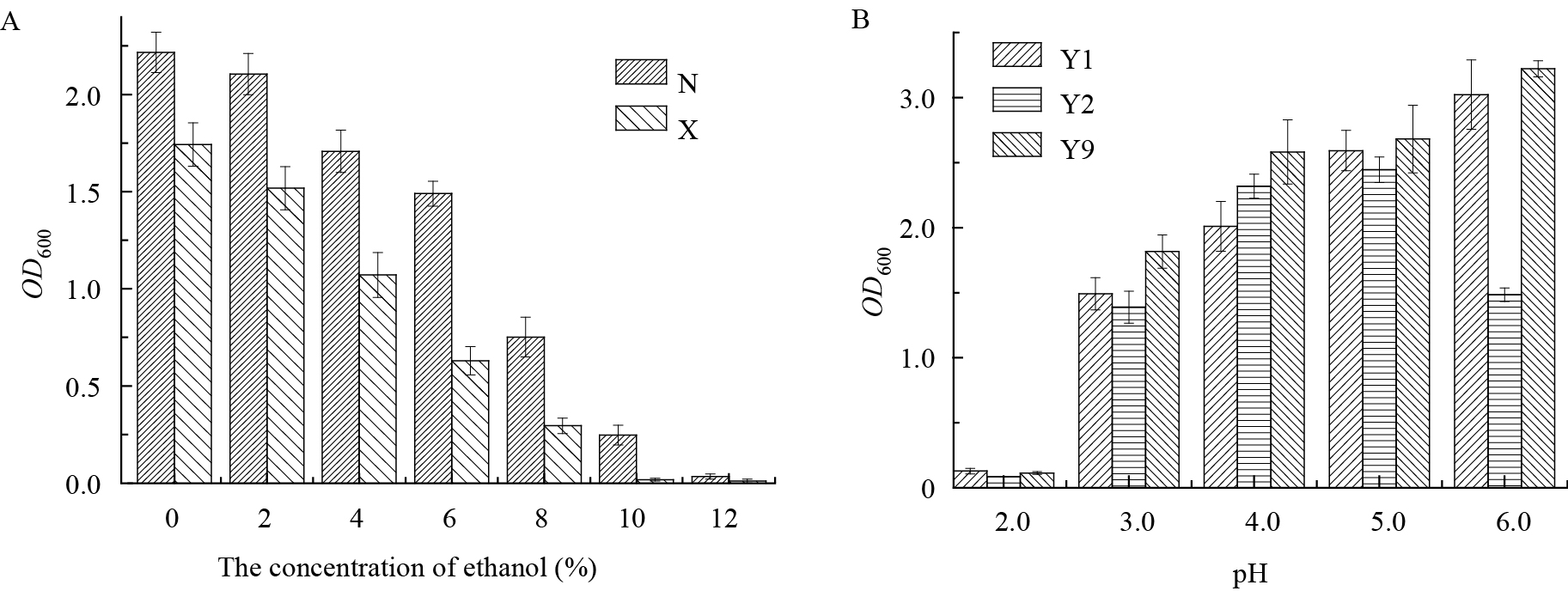
2.3 酵母的乳酸耐受性和乳酸菌的乙醇耐受性研究由图 4A可知,随乙醇浓度递增,2株乳酸菌生长受抑制程度上升,L. homohiochii XJ-L1在乙醇浓度为8%时几乎不生长,L. buchneri XJ-L2在乙醇浓度为10%时几乎不生长,说明2株乳酸菌对乙醇具有较强的耐受性。因此,酵母代谢物中低浓度的乙醇不能抑制乳酸菌的生长,而且代谢物中丰富的氨基酸等营养物质能够促进乳酸菌的生长。由图 4B可知,pH为3.0时3株酵母仍能大量繁殖,而Z. bailii XJ-Y2在pH为5.0时的生长较pH为6.0时好,另外2株酵母在pH为5.0时其生长也未受到明显抑制,说明此3株酵母均具有较强的乳酸耐受性。因此,乳酸菌代谢产物中以乳酸为主的有机酸未能抑制酵母生长,其形成的弱酸性环境促进了酵母的生长。研究表明酵母对乳酸菌的主要产物乳酸以及乳酸菌对酵母的产物乙醇均具有较好的耐受性。
|
|
图 4
乳酸菌的乙醇耐受性和酵母的乳酸耐受性情况
Figure 4
Ethanol tolerance of two lactic acid bacteria strains andlactic acid tolerance of three yeasts
注:A:2株乳酸菌的乙醇耐受性;B:3株酵母的乳酸耐受性;N:L. buchneri XJ-L2的纯培养;X:L. homohiochii XJ-L1的纯培养;Y1:P. galeiformis XJ-Y3的纯培养;Y2:Z. bailii XJ-Y2的纯培养;Y9:S. cerevisiae XJ-Y1的纯培养.
Note: A: Ethanol tolerance of two lactic acid bacteria; B: Lactic acid tolerance of three yeasts. N: pure culture of L. buchneri XJ-L2; X: pure culture of L. homohiochii XJ-L1; Y1: pure culture of P. galeiformis XJ-Y3; Y2: pure culture of Z. bailii XJ-Y2; Y9: pure culture of S. cerevisiae XJ-Y1. |
为验证酵母的耐乙醇能力和乳酸菌的耐乳酸能力,同时探究第3天发酵结束时混合培养体系中乳酸菌和酵母对各自主要代谢物产量的影响,分析发酵结束后发酵体系中乙醇和乳酸的含量。
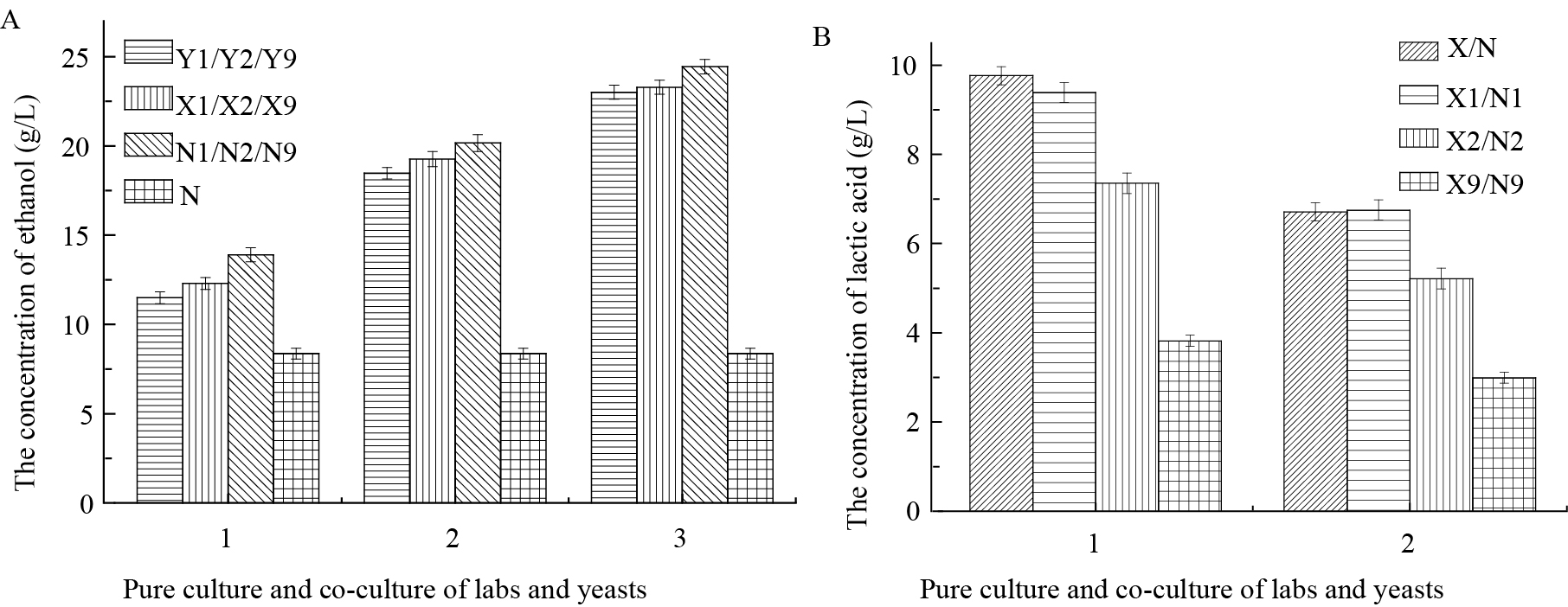
混合培养体系与纯培养体系中乙醇的产量基本一致,且仅在乳酸菌与S. cerevisiae XJ-Y1的混合培养体系中乙醇浓度超过20 g/L (图 5A)。与纯培养时相比,2株乳酸菌与P. galeiformis XJ-Y3混合培养体系中乳酸产量未有明显变化,而与Z. bailii XJ-Y2共培养时乳酸产量降低了20%左右,与S. cerevisiae XJ-Y1共培养时降低了50%左右,混合培养体系中乳酸最高浓度不足10 g/L (图 5B)。实验结果进一步证实乳酸菌对乙醇和酵母对乳酸均具有较强的耐受性,同时说明乳酸菌的生长对酵母代谢乙醇的能力几乎无影响,而部分酵母菌的生长可抑制乳酸菌代谢乳酸的能力。但是乳酸菌对酵母代谢白酒风味物质的影响尚不清楚,因此,进一步对混合发酵体系中与白酒风味相关的其他代谢物进行研究。
|
|
图 5
纯培养和共培养体系中乙醇和乳酸含量变化情况
Figure 5
The concentration of ethanol and lactic acid in pure culture and co-culture systems
注:A:纯培养和共培养体系中乙醇含量;1:P. galeiformis XJ-Y3的纯培养和共培养体系;2:Z. bailii XJ-Y2的纯培养和共培养体系;3:S. cerevisiae XJ-Y1的纯培养和共培养体系. B:纯培养和共培养体系中乳酸含量;1:L. homohiochii XJ-L1的纯培养和共培养体系;2:L. buchneri XJ-L2的纯培养和共培养体系. Y1:P. galeiformis XJ-Y3的纯培养;Y2:Z. bailii XJ-Y2的纯培养;Y9:S. cerevisiae XJ-Y1的纯培养;X1:P. galeiformis XJ-Y3和L. homohiochii XJ-L1的共培养;X2:Z. bailii XJ-Y2和L. homohiochii XJ-L1的共培养;X9:S. cerevisiae XJ-Y1和L. homohiochii XJ-L1的共培养;N1:P. galeiformis XJ-Y3和L. buchneri XJ-L2的共培养;N2:Z. bailii XJ-Y2和L. buchneri XJ-L2的共培养;N9:S. cerevisiae XJ-Y1和L. buchneri XJ-L2的共培养;N:L. buchneri XJ-L2的纯培养;X:L. homohiochii XJ-L1的纯培养.
Note: A: The concentration of ethanol in pure culture and co-culture systems; 1: Pure culture and co-culture systems of P. galeiformis XJ-Y3; 2: Pure culture and co-culture systems of Z. bailii XJ-Y2; 3: Pure culture and co-culture systems of S. cerevisiae XJ-Y1. B: The concentration of lactic acid in pure culture and co-culture systems; 1: Pure culture and co-culture systems of L. homohiochii XJ-L1; 2: Pure culture and co-culture systems of L. buchneri XJ-L2. Y1: Pure culture of P. galeiformis XJ-Y3; Y2: Pure culture of Z. bailii XJ-Y2; Y9: Pure culture of S. cerevisiae XJ-Y1; X1: Co-culture of P. galeiformis XJ-Y3 and L. homohiochii XJ-L1; X2: Co-culture of Z. bailii XJ-Y2 and L. homohiochii XJ-L1; X9: Co-culture of S. cerevisiae XJ-Y1 and L. homohiochii XJ-L1; N1: Co-culture of P. galeiformis XJ-Y3 and L. buchneri XJ-L2; N2: Co-culture of Z. bailii XJ-Y2 and L. buchneri XJ-L2; N9: Co-culture of S. cerevisiae XJ-Y1 and L. buchneri XJ-L2; N: Pure culture of L. buchneri XJ-L2; X: Pure culture of L. homohiochii XJ-L1. |
2.5 共培养体系中乳酸菌对重要挥发性风味物质的影响
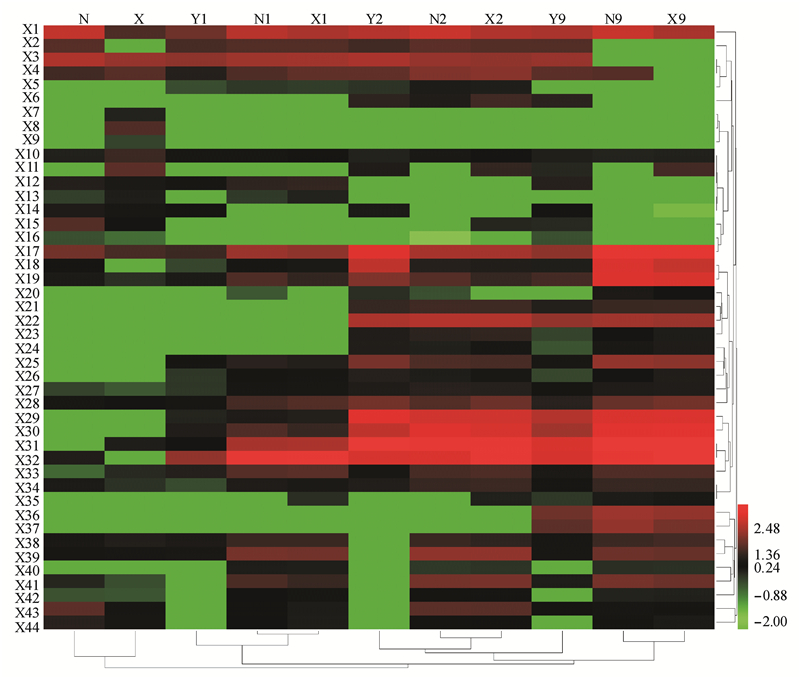
分析第3天发酵结束时2株乳酸菌与3株酵母纯培养和混合培养体系中挥发性风味组分,选择色谱峰面积大于10 000的共44种风味物质进行分析,其中含有酯类8种,醇类17种,酸类8种,酮类6种,醛类2种和酚类3种。聚类分析后绘制风味物质热图(图 6)。
|
| 图 6 2株乳酸菌与3株酵母纯培养和共培养体系中挥发性风味物质含量热图 Figure 6 Heat map of volatile flavor substances in the pure culture and co-culture systems of two lactic acid bacteria strains and three yeast strains Note: N: Pure culture of L. buchneri XJ-L2; X: Pure culture of L. homohiochii XJ-L1; Y1: Pure culture of P. galeiformis XJ-Y3; N1: Co-culture of P. galeiformis XJ-Y3 and L. buchneri XJ-L2; X1: Co-culture of P. galeiformis XJ-Y3 and L. homohiochii XJ-L1; Y2: Pure culture of Z. bailii XJ-Y2; N2: Co-culture of Z. bailii XJ-Y2 and L. buchneri XJ-L2; X2: Co-culture of Z. bailii XJ-Y2 and L. homohiochii XJ-L1; Y9: Pure culture of S. cerevisiae XJ-Y1; N9: Co-culture of S. cerevisiae XJ-Y1 and L. buchneri XJ-L2; X9: Co-culture of S. cerevisiae XJ-Y1 and L. homohiochii XJ-L1; X1: Acetic acid; X2: Butanoic acid; X3: Hexanoic acid; X4: 3,5-Dimethyl-benzaldehyde ; X5: 2-Octanol; X6: Benzaldehyde; X7: 4-Methyl-pentanoic acid; X8: 2,3-Butanedione; X9: 2,3-Dihydro-benzofuran; X10: 2-Methoxy-phenol; X11: 3-Hydroxy-2-butanone; X12: Heptanoic acid; X13: 4-Ethyl-2-methoxy-phenol; X14: 2-Furanmethanol; X15: 2-Heptanone; X16: 2-Nonanone; X17: Octanoic acid; X18: Ethyl octanoate; X19: n-Decanoic acid; X20: Ethyl nonanoate; X21: 2-Methyl-1-propanol; X22: Ethyl acetate; X23: Ethyl heptanoate; X24: 3-(Methylthio)-1-propanol; X25: 3-Methyl-1-butanol; X26: Ethyl benzeneacetate; X27: 1-Hexanol; X28: Dihydro-5-pentyl-2(3H)-furanone; X29: 2-Methyl-1-butanol; X30: 2-Phenylethyl acetate; X31: 3-Methyl-1-butanol; X32: Phenylethyl alcohol; X33: Phenol; X34: 1-Octanol; X35: 1-Heptanol; X36: Ethyl decanoate; X37: Ethyl 9-decenoate; X38: Nonanoic acid; X39: 2-Ethyl-1-hexanol; X40: 3-Ethyl-3-heptanol; X41: 1-Nonanol; X42: Benzyl alcohol; X43: 2-Nonanol; X44: 2-Undecanol. |
由图 6可知,2株乳酸菌代谢物中酸类、醇类、酮类、酚类物质的种类丰富。L. homohiochii XJ-L1代谢酮类和酚类的种类较多,其中双乙酰、3-羟基-2-丁酮、2-甲氧基苯酚的含量均大于30 µg/L。而双乙酰、3-羟基-2-丁酮、2-甲氧基苯酚在白酒呈香中发挥一定的作用[25]。L. buchneri XJ-L2的代谢物中酸类和醇类的种类较多,其中乙酸、丁酸、己酸、辛酸、2-壬醇和2-十一醇的含量均大于50 µg/L。而这些饱和脂肪酸和饱和脂肪醇作为重要的风味化合物都曾在白酒中检测到[25]。
3株酵母的纯培养和混合培养体系中的挥发性风味物质明显聚为两类,且S. cerevisiae XJ-Y1的纯培养和混合培养代谢物含量差异最为明显。与纯培养时相比,3株酵母的混合培养体系中,P. galeiformis XJ-Y3的代谢物中乙酸、己酸、庚酸等有机酸含量提高,Z. bailii XJ-Y2的代谢物中苯乙醇、2-乙基-正己醇、3-乙基-3-庚醇等醇类含量增加最显著,而S. cerevisiae XJ-Y1的代谢物中酯类含量显著增加,如乙酸乙酯、乙酸-2-苯乙酯、9-癸酸乙酯等。综上可知,与纯培养时相比,酵母与乳酸菌的混合培养体系中部分酸类、醇类和酯类的含量增多。而此3类物质是白酒中的主要呈味物质,其含量变化对白酒品质有着重要的影响[26]。
因此,酱香型白酒发酵中乳酸菌除通过自身代谢物直接对白酒风味产生影响外,还可以通过影响酵母的生长和代谢,促进白酒呈味物质的产生,间接提升白酒风味进而影响优质酱香型白酒的酿造。
3 结论通过对酱香型白酒发酵中2株主要乳酸菌对酿造微生物群体影响的研究,初步阐述了乳酸菌如何通过影响酿造微生物群体的生长间接影响酱香型白酒品质。L. homohiochii XJ-L1和L. buchneri XJ-L2通过对酱香型白酒发酵中酿造微生物群体尤其是酵母的抑制或促进作用,维持了酿造微生物区系平衡,从而影响了白酒风味物质的形成,保证了酱香型白酒的品质。
酱香型白酒具有二次投料、多轮次发酵、一年一个大轮回的工艺特点[9]。随着轮次的增加,发酵酒醅中的营养物质不断减少,有机酸等难挥发性代谢产物不断积累,发酵环境越来越不利于环境耐受性较差、营养要求较高的菌种生长。发酵到最后两个轮次,乳酸菌大量繁殖,严重抑制了其他微生物的生长,使酿造微生物区系失去平衡,导致最后两轮次所产酒品质变差,不再具有典型的酱香型特征。因此,适当比例的乳酸菌对维持酿造微生物区系平衡、生产典型酱香品质白酒有一定贡献。后续将对乳酸菌在何种条件下会快速大量繁殖导致酿造体系的失衡,以及如何提前预防乳酸菌造成负面影响进行深入研究,这将对提高酱香型酒的产品质量有重要的指导意义。
| [1] | Liu SN, Han Y, Zhou ZJ. Lactic acid bacteria in traditional fermented Chinese foods[J]. Food Research International, 2011, 44(3): 643-651 |
| [2] | Minervini F, Di Cagno R, Lattanzi A, et al. Lactic acid bacterium and yeast microbiotas of 19 sourdoughs used for traditional/typical Italian breads: interactions between ingredients and microbial species diversity[J]. Applied and Environmental Microbiology, 2012, 78(4): 1251-1264 |
| [3] | Wullschleger S, Lacroix C, Bonfoh B, et al. Analysis of lactic acid bacteria communities and their seasonal variations in aspontaneously fermented dairy product (Malian fènè) by applying a cultivation/genotype-based binary model[J]. International Dairy Journal, 2013, 29(1): 28-35 |
| [4] | Bujňáková D, Kme? V. Functional properties of Lactobacillus strains isolated from dairy products[J]. Folia Microbiologica, 2012, 57(4): 263-267 |
| [5] | Zotta T, Ricciardi A, Parente E. Enzymatic activities of lactic acid bacteria isolated from Cornetto di Matera sourdoughs[J]. International Journal of Food Microbiology, 2007, 115(2): 165-172 |
| [6] | Dalié DKD, Deschamps AM, Richard-Forget F. Lactic acid bacteria–potential for control of mould growth and mycotoxins: a review[J]. Food Control, 2010, 21(4): 370-380 |
| [7] | Gobbetti M, de Angelis M, Corsetti A, et al. Biochemistry and physiology of sourdough lactic acid bacteria[J]. Trends in Food Science & Technology, 2005, 16(1/3): 57-69 |
| [8] | Li WQ. Relationship between Luzhou flavor liquor, lactic acid bacteria, lactic acid and ethyl lactate[J]. Liquor Making, 2010, 37(3): 90-93 (in Chinese) 李维青. 浓香型白酒与乳酸菌、乳酸、乳酸乙酯[J]. 酿酒, 2010, 37(3): 90-93 |
| [9] | Shen YF. Liquor Production Technology Encyclopedia[M]. Beijing: Chinese Light Industry Press, 1998: 357-359 (in Chinese) 沈怡方. 白酒生产技术全书[M]. 北京: 中国轻工业出版社, 1998: 357-359 |
| [10] | Wu LL, Wang HY, Xu Y, et al. Differences of lactic acid bacteria community between soy sauce aroma style and light aroma style liquor fermentation[J]. Microbiology China, 2013, 40(12): 2182-2188 (in Chinese) 吴莉莉, 王海燕, 徐岩, 等. 酱香型与清香型白酒发酵过程 中乳酸菌菌群的差异性分析[J]. 微生物学通报, 2013, 40(12): 2182-2188 |
| [11] | Shao MK. Bacterium and yeast community structures and their implications for flavor components during the fermentation process of Chinese Maotai-flavor liquor[D]. Wuxi: Master’s Thesis of Jiangnan University, 2014 (in Chinese) 邵明凯. 酱香型白酒发酵中细菌和酵母群落结构及其对风味 组分影响的研究[D]. 无锡: 江南大学硕士学位论文, 2014 |
| [12] | Wu Q, Xu Y, Chen LQ. Diversity of yeast species during fermentative process contributing to Chinese Maotai-flavor liquor making[J]. Letters in Applied Microbiology, 2012, 55(4): 301-307 |
| [13] | Xu Y, Ji KL. Moutai (Maotai): production and sensory properties[A]//Piggott J. Alcoholic Beverages: Sensory Evaluation and Consumer Research[M]. Cambridge: Woodhead Publishing, 2012: 315-330 |
| [14] | Zheng XW, Tabrizi MR, Nout MJR, et al. Daqu-a traditional Chinese liquor fermentation starter[J]. Journal of the Institute of Brewing, 2011, 117(1): 82-90 |
| [15] | Hang WX, Qiao ZW, Shigematsu T, et al. Analysis of the bacterial community in Zaopei during production of Chinese Luzhou-flavor Liquor[J]. Journal of the Institute of Brewing, 2005, 111(2): 215-222 |
| [16] | Schillinger U, Lücke FK. Antibacterial activity of Lactobacillus sake isolated from meat[J]. Applied and Environmental Microbiology, 1989, 55(8): 1901-1906 |
| [17] | Magnusson J, Ström K, Roos S, et al. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria[J]. FEMS Microbiology Letters, 2003, 219(1): 129-135 |
| [18] | Stadie J, Gulitz A, Ehrmann MA, et al. Metabolic activity and symbiotic interactions of lactic acid bacteria and yeasts isolated from water kefir[J]. Food Microbiology, 2013, 35(2): 92-98 |
| [19] | Duan YT. Establishment and related research about nine kinds of organic RP-HPLC detection systems in grapes and wine[D]. Beijing: Master’s Thesis of China Agricultural University, 2007 (in Chinese) 段云涛. 葡萄和葡萄酒中9种有机酸RP-HPLC 检测体系的建 立及其相关研究[D]. 北京: 中国农业大学硕士学位论文, 2007 |
| [20] | Kong Y, Wu Q, Zhang Y, et al. In situ analysis of metabolic characteristics reveals the key yeast in the spontaneous and solid-state fermentation process of Chinese light-style liquor[J]. Applied and Environmental Microbiology, 2014, 80(12): 3667-3676 |
| [21] | Chen B, Wu Q, Xu Y. Filamentous fungal diversity and community structure associated with the solid state fermentation of Chinese Maotai-flavor liquor[J]. International Journal of Food Microbiology, 2014, 179: 80-84 |
| [22] | Gong HS, Meng XC, Liu HJ. Purification and biological characteristics of bacteriocin-like substance produced by Lactobacillus buchneri KLDS1. 0364[J]. Microbiology China, 2008, 35(2): 193-199 (in Chinese) 贡汉生, 孟祥晨, 刘红娟. 一株布氏乳杆菌所产类细菌素的 初步纯化与部分特性[J]. 微生物学通报, 2008, 35(2): 193-199 |
| [23] | Yildirim Z, Yildirim M. Characterization of buchnericin LB produced by Lactobacillus buchneri LB[J]. Turkish Journal of Biology, 2001, 25(1): 73-82 |
| [24] | Smid EJ, Lacroix C. Microbe–microbe interactions in mixed culture food fermentations[J]. Current Opinion in Biotechnology, 2013, 24(2): 148-154 |
| [25] | Fan WL, Xu Y. Flavor Chemistry of Alcoholic Beverage[M]. Beijing: Chinese Light Industry Press, 2014: 99-102 (in Chinese) 范文来, 徐岩. 酒类风味化学[M]. 北京: 中国轻工业出版社, 2014: 99-102 |
| [26] | Fan WL, Shen HY, Xu Y. Quantification of volatile compounds in Chinese soy sauce aroma type liquor by stir bar sorptive extraction and gas chromatography–mass spectrometry[J]. Journal of the Science of Food and Agriculture, 2011, 91(7): 1187-1198 |
 2015, Vol. 42
2015, Vol. 42




